Abstract
Inflammatory responses to implanted biomedical devices elicit a foreign body fibrotic reaction that limits device integration and performance in various biomedical applications. We examined chronic inflammatory responses to microgel conformal coatings consisting of thin films of poly(N-isopropylacrylamide) hydrogel microparticles cross-linked with poly(ethylene glycol) diacrylate deposited on poly(ethylene terephthalate) (PET). Unmodified and microgel-coated PET disks were implanted subcutaneously in rats for 4 weeks and explants were analyzed by histology and immunohistochemistry. Microgel coatings reduced chronic inflammation and resulted in a more mature/organized fibrous capsule. Microgel-coated samples exhibited 22% thinner fibrous capsules that contained 40% fewer cells compared to unmodified PET disks. Furthermore, microgel-coated samples contained significantly higher levels of macrophages (40%) than unmodified PET controls. These results demonstrate that microgel coatings reduce chronic inflammation to implanted biomaterials.
Keywords: foreign body response, macrophage, hydrogel, polyethylene terephthalate, fibrous capsule
INTRODUCTION
Biomaterials and implantable devices elicit a host foreign body response that often impairs wound healing and tissue remodeling.1 Implantation of these synthetic materials triggers dynamic, multi-component responses involving protein adsorption, leukocyte recruitment, adhesion and activation, cytokine expression/release, macrophage fusion into multi-nucleated foreign body giant cells (FBGCs), tissue remodeling and fibrous encapsulation of the implant.2 These inflammatory responses significantly interfere with the biological performance of these devices, often resulting in inadequate performance and failures that may require secondary interventions. Examples of chronic inflammatory responses to biomedical devices include thrombogenic responses on vascular grafts,3,4 degradation and stress cracking of pacemaker leads,5,6 tissue fibrosis surrounding mammary prostheses,7 reactive gliosis around neural probes,8 degradation in glucose biosensor function,9 and generation of wear debris around orthopedic joint prostheses.10
Fibrous capsule formation around the implant and the presence of macrophages and FBGCs at the tissue-material interface are the hallmarks of a chronic inflammatory response. The αMβ2 integrin and macrophage mannose receptor have been identified as critical components for FBGC formation.11 Although the molecular mechanisms leading to macrophage fusion have not been fully elucidated, soluble molecules, signal transducers, and numerous receptors are likely involved.2 FBGCs have been implicated in biodegradation of polymeric implants through surface oxidation and enzymatic degradation.12,13 Multi-nucleated giant cells have been observed in chronically inflamed tissues induced by a foreign stimulus, yet the physiological significance and precise role of FBGCs at the tissue-material interface remains poorly understood. The cell-cell interactions of the foreign body response are quite complex, and the overall biological response to implanted materials is likely a composite of macrophages, fibroblasts, lymphocytes, and FBGCs. Further elucidation of the molecular events governing inflammation will aid in the development of implantable materials with more appropriate host responses.
Significant research efforts have focused on modifying material properties to generate implants that appropriately integrate with the host tissue while eliciting minimal undesirable effects. A common approach to reduce inflammatory responses is the use of non-fouling (protein adsorption-resistant) polymeric coatings, which have been developed in various forms including polymer brushes and thin or bulk hydrogels. Although many of these methods have been effective when tested in vitro, these coatings usually exhibit high levels of adherent leukocytes, persistent inflammation, and fibrous encapsulation of the implant.14–16 Long-term tissue fibrosis is particularly limiting for interactive implants such as biosensors, biomedical leads and electrodes, encapsulated cells, and drug delivery systems, because it impedes analyte transport and exchange of nutrients and cellular byproducts with the surrounding medium.9,17–21 By controlling capsule thickness, implant coatings may have the ability to maintain an open exchange of key biomolecules and extend the in vivo lifetime of these constructs.
We recently engineered a hydrogel-based coating composed of pNIPAm-co-AA microgel particles cross-linked with PEG diacrylate tethered onto a poly(ethylene terephthalate) (PET) substrate.22 PET was chosen as the base material because this polymer is used in many biomedical devices including sutures, vascular grafts, sewing cuffs for heart valves, and components for percutaneous access devices. PET elicits acute and chronic inflammatory responses, characterized by leukocyte adhesion and fibrous encapsulation.23,24 We have shown that these microgel coatings modulate events associated with acute inflammation (i.e. protein adsorption and cell adhesion) and significantly reduce leukocyte recruitment and cytokine expression in vivo at early time points.22 In the present study, we evaluated chronic host responses to these microgel coatings. We demonstrate that these conformal microgel coatings reduce chronic inflammation to implanted materials.
MATERIALS AND METHODS
Sample preparation
Thin sheets of poly(ethylene terephthalate) (AIN Plastics/ThyssenKrupp Materials NA, Madison Heights, MI) were cut into disks (8 mm diameter) using a sterile biopsy punch (Miltex Inc., York, PA) and extensively rinsed in 70% ethanol to remove contaminants introduced during the manufacturing process. Microgel particles were synthesized with 10 mol% acrylic acid as a co-monomer to incorporate functional groups for future modification. pNIPAm-co-AA microgel particles (100 mM total monomer concentration) were synthesized with 2 mol% PEG diacrylate (M.W. 575) by a free radical precipitation polymerization method.25 Particle composition and size (hydrodynamic radius 334 ± 30 nm) were confirmed by NMR and dynamic light scattering, respectively. Microgel particles were deposited on both sides of PET disks using a spin coating and photo-crosslinking process as previously described.22,25 AFM and XPS analyses demonstrated uniform conformal microgel coatings, in excellent agreement with previous analyses.22,25 Unmodified PET disks were used as controls.
After surface functionalization, all samples were rinsed in 70% ethanol on a rocker plate for 4 days, changing the solution daily to remove endotoxin contaminants, and were stored in 70% ethanol until use. Samples contained 10-fold lower levels of endotoxin than the United States Food and Drug Administration’s recommended 0.5 EU/mL, as determined by the LAL chromogenic assay (Cambrex, East Rutherford, NJ). Prior to use, samples were rinsed three times in sterile phosphate buffered saline (PBS) and allowed to rehydrate in PBS for at least 1 hour.
Subcutaneous implantation
NIH guidelines for the care and use of laboratory animals (NIH publication #85-23 Rev. 1985) have been observed. Samples (unmodified PET, microgel-coated PET; n = 8 samples/group) were implanted subcutaneously following IACUC-approved procedures to evaluate the chronic phase foreign body response. Male 5–6 wk old Wistar rats (Charles River Laboratories, Wilmington, MA) were anesthetized by isofluorane. A single 1-cm incision was made on the dorsum proximal to the spine, and a subcutaneous pocket laterally spanning the dorsum was created. Sterile samples (two per subject, one on either side of the spine) were implanted, and the incision was closed using sterile wound clips. After four weeks, rats were sacrificed using a CO2 chamber and samples were explanted, rinsed in sterile PBS, and fixed in formalin. Samples were carefully explanted with the surrounding tissue intact to avoid disrupting the cell-material interface. Explants were bisected in order to avoid edge effects and to standardize the sectioning location for analysis, and they were paraffin-embedded for histological processing.
Histological staining of explants
Histological sections (5 μm thick) were stained for various markers. A Verhoeff-van Gieson kit (Accustain® Elastic Stain kit from Sigma-Aldrich, St. Louis, MO) was used to stain collagen (pink), elastin fibers (black), and nuclei (dark blue). Sixteen total fields per sample (eight fields on both the muscle and skin sides of the implant) were acquired using a high magnification 60X Plan Apo Nikon objective (1.40 NA). ImagePro software (Media Cybernetics, Silver Spring, MD) was used to quantify fibrous capsule thickness.
Sections were also stained using immunohistochemical methods to determine the inflammatory cellular profile at the cell-material interface. Following proteolytic antigen retrieval with pronase (1 mg/mL solution for 10 min), sections were incubated in a mouse monoclonal antibody against the CD68 antigen of macrophages (clone ED1, AbD Serotec, Raleigh, NC), a biotinylated secondary antibody, and an avidin-linked alkaline phosphatase-based developing reagent (Vectastain® ABC-AP Kit, Vector Labs, Burlingame, CA), and counterstained with hematoxylin. Control sections (secondary antibody-only controls and tissue-specific controls) confirmed specificity of the primary antibody for this marker. Sixteen total fields per sample (eight fields on both the muscle and skin sides of the implant) were acquired using a high magnification 60X Plan Apo Nikon objective (1.40 NA). Images were blindly scored for total nuclei, CD68+ cells with one nucleus (macrophages), and CD68+ multi-nucleated cells (foreign body giant cells).
Statistical analysis
Data are presented as mean ± standard error. Statistical analysis (t-tests, 95% confidence level considered significant) was performed by ANOVA using Systat 11.0 (Systat Software Inc., San Jose, CA).
RESULTS
Fibrous capsule formation surrounding implants
PET disks were functionalized with p(NIPAM-co-AAc-co-PEGDA) microgel particles via a spin coating and photo-crosslinking method to generate uniform, conformal coatings. XPS and AFM analyses confirmed the chemical composition and the uniformity of microgel coating, in excellent agreement with previous studies.22 Tissue responses to these materials were evaluated using an established subcutaneous rat model to determine the extent of chronic inflammation.1 Unmodified PET and microgel-coated PET disks were implanted for 4 weeks. Explants were processed histologically, and sections were analyzed for fibrous capsule development using a Verhoeff van Gieson kit to stain collagen and elastin fibers; all nuclei were counterstained for reference (Figure 1). The capsule was defined as the dense tissue adjacent to the implant, and image analysis of high magnification images was used to measure capsule thickness as the perpendicular distance starting at the capsule-implant interface and moving outward. Measurement of fibrous capsule thickness following subcutaneous implantation is a standard measure of chronic inflammation to synthetic materials.1
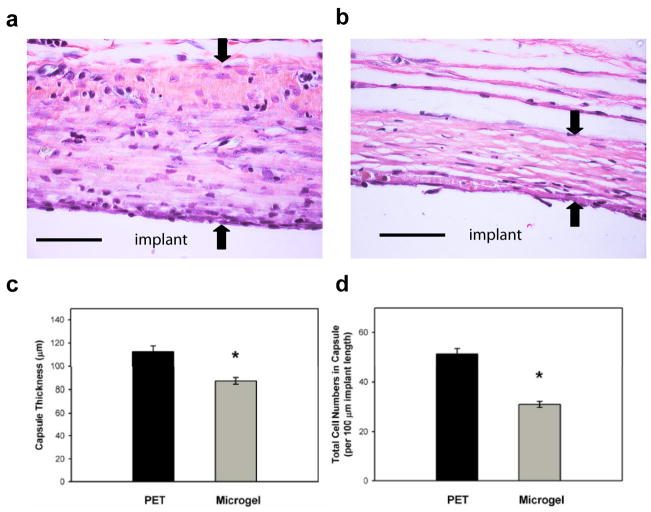
Figure 1.
Microgel coatings reduce chronic inflammation associated with materials implanted subcutaneously in the rat dorsum for 4 wk. Explants were evaluated for fibrous encapsulation by staining collagen (pink), elastin (black), and nuclei (black). Representative images for unmodified PET (a) and microgel-coated PET (b) disks, and the original implant location is designated. Black arrows indicate capsule measurements. Microgel coatings reduced fibrous capsule thickness by 22% compared to unmodified PET controls as quantified in (c), * p < 0.04. The density of capsule-associated cells was also significantly reduced in microgel-coated samples (* p < 5.6×10−3) compared to unmodified PET controls as quantified in (d). Data is represented as the average value ± standard error of the mean using n = 4–7 samples per treatment group. Scale bar is 50 μm.
Unmodified PET controls exhibited a tissue reaction characteristic of chronic inflammation with a thick collagenous capsule containing high numbers of cells (Figure 1a). Microgel-coated samples exhibited thinner and more compact capsules with more organized collagen fibrils (Figure 1b). Image analysis demonstrated significantly thinner capsules (22%) for microgel-coated PET compared to unmodified PET controls (p < 0.04, Figure 1c). The average capsule thickness was 112.3 ± 5.1 and 87.3 ± 2.9 μm for PET controls and microgel-coated samples, respectively.
The density of total cells present in the tissue capsules was scored using counterstained nuclei, and sections were quantified in 100 μm increments along the implant interface (Figure 1d). Microgel-coated samples contained approximately 40% fewer capsule-associated cells than their unmodified PET control counterparts (p < 0.01). The average cell density was 51.2 ± 2.2 and 31.1 ± 1.2 cells per 100 μm length of implant for PET controls and microgel-coated samples, respectively. These results demonstrate that microgel coatings reduce the thickness and cell density of tissue capsules surrounding implanted biomaterials.
Inflammatory cell profile at the implant interface
Explant sections were processed to evaluate the composition of cells at the implant-tissue interface (Figure 2). Immunohistochemistry was used stain for the CD68 antigen, a marker of monocytes and tissue macrophages, and all nuclei were counterstained for reference. Images were scored for total CD68+ cells containing one nucleus (macrophages) and CD68+ cells fused to form multi-nucleated FBGCs.
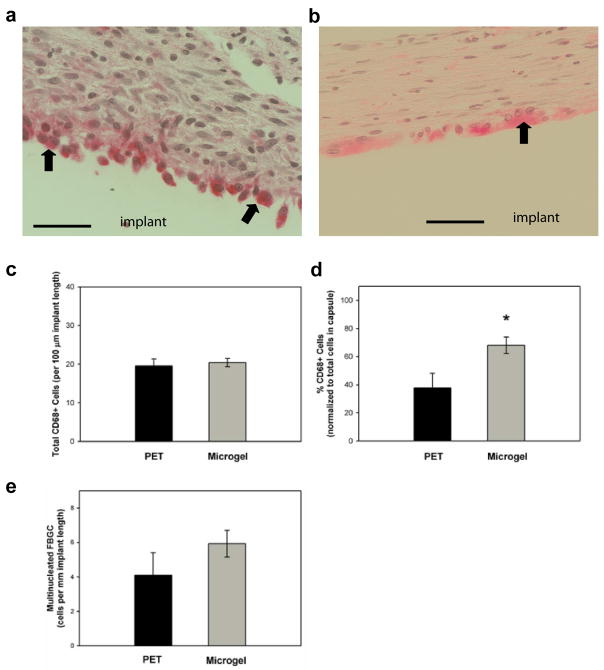
Figure 2.
Inflammatory cell profiles associated with biomaterials implanted subcutaneously in the rat dorsum for 4 wk. Explant sections were stained via immunohistochemical methods for macrophage marker CD68 (pink) and counter-stained with hematoxylin to label nuclei (blue). Representative images for unmodified PET (a) and microgel-coated PET (b) disks, and the original implant location is designated. Total CD68+ cells were quantified in (c), but no statistical differences were found between treatment groups. (d) When normalized to total capsule-associated cells (from Fig. 1d), unmodified PET controls contained proportionately fewer CD68+ cells than microgel-coated PET (* p < 0.02). Multinucleated CD68+ cells (FBGCs) at the cell-implant interface were also quantified (e), but no statistical differences were found between treatment groups. FBGCs are designated by black arrows. Data is represented as the average value ± standard error of the mean using n = 4–7 samples per treatment group. Scale bar is 50 μm.
High magnification images of unmodified PET controls (Figure 2a) and microgel-coated disks (Figure 2b) revealed that CD68 staining was localized to the capsule, primarily along the capsule-implant interface. All implanted samples, regardless of coating, contained similar levels of CD68+ cells as quantified in Figure 2c (no differences between groups). The average number was 19.5 ± 1.8 and 20.4 ± 1.1 CD68+ cells per 100 μm of implant length for PET controls and microgel-coated samples, respectively. CD68+ cell counts were then normalized to total cells in the fibrous capsule (as quantified in Figure 1d) to determine the relative numbers of macrophages in the capsule. Microgel-coated samples contained significantly higher relative levels of macrophages than unmodified PET controls (Figure 2d, p < 0.02). The average values were 37.8 ± 10.4 and 68.1 ± 5.8 % CD68+ cells for PET controls and microgel-coated samples, respectively. We note that this antibody can potentially stain CD68 antigens in both adipose tissue26 and fibroblasts,27 the latter of which are also localized in the fibrous capsule and participate in collagen deposition.
Sections were also scored for multi-nucleated FBGC, designated by black arrows (Figure 2). Few samples contained extensive development of multi-nucleated FBGC. The average values were 4.1 ± 1.3 and 5.9 ± 0.8 FBGCs per mm of implant length for PET controls and microgel-coated samples, respectively. Numbers of FBGC per sample ranged from 1.4–11.1 and 3.0–11.8 cells/mm implant length for PET controls and microgel-coated disks, respectively. No statistical differences were found between groups.
DISCUSSION
We have engineered a hydrogel-based polymeric coating composed of PEG-crosslinked pNIPAm-co-AA microparticles, which are applied to PET substrates using a spin coating and photo-crosslinking method to generate a conformal monolayer.25 This coating strategy offers many advantages over traditional surface modification methods, including precise control over particle synthesis, the ability to generate complex architectures including “mosaic” coatings containing variations in particle composition or spatial arrangement, and deposition onto biomedically-relevant materials. We previously demonstrated these coatings reduce protein adsorption and cell adhesion.25,28 In addition, these microgel coatings reduce leukocyte adhesion and activation, as well as expression of pro-inflammatory cytokines, to biomedical polymer implants in vivo at acute time points.22 The results of the present study demonstrate that the microgel coatings also modulate chronic inflammatory events, such as reductions in fibrous capsule thickness and cell density within the capsule.
Microgel coatings reduced chronic inflammation compared to uncoated PET controls as determined by the organization and thickness of the tissue capsule. The more compact and organized structure of the fibrous capsule associated with microgel-coated samples suggests that these coatings lead to faster resolution of the tissue reaction and more mature and thinner capsules. This reduced chronic inflammation is likely related to the reduced monocyte/macrophage adhesion and pro-inflammatory cytokine expression observed at acute time points.22 Furthermore, tissue associated with microgel-coated samples contained less total cells but proportionately more macrophages than unmodified PET controls. The reduction in total number of cells is consistent with a reduced chronic inflammatory response for microgel-coated samples. The relevance of the relative increase in monocytes/macrophages for the microgel-coated implants compared to the PET controls is not clear at this point. An intriguing possibility is that microgel coatings modulate macrophage profiles towards an “alternatively activated”/anti-inflammatory phenotype29 that results in reduced chronic inflammation and a faster resolution of the tissue response. Further studies are necessary to characterize macrophage phenotypes and cytokines associated with these biomaterials. Moreover, it will be important to conduct more extensive studies in order to determine inflammatory responses at longer, clinically-relevant implantation times as well as the in vivo stability of these coatings.
Previous efforts have focused on hydrogel coatings that exhibit in vitro resistance to protein adsorption and leukocyte adhesion to reduce biomaterial-mediated related inflammation.14,15,30–35 Although these coatings reduce biofouling in vitro, some of these materials still exhibit high levels of adherent leukocytes and continued inflammation in vivo with significant fibrous encapsulation of the implant. For instance, in vitro protein adsorption was significantly suppressed by photochemically immobilized polymer coatings on silicone rubber substrates and by polyethylene oxide-like tetraglyme coatings, yet neither treatment significantly reduced fibrous capsule thickness when implanted subcutaneously.14,34 In contrast, other coatings, such as dihydroxypropyl methacrylate, PEG, and phosphorylcholine-based polymers, have shown reductions in fibrous encapsulation compared to the base substrates.30–33,35 The reductions in fibrous capsule thickness elicited by these coatings are comparable to those observed in the present study. Taken together, these studies do not reveal a clear correlation between in vitro fouling behavior and in vivo leukocyte adhesion and tissue response. However, it is possible that differences in the surface density, composition, and structure of the non-fouling polymer, material stability, and implantation time point, site and species complicate this relationship.
In the present study, microgel coatings reduced fibrous capsule thickness by 22% compared to unmodified control samples. Whether such a reduction in fibrous capsule thickness translates into improved biological performance remains to be determined. Functional testing in specific applications (e.g. glucose sensors, pacing leads, neural electrodes) is required to evaluate the potential of these microgel coatings to ameliorate chronic inflammatory responses to implanted devices. Fibrous capsules on the order of 85 μm thick (as in our current study) may still pose a significant barrier to certain implanted devices or therapeutics by blocking the exchange of nutrients or impeding signal transduction to an external medium. For example, Moussy and colleagues recently demonstrated a correlation between increased collagen deposition surrounding implanted glucose sensors and decreased sensor sensitivity; natural angiogenesis failed to overcome the barrier to glucose diffusion caused by the associated fibrous capsule.36
The present work provides the foundation for developing a microgel-based coating system incorporating various signaling agents and bioactive therapeutics within a low-fouling background. These biotherapeutic delivery systems offer several advantages over approaches relying on passive non-fouling behavior, including highly controlled presentation/release of immunomodulatory agents, control over reaction kinetics, and versatility through hybrid designs. Biomaterial-associated inflammation/fibrosis and/or implant integration could be further improved by using such complex coatings with mechanisms to deliver immunomodulatory agents, such as IL-1Ra, angiostatin, or dexamethasone, which have improved biological responses to implanted materials.15,37–40
CONCLUSION
Using a model of chronic biomaterial-mediated inflammation, we demonstrate that surface coatings comprised of pNIPAm-co-PEG hydrogel microparticles reduce chronic inflammation. Microgel coatings elicited thinner and more compact capsules with more organized collagen fibrils and fewer total cells within the capsule compared to uncoated PET. Our current results demonstrate that microgel particles can be applied as implant coatings to modulate inflammation and achieve more desirable chronic host responses in vivo, with the potential to extend implant performance and lifetime.
Acknowledgments
This work was supported by the Georgia Tech/Emory Center (GTEC) for the Engineering of Living Tissues and the Atlanta Clinical and Translational Science Institute (ACTSI) and the National Institutes of Health (R01-EB004496). A.W.B. and R.E.W. were supported by National Science Foundation Graduate Fellowships.
Footnotes
No benefit of any kind will be received either directly or indirectly by the author(s).
References
- 1.Anderson JM. Biological responses to materials. Annu Rev Mater Res. 2001;31:81–110. [Google Scholar]
- 2.Anderson JM, Rodriguez A, Chang DT. Foreign body reaction to biomaterials. Semin Immunol. 2008;20(2):86–100. doi: 10.1016/j.smim.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gorbet MB, Sefton MV. Biomaterial-associated thrombosis: roles of coagulation factors, complement, platelets and leukocytes. Biomaterials. 2004;25(26):5681–703. doi: 10.1016/j.biomaterials.2004.01.023. [DOI] [PubMed] [Google Scholar]
- 4.Kottke-Marchant K, Anderson JM, Umemura Y, Marchant RE. Effect of albumin coating on the in vitro blood compatibility of Dacron arterial prostheses. Biomaterials. 1989;10(3):147–55. doi: 10.1016/0142-9612(89)90017-3. [DOI] [PubMed] [Google Scholar]
- 5.Zhao Q, Topham N, Anderson JM, Hiltner A, Lodoen G, Payet CR. Foreign-body giant cells and polyurethane biostability: in vivo correlation of cell adhesion and surface cracking. J Biomed Mater Res. 1991;25(2):177–83. doi: 10.1002/jbm.820250205. [DOI] [PubMed] [Google Scholar]
- 6.Sutherland K, Mahoney JR, 2nd, Coury AJ, Eaton JW. Degradation of biomaterials by phagocyte-derived oxidants. J Clin Invest. 1993;92(5):2360–7. doi: 10.1172/JCI116841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Destouet JM, Monsees BS, Oser RF, Nemecek JR, Young VL, Pilgram TK. Screening mammography in 350 women with breast implants: prevalence and findings of implant complications. AJR Am J Roentgenol. 1992;159(5):973–8. doi: 10.2214/ajr.159.5.1414810. discussion 979–81. [DOI] [PubMed] [Google Scholar]
- 8.McGraw J, Hiebert GW, Steeves JD. Modulating astrogliosis after neurotrauma. J Neurosci Res. 2001;63(2):109–15. doi: 10.1002/1097-4547(20010115)63:2<109::AID-JNR1002>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 9.Wisniewski N, Moussy F, Reichert WM. Characterization of implantable biosensor membrane biofouling. Fresenius Journal of Analytical Chemistry. 2000;366(6–7):611–621. doi: 10.1007/s002160051556. [DOI] [PubMed] [Google Scholar]
- 10.Voronov I, Santerre JP, Hinek A, Callahan JW, Sandhu J, Boynton EL. Macrophage phagocytosis of polyethylene particulate in vitro. J Biomed Mater Res. 1998;39(1):40–51. doi: 10.1002/(sici)1097-4636(199801)39:1<40::aid-jbm6>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 11.McNally AK, Anderson JM. Beta1 and beta2 integrins mediate adhesion during macrophage fusion and multinucleated foreign body giant cell formation. Am J Pathol. 2002;160(2):621–30. doi: 10.1016/s0002-9440(10)64882-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Henson PM. The immunologic release of constituents from neutrophil leukocytes. I. The role of antibody and complement on nonphagocytosable surfaces or phagocytosable particles. J Immunol. 1971;107(6):1535–46. [PubMed] [Google Scholar]
- 13.Mathur AB, Collier TO, Kao WJ, Wiggins M, Schubert MA, Hiltner A, Anderson JM. In vivo biocompatibility and biostability of modified polyurethanes. J Biomed Mater Res. 1997;36(2):246–57. doi: 10.1002/(sici)1097-4636(199708)36:2<246::aid-jbm14>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 14.Shen M, Martinson L, Wagner MS, Castner DG, Ratner BD, Horbett TA. PEO-like plasma polymerized tetraglyme surface interactions with leukocytes and proteins: in vitro and in vivo studies. J Biomater Sci Polym Ed. 2002;13(4):367–90. doi: 10.1163/156856202320253910. [DOI] [PubMed] [Google Scholar]
- 15.Norton LW, Koschwanez HE, Wisniewski NA, Klitzman B, Reichert WM. Vascular endothelial growth factor and dexamethasone release from nonfouling sensor coatings affect the foreign body response. J Biomed Mater Res A. 2007;81(4):858–69. doi: 10.1002/jbm.a.31088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Bilsen PH, Popa ER, Brouwer LA, Vincent J, Taylor CE, de Leij LF, Hendriks M, van Luyn MJ. Ongoing foreign body reaction to subcutaneous implanted (heparin) modified Dacron in rats. J Biomed Mater Res A. 2004;68(3):423–7. doi: 10.1002/jbm.a.20069. [DOI] [PubMed] [Google Scholar]
- 17.Gilligan BJ, Shults MC, Rhodes RK, Updike SJ. Evaluation of a subcutaneous glucose sensor out to 3 months in a dog model. Diabetes Care. 1994;17(8):882–7. doi: 10.2337/diacare.17.8.882. [DOI] [PubMed] [Google Scholar]
- 18.Biran R, Martin DC, Tresco PA. Neuronal cell loss accompanies the brain tissue response to chronically implanted silicon microelectrode arrays. Exp Neurol. 2005;195(1):115–26. doi: 10.1016/j.expneurol.2005.04.020. [DOI] [PubMed] [Google Scholar]
- 19.Zekorn TD, Horcher A, Mellert J, Siebers U, Altug T, Emre A, Hahn HJ, Federlin K. Biocompatibility and immunology in the encapsulation of islets of Langerhans (bioartificial pancreas) Int J Artif Organs. 1996;19(4):251–7. [PubMed] [Google Scholar]
- 20.Peek LJ, Middaugh CR, Berkland C. Nanotechnology in vaccine delivery. Adv Drug Deliv Rev. 2008;60(8):915–28. doi: 10.1016/j.addr.2007.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anderson JM. In-vivo biocompatibility of implantable delivery systems and biomaterials. European Journal of Pharmaceutics and Biopharmaceutics. 1994;40(1):1–8. [Google Scholar]
- 22.Bridges AW, Singh N, Burns KL, Babensee JE, Andrew Lyon L, Garcia AJ. Reduced acute inflammatory responses to microgel conformal coatings. Biomaterials. 2008;29(35):4605–15. doi: 10.1016/j.biomaterials.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tang L, Eaton JW. Fibrin(ogen) mediates acute inflammatory responses to biomaterials. J Exp Med. 1993;178(6):2147–56. doi: 10.1084/jem.178.6.2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van der Giessen WJ, Lincoff AM, Schwartz RS, van Beusekom HM, Serruys PW, Holmes DR, Jr, Ellis SG, Topol EJ. Marked inflammatory sequelae to implantation of biodegradable and nonbiodegradable polymers in porcine coronary arteries. Circulation. 1996;94(7):1690–7. doi: 10.1161/01.cir.94.7.1690. [DOI] [PubMed] [Google Scholar]
- 25.Singh N, Bridges AW, Garcia AJ, Lyon LA. Covalent tethering of functional microgel films onto poly(ethylene terephthalate) surfaces. Biomacromolecules. 2007;8(10):3271–5. doi: 10.1021/bm700516v. [DOI] [PubMed] [Google Scholar]
- 26.Khazen W, M’Bika JP, Tomkiewicz C, Benelli C, Chany C, Achour A, Forest C. Expression of macrophage-selective markers in human and rodent adipocytes. FEBS Lett. 2005;579(25):5631–4. doi: 10.1016/j.febslet.2005.09.032. [DOI] [PubMed] [Google Scholar]
- 27.Kunz-Schughart LA, Weber A, Rehli M, Gottfried E, Brockhoff G, Krause SW, Andreesen R, Kreutz M. The “classical” macrophage marker CD68 is strongly expressed in primary human fibroblasts. Verh Dtsch Ges Pathol. 2003;87:215–23. [PubMed] [Google Scholar]
- 28.Nolan CM, Reyes CD, Debord JD, Garcia AJ, Lyon LA. Phase transition behavior, protein adsorption, and cell adhesion resistance of poly(ethylene glycol) cross-linked microgel particles. Biomacromolecules. 2005;6(4):2032–9. doi: 10.1021/bm0500087. [DOI] [PubMed] [Google Scholar]
- 29.Mills CD, Kincaid K, Alt JM, Heilman MJ, Hill AM. M-1/M-2 macrophages and the Th1/Th2 paradigm. J Immunol. 2000;164(12):6166–73. doi: 10.4049/jimmunol.164.12.6166. [DOI] [PubMed] [Google Scholar]
- 30.Moro T, Takatori Y, Ishihara K, Konno T, Takigawa Y, Matsushita T, Chung UI, Nakamura K, Kawaguchi H. Surface grafting of artificial joints with a biocompatible polymer for preventing periprosthetic osteolysis. Nat Mater. 2004;3(11):829–36. doi: 10.1038/nmat1233. [DOI] [PubMed] [Google Scholar]
- 31.Wang C, Yu B, Knudsen B, Harmon J, Moussy F, Moussy Y. Synthesis and performance of novel hydrogels coatings for implantable glucose sensors. Biomacromolecules. 2008;9(2):561–7. doi: 10.1021/bm701102y. [DOI] [PubMed] [Google Scholar]
- 32.Quinn CP, Pathak CP, Heller A, Hubbell JA. Photo-crosslinked copolymers of 2-hydroxyethyl methacrylate, poly(ethylene glycol) tetra-acrylate and ethylene dimethacrylate for improving biocompatibility of biosensors. Biomaterials. 1995;16(5):389–96. doi: 10.1016/0142-9612(95)98856-9. [DOI] [PubMed] [Google Scholar]
- 33.Goreish HH, Lewis AL, Rose S, Lloyd AW. The effect of phosphorylcholine-coated materials on the inflammatory response and fibrous capsule formation: in vitro and in vivo observations. J Biomed Mater Res A. 2004;68(1):1–9. doi: 10.1002/jbm.a.10141. [DOI] [PubMed] [Google Scholar]
- 34.DeFife KM, Shive MS, Hagen KM, Clapper DL, Anderson JM. Effects of photochemically immobilized polymer coatings on protein adsorption, cell adhesion, and the foreign body reaction to silicone rubber. J Biomed Mater Res. 1999;44(3):298–307. doi: 10.1002/(sici)1097-4636(19990305)44:3<298::aid-jbm8>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 35.Hyung Park J, Bae YH. Hydrogels based on poly(ethylene oxide) and poly(tetramethylene oxide) or poly(dimethyl siloxane). III. In vivo biocompatibility and biostability. J Biomed Mater Res A. 2003;64(2):309–19. doi: 10.1002/jbm.a.10424. [DOI] [PubMed] [Google Scholar]
- 36.Dungel P, Long N, Yu B, Moussy Y, Moussy F. Study of the effects of tissue reactions on the function of implanted glucose sensors. J Biomed Mater Res A. 2008;85(3):699–706. doi: 10.1002/jbm.a.31593. [DOI] [PubMed] [Google Scholar]
- 37.Zhong Y, Bellamkonda RV. Dexamethasone-coated neural probes elicit attenuated inflammatory response and neuronal loss compared to uncoated neural probes. Brain Res. 2007;1148:15–27. doi: 10.1016/j.brainres.2007.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim DH, Smith JT, Chilkoti A, Reichert WM. The effect of covalently immobilized rhIL-1ra-ELP fusion protein on the inflammatory profile of LPS-stimulated human monocytes. Biomaterials. 2007;28(23):3369–77. doi: 10.1016/j.biomaterials.2007.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mantovani A, Locati M, Vecchi A, Sozzani S, Allavena P. Decoy receptors: a strategy to regulate inflammatory cytokines and chemokines. Trends Immunol. 2001;22(6):328–36. doi: 10.1016/s1471-4906(01)01941-x. [DOI] [PubMed] [Google Scholar]
- 40.Chavakis T, Athanasopoulos A, Rhee JS, Orlova V, Schmidt-Woll T, Bierhaus A, May AE, Celik I, Nawroth PP, Preissner KT. Angiostatin is a novel anti-inflammatory factor by inhibiting leukocyte recruitment. Blood. 2005;105(3):1036–43. doi: 10.1182/blood-2004-01-0166. [DOI] [PubMed] [Google Scholar]