Abstract
The antioxidant and anti-inflammatory compound AGI-1067 (succinobucol) has potential as an oral anti-diabetic agent. AGI-1067 reduces HbA1c, improves fasting plasma glucose, and reduces new-onset diabetes. We investigated AGI-1067 for possible effects on mouse pancreatic islets in vitro. Pretreatment with 10uM AGI-1067 increased glucose-stimulated insulin secretion (11mM) without affecting secretion in basal (3mM) glucose. AGI-1067 enhanced the intracellular calcium response to glucose stimulation in 7mM and 11mM glucose, but had no effect in 28mM or basal glucose. AGI-1067-pretreated islets also showed enhanced calcium responses to methyl pyruvate and alpha-ketoisocaproate at low doses, but not high doses. The AGI-1067-mediated effects on glucose-stimulated calcium were maintained during continuous diazoxide exposure, suggesting effects on the KATP-channel-independent pathway. AGI-1067 also reduced cytokine-induced islet cell death and expression of iNOS, a key component in cytokine signaling. This is the first report of direct stimulatory and protective effects of a first-in-class potential anti-diabetic agent on pancreatic islets.
Keywords: succinobucol, probucol, cytokines, inflammation, anti-oxidant, oxidative stress, anti-inflammatory, inflammation, glucose sensitivity, glucokinase, calcium, biphasic, insulin, beta-cell, beta cells, ER stress
Introduction
Type 2 diabetes mellitus (T2D) is a disease characterized by insulin resistance and the progressive failure of pancreatic beta cells to secrete sufficient amounts of insulin to regulate blood glucose. The increased incidence of T2D world-wide has led the CDC to classify it as an epidemic (2008). Insufficient levels of insulin can lead to hyperglycemia, which promotes the progressive deterioration of insulin-producing beta cells and leads to more severe and chronic health problems including high blood pressure, neuropathy, vision loss, renal failure, stroke, coma, and death. Although the pathophysiological mechanisms involved in the development of T2D are complex, oxidative stress and inflammation are known key contributors to the development of the disease (Evans et al., 2002, Hotamisligil, 2006). Prolonged hyperglycemia can lead to oxidative stress and activation of resultant inflammatory pathways, which is important not only in insulin resistance (Shoelson et al., 2006), but also pancreatic beta cell failure (Robertson et al., 2004, Kaneto et al., 2005, Oprescu et al., 2007).
Several antioxidants, including the anti-hyperlipidemic drug probucol, have been shown to preserve beta-cell mass and function, improve glucose tolerance, and prevent the development of diabetes in several preclinical models of diabetes by reducing the oxidative stress that promotes beta-cell apoptosis (Drash et al., 1988, Uehara et al., 1991, Fukuda et al., 1995, Gorogawa et al., 2002, Takatori et al., 2003, Lankin et al., 2004). The clinical use of probucol, however, has been associated with side effects, including reduced high-density lipoprotein cholesterol (HDLc) and prolonged QT interval, thus limiting its usage (Tardif et al., 1997). AGI-1067 [mono[4-[[1-[[3,5-bis(1,1-dimethylethyl)-4-hydroxyphenyl]thio]-1-methylethyl]thio]-2,6-bis(1,1-dimethylethyl)phenyl]ester](butanedioic acid), also called succinobucol, is a metabolically stable derivative of probucol (Meng et al., 2002, Meng et al., 2004) that is currently in clinical development for the treatment of diabetes. Clinical studies have shown that AGI-1067 inhibits both restenosis (Tardif et al., 2003) and atherosclerosis (Tardif et al., 2008a) and does not show QTc prolongation (Tardif et al., 2003). More recently, a double-blind, placebo-controlled trial in subjects with established cardiovascular disease demonstrated that AGI-1067 significantly reduced the composite of cardiovascular death, cardiovascular arrest, myocardial infarction or stroke (Tardif et al., 2008b). Furthermore, in the diabetic subpopulation of this study, AGI-1067 significantly reduced glycated hemoglobin levels and improved fasting plasma glucose levels. In addition, AGI-1067 substantially reduced the incidence of new-onset diabetes. The magnitude of the preventative effect was greater than that seen with the anti-diabetic drugs acarbose and metformin and comparable to thiazolidinediones. Unlike thiazolidinediones however, AGI-1067 did not cause increased weight or waist circumference (Tardif et al., 2008b).
Preclinical mechanistic studies have shown that AGI-1067 is not only a potent lipid antioxidant, but also an anti-inflammatory agent. Specifically, AGI-1067 inhibits inflammatory signaling pathways and reduces the expression of inflammatory adhesion molecules and cytokines in several cell types including endothelial cells and macrophages (Kunsch et al., 2004, Luyendyk et al., 2007). In addition to those properties, AGI-1067 has also been shown to enhance insulin sensitivity in mouse adipocytes by blocking inflammatory signaling pathways known to contribute to peripheral insulin resistance (Chen et al., 2008). Furthermore, in animal studies, AGI-1067 inhibited the progression of atherosclerosis (Sundell et al., 2003) and improves insulin resistance (Sundell et al., 2008).
To date, AGI-1067 has not been studied at the level of the pancreatic islet. Given the potential importance of oxidative stress and inflammatory processes on islet cell dysfunction in diabetes, we investigated AGI-1067 for effects on islets in vitro using a newly developed fluorescent imaging technique. By fluorescently labeling one set of islets with Cell Tracker Red (CTR) for positive identification, we could distinguish between unlabeled and CTR-labeled islets to record both control and AGI-1067-treated islets simultaneously and under identical experimental conditions to monitor changes in intracellular [Ca2+]i as a measure of normal islet function (Henquin et al., 2006, Jahanshahi et al., 2009). This technique improved our sensitivity in evaluating this potential therapeutic compound for effects on islet function. Our results indicate that pretreatment with AGI-1067 protects islets against cytokine-induced damage and also increased calcium influx and insulin secretion in response to glucose stimulation among control islets. Our study suggests AGI-1067 has multiple direct effects on islet function and demonstrates the utility of a dual-labeling technique for testing potential therapeutic compounds on islet function.
Materials and Methods
Mice and islet isolation
Male C57BL/6J mice weighing 20-35 g were purchased from Jackson Laboratories (Bar Harbor, ME) and housed in a pathogen-free facility at the University of Virginia (UVA) for use in all studies. Mice were euthanized according to IACUC approved protocol, and their pancreatic islets were isolated by collagenase digestion and Histopaque centrifugation as previously published (Carter et al., 2009). Following isolation, islets were transferred to a Petri dish containing RPMI 1640 (Invitrogen Inc., Carlsbad, CA) supplemented with 10% fetal bovine serum and penicillin/streptomycin. All islets were incubated overnight to allow sufficient recovery time from collagenase digestion before any experiments were performed.
Drug Treatments
AGI-1067 was supplied by AtheroGenics, Inc. and solubilized in DMSO (0.1% DMSO for final concentration). Cell Tracker Red (CTR, a cell permeable mildly thiol-reactive vital probe used to label cells) and fura-2 AM (a probe for [Ca2+]i) were purchased from Invitrogen. Murine cytokines (B&D Scientific, Mountain Lakes, NJ) were solubilized in sterile H20 at 1000x for use at the following final concentrations: 100 pg/ml for TNF-alpha, 1000 pg/ml for IFN-gamma, and 50 pg/ml for IL-1beta in KRB. This combination of cytokines is within the range of concentrations we have previously published (Yang et al., 2005, Jahanshahi et al., 2009). Unless otherwise stated, all other drugs used were purchased from Sigma-Aldrich (St. Louis, MO) and made soluble in 0.1% DMSO.
Cell Tracker Red labeling and [Ca2+]i measurements of islet function
[Ca2+]i was measured using the ratiometric [Ca2+]i indicator fura-2 AM using previously described methods (Jahanshahi et al., 2009). Islets were dye-loaded and recorded in a modified Krebs-Ringer buffer (Nunemaker et al., 2004) containing (in mM): 11 glucose, 130.5 NaCl, 3 CaCl2, 5 KCl, 2 MgCl2, 10 HEPES, pH 7.3 (3, 7, or 28 mM glucose was used in place of 11 mM as indicated below). Islets were loaded for 30-40 min with 1 μM fura-2 AM or with 1 μM fura-2 AM + 0.2 μM CTR, washed, and then transferred to a small volume chamber (Warner Instruments, Hamden, CT) mounted on the stage of an Olympus BX51WI fluorescence microscope (Olympus, Tokyo, Japan). Islets were recorded in 3 mM (low) glucose for 3 min and then exposed to 7, 11, or 28 mM (high) glucose stimulation, or other treatments as described. Every experiment was performed with at least two trials using islets isolated from different mice on separate occasions and with CTR labels flipped to further verify CTR labeling did not interfere with islet calcium handling. The glucose-stimulation [Ca2+]i response (GSCa) is defined as the change in [Ca2+]i levels between high vs. low glucose as measured by fura-2 AM ratio (340/380 nm fluorescence). Data were analyzed with IP Lab software Version 4.0 (Scanalytics, Rockville, MD).
Cell death measurements
Measurements of cell death were performed by treating islets with 20 μg/ml of propidium iodide (PI) for 10 min. Islets were imaged once under brightfield illumination to determine the islet borders and imaged again to measure PI fluorescence using 535 nm excitation and 617 nm emission. AnnexinV (Invitrogen), which detects cells that have expressed phosphatidylserine on the cell surface, was also used (488 nm excitation, 535 nm emission).
Islet insulin secretion
After overnight incubation, islets were tested for insulin secretion as described previously (Chen et al., 2002, Nunemaker et al., 2008). Briefly, islets were preincubated at 37 °C and 5% CO2 for 1 hour in a modified Krebs-Ringer Buffer (KRB) solution containing 0 mM glucose, then washed and incubated in KRB supplemented with 3 mM glucose for 1 hour followed by a 1-hour treatment with KRB containing 11 mM glucose. The supernatant was collected after each treatment, and insulin concentration in the supernatant was measured by an EIA method (Mercodia, Uppsala, Sweden) with a mouse insulin standard. The intra-assay variation was 3.6% and inter-assay variation was <10%.
RT-PCR
RNA from islets was prepared using the RNeasy kit (Qiagen, Valencia, CA). cDNA was made from 5 μg of total RNA using MMLV reverse transcriptase in 20 μL reaction volume using random hexamers (Invitrogen). For quantitative measurement of PCR products, 3 μL of the cDNA reaction (five-fold diluted) was used as template for PCR with Jump Start Taq-Polymerase (Sigma-Aldrich, St. Louis, MO) in a reaction volume of 25 μL for PCR (Chakrabarti et al., 2002). Taqman probes were purchased from Applied Biosystems (Applied Biosystems, Carlsbad, CA) and real-time PCR was performed according to manufacturer’s instructions. All thermal cycling was performed using the CFX96 Thermal Cycler (Bio-Rad, Hercules, CA). All reactions were performed in triplicate and the data was normalized to the housekeeping gene actin and evaluated using the 2−ΔΔ CT method. Expression levels are presented as fold induction/downregulation of transcripts of respective genes relative to control.
Statistics
A two-tailed Student t-test was used for comparisons of control vs. AGI–treated conditions and for cytokine vs. cytokine+AGI-1067 conditions. For gene expression studies, we used one-way ANOVA with Tukey’s multiple comparison test. P<0.05 was considered significant.
Results
Acute effects of AGI-1067 and tolbutamide on [Ca2+]i
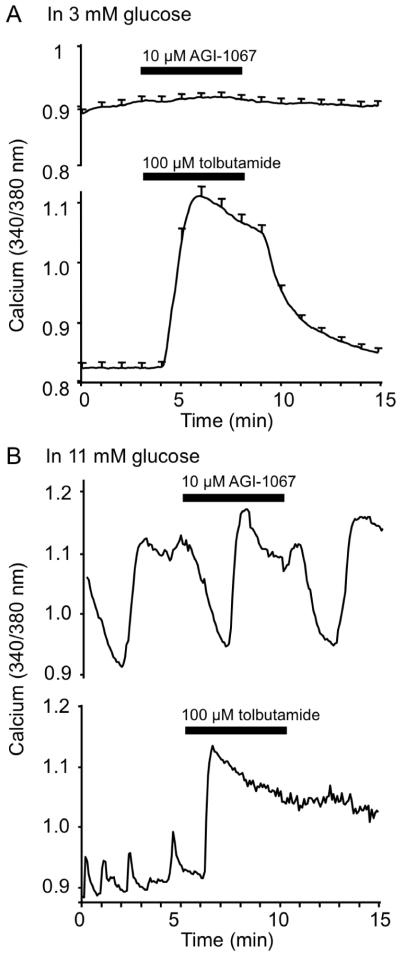
We initially examined whether AGI-1067 acutely altered [Ca2+]i, an upstream component of the insulin secretion pathway and a common indicator of overall islet health (Henquin et al., 2006, Jahanshahi et al., 2009). For all studies, islets were treated with either 0.1% DMSO alone (control) or 2, 5, or 10 μM AGI-1067 in 0.1% DMSO. Concentrations were based on previously established effective concentrations in other cell types (Sundell et al., 2003, Kunsch et al., 2004, Chen et al., 2008); these concentrations approximate the efficacious plasma levels that are achieved in rodents and humans. As shown in Figure 1A (upper panel), acute treatment with 10 μM AGI-1067 for 5 min in 3 mM glucose (a basal, non-stimulatory glucose concentration) had no immediate effect on islet [Ca2+]i. In contrast, the anti-diabetic drug tolbutamide, a sulphonylurea, induced substantial calcium influx by blocking adenosine triphosphate sensitive potassium (KATP) channels (lower panel). Additional tests confirmed that islets that did not respond to AGI-1067 were still capable of responding to subsequent tolbutamide stimulation and were therefore functional (data not shown). Acute AGI-1067 treatment also had no effect on [Ca2+]i in glucose-stimulated conditions of 11 mM glucose as shown in Figure 1B. AGI-1067 did not appear to alter endogenous oscillations in [Ca2+]i, whereas tolbutamide acutely increased [Ca2+]i and disrupted oscillatory patterns (Figure 1B).
Figure 1.
No acute effects of AGI-1067 on [Ca2+]i in islets. (A) [Ca2+]i was recorded for 3 min in 3 mM glucose, and then islets were treated with either 10 μM AGI-1067 (upper panel) or 100 μM tolbutamide (lower panel) for 5 min. [Ca2+]i did not significantly change during AGI-1067 treatment (p=0.20, n=23), whereas a very large response to tolbutamide was observed (p<0.001, n=16). (B) Ca2+]i was recorded for 10 min in 11 mM glucose (only 5 min are displayed), and then islets were treated with either 10 μM AGI-1067 (upper panel) or 100 μM tolbutamide (lower panel). AGI-1067 treatment did not appreciably change oscillatory patterns (n=11), whereas tolbutamide raised [Ca2+]i and eliminated oscillations (n=22).
CTR-labeling to improve measurements of islet [Ca2+]i
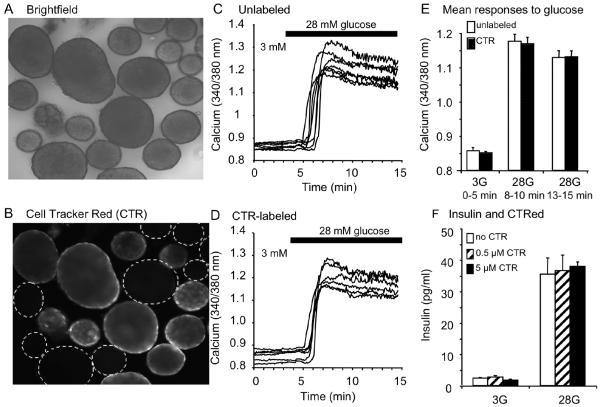
To investigate potentially more subtle effects of AGI-1067 on islet [Ca2+]i handling, we developed a dual-labeling technique to make more sensitive comparisons between control and AGI-1067-pretreated islets. One set of islets was loaded for 30 min with CTR, a cell-permeable mildly thiol-reactive vital dye, and another set of islets was not. Islets in monochrome visible light (Figure 2A) exposed to 1 μM CTR were clearly visible using 535 nm excitation and 630 nm emission, whereas islets not exposed to CTR (unlabeled) were not visible (outlined in dotted circles, Figure 2B). CTR showed no spectral overlap with the excitations (340 and 380 nm) and emission (510 nm) used to measure [Ca2+]i with fura-2 AM.
Figure 2.
CTR shows no spectral overlap with fura-2 AM and does not interfere with islet physiological function. (A) Brightfield, (B) Image of CTR vs. unlabeled islets with 535 nm excitation (Ex) and 630 nm emission (Em). (C-D) Unlabeled (C, n=7) and CTR-labeled islets (D, n=6) showed similar [Ca2+]i responses to glucose stimulation (3 to 28 mM). (E) No differences in [Ca2+]i were observed during the glucose stimulation tests involving >100 islets in total. (F) CTR-labeling does not interfere with glucose-stimulated insulin secretion (n=6 sets of 50 islets per treatment group).
We also tested CTR for possible interference with islet physiology. Islets loaded with 1 μM CTR plus 3 μM fura-2 AM (Figure 2C) or fura-2 AM alone (Figure 2D) displayed a similar GSCa. As summarized in Figure 2E, [Ca2+]i did not differ between labeled and unlabeled islets in low (3 mM) or high glucose (28 mM). Similarly, 0.5 or 5 μM CTR did not interfere with glucose-stimulated insulin secretion (GSIS) in low or high glucose (Figure 2F). CTR also did not alter other features of normal islet function including responses to depolarization with potassium chloride or intrinsic islet [Ca2+]i oscillations (data not shown). Although we found no evidence that CTR interfered with the physiological function of islets, we nevertheless used 200 nM CTR, the lowest concentration that permitted consistent labeling (Goebel et al., 2006), to minimize any possible interference.
AGI-1067 pretreatment augments GSCa and GSIS
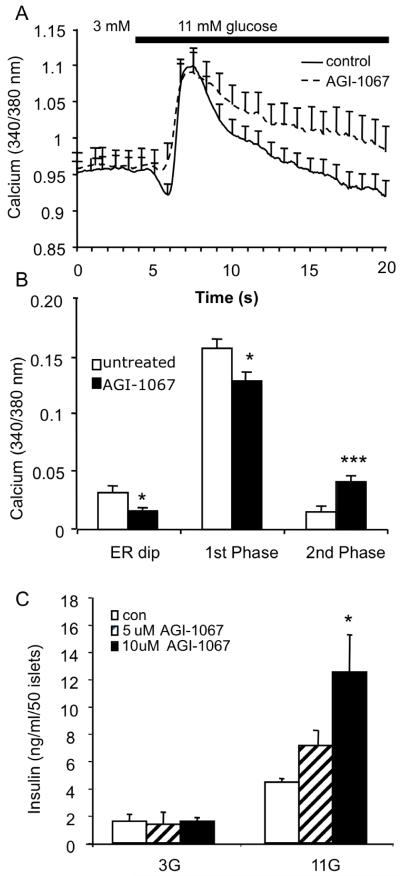
With this new imaging technique, we examined the effects of pretreatment with AGI-1067 on GSCa. Islets were pretreated with either 10 μM AGI-1067 or vehicle (control) for 60 min in a KRB solution, and then control islets were loaded with 3 μM fura-2 AM and AGI-1067-treated islets were loaded with 3 μM fura-2 AM + 200 nM CTR for 30 min. Both sets of individually loaded islets were then combined, and all islets were imaged simultaneously to measure changes in [Ca2+]i during stimulation from 3 to 11 mM glucose. The standard islet [Ca2+]i response to glucose stimulation for both rodent and human islets involves three phases (Roe et al., 1994a, Martin and Soria, 1996): an initial drop in [Ca2+]i due to ER sequestration (phase 0), a sharp rise to a peak (phase 1 or first phase) related to the initial burst of calcium influx and release of docked insulin granules, and then a plateau associated with an elevated [Ca2+]i and insulin release rate (phase 2 or second phase). This is similar to the kinetics of biphasic insulin secretion (without phase 0) in response to glucose stimulation both in vivo and in vitro (Henquin et al., 2006, Nunemaker et al., 2006b). As shown in Figure 3A, the mean responses from simultaneous [Ca2+]i recordings of control (n=12) and test groups (n=10) show that islets treated with AGI-1067 differed from the control group. Most notably, AGI-1067-treated islets showed a significantly elevated second phase [Ca2+]i response (p<0.001) and greatly reduced phase 0 dip (p<0.002) compared to controls. Similar findings were observed when islets were pretreated with AGI-1067 for only 30 min (data not shown). Consistent with the data in Figure 1, AGI-1067 pretreatment did not affect basal [Ca2+]i and slightly reduced the acute first phase response to glucose stimulation (p<0.05). Mean changes in [Ca2+]i from basal levels are displayed in Figure 3B, highlighting the differences between AGI-1067-treated (n=27) and vehicle-treated islets (n=37). Note that for each set of studies, at least two trials were made, and CTR was used to alternatively label the control or AGI-1067 groups to compensate for any potential confounding effects of CTR.
Figure 3.
AGI-1067 augments GSCa and GSIS. (A) Representative examples of control (solid, n=12 islets) and AGI-1067-treated islet (dashed, n=10 islets). (B) Comparisons of mean [Ca2+]i response for control or AGI-pretreated islets during phase 0 (ER dip), phase 1, and phase 2 of glucose stimulation. A total of 6 trials were preformed using n=37 control and 27 AGI-pretreated islets. (C) Insulin secretion from islets pretreated with 0, 5, or 10 μM AGI-1067. AGI-1067 significantly increased insulin secretion in 11 mM glucose, but not basal (3 mM) glucose (n=9 replicates using islets from 12 mice, *p<0.05, ***p<0.001).
To determine if these differences in [Ca2+]i corresponded with insulin secretion, we pretreated islet cells with either 0, 5, or 10 μM AGI-1067 for 30 min and then measured static GSIS as described in the Materials and Methods using consecutive one-hour treatments in 3 and 11 mM glucose. As shown in Figure 3C, AGI-1067 augmented GSIS in a dose-dependent fashion. AGI-1067 did not affect basal insulin secretion (p=0.50). These data support the [Ca2+]i findings indicating that AGI-1067 augments responses to stimulatory glucose without altering activity under basal glucose conditions.
AGI-1067 shifts islet glucose sensitivity
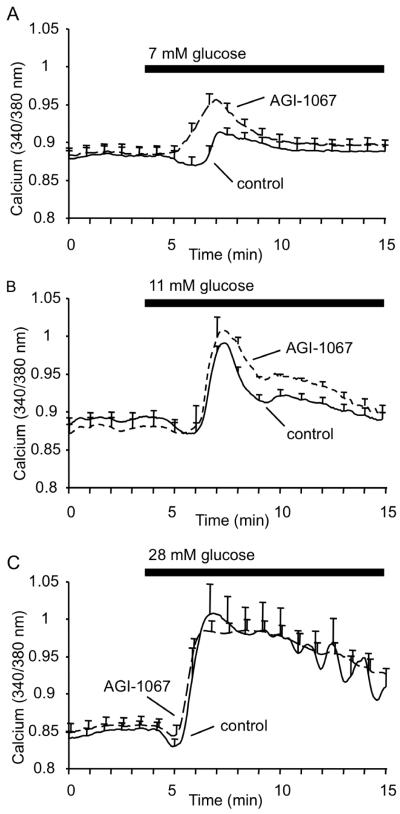
We also examined the effects of AGI-1067 pretreatment on GSCa using different glucose concentrations as an indicator of islet glucose sensitivity. We repeated the GSCa protocol, pretreating islets in either KRB or 10 μM AGI-1067 and simultaneously comparing their responses to glucose stimulation using 7, 11, or 28 mM glucose. We chose these concentrations because 7 mM glucose is at the cusp of islet activation, 11 mM is the half-maximal concentration, and 28 mM is sufficient to saturate glucose metabolism rates (Scott et al., 1981, Nunemaker et al., 2006a).
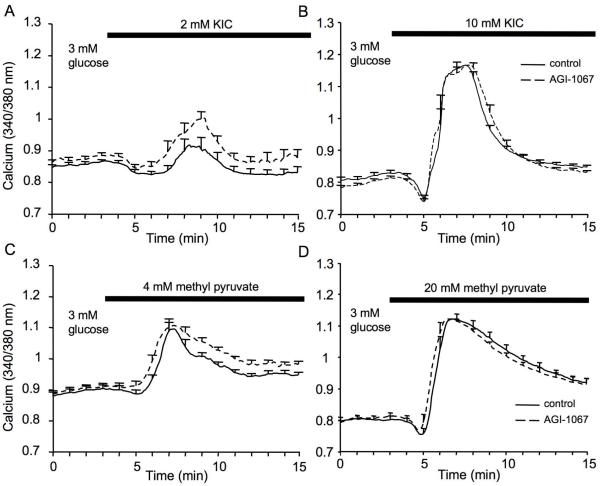
As shown in Figure 4A, AGI-1067 augmented [Ca2+]i levels significantly higher than the control group, which showed only a slight response to 7 mM glucose. We then repeated our previous trial with a stimulatory glucose concentration of 11 mM as shown in Figure 4B, which confirmed our previous GSCa findings (Figure 3) showing a reduced phase 0 dip and an enhanced second phase [Ca2+]i response. With 28 mM glucose stimulation (Figure 4C), however, there were no significant differences (p=0.50) in [Ca2+]i response, which suggests saturation of glucose stimulation of [Ca2+]i for both control and AGI-1067-treated conditions. These findings are summarized in Table 1 and suggest that AGI-1067 enhances the sensitivity of islets to physiological levels of glucose.
Figure 4.
Effects of AGI-1067 on glucose sensitivity. Islets were pretreated with 10 μM AGI-1067 or vehicle (control), and GSCa was recorded using stimulation of 7 mM glucose (A), 11 mM glucose (B), or 28 mM glucose (C). AGI-1067-pretreated islets showed a reduced phase 0 dip and an enhanced 1st phase response compared to the control group of islets in 7 mM glucose; a reduced ER dip and an elevated 2nd phase response compared to the control group in 11 mM glucose; no significant differences in 28 mM glucose. Traces in each panel consist of mean values of n=7 islets or more for each treatment. At least two trials were performed for each concentration of glucose with total islets numbers listed in Table 1.
Table 1.
Mean values for islet [Ca2+]i response to stimulation (phase 0, phase 1, and phase 2)
Effects of AGI-1067 on calcium response to glucose and other fuels
| Phase 0 | Phase 1 | Phase 2 | ||||
|---|---|---|---|---|---|---|
| Treatment | Control | AGI-1067 | Control | AGI-1067 | Control | AGI-1067 |
| 7 mM glucose | 0.016 ± 0.004 (n=21) |
0.013 ± 0.008 (n=13) |
0.038 ± 0.012 | 0.078 ± 0.014* | 0.006 ± 0.003 | 0.011 ± 0.006 |
| 11 mM glucose | 0.032 ± 0.006 (n=37) |
0.016 ± 0.003* (n=27) |
0.156 ± 0.008 | 0.128 ± 0.008* | 0.015 ± 0.005 | 0.041 ± 0.005*** |
| 28 mM glucose | 0.020 ± 0.003 (n=12) |
0.027 ± 0.009 (n=22) |
0.171 ± 0.023 | 0.146 ± 0.011 | 0.073 ± 0.014 | 0.065 ± 0.010 |
| 2 mM KIC | 0.017 ± 0.002 n=24 |
0.014 ± 0.002 n=25 |
0.048 ± 0.004 | 0.108 ± 0.015** | 0.018 ± 0.012 | 0.036 ± 0.005* |
| 10 mM KIC | 0.037 ± 0.006 (n=26) |
0.032 ± 0.004 (n=24) |
0.387 ± 0.015 | 0.367 ± 0.010 | 0.068 ± 0.006 | 0.088 ± 0.009 |
| 4 mM MP | 0.019 ± 0.002 (n=17) |
0.019 ± 0.002 (n=18) |
0.221 ± 0.027 | 0.234 ± 0.035 | 0.057 ± 0.009 | 0.081 ± 0.012** |
| 20 mM MP | 0.050 ± 0.003 (n=31) |
0.051 ± 0.003 (n=30) |
0.278 ± 0.011 | 0.259 ± 0.009 | 0.119 ± 0.006 | 0.119 ± 0.005 |
n= number of islets (same value for phase 0, 1 and 2).
KIC = alpha-ketoisocaproate, MP = methyl pyruvate.
p<0.05
p<0.01
p<0.001
Effects of AGI-1067 on the [Ca2+]i response to alternative fuels
We next stimulated islets with alpha-ketoisocaproic acid (KIC) and methyl pyruvate as alternative fuels to glucose. KIC is a direct mitochondrial activator that acts on the tricarboxylic acid cycle (Pralong et al., 1990). Following 30-min incubation with 10 uM AGI-1067 or vehicle, [Ca2+]i was recorded in 3 mM glucose and then switched to 3 mM glucose + 2 mM KIC to mildly stimulate mitochondrial activity. As shown in Figure 5A, islets that were pretreated with AGI-1067 showed a more robust response to stimulation with 2 mM KIC. As shown in Figure 5B, AGI-1067 did not significantly affect the calcium response to a much higher dose of 10 mM KIC. Similar tests were performed using methyl pyruvate, a membrane permeable form of the glycolytic product pyruvate and a potent insulin secretagogue (Dufer et al., 2002). As shown in Figure 5C-D, AGI-1067 enhanced the phase 2 response to stimulation with 4 mM methyl pyruvate (Figure 5C), but AGI-1067 did not significantly affect the calcium response to 20 mM methyl pyruvate (Figure 5D). These findings are summarized in Table 1 and suggest that, as with glucose, AGI-1067 increases islet sensitivity to these alternative fuels.
Figure 5.
Effects of AGI-1067 on islet stimulation by alternative fuels. (A-B) Islets were pretreated with 10 μM AGI or vehicle (control) and then recorded for [Ca2+]i changes in response to stimulation by alpha-ketoisocaproate (KIC) or methyl pyruvate. (A-B) AGI-pretreated islets (dashed) showed a greater response to 2 mM KIC than untreated control (solid) islets (A), but no differences were observed with 10 mM KIC (B). (C-D) AGI-pretreated islets (dashed) also showed a greater response to 4 mM methyl pyruvate than untreated control (solid) islets (C), but no differences were observed with 20 mM methyl pyruvate (D). Data represent 1 of 3 trials that were conducted for each fuel. Mean values among combined trials and total islets numbers are shown in Table 1.
AGI-1067 diminishes KCl-induced calcium influx
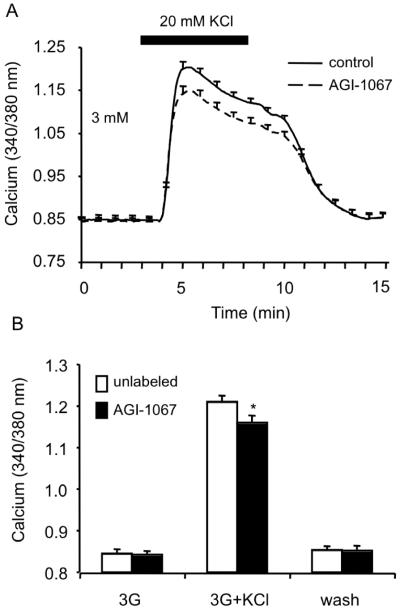
In an attempt to establish a possible mechanism of action for AGI-1067, we investigated the general effects of AGI-1067 treatment on ion channel activity using potassium chloride (KCl) as a depolarizing agent to induce calcium influx and secretion. Islets that were pretreated with 10 μM AGI-1067 showed a small but significant decrease (p<0.05) in [Ca2+]i response compared to control (Figure 6A). Mean changes in [Ca2+]i from 5 independent trials are displayed in Figure 6B and highlight the differences between AGI-1067-treated (n=35 total) and vehicle-treated islets (n=29 total). These results suggest that AGI-1067 may impact ion channel activity and calcium handling, however, the diminished KCl-induced calcium influx is not consistent with the enhancement in glucose-stimulated [Ca2+]i.
Figure 6.
Effects of AGI-1067 on KCl-stimulated [Ca2+]i response. (A) Islets pretreated with 10 μM AGI-1067 showed a reduced response to KCl stimulation compared to the control islets. (B) Mean [Ca2+]i values before, during, and after KCl treatment. *p<0.05. A total of 6 trials were preformed using n=29 control and n=35 AGI-pretreated islets.
AGI-1067 augments GSCa in the presence of the KATP-channel opener diazoxide
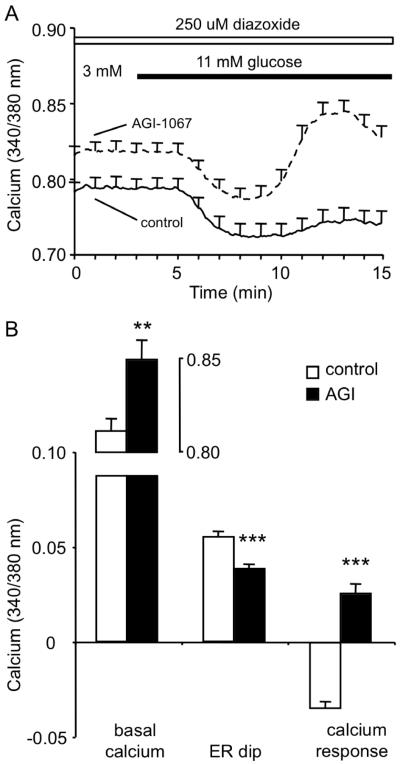
The KATP channel plays a crucial role in coupling glucose metabolism with insulin secretion. We examined whether the effects of AGI-1067 on GSCa occurred under conditions in which KATP-channel were constitutively active by maintaining islets in 250 μM diazoxide throughout the experiment. Islets were pretreated with 10 μM AGI-1067 or untreated and then recorded in 3 mM glucose + 250 μM diazoxide for 3 min before switching to 11 mM glucose + 250 μM diazoxide. As shown in Figure 7A, islets pretreated with AGI-1067 consistently showed elevated basal [Ca2+]i. Both control and AGI-1067-pretreated islets showed similar phase 0 responses caused by calcium sequestration by the ER. Following the phase 0 dip, the control group showed virtually no change in [Ca2+]i, whereas islets pretreated with AGI-1067 showed a significant increase in [Ca2+]i above basal levels (Figure 7A). Mean values for basal [Ca2+]i, the ER dip (phase 0), and the peak [Ca2+]i response are shown in Figure 7B. Note that the delayed timing of the [Ca2+]i increase corresponded with the second phase response (peak response at ~13 min compared to the ~8-min mark shown in Figures 3 and 4). Because KATP channels could not close in response to glucose stimulation in the presence of diazoxide, the delayed response provides further evidence that AGI-1067 enhances the second phase response.
Figure 7.
AGI-1067 pretreatment enhances GSCa in the presence of diazoxide. (A) Examples of GSCa for control (solid, n=10) and AGI-pretreated (dashed, n=11) islets in the presence of 250 uM diazoxide (a KATP channel opener) throughout the recording. Due to the presence of diazoxide the normal calcium response to glucose is disrupted. Traces are representative of data from 1 of 3 trials. (B) Mean values for basal [Ca2+]i in 3 mM glucose + diazoxide, ER dip (phase 0), and [Ca2+]i response to stimulation. Control (white, n=33) and AGI-1067-pretreated (black, n=35) islets differed markedly in their responses. Data are combined values from 3 separate trials. **p<0.01, ***p<0.001,
AGI-1067 does not affect calcium handling during thapsigargin treatment
The endoplasmic reticulum (ER) is a major source and store of [Ca2+]i. Since the GSCa data showed that AGI-1067 prevented the phase 0 dip (see Figure 3), this suggests that AGI-1067 may alter ER calcium handling (Roe et al., 1994b, Eizirik et al., 2008) in one of two ways: (1) the ER may be impaired by AGI-1067 and thus unable to sequester calcium adequately or (2) AGI-1067 may enhance ER calcium sequestration such that little additional calcium would enter in response to glucose. To test this hypothesis, we treated islets with 1 μM thapsigargin, a sarco(endo)plasmic reticulum ATPase (SERCA) inhibitor that prevents calcium transport through the SERCA pumps into the ER. The subsequent increase [Ca2+]i due to the release of calcium from the ER is thus an indirect measure of stored ER calcium (Jahanshahi et al., 2009, Roe et al., 1994a). Islets pretreated with 10 μM AGI-1067 did not differ in response to thapsigargin as compared with controls (control: 0.017 +/− 0.008 ratio, n=14 islets vs. AGI-pretreated: 0.019 +/− 0.008 ratio, n=12 islets). A total of 3 trials were performed (p=0.87). . This suggests that AGI-1067 does not affect ER calcium handling. It should be noted, however, that store-operated ion channels, such as the calcium-activated calcium release (CRAC) channel in the plasma membrane (Bertram et al., 1995), may have contributed to the calcium response since the experiment was conducted in the presence of extracellular calcium. Since no effect of AGI-1067 was observed in the amplitude or trajectory of the response during thapsigargin treatment, we chose not to pursue this mechanism.
AGI-1067 partially protects islets from cytokine-induced cell death
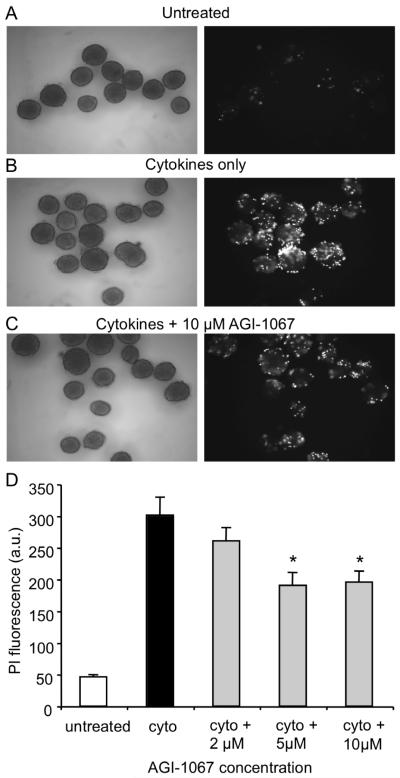
Previous studies have shown that AGI-1067 inhibits cytokine signaling and protects against oxidative stress in various cell types (Sundell et al., 2003, Kunsch et al., 2004, Chen et al., 2008), so we examined AGI-1067 for protective effects against pro-inflammatory cytokine-induced damage. Islets were preincubated for 30 min with 0, 2, 5, and 10 μM AGI-1067 and then treated overnight with a combination of proinflammatory cytokines known to induce inflammatory and cytotoxic responses in islets (Mandrup-Poulsen et al., 1987, Campbell et al., 1988, Rabinovitch et al., 1994, Cardozo et al., 2005, Yang et al., 2005, Lee et al., 2004, Jahanshahi et al., 2009). It should be noted that human islets are less susceptible to certain insults than rodent islets in vitro (Eizirik et al., 1994), however the combination of cytokines used in these experiments has been shown to induce cell death in both rodent and human islets (Rabinovitch, 1998). AGI-1067 remained in the culture medium during overnight cytokine treatment. Cell viability was assessed with PI. In Figure 8, representative images of islets from the various treatment groups are shown in the left panel. PI fluorescence in the corresponding right panel indicates nearly no fluorescence among control islets (Figure 8A), but substantial PI fluorescence among cytokine-treated islets (Figure 8B), which was attenuated by AGI-1067 pretreatment (Figure 8C). As summarized quantitatively in Figure 8D, AGI-1067 reduced cytokine-induced cell death by ~35-40% at the 5 and 10 μM concentrations (n=33-40 islets/condition total, 3 replicates). We also examined islets for annexinV, which detects cells that have expressed phosphatidylserine on the cell surface, a feature found in apoptosis as well as other forms of cell death. Islets that were pretreated with AGI-1067 completely inhibited cytokine-induced annexinV expression: annexinV among cytokine treated islets was 129 ± 9% of untreated control islets vs. 101 ± 9% for AGI-pretreated islets exposed to overnight cytokine treatment (n>22 islets per treatment group).
Figure 8.
AGI-1067 protects against cytokine-induced cell death. (A-C) Images of islets (left panel) and their respective cell death rates indicated by PI fluorescence (right panel). Control islets (A) showed very low PI fluorescence compared to cytokine-treated islets (B); PI fluorescence was reduced but not completely eliminated among islets pretreated with 10 μM AGI-1067 and then exposed to cytokines (C). (D) Mean PI values in arbitrary units (a.u.) for each condition taken from 3 separate trials (n=33-40 islets per condition). *p < 0.01.
Changes in islet gene expression
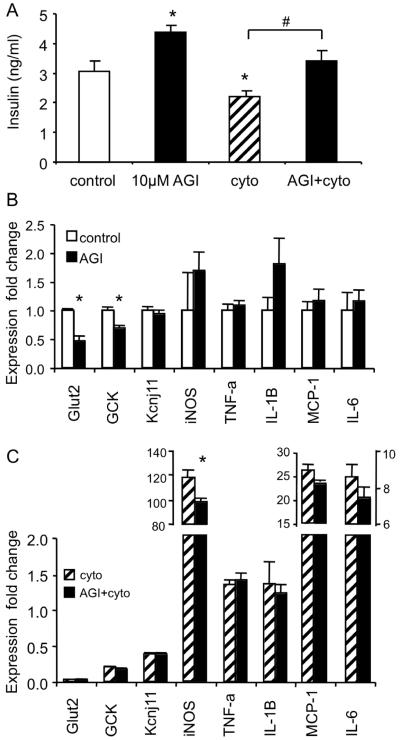
We examined possible mechanisms of action for AGI-1067 by examining changes in gene expression using RT-PCR. We investigated several genes linked to stimulus-secretion coupling (glut-2: glucose transporter; GCK: glucokinase, involved with glucose metabolism; KCNJ11: KATP-channel subunit) and cytokine signaling (iNOS; TNF-alpha; IL-1beta; MCP-1; IL-6). Four conditions were used: (1) control, (2) treated with 10 μM AGI-1067 overnight, (3) pretreated with 10 μM AGI-1067 and then treated with cytokines overnight, or (4) treated only with cytokines overnight. Following treatment, islets were then washed and cultured in RPMI containing 11 mM glucose for 30 min to collect the supernatant for insulin measurements and islets for mRNA. As shown in Figure 9A, insulin secretion was significantly elevated among AGI-1067-treated islets compared to controls. Also, the cytokine treatment reduced insulin secretion, an effect that was reversed by AGI-1067 pretreatment.
Figure 9.
AGI-1067 effects on islet gene expression. (A) Insulin secretion from islets in 11 mM glucose following overnight treatment with pro-inflammatory cytokines and/or 10 μM AGI-1067. (B) Gene expression differences between control and AGI-1067-treated islets. (C) Gene expression for islets treated with cytokines alone and islets treated with AGI-1067 prior to overnight cytokine treatment. Fold differences are compared to expression levels in control islets as shown in (B). 50 islets were used for each replicate of each condition (n=6 replicates, *p<0.05).
The same islets used to assess insulin secretion were also used to assess changes in gene expression. As shown in Figure 9B, AGI-1067 treatment alone did not alter the expression of cytokine signaling genes, but AGI-1067 downregulated both glut-2 and GCK. Among the islets treated with cytokines shown in Figure 9C, cytokine treatment downregulated GCK, Kcnj11, and glut-2, as expected. AGI-1067 pretreatment also provided some protection against iNOS induction, an important early component of cytokine signaling as shown in Figure 9C (p<0.01). An additional trial consisting of n=3 sets of islets/condition produced similar results (cytokines: 117-fold iNOS; AGI + cytokines: 84-fold iNOS induction. Other cytokine signaling genes, however, were not significantly attenuated by AGI-1067 pretreatment.
Discussion
In this study, we set out to accomplish two primary goals: (1) to show the utility of a new dual-labeling technique in evaluating pancreatic islet function and (2) to employ that technique to evaluate a novel, first-in-class potential anti-diabetic agent, AGI-1067, for direct effects on islet function. We demonstrate that CTR can fluorescently label cells to make simultaneous measurements of control and test islets without impacting their function. Simultaneous measurement allows for the detection of different latencies, trajectories, and amplitudes in response to stimulation, ultimately generating near uniformity of experimental conditions. This labeling approach can be adapted to make direct comparisons between control and test groups of other cellular processes that utilize fluorescent probes such as mitochondrial activity, insulin secretion, metabolism, generation of reactive oxygen species, and rates of cell death. We applied this labeling technique to investigate effects of AGI-1067 on [Ca2+]i dynamics in islets.
This is the first report to show direct effects of the anti-oxidant and anti-inflammatory compound AGI-1067 on pancreatic islet physiology. We found that AGI-1067 enhanced insulin secretion and calcium influx in stimulatory glucose, but did not have any apparent effects in low glucose (3 mM). The effects of AGI-1067 were different from more traditional treatments like sulphonylureas, which close KATP-channels to trigger calcium influx and insulin secretion. Our observed lack of direct immediate effects (see Figure 1) or any enhancement of KCl-mediated [Ca2+]i flux further suggests that AGI-1067 acts differently from the direct action of sulfonylureas on KATP-channels. Therapeutically, the glucose-dependent effect of AGI-1067 reduces the likelihood of hypoglycemic episodes and suggests continuous AGI-1067 treatment would not lead to excess insulin release, which is an important concern with sulphonylureas (Amiel et al., 2008). In fact, clinical studies of AGI-1067 in diabetic subjects showed no increased incidence of hypoglycemia (Tardif et al., 2008b). In addition, in diabetic subjects that were receiving treatment with sulphonylureas, treatment with AGI-1067 provided a significant additive effect compared with those subjects treated with placebo (Klug et al., 2008). This observation supports the notion that the mechanism of glycemic control by AGI-1067 is different from the sulphonylurea class of drugs.
Our findings suggest that AGI-1067 primarily affects second phase insulin secretion, which is variously referred to as the “KATP-channel independent pathway” (Straub et al., 1998, Sato and Henquin, 1998), the “non-ionic pathway” (Aizawa et al., 2002), and the “amplifying pathway” (Henquin, 2000). First phase insulin release involves the secretion of a readily releasable pool of insulin granules, whereas second phase is governed by the rate at which granules can be transported to the plasma membrane and released. In addition to elevating second phase [Ca2+]i and insulin release during normal glucose stimulation, AGI-1067 pretreatment markedly enhanced GSCa in the presence of diazoxide. Diazoxide prevents the normal closure of KATP-channels that occurs when glucose metabolism increases ATP/ADP, thus blocking the trigger of first phase insulin exocytosis. AGI-1067 appears to raise [Ca2+]i independently of this KATP-dependent pathway. This response could be caused by release of [Ca2+]i stores or by activity of plasma membrane ion channels, pumps, and/or exchangers (Herchuelz et al., 2002, Mears, 2004). Alternatively, AGI-1067 could act in some manner to reverse the action of diazoxide and close KATP-channels. However, if AGI-1067 acted on diazoxide binding to reverse its effects, this would not explain the second phase [Ca2+]i response we observed in response to glucose and other fuels. Our data collectively suggest a KATP-dependent effect of AGI-1067 on [Ca2+]i handling.
Our findings also suggest that AGI-1067 protects against potential inflammatory-mediated damage in islets. Based on prior studies of AGI-1067 and its parent compound probucol (Gorogawa et al., 2002, Tardif, 2006), we investigated possible protective mechanisms by examining expression patterns of several key gene related to inflammatory signaling in islets following treatment with pro-inflammatory cytokines. AGI-1067 significantly reduced iNOS expression, a cytokine-induced gene associated with the nuclear factor kappa-beta pathway, endoplasmic reticulum stress, and activation of apoptotic pathways (Lee et al., 2004, Eizirik et al., 2008). The relatively modest attenuation in cytokine-induced iNOS expression by AGI-1067 pretreatment is thus consistent with reduced cytokine-induced cell death, but it is likely not the direct target of AGI-1067. Effects of AGI-1067 on genes related to glucose metabolism, glut-2 and GCK were also identified. Reduced expression of these genes is often associated with diabetic phenotypes (Weir et al., 1997, Terauchi et al., 2007, Evans-Molina et al., 2009), which is seemingly contrary to AGI-1067’s sustained enhancement of insulin secretion and reduced rates of diabetes in clinical trials. Any explanation for this contradiction would be speculative at this time, however, these data do establish that this novel class of drugs has effects on islet gene expression related to glucose metabolism. Candidate pathways that produce both stimulatory and protective effects on islets include vitamin E/tocopherols (Sjoholm et al., 2000), hemoxygenases (Ye and Laychock, 1998, Stocker, 2009), and MAP kinases (Luyendyk et al., 2007) and will be investigated in ongoing research.
In addition to the multiple effects on islet function that we have identified, AGI-1067 also has a number of direct or indirect effects on other target tissues that combine to produce the overall in vivo effect. For example, AGI-1067 has potent anti-oxidant effects in aortic endothelial cells and reduces TNF-alpha-induced expression of MCP-1 and VCAM-1 (Sundell et al., 2003). Recent work suggests that AGI-1067 also may reduce the production of pro-inflammatory cytokines in adipocytes by inhibiting activation of JNK and IRS-1 serine phosphorylation (Chen et al., 2008). Clinical studies of AGI-1067 in diabetic subjects showed a measurable decrease in blood serum insulin levels (Tardif et al., 2008a, Tardif et al., 2008b), which supports data in animal models showing improvement in insulin sensitivity (Sundell et al., 2008). Thus, AGI-1067 may target multiple tissues to improve insulin sensitivity in peripheral tissues such as fat and muscle, as well as enhancing insulin release from the pancreas when glucose is elevated.
In conclusion, our findings suggest AGI-1067 has multiple effects on islet function that include protection against damage from proinflammatory cytokines, increased glucose sensitivity, and enhanced insulin secretion. AGI-1067 thus has a number of distinct therapeutic advantages and great potential as a treatment for type 2 diabetes because it appears to protect pancreatic islets and augment beta-cell secretory function. Future studies will focus on determining how AGI-1067 acts mechanistically at the islet level.
Acknowledgments
This work was funded by an investigator-initiated research grant from AtheroGenics Inc., by NIH grant 1K01 DK081621 to C.S.N., Mouse Metabolic Phenotyping Center (MMPC) Pilot and Feasibility grant 07-MCG-22 to C.S.N., and NIH RO1 DK 55240 to J.L.N. Mouse islets were acquired through the Cell and Islet Isolation Core facility at the UVA DERC (DK063609). A special thanks to Stacey Dula, Kathryn Corbin, and Ruth Haile for assisting with these studies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- MMWR Morb Mortal Wkly Rep. 2008;57:1169–73. [PubMed] [Google Scholar]
- Aizawa T, Sato Y, Komatsu M. Diabetes. 2002;51(Suppl 1):S96–8. doi: 10.2337/diabetes.51.2007.s96. [DOI] [PubMed] [Google Scholar]
- Amiel SA, Dixon T, Mann R, Jameson K. Diabet Med. 2008;25:245–54. doi: 10.1111/j.1464-5491.2007.02341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertram R, Smolen P, Sherman A, Mears D, Atwater I, Martin F, Soria B. Biophys J. 1995;68:2323–32. doi: 10.1016/S0006-3495(95)80414-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell IL, Iscaro A, Harrison LC. J Immunol. 1988;141:2325–9. [PubMed] [Google Scholar]
- Cardozo AK, Ortis F, Storling J, Feng YM, Rasschaert J, Tonnesen M, Van Eylen F, Mandrup-Poulsen T, Herchuelz A, Eizirik DL. Diabetes. 2005;54:452–61. doi: 10.2337/diabetes.54.2.452. [DOI] [PubMed] [Google Scholar]
- Carter JD, Dula SB, Corbin KL, Wu R, Nunemaker CS. Biol Proced Online. 2009 doi: 10.1007/s12575-009-9021-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti SK, James JC, Mirmira RG. J Biol Chem. 2002;277:13286–93. doi: 10.1074/jbc.M111857200. [DOI] [PubMed] [Google Scholar]
- Chen M, Yang Z, Wu R, Nadler JL. Endocrinology. 2002;143:2341–8. doi: 10.1210/endo.143.6.8841. [DOI] [PubMed] [Google Scholar]
- Chen XL, Dodd G, Sundell CL, Kunsch C. Diabetes. 2008;57:A359–A359. [Google Scholar]
- Drash AL, Rudert WA, Borquaye S, Wang R, Lieberman I. Am J Cardiol. 1988;62:27B–30B. doi: 10.1016/s0002-9149(88)80047-x. [DOI] [PubMed] [Google Scholar]
- Dufer M, Krippeit-Drews P, Buntinas L, Siemen D, Drews G. Biochem J. 2002;368:817–25. doi: 10.1042/BJ20020657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eizirik DL, Cardozo AK, Cnop M. Endocr Rev. 2008;29:42–61. doi: 10.1210/er.2007-0015. [DOI] [PubMed] [Google Scholar]
- Eizirik DL, Pipeleers DG, Ling Z, Welsh N, Hellerstrom C, Andersson A. Proc Natl Acad Sci U S A. 1994;91:9253–6. doi: 10.1073/pnas.91.20.9253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans JL, Goldfine ID, Maddux BA, Grodsky GM. Endocr Rev. 2002;23:599–622. doi: 10.1210/er.2001-0039. [DOI] [PubMed] [Google Scholar]
- Evans-Molina C, Robbins RD, Kono T, Tersey SA, Vestermark GL, Nunemaker CS, Garmey JC, Deering TG, Keller SR, Maier B, Mirmira RG. Mol Cell Biol. 2009 doi: 10.1128/MCB.01179-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda M, Ikegami H, Kawaguchi Y, Sano T, Ogihara T. Biochem Biophys Res Commun. 1995;209:953–8. doi: 10.1006/bbrc.1995.1590. [DOI] [PubMed] [Google Scholar]
- Goebel S, Huang M, Davis WC, Jennings M, Siahaan TJ, Alexander JS, Kevil CG. Am J Physiol Gastrointest Liver Physiol. 2006;290:G648–54. doi: 10.1152/ajpgi.00466.2005. [DOI] [PubMed] [Google Scholar]
- Gorogawa S, Kajimoto Y, Umayahara Y, Kaneto H, Watada H, Kuroda A, Kawamori D, Yasuda T, Matsuhisa M, Yamasaki Y, Hori M. Diabetes Res Clin Pract. 2002;57:1–10. doi: 10.1016/s0168-8227(02)00005-0. [DOI] [PubMed] [Google Scholar]
- Henquin JC. Diabetes. 2000;49:1751–60. doi: 10.2337/diabetes.49.11.1751. [DOI] [PubMed] [Google Scholar]
- Henquin JC, Nenquin M, Stiernet P, Ahren B. Diabetes. 2006;55:441–51. doi: 10.2337/diabetes.55.02.06.db05-1051. [DOI] [PubMed] [Google Scholar]
- Herchuelz A, Diaz-Horta O, van Eylen F. Diabetes Metab. 2002;28:3S54–60. discussion 3S108-12. [PubMed] [Google Scholar]
- Hotamisligil GS. Nature. 2006;444:860–7. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- Jahanshahi P, Wu R, Carter JD, Nunemaker CS. Endocrinology. 2009;150:607–15. doi: 10.1210/en.2008-0773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneto H, Kawamori D, Matsuoka TA, Kajimoto Y, Yamasaki Y. Am J Ther. 2005;12:529–33. doi: 10.1097/01.mjt.0000178773.31525.c2. [DOI] [PubMed] [Google Scholar]
- Klug E, Pfeffer MA, McMurray JJ, Fleming AG, Long WA, Small R, Tardif JC. Diabetes. 2008;57:A132–A132. [Google Scholar]
- Kunsch C, Luchoomun J, Grey JY, Olliff LK, Saint LB, Arrendale RF, Wasserman MA, Saxena U, Medford RM. J Pharmacol Exp Ther. 2004;308:820–9. doi: 10.1124/jpet.103.059733. [DOI] [PubMed] [Google Scholar]
- Lankin VZ, Korchin VI, Konovalova GG, Lisina MO, Tikhaze AK, Akmaev IG. Bull Exp Biol Med. 2004;137:20–3. doi: 10.1023/b:bebm.0000024376.31288.fc. [DOI] [PubMed] [Google Scholar]
- Lee MS, Chang I, Kim S. Mol Genet Metab. 2004;83:82–92. doi: 10.1016/j.ymgme.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Luyendyk JP, Piper JD, Tencati M, Reddy KV, Holscher T, Zhang R, Luchoomun J, Chen X, Min W, Kunsch C, Mackman N. Arterioscler Thromb Vasc Biol. 2007;27:1857–63. doi: 10.1161/ATVBAHA.107.143552. [DOI] [PubMed] [Google Scholar]
- Mandrup-Poulsen T, Bendtzen K, Dinarello CA, Nerup J. J Immunol. 1987;139:4077–82. [PubMed] [Google Scholar]
- Martin F, Soria B. Cell Calcium. 1996;20:409–14. doi: 10.1016/s0143-4160(96)90003-2. [DOI] [PubMed] [Google Scholar]
- Mears D. J Membr Biol. 2004;200:57–66. doi: 10.1007/s00232-004-0692-9. [DOI] [PubMed] [Google Scholar]
- Meng CQ, Somers PK, Hoong LK, Zheng XS, Ye Z, Worsencroft KJ, Simpson JE, Hotema MR, Weingarten MD, Mac DML, Hill RR, Marino EM, Suen KL, Luchoomun J, Kunsch C, Landers LK, Stefanopoulos D, Howard RB, Sundell CL, Saxena U, Wasserman MA, Sikorski JA. J Med Chem. 2004;47:6420–32. doi: 10.1021/jm049685u. [DOI] [PubMed] [Google Scholar]
- Meng CQ, Somers PK, Rachita CL, Holt LA, Hoong LK, Zheng XS, Simpson JE, Hill RR, Olliff LK, Kunsch C, Sundell CL, Parthasarathy S, Saxena U, Sikorski JA, Wasserman MA. Bioorg Med Chem Lett. 2002;12:2545–8. doi: 10.1016/s0960-894x(02)00516-4. [DOI] [PubMed] [Google Scholar]
- Nunemaker CS, Bertram R, Sherman A, Tsaneva-Atanasova K, Daniel CR, Satin LS. Biophys J. 2006a;91:2082–96. doi: 10.1529/biophysj.106.087296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunemaker CS, Chen M, Pei H, Kimble SD, Keller SR, Carter JD, Yang Z, Smith KM, Wu R, Bevard MH, Garmey JC, Nadler JL. Am J Physiol Endocrinol Metab. 2008 doi: 10.1152/ajpendo.90371.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunemaker CS, Wasserman DH, McGuinness OP, Sweet IR, Teague JC, Satin LS. Am J Physiol Endocrinol Metab. 2006b;290:E523–9. doi: 10.1152/ajpendo.00392.2005. [DOI] [PubMed] [Google Scholar]
- Nunemaker CS, Zhang M, Satin LS. Diabetes. 2004;53:1765–72. doi: 10.2337/diabetes.53.7.1765. [DOI] [PubMed] [Google Scholar]
- Oprescu AI, Bikopoulos G, Naassan A, Allister EM, Tang C, Park E, Uchino H, Lewis GF, Fantus IG, Rozakis-Adcock M, Wheeler MB, Giacca A. Diabetes. 2007;56:2927–37. doi: 10.2337/db07-0075. [DOI] [PubMed] [Google Scholar]
- Pralong WF, Bartley C, Wollheim CB. Embo J. 1990;9:53–60. doi: 10.1002/j.1460-2075.1990.tb08079.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinovitch A. Diabetes Metab Rev. 1998;14:129–51. doi: 10.1002/(sici)1099-0895(199806)14:2<129::aid-dmr208>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Rabinovitch A, Suarez-Pinzon WL, Strynadka K, Schulz R, Lakey JR, Warnock GL, Rajotte RV. J Clin Endocrinol Metab. 1994;79:1058–62. doi: 10.1210/jcem.79.4.7962274. [DOI] [PubMed] [Google Scholar]
- Robertson RP, Harmon J, Tran PO, Poitout V. Diabetes. 2004;53(Suppl 1):S119–24. doi: 10.2337/diabetes.53.2007.s119. [DOI] [PubMed] [Google Scholar]
- Roe MW, Mertz RJ, Lancaster ME, Worley JF, 3rd, Dukes ID. Am J Physiol. 1994a;266:E852–62. doi: 10.1152/ajpendo.1994.266.6.E852. [DOI] [PubMed] [Google Scholar]
- Roe MW, Philipson LH, Frangakis CJ, Kuznetsov A, Mertz RJ, Lancaster ME, Spencer B, Worley JF, 3rd, Dukes ID. J Biol Chem. 1994b;269:18279–82. [PubMed] [Google Scholar]
- Sato Y, Henquin JC. Diabetes. 1998;47:1713–21. doi: 10.2337/diabetes.47.11.1713. [DOI] [PubMed] [Google Scholar]
- Scott AM, Atwater I, Rojas E. Diabetologia. 1981;21:470–5. doi: 10.1007/BF00257788. [DOI] [PubMed] [Google Scholar]
- Shoelson SE, Lee J, Goldfine AB. J Clin Invest. 2006;116:1793–801. doi: 10.1172/JCI29069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjoholm A, Berggren PO, Cooney RV. Biochem Biophys Res Commun. 2000;277:334–40. doi: 10.1006/bbrc.2000.3650. [DOI] [PubMed] [Google Scholar]
- Stocker R. Curr Opin Lipidol. 2009;20:227–35. doi: 10.1097/MOL.0b013e32832aee68. [DOI] [PubMed] [Google Scholar]
- Straub SG, James RF, Dunne MJ, Sharp GW. Diabetes. 1998;47:758–63. doi: 10.2337/diabetes.47.5.758. [DOI] [PubMed] [Google Scholar]
- Sundell CL, Fosgerau K, Chen XL, Dodd G, Tang-Christensen M, Kunsch C. Diabetes. 2008;57:A167–A167. [Google Scholar]
- Sundell CL, Somers PK, Meng CQ, Hoong LK, Suen KL, Hill RR, Landers LK, Chapman A, Butteiger D, Jones M, Edwards D, Daugherty A, Wasserman MA, Alexander RW, Medford RM, Saxena U. J Pharmacol Exp Ther. 2003;305:1116–23. doi: 10.1124/jpet.102.048132. [DOI] [PubMed] [Google Scholar]
- Takatori A, Ohta E, Inenaga T, Horiuchi K, Ishii Y, Itagaki S, Kyuwa S, Yoshikawa Y. Exp Anim. 2003;52:317–27. doi: 10.1538/expanim.52.317. [DOI] [PubMed] [Google Scholar]
- Tardif JC. Can J Cardiol. 2006;22(Suppl B):61B–65B. doi: 10.1016/s0828-282x(06)70988-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tardif JC, Cote G, Lesperance J, Bourassa M, Lambert J, Doucet S, Bilodeau L, Nattel S, de Guise P. N Engl J Med. 1997;337:365–72. doi: 10.1056/NEJM199708073370601. [DOI] [PubMed] [Google Scholar]
- Tardif JC, Gregoire J, L’Allier PL, Ibrahim R, Anderson TJ, Reeves F, Title LM, Schampaert E, LeMay M, Lesperance J, Scott R, Guertin MC, Brennan ML, Hazen SL, Bertrand OF. Atherosclerosis. 2008a;197:480–6. doi: 10.1016/j.atherosclerosis.2006.11.039. [DOI] [PubMed] [Google Scholar]
- Tardif JC, Gregoire J, Schwartz L, Title L, Laramee L, Reeves F, Lesperance J, Bourassa MG, L’Allier PL, Glass M, Lambert J, Guertin MC. Circulation. 2003;107:552–8. doi: 10.1161/01.cir.0000047525.58618.3c. [DOI] [PubMed] [Google Scholar]
- Tardif JC, McMurray JJ, Klug E, Small R, Schumi J, Choi J, Cooper J, Scott R, Lewis EF, L’Allier PL, Pfeffer MA. Lancet. 2008b;371:1761–8. doi: 10.1016/S0140-6736(08)60763-1. [DOI] [PubMed] [Google Scholar]
- Terauchi Y, Takamoto I, Kubota N, Matsui J, Suzuki R, Komeda K, Hara A, Toyoda Y, Miwa I, Aizawa S, Tsutsumi S, Tsubamoto Y, Hashimoto S, Eto K, Nakamura A, Noda M, Tobe K, Aburatani H, Nagai R, Kadowaki T. J Clin Invest. 2007;117:246–57. doi: 10.1172/JCI17645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uehara Y, Shimizu H, Sato N, Shimomura Y, Mori M, Kobayashi I. Diabetes Res. 1991;17:131–4. [PubMed] [Google Scholar]
- Weir GC, Sharma A, Zangen DH, Bonner-Weir S. Acta Diabetol. 1997;34:177–84. doi: 10.1007/s005920050071. [DOI] [PubMed] [Google Scholar]
- Yang Z, Chen M, Ellett JD, Carter JD, Brayman KL, Nadler JL. Am J Transplant. 2005;5:475–83. doi: 10.1111/j.1600-6143.2005.00707.x. [DOI] [PubMed] [Google Scholar]
- Ye J, Laychock SG. Endocrinology. 1998;139:4155–63. doi: 10.1210/endo.139.10.6244. [DOI] [PubMed] [Google Scholar]