Abstract
The purpose of this study was to determine the measurement error associated with antagonist muscle activity in isometric knee strength testing at 60° of knee flexion in both sexes. Muscle specific EMG–contraction intensity relationships were obtained from 22 young people by having them match moment targets ranging from 10% to 100% peak moment. The moments attributed to each of the quadriceps and hamstrings muscles were partitioned using a practical mathematical model. Subject specific EMG–moment relationships were defined for each muscle using second-order polynomial equations. These equations were subsequently used to predict the countermoment associated with antagonist muscle activity. Error during strength testing was calculated by expressing net antagonist moments as a percentage of net agonist moments. The net antagonist moments associated with quadriceps and hamstrings muscle activity were 11.0% and 8.7% of the peak moment values recorded when the same muscle groups were acting as agonists. The error associated with antagonist activity was significantly higher in knee flexion (20.1%) than in knee extension (4.5%). Females displayed significantly higher error in knee flexor testing (P < 0.001). Limb symmetry indices did not change significantly when the countermoments generated by the antagonist muscles were accounted for (P > 0.05). The results of this study indicate that the error associated with antagonist activity in knee extensor testing is relatively small, whereas the error in knee flexor testing is larger. This is due to the quadriceps being much stronger than the hamstrings muscles while displaying similar levels of antagonist activity.
Keywords: Coactivation, Electromyography, Quadriceps, Hamstrings, Moment, Force
Introduction
Evidence suggests that knee strength is important to knee joint stability and health (Hurley 1999; Keays et al. 2003; Lewek et al. 2002; Williams et al. 2005b). The influence of muscle strength on knee health argues for strength testing being part of the clinical management of people with knee pathology. Accordingly, knee strength tests are often used by clinicians to guide the rehabilitation process (Myer et al. 2006). The most common method of assessing knee strength clinically is to assess the strength ratio of the involved side with respect to the uninvolved side. This is a practical selection based on the fact that preinjury strength data are rarely present.
The net moment generated at a joint is the sum of the moments produced by the agonist and antagonist muscles (Kellis 1998). Previous studies indicate that active young people exhibit considerable antagonist activity during maximal voluntary agonist contraction (Aagaard et al. 2000; Kellis 1998; Krishnan and Williams 2009b). This finding is consistent irrespective of the testing mode (isometric, isokinetic, or isotonic) and testing direction (extension or flexion) (Aagaard et al. 2000; Kellis 2003; Kellis and Baltzopoulos 1997; Krishnan and Williams 2009b). Antagonist muscle activity theoretically produces force/moment that opposes the moment of interest. It is unlikely that the countermoments associated with this antagonist activity mathematically cancel out across sides as evidence indicates that there is significant variability in antagonist activity between legs during isometric knee strength testing (Krishnan and Williams 2009b). Inaccuracy associated with measurement error in strength tests can impact research results and clinical decision making as strength test results are often used to evaluate patient status and as guidelines for patient progression including return to play.
The magnitude and variability of antagonist muscle activity are likely to be higher after knee joint injury and surgery. This idea is supported by the finding that people with ACL pathology display higher agonist–antagonist coactivation in their injured legs than they do in their uninvolved limbs (Hurd and Snyder-Mackler 2007; Williams et al. 2003). Such inter-limb differences in coactivation could contribute to the strength discrepancies commonly observed between the legs of knee injury patients. Since most clinicians rely on the contralateral leg for strength comparisons, knowledge of the effects of antagonist muscle activity on strength test accuracy is meaningful. Direct quantification of agonist and antagonist muscle forces during knee joint action is not possible without invasive procedures. Hence, estimation of antagonist moment requires a mathematical model that incorporates several musculoskeletal parameters. Numerous approaches have been documented to predict individual muscle forces during joint action (Kellis 1998). Most mathematical muscle models are complex, time intensive, and unable to be practically applied in clinical settings. For this reason, some scientists have recommended simpler practical EMG-based models, which have demonstrated sufficient reliability (Doorenbosch and Harlaar 2003; Kellis and Katis 2008; Kellis et al. 2005).
Electromyography provides a reasonable estimate on the magnitude of antagonist muscle activity during strength testing. The magnitude of antagonist activity, however, may not provide a reliable estimate of the moment associated with the antagonist muscle activity because even in a simple isometric set-up, the relative contribution of each muscle to the externally measured moment (i.e., the EMG–moment relationship) differs based on the anatomical, physiological, and neural activation parameters of the muscle (Dowling 1997; Kellis 1998). Therefore, in this study we used a practical EMG–moment model that incorporated age and sex specific anatomical and physiological parameters for the quadriceps and hamstrings muscles to answer the following research questions: (1) does antagonist activity during isometric knee strength testing lead to clinically meaningful measurement error? and, (2) are there sex differences in the magnitudes of antagonist muscle moment and associated measurement error during isometric knee strength testing at 60° of knee flexion? Knee position was selected based on the fact that the quadriceps muscle group is generally more severely affected than the hamstrings muscle group and because 60° of knee flexion is in the optimal working range for the quadriceps muscles. Based on pilot data, we hypothesized that antagonist muscle activity would produce a countermoment that was approximately 10% of the peak moment values observed when the same muscle groups were agonists. Evidence suggests that the magnitudes of antagonist muscle activity are relatively similar between the quadriceps and hamstring muscles at 60° of knee flexion; however, the quadriceps muscles are much stronger than the hamstrings (Krishnan and Williams 2009b). Therefore, we hypothesized that antagonist muscle activity would have a significantly greater impact on knee flexor strength test results than on extensor strength test results (i.e., error in flexion would be significantly greater than error in extension). We also hypothesized that females would exhibit significantly higher error during knee flexor strength testing based on the observation that females demonstrate greater antagonistic quadriceps activity during knee flexion (Krishnan et al. 2008; Krishnan and Williams 2009b).
Materials and methods
Subjects
Twenty-two young people (11 males, 11 females) between 20 and 31 years of age (mean age: 24.2 ± 2.5 years) volunteered to participate in this study. All subjects were regular participants in land-based fitness activities or sports (Tegner Activity Score: median 6.0, range 4.0–8.0) (Tegner and Lysholm 1985). The sample size was based on an a priori power analysis using pilot data for predicted antagonist quadriceps moment (expected sex differences in mean = 5.5%, expected pooled standard deviation = 0.87%) and predicted error in flexor strength testing (expected sex differences in mean = 11.66%, expected pooled standard deviation = 3.1%). This analysis indicated that 11 subjects in each group would yield >95% power at α = 0.05 with either variable. Prior to enrollment, subjects completed a brief questionnaire regarding their activity profile and lower extremity health status. A basic lower extremity physical examination was performed bilaterally to confirm the subjects had no signs of pathology that would exclude them from participation. Exclusion criteria included a history of significant lower extremity muscle injury, major knee ligament injury, knee surgery, abnormal KT-2000™ evaluation (≥3 mm side-to-side difference in laxity), ankle sprain or fracture within the prior 6 months, lower extremity nerve injury, knee joint effusion, abnormal gait pattern, or evidence of any health condition that would adversely impact the validity of the study. No potential subjects were excluded from the study based on physical exam or medical history. All subjects provided written informed consent to participation using a form approved by the University of Iowa Human Subjects Research Institutional Review Board.
Testing procedures
Prior to testing, subjects performed a 5-min warm-up on a cycle ergometer followed by self-directed stretching of the quadriceps, hamstrings, and gastrocnemius musculature. The leg that subjects began testing with was randomized a priori to minimize the effects of testing order. Electromyographic (EMG) preamplifiers (model 544, Therapeutics Unlimited, Inc., Iowa City, IA, USA; 35× differential gain, 87 dB common-mode rejection at 60 Hz, input impedance >25 MΩ, noise <2 μV RMS) were applied over the muscle bellies of the semitendinosus (ST), biceps femoris longus (BFL), vastus lateralis (VL), rectus femoris (RF), and vastus medialis (VM) muscles after cleaning the skin with alcohol swabs. Electrode placement sites were selected according to recommendations described by Perotto and Delagi (1994). Special consideration was given to standardization of the electrode placement sites and to minimize the susceptibility of EMG electrodes to cross talk. A common ground was placed on the skin over the patella. Subjects were then positioned on and secured to a HUMAC NORM Testing and Rehabilitation System (Computer Sports Medicine, Inc., Stoughton, MA, USA) according to the manufacturer’s testing guidelines (Fig. 1a). Subjects sat on a small platform placed on the test system’s chair in order to minimize the potential for noise resulting from pressure on the EMG preamplifiers fixed over the hamstrings muscles. Knee and hip position were standardized at 60° and 90° of flexion, respectively. The moment arm pad was fixed to the shank approximately 7 cm proximal to the medial malleolus. Maximal voluntary isometric knee strength was tested by having subjects perform three maximum voluntary isometric contractions (MVICs) of their knee extensor and knee flexor muscles in an alternating fashion. Three minutes of rest was provided between each like trial. Both legs were tested independently.
Fig. 1.
A subject positioned on the test system for an isometric knee strength test (a) and EMG–contraction intensity testing (b)
After testing knee strength, we determined muscle specific EMG–contraction intensity relationships by having subjects match knee extension and flexion moment targets at 10 normalized magnitudes (10–100% MVIC in 10% increments). This part of the experiment began by applying a 3-in. fiberglass cylinder cast to the distal shank with its mid-point approximately 7.5 cm proximal to the medial malleolus (Fig. 1b). This cast and a custom-designed clamping device attached to the test system dynamometer were used to rigidly fix the distal shank to a 6-axis force/moment transducer (model Delta SI-660-60 F/T DAQ, ATI Industrial Automation, Apex, NC, USA). The force transducer haptically controlled a cursor during target-matching at the various contraction intensities. Subjects viewed the moment targets and real-time feedback of their target-matching efforts on a liquid crystal display (LCD) monitor placed in front of them. To match the targets, subjects applied loads against the force/moment transducer to move a cursor positioned in the center of the monitor over targets that appeared at the periphery of the monitor. Subjects were provided several practice trials to familiarize them with the methods and minimize the effects of task novelty and learning. Subjects then matched targets at the 10 moment magnitudes. These moment magnitudes appeared one at a time, with targets alternating between the 12 o’clock position (extension) and the 6 o’clock position (flexion) until the entire range of moments had been tested. The moment magnitudes were presented in random order to minimize systematic error associated with the presentation of target loads. Two trials in each direction (extension, flexion) were performed at each moment magnitude and the average of the two trials was used in analysis. Repeat MVIC tests were performed after subjects had completed testing over the entire range of moment magnitudes to assess whether subjects experienced muscle fatigue during the experiment. After testing was completed on the first leg, the subjects’ opposite leg was tested in the same fashion.
Signal sampling, conditioning, and processing
Software written in Visual Basic 6.0 (Microsoft Corp., Redmond, WA, USA) and LabVIEW 7.0 (National Instruments Corp., Austin, TX, USA) was used to collect and analyze the data. Signals were sampled at 1000 Hz using two 16-bit A-to-D conversion boards (NI PCI-6032E and NI PCI-6034E, National Instruments Corp., Austin, TX, USA) that were synchronized using the boards’ Real Time System Integration (RTSI) bus. The EMG signals were conditioned using an eighth order analog Butterworth low-pass filter (SCXI-1143, National Instruments Corp., Austin, TX, USA) with a 500 Hz cut-off frequency. Normalization of the EMG recordings was performed using maximal EMG values recorded during the MVIC trials. The signals from the HUMAC NORM Testing and Rehabilitation System were passed through an eighth order analog Butterworth low-pass filter with a cut-off frequency of 10 Hz and then converted to moment values (N m) using calibrated conversion factors that were validated onsite prior to testing. The EMG signals were baseline removed, full-wave rectified, and averaged during the 200 ms target-matching period to obtain a mean EMG value at each target. The magnitude of antagonist muscle activity present during testing (hamstrings muscle activity in extension trials and quadriceps muscle activity in flexion trials) was determined by representing the values recorded when the muscles were antagonists as a percentage of the respective values recorded when the muscles were agonists. A limb symmetry index was calculated for each subject using the following equation: (peak moment produced by the weaker leg/peak moment produced by the stronger leg) × 100.
Antagonist moment and error associated with strength testing
The contribution of each muscle to the total agonist moment at each target load was partitioned based on the relative size, activation, and moment arm of the muscles using the following equation:
| (1) |
where Mk is the moment contribution of the kth individual muscle, Vk is the relative (with respect to the total quadriceps or hamstrings muscle group) muscle volume of the kth muscle, ak is the relative muscle activation of the kth muscle from EMG measurement, rk is the moment arm of the kth muscle, and Mago is the net moment of the agonist muscle group (Dowling 1997; Kellis and Katis 2008). The muscle volume (relative volume in %: VL = 48%, RF = 20%, VM = 32%, ST = 50%, and BFL = 50%) and the moment arm data (Females: ST = 36.1 mm, BFL = 20.2 mm; Males: ST = 41.3 mm, BFL = 24.4 mm) used in the equation were based on age and sex-specific values from the literature (Tate et al. 2006; Wretenberg et al. 1996). We assumed that the five muscles tested were the only muscles contributing to the net moment during knee extension and flexion. The total muscle volume for the quadriceps and hamstrings muscles was determined by summing the respective agonist group muscle volumes (i.e., quadriceps muscle volume was determined by summing VL, RF, and VM muscle volumes, and hamstrings muscle volume was determined by summing ST and BFL muscle volumes). After partitioning the moment contributed by each muscle, subject-specific EMG–moment relationships were defined for each muscle using second-order polynomial equations. The countermoment associated with each muscle was determined by inputting the normalized antagonist activity values recorded during maximal testing into the equations. The net antagonist moments were determined by summing the values for the respective muscles in each muscle group. For example, quadriceps antagonist moment was calculated by summing VL, RF, and VM antagonist moments. The error associated with antagonist moment during strength testing was calculated using the following equation:
| (2) |
where Mant is the net moment of the antagonist muscle group and Mago is the net moment of the agonist muscle group. For example, error during knee extensor strength testing was calculated by expressing the antagonist moment generated by the hamstrings muscles as a percentage of quadriceps agonist moment.
In order to determine if the countermoments associated with antagonist muscle activity significantly affect limb symmetry indices, the antagonist moment values generated by the hamstrings and quadriceps muscles were used to mathematically “correct” the voluntary peak knee extensor and the flexor moment values observed during knee strength testing. This was performed using the following equation:
| (3) |
where corrected moment is the peak agonist moment after correcting for the antagonist moment, observed moment is the observed peak moment during isometric knee strength testing, and antagonist moment is the net antagonist moment generated by the antagonist muscles. For example, corrected knee extensor moment was calculated by summing observed knee extensor moment and net antagonistic hamstrings moment during knee extensor strength testing.
Data analysis
All statistical analyses were performed using SPSS for Windows (SPSS Inc., Chicago, IL, USA). Descriptive statistics were calculated for each variable. Descriptive statistics were also used to report the coefficients of determination (R2 values) obtained from fitting the subject-specific EMG–moment curves with second-order polynomial equations. Two-sample t-tests were used to evaluate whether significant differences existed in the demographic profiles of the male and female participants. Repeated measures ANOVA with side as a within-subjects factor and sex of subject as a between-subjects factor was used to evaluate differences by side and sex for each dependent variable (quadriceps antagonist moment, hamstrings antagonist moment, error during extension, and error during flexion). Repeated measures ANOVA was also used to evaluate whether or not error values differed by movement direction (extension or flexion). Paired t-tests were used to determine if significant differences were present between limb symmetry indices calculated from the peak observed agonist moments and the associated corrected moments. A significance level of α = 0.05 was used for all analyses. We recently published a detailed report on sex-based differences in agonist and antagonist muscle activity across the range of contraction intensity based on this sample of subjects (Krishnan and Williams 2009a); therefore, these data are not presented in the current report.
Results
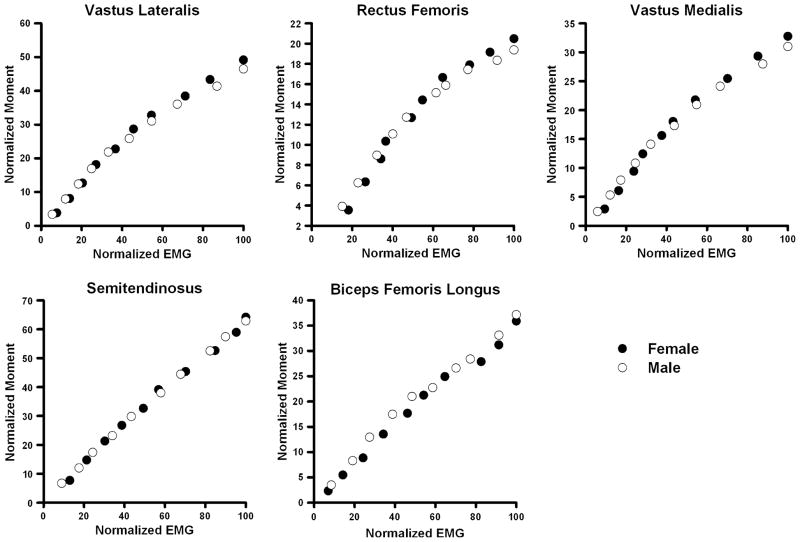
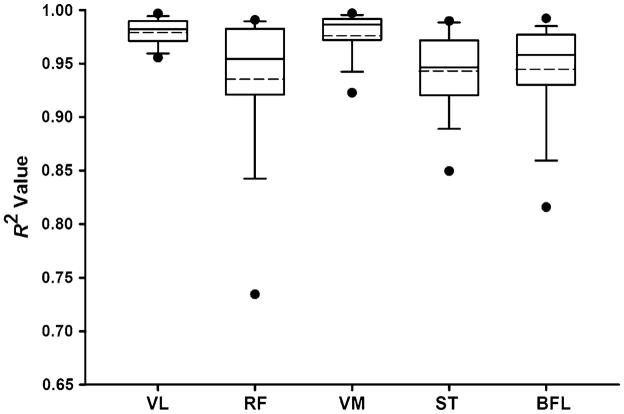
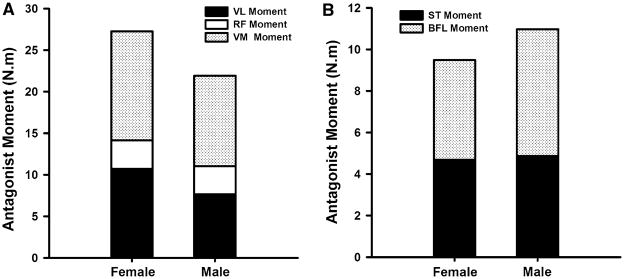
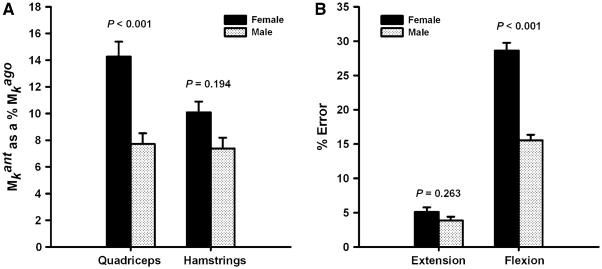
There were no significant differences in the age (males: 24.0 ± 2.2 years, females: 24.4 ± 2.8 years; P = 0.740), activity level (males: 6.5 ± 0.7, females: 6.0 ± 1.0; P = 0.229), or BMI (males: 24.06 ± 2.2 kg/m2, females: 23.2 ± 2.2 kg/m2; P = 0.157) of the male and female participants. The magnitudes of antagonist muscle activity observed during maximal knee extension and flexion trials averaged between 5% and 12% of maximum values (Table 1). The EMG–moment curves derived by mathematical modeling were similar between the sexes (Fig. 2). The coefficients of determination (R2 values) for the subject-specific EMG–moment curves were strong; hence, the second-order polynomial equations provided a good fit for the EMG–moment curves (Fig. 3). The spread of the R2 values, however, was greater for the biarticular muscles (RF, ST, & BFL) indicating that the patterns of the EMG–moment curves were less variable in the uniarticular VL and VM muscles (Fig. 3). The net moments associated with antagonistic quadriceps (P = 0.197) and hamstrings (P = 0.539) muscle activity were not significantly different between males and females (Fig. 4). However, when antagonist moment was expressed as a percentage of same muscle groups’ agonist moment, quadriceps antagonist moment was significantly higher in females than in males (P < 0.001; Fig. 5a). This was not the case for antagonist hamstrings moment (P = 0.194; Fig. 5a). The net moments associated with antagonistic quadriceps muscle activity averaged 11.0 ± 5.6% of peak moment values recorded during knee extension MVICs. The net moments associated with antagonistic hamstring muscle activity averaged 8.7 ± 5.3% of peak moment values recorded during knee flexion MVICs. The measurement error associated with antagonist activity was 4.5 ± 2.9% during knee extension and 22.1 ± 12.1% during flexion. This difference in error magnitude was statistically significant (P < 0.001, Fig. 5b). Females had significantly higher error than males in knee flexion trials (P < 0.001, Fig. 5b); however, the error during knee extension trials was not significantly different between the sexes (P = 0.263, Fig. 5b). None of the muscle groups displayed significant differences in the antagonist moments or measurement error by side (P = 0.230 to P = 0.555). There were no significant side by sex interaction effects (P = 0.126 to P = 0.910). The mean knee symmetry index was 90.8% for both the knee extensors and the knee flexors. The limb symmetry indices did not change significantly when antagonist moment values were accounted for (extension: 90.8 ± 6.4% versus 91.3 ± 7.0%, P = 0.273; flexion: 90.8 ± 6.9% versus 90.7 ± 6.8%, P = 0.905). There was no indication that the subjects experienced muscle fatigue during the experimental protocol as most subjects produced slightly higher MVIC values after testing (10% higher moment in the knee extensors, 11% higher moment in the knee extensors).
Table 1.
Mean antagonist muscle activity (% maximum) during isometric knee strength testing
| VL | RF | VM | ST | BFL | |
|---|---|---|---|---|---|
| Female | 8.65 ± 4.13 | 6.10 ± 3.48 | 15.83 ± 7.02 | 6.15 ± 3.60 | 10.62 ± 6.76 |
| Male | 3.71 ± 1.68 | 3.72 ± 2.08 | 7.92 ± 4.45 | 4.40 ± 1.88 | 9.22 ± 6.37 |
| Total | 6.18 ± 3.99 | 4.91 ± 3.08 | 11.88 ± 7.05 | 5.28 ± 2.97 | 9.92 ± 6.53 |
Data are presented as mean ± standard deviation
VL vastus lateralis, RF rectus femoris, VM vastus medialis, ST semitendinosus, BFL long head of the biceps femoris
Fig. 2.
EMG–moment curves for the five knee muscles tested. The values on the x-axis represent EMG activity normalized as a percentage of maximum. The values on the y-axis represent predicted agonist moment (i.e., the partitioned moment for each muscle based on Eq. 1) normalized as a percentage of the peak agonist net moment measured during strength testing. Note that the maximum values on the y-axes of the quadriceps muscles (VL, RF, & VM) approximate relative volume data used in Eq. 1. This is because the individual quadriceps muscles have the same relative activation and moment arms during maximal contractions. Males and females had similar EMG–moment curves
Fig. 3.
Box plots representing the coefficients of determination (R2) obtained from the subject-specific EMG–moment curves, which were fitted using second-order polynomial equations. The boundary of the box closest to zero indicates the 25th percentile, the black line within the box marks the median, the dashed line within the box marks the mean, and the boundary of the box farthest from zero indicates the 75th percentile. Whiskers (error bars) above and below the box indicate the 90th and 10th percentiles, respectively. Data points outside the whiskers represent values that were outside the 95th and 5th percentile values. VL vastus lateralis, RF rectus femoris, VM vastus medialis, ST semitendinosus, BFL long head of the biceps femoris
Fig. 4.
Stacked bars representing the mean antagonist moment associated with the quadriceps (a) and hamstrings (b) muscle antagonist activity of males and females during isometric knee strength testing. The net antagonist moment was not significantly different between the sexes. VL vastus lateralis, RF rectus femoris, VM vastus medialis, ST semitendinosus, BFL long head of the biceps femoris
Fig. 5.
Females had significantly higher quadriceps moments when mean antagonist moments were expressed as a percentage of same muscle groups’ agonist moment (a). Error during knee flexor strength testing was significantly higher in females when compared to males, whereas error during knee extensor strength testing was similar (b)
Discussion
This study contributes the following information to the literature: (1) a detailed report of the moments and measurement error associated with antagonistic quadriceps and hamstrings muscle activity during isometric knee strength testing at 60° of knee flexion, (2) evidence indicating that the effect of antagonist activity on knee extensor strength testing is much smaller than the effect on flexor strength testing at 60° of knee flexion, (3) evidence suggesting side-to-side differences in antagonist activity may not have a meaningful impact on side-to-side strength comparisons, and (4) evidence indicating that there are some sex-based differences in the magnitude of antagonist muscle moment and related error during knee strength testing.
The results indicate that the countermoments associated with antagonist muscle activity lead to an underestimation of true agonist muscle strength. The magnitudes of the moments associated with quadriceps and hamstrings antagonist activity were approximately 10% of the peak moment values recorded when the same muscle groups were acting as agonists (11% for the quadriceps, 8.7% for the hamstrings). This finding confirms our primary hypothesis and is in agreement with Kellis et al. (Kellis and Katis 2008) who recently reported a hamstrings countermoment that was approximately 9% of peak knee flexor moment values at 45° of knee flexion. The predicted antagonist moment during knee extension was lower than those reported in the isokinetic designs; however, the predicted antagonist moment during knee flexion was similar to those reported in an isokinetic designs (Aagaard et al. 2000). The observation of greater error in knee flexion is attributable to the presence of similar levels of antagonist activity in the quadriceps and hamstrings muscle while the quadriceps muscles are much stronger than the hamstrings group at 60° of knee flexion. The combination of these factors results in a higher antagonist moment for the quadriceps muscles despite similar levels of antagonist activity. It is important to note, however, that the error values reported may be specific to the 60° of knee flexion position. It is expected that error values would change somewhat in different knee positions due to changes in the length–tension relationships and activation patterns of the muscles involved. The quadriceps muscle group produces peak moment between 60° and 70° of knee flexion. This is not true, however, for the hamstrings muscle group, which produces peak moment between 20° and 30° of knee flexion (Knapik et al. 1983). We elected to test both muscle groups at 60° of knee flexion in an effort to provide clinically relevant data. One of the primary challenges to regular use of strength tests in clinical practice is the time involved. Testing both muscle groups at a single angle is, therefore, advantageous. Evidence suggests that one can test knee extensor and knee flexor strength at 60° of flexion without compromising test validity if side-to-side strength comparisons are of interest. This is possible because knee angle has a relatively small effect on knee flexor moment, but a large effect on knee extensor moment. Testing strength in deeper flexion (60°–90° of flexion) is advisable as this is the optimal range to test the quadriceps muscle group, which is much more profoundly affected by knee trauma and surgery than the hamstrings muscle group. Yet, we acknowledge that testing knee flexor strength closer to the optimal working range for the hamstrings muscles may yield different results and be advisable if ipsilateral comparisons are being used rather than side-to-side strength ratios.
Subjects in this study exhibited side-to-side strength differences of 9.2% by side. This side-to-side strength differences in strength is most likely the product of multiple factors. Scientists have theorized that inter-limb variations in antagonist muscle activity may explain some of the observed strength differences by side (Maganaris et al. 1998). This theory is based on the premise that side-to-side variability in antagonist muscle activity would significantly affect the side-to-side moment ratios. The results of this study indicate that the limb symmetry indices were similar even after accounting for the antagonist muscle moment. This calls into question the meaningfulness of this antagonist activity when assessing strength difference across sides and suggests that the observed strength differences across sides are related to other factors such as muscle morphology and voluntary activation levels (Krishnan and Williams 2009a). It is to be noted, however, that the subjects tested in this study were active young people with no history of serious lower extremity injuries. It is likely that people with knee pathology have greater variability in antagonist muscle activity between their involved and uninvolved legs. Hence, antagonist activity may have a more significant impact on side-to-side strength differences in people with knee pathology.
Female subjects displayed significantly higher error than male subjects during knee flexor strength testing due to the presence of greater quadriceps antagonist activity. The magnitude of antagonistic quadriceps muscle activity in the female subjects was about twice the magnitude observed in the males (Table 1). This finding compliments reports indicating that females exhibit significantly higher antagonistic vastus medialis activity during both maximal and submaximal flexion contractions (Krishnan et al. 2008; Krishnan and Williams 2009b; White et al. 2003). The fact that females have consistently exhibited higher activation than males when their quadriceps muscles act as antagonists suggests that there are differences in the way females and males use their thigh muscles. This observation is particularly interesting when considering the female predisposition to ACL injuries, patellofemoral pain, and knee osteoarthritis. We note, however, that there is currently no evidence indicating that the observed sex-based differences in quadriceps muscle activation patterns are related to knee injury or clinically important beyond providing a greater understanding of human physiology. Future research should focus on elucidating the mechanisms underlying these sex-based differences and define the functional importance of the observation.
The mathematical model used in this study differed from prior practical models (Aagaard et al. 2000; Kellis and Katis 2008) in that we used age- and sex-specific anatomical and morphological muscle parameters as inputs for the predictive model. This is particularly important considering the fact that the test sample was a group of young people who regularly participated in fit-ness and sports activities. Evidence indicates that the muscle morphology values of young athletes are substantially different from the data derived from cadaver studies (Tate et al. 2006). Hence, using morphological data from cadaver studies is likely to lead to error in biomechanical models of active young people’s muscle function. The use of relative values instead of absolute values also strengthened our analysis as variations in the relative size of a muscle with respect to the entire agonist muscle group are considerably lower than the variations in the absolute size of the muscles (Dowling and Cardone 1994). We acknowledge that it would have been even better to use subject-specific values as inputs into the model; this was not possible in the current study.
We assessed whether fatigue was a confounding factor in the study by having subjects perform repeat MVICs at the end of the experiment. This was done because fatigue can alter the activation patterns and thereby affect EMG–moment relationships (Smyth et al. 1990). Moment values recorded after the subjects completed the experimental protocol were in general higher than those recorded just prior to testing, which suggests that fatigue did not adversely affect the results of the study. The small increase in moment measured following completion of the experimental protocol is not surprising because skeletal muscles are known to exhibit post-activation potentiation as a result of phosphorylation of the myosin regulatory light chain, which increases the sensitivity of the actin–myosin complex to calcium ions released from sarcoplasmic reticulum (Hodgson et al. 2005).
This study has some potential limitations that warrant discussion. The predictive mathematical model used in this study was a practical model that included five knee muscles. Other muscles that are known to contribute to knee flexion or extension moments include the gracilis, sartorius, semimembranosus, short head of the biceps femoris, and the vastus intermedius. These muscles were excluded because examining them would have required intramuscular EMG and increased the data collection time. Moreover, the use of a practical mathematical model offers advantages in terms of applicability and has strong support in the literature with evidence of sufficient reliability (Doorenbosch and Harlaar 2003; Kellis and Katis 2008; Kellis et al. 2005). We also assumed that the observed antagonist muscle activity and its associated countermoments were entirely directed at the knee joint. While this may be a valid assumption for the uniarticular vastus medialis and vastus lateralis muscles, the extent to which this is true for the biarticular rectus femoris and hamstrings muscles is less clear. The subjects’ hip and trunk were tightly secured to the test system with straps as per typical testing protocol in order to provide stability and minimize compensatory muscle activity; however, we acknowledge that it is possible that some of the observed antagonist muscle activity was directed at stabilizing the hip rather than acting at the knee.
Although we used a high-quality electromyography equipment, carefully placed EMG electrodes according to the established guidelines, and judiciously examined the signals we obtained during manual muscle tests for cross talk prior to running the experiment, we cannot rule out cross talk from neighboring muscles. If cross talk was present, the magnitudes of antagonist moment and the error associated with antagonist activity could have been overestimated to a small degree. However, any effect of cross talk is expected to be similar between legs (right vs. left), between subjects (males vs. females) and between muscle groups (extensors vs. flexors). Therefore, we expect that cross talk would have negligible effects on the study’s findings.
Finally, the subjects who participated in this study were active young people with no history of serious lower extremity injuries. It is unclear if the results of this study are generalizable to people with knee pathology. The literature suggests that higher antagonistic muscle activity is often observed in the involved legs of people with knee pathology (e.g., anterior cruciate ligament injuries) (Grabiner and Weiker 1993). Therefore, it is reasonable to presume that the error associated with antagonist muscle activity is higher in the involved legs of these individuals. Yet, there is also evidence suggesting that antagonist muscle activity varies proportionately with agonist activity (De Luca and Mambrito 1987) and evidence indicating that people with knee pathology commonly have diminished voluntary quadriceps muscle activation in their involved legs (Chmielewski et al. 2004; Lewek et al. 2004; Williams et al. 2005a). Considering these findings, it seems equally likely that antagonist muscle activity and associated error may be lower in the involved legs of people with intra-articular knee pathology. Regardless, we recommend caution when extrapolating the results of this study to people with knee pathology.
Conclusions
In summary, the results of this study indicate that antagonist muscle activity produces a countermoment that results in underestimation of agonist muscle strength during isometric knee strength testing at 60° of flexion. The error associated with the countermoments was relatively small in knee extensor testing, but fairly large in knee flexor testing. The difference in error between muscle groups is a result of the quadriceps being much stronger than the hamstrings muscles while displaying similar levels of antagonist activity at 60° of knee flexion. Females exhibited greater quadriceps antagonist activity than males in knee flexion tests and therefore had significantly higher error than their male counterparts in these tests. Limb symmetry indices did not change significantly when the countermoments generated by the antagonist muscles were accounted for. Hence, antagonist activity does not appear to have a significant effect on side-to-side strength comparisons. The knowledge gained in this study is meaningful as isometric knee strength tests are commonly used in clinical treatment and research related to knee pathology and human performance. If the absolute force generating capacity of a muscle is of interest, mathematical modeling should be considered as some error is inherent with strength testing. However, in clinical practice and physiology research strength testing is more commonly used to assess changes in strength associated with injury or an intervention (i.e., a treatment approach, experimental protocol, or experimentally induced condition). In these scenarios, strength is generally analyzed using side-to-side comparisons or by evaluating values collected from the same leg at different points in time. The error associated with antagonist muscle activity is unlikely to have a significant impact on such analyses because the observed countermoments are similar across sides and antagonist activity is fairly stable across time in healthy people.
Acknowledgments
The authors would like to acknowledge Jacob Moore and Kellen Huston for their assistance with data collection. This work was supported in part by United States National Institutes of Health Grant K12 HD055931 and funds from the Davee Foundation.
Footnotes
Conflict of interest statement The authors declare that they have no conflict of interest.
Contributor Information
Chandramouli Krishnan, Searle Laboratory, Sensory Motor Performance Program, Rehabilitation Institute of Chicago, Chicago, IL, USA.
Glenn N. Williams, Email: glenn-williams@uiowa.edu, Physical Therapy and Rehabilitation Science, Carver College of Medicine, University of Iowa, Iowa City, IA 52242-1190, USA
References
- Aagaard P, Simonsen EB, Andersen JL, Magnusson SP, Bojsen-Moller F, Dyhre-Poulsen P. Antagonist muscle coactivation during isokinetic knee extension. Scand J Med Sci Sport. 2000;10:58–67. doi: 10.1034/j.1600-0838.2000.010002058.x. [DOI] [PubMed] [Google Scholar]
- Chmielewski TL, Stackhouse S, Axe MJ, Snyder-Mackler L. A prospective analysis of incidence and severity of quadriceps inhibition in a consecutive sample of 100 patients with complete acute anterior cruciate ligament rupture. J Orthop Res. 2004;22:925–930. doi: 10.1016/j.orthres.2004.01.007. [DOI] [PubMed] [Google Scholar]
- De Luca CJ, Mambrito B. Voluntary control of motor units in human antagonist muscles: coactivation and reciprocal activation. J Neurophysiol. 1987;58:525–542. doi: 10.1152/jn.1987.58.3.525. [DOI] [PubMed] [Google Scholar]
- Doorenbosch CA, Harlaar J. A clinically applicable EMG-force model to quantify active stabilization of the knee after a lesion of the anterior cruciate ligament. Clin Biomech. 2003;18:142–149. doi: 10.1016/s0268-0033(02)00183-3. [DOI] [PubMed] [Google Scholar]
- Dowling JJ. The use of electromyography for the noninvasive prediction of muscle forces current issues. Sports Med. 1997;24:82–96. doi: 10.2165/00007256-199724020-00002. [DOI] [PubMed] [Google Scholar]
- Dowling JJ, Cardone N. Relative cross-sectional areas of upper and lower extremity muscles and implications for force prediction. Int J Sports Med. 1994;15:453–459. doi: 10.1055/s-2007-1021087. [DOI] [PubMed] [Google Scholar]
- Grabiner MD, Weiker GG. Anterior cruciate ligament injury and hamstrings coactivation. Clin Biomech. 1993;8:215–219. doi: 10.1016/0268-0033(93)90017-C. [DOI] [PubMed] [Google Scholar]
- Hodgson M, Docherty D, Robbins D. Post-activation potentiation: underlying physiology and implications for motor performance. Sports Med. 2005;35:585–595. doi: 10.2165/00007256-200535070-00004. [DOI] [PubMed] [Google Scholar]
- Hurd WJ, Snyder-Mackler L. Knee instability after acute ACL rupture affects movement patterns during the mid-stance phase of gait. J Orthop Res. 2007;25:1369–1377. doi: 10.1002/jor.20440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley MV. The role of muscle weakness in the pathogenesis of osteoarthritis. Rheum Dis Clin North Am. 1999;25:283–298. doi: 10.1016/s0889-857x(05)70068-5. [DOI] [PubMed] [Google Scholar]
- Keays SL, Bullock-Saxton JE, Newcombe P, Keays AC. The relationship between knee strength and functional stability before and after anterior cruciate ligament reconstruction. J Orthop Res. 2003;21:231–237. doi: 10.1016/S0736-0266(02)00160-2. [DOI] [PubMed] [Google Scholar]
- Kellis E. Quantification of quadriceps and hamstring antagonist activity. Sports Med. 1998;25:37–62. doi: 10.2165/00007256-199825010-00004. [DOI] [PubMed] [Google Scholar]
- Kellis E. Antagonist moment of force during maximal knee extension in pubertal boys: effects of quadriceps fatigue. Eur J Appl Physiol. 2003;89:271–280. doi: 10.1007/s00421-003-0795-5. [DOI] [PubMed] [Google Scholar]
- Kellis E, Baltzopoulos V. The effects of antagonist moment on the resultant knee joint moment during isokinetic testing of the knee extensors. Eur J Appl Physiol Occup Physiol. 1997;76:253–259. doi: 10.1007/s004210050244. [DOI] [PubMed] [Google Scholar]
- Kellis E, Katis A. Hamstring antagonist moment estimation using clinically applicable models: muscle dependency and synergy effects. J Electromyogr Kinesiol. 2008;18:144–153. doi: 10.1016/j.jelekin.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Kellis E, Kouvelioti V, Ioakimidis P. Reliability of a practicable EMG–moment model for antagonist moment prediction. Neurosci Lett. 2005;383:266–271. doi: 10.1016/j.neulet.2005.04.038. [DOI] [PubMed] [Google Scholar]
- Knapik JJ, Wright JE, Mawdsley RH, Braun J. Isometric, isotonic, and isokinetic torque variations in four muscle groups through a range of joint motion. Phys Ther. 1983;63:938–947. doi: 10.1093/ptj/63.6.938. [DOI] [PubMed] [Google Scholar]
- Krishnan C, Williams GN. Sex differences in quadriceps and hamstrings EMG–moment relationships. Med Sci Sports Exerc. 2009a;41:1652–1660. doi: 10.1249/MSS.0b013e31819e8e5d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan C, Williams GN. Variability in antagonist muscle activity and peak torque during isometric knee strength testing. Iowa Orthop J. 2009b;29:149–158. [PMC free article] [PubMed] [Google Scholar]
- Krishnan C, Huston K, Amendola A, Williams GN. Quadriceps and hamstrings muscle control in athletic males and females. J Orthop Res. 2008;26:800–808. doi: 10.1002/jor.20592. [DOI] [PubMed] [Google Scholar]
- Lewek M, Rudolph K, Axe M, Snyder-Mackler L. The effect of insufficient quadriceps strength on gait after anterior cruciate ligament reconstruction. Clin Biomech. 2002;17:56–63. doi: 10.1016/s0268-0033(01)00097-3. [DOI] [PubMed] [Google Scholar]
- Lewek MD, Rudolph KS, Snyder-Mackler L. Quadriceps femoris muscle weakness and activation failure in patients with symptomatic knee osteoarthritis. J Orthop Res. 2004;22:110–115. doi: 10.1016/S0736-0266(03)00154-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maganaris CN, Baltzopoulos V, Sargeant AJ. Differences in human antagonistic ankle dorsiflexor coactivation between legs; can they explain the moment deficit in the weaker plantarflexor leg? Exp Physiol. 1998;83:843–855. doi: 10.1113/expphysiol.1998.sp004164. [DOI] [PubMed] [Google Scholar]
- Myer GD, Paterno MV, Ford KR, Quatman CE, Hewett TE. Rehabilitation after anterior cruciate ligament reconstruction: criteria-based progression through the return-to-sport phase. J Orthop Sports Phys Ther. 2006;36:385–402. doi: 10.2519/jospt.2006.2222. [DOI] [PubMed] [Google Scholar]
- Perotto A, Delagi EF. Anatomical guide for the electromyographer: the limbs and trunk. Charles C Thomas; Springfield, IL: 1994. [Google Scholar]
- Smyth G, Arsenault AB, Nagata S, Gagnon D, Mathieu PA. Slope of the EMG/moment relationship as a measure of muscular fatigue: a validation study. Med Biol Eng Comput. 1990;28:379–383. doi: 10.1007/BF02446158. [DOI] [PubMed] [Google Scholar]
- Tate CM, Williams GN, Barrance PJ, Buchanan TS. Lower extremity muscle morphology in young athletes: an MRI-based analysis. Med Sci Sports Exerc. 2006;38:122–128. doi: 10.1249/01.mss.0000179400.67734.01. [DOI] [PubMed] [Google Scholar]
- Tegner Y, Lysholm J. Rating systems in the evaluation of knee ligament injuries. Clin Orthop Relat Res. 1985;198:43–49. [PubMed] [Google Scholar]
- White KK, Lee SS, Cutuk A, Hargens AR, Pedowitz RA. EMG power spectra of intercollegiate athletes and anterior cruciate ligament injury risk in females. Med Sci Sports Exerc. 2003;35:371–376. doi: 10.1249/01.MSS.0000053703.65057.31. [DOI] [PubMed] [Google Scholar]
- Williams GN, Barrance PJ, Snyder-Mackler L, Axe MJ, Buchanan TS. Specificity of muscle action after anterior cruciate ligament injury. J Orthop Res. 2003;21:1131–1137. doi: 10.1016/S0736-0266(03)00106-2. [DOI] [PubMed] [Google Scholar]
- Williams GN, Buchanan TS, Barrance PJ, Axe MJ, Snyder-Mackler L. Quadriceps weakness, atrophy, and activation failure in predicted noncopers after anterior cruciate ligament injury. Am J Sports Med. 2005a;33:402–407. doi: 10.1177/0363546504268042. [DOI] [PubMed] [Google Scholar]
- Williams GN, Snyder-Mackler L, Barrance PJ, Buchanan TS. Quadriceps femoris muscle morphology and function after ACL injury: a differential response in copers versus non-copers. J Biomech. 2005b;38:685–693. doi: 10.1016/j.jbiomech.2004.04.004. [DOI] [PubMed] [Google Scholar]
- Wretenberg P, Nemeth G, Lamontagne M, Lundin B. Passive knee muscle moment arms measured in vivo with MRI. Clin Biomech. 1996;11:439–446. doi: 10.1016/s0268-0033(96)00030-7. [DOI] [PubMed] [Google Scholar]