Abstract
The parasite Trypanasoma cruzi is responsible for Chagas disease and its triatomine vector, Rhodnius prolixus, has a symbiotic relationship with the soil bacterium, Rhodococcus rhodnii.
R. rhodnii that was previously genetically engineered to produce the anti-microbial peptide, cecropin A was co-infected with T. cruzi into R. prolixus resulting in clearance of the infectious T. cruzi in 65% of the vectors. Similar anti-microbial peptides have been isolated elsewhere and were studied for differential toxicity against T. cruzi and R. rhodnii. Of the six anti-microbial peptides tested, apidaecin, magainin II, melittin, and cecropin A were deemed potential candidates for the Chagas paratransgenic system as they were capable of killing T. cruzi at concentrations that exhibit little or no toxic effects on R. rhodnii. Subsequent treatments of T. cruzi with these peptides in pair-wise combinations resulted in synergistic killing, indicating that improvement of the 65% parasite clearance seen in previous experiments may be possible utilizing combinations of different anti-microbial peptides.
Keywords: Trypanosoma cruzi, Anti-microbial Peptides, Rhodnius prolixus, Rhodococcus rhodnii, Lethal Concentration 100 (LC100), Fractional Inhibitory Concentration Index (FIC), Minimal Inhibitory Concentration (MIC), Minimal Bactericidal Concentration (MBC), Paratransgenesis, Chagas disease
1. Introduction
Trypanosoma cruzi is an intracellular protozoan parasite responsible for Chagas disease, also known as American trypanosomiasis. Chagas disease is endemic throughout regions of Mexico, Central America, and South America where an estimated eight to eleven million people are infected and twenty-five million are at risk (Dias, et al., 2002). Approximately 30% of infected people develop debilitating and sometimes life-threatening medical conditions resulting from abnormalities in heart, colon, and esophageal structures (Bern, et al., 2007). These medical conditions range from megaesophagus and megacolon to heart arrhythmias and sudden death.
Methods of Chagas disease control employed to date have had varying degrees of success and include those that address vector-borne transmission and transmission resulting from T. cruzi-tainted blood transfusions. Vector-targeted strategies such as pesticide regimens have significantly decreased the transmission of T. cruzi in areas where employed (Costa, et al., 1998, Schofield, 1985), but have severe practical limitations for long-term use. The greatest limitation of these treatments is the selection of pesticide-resistant insect vectors that render future applications ineffective (WHO, 1992). In addition, pesticide treatments as well as other vector-directed strategies such as housing improvements are expensive, and frequently out of the means of the communities in the impoverished regions affected by Chagas disease.
Infection occurs primarily upon transmission of T. cruzi parasites to humans via contact with feces of an infected triatomine vector. Within the vector, trypomastigotes differentiate into replicative epimastigotes that divide by binary fission in the midgut. Upon passage to the hindgut, epimastigotes differentiate into infective bloodstream trypomastigotes. As the insect feeds on a human and engorges, it defecates, and trypomastigotes that are consequently deposited adjacent to bite wound can enter the vertebrate host (Brener, 1973).
Common triatomines that vector trypanosomiasis belong to the genera Triatoma, Rhodnius, and Panstrongylus. Rhodnius prolixus is an important vector of Chagas disease in northern parts of South America and Central America. This vector harbors a obligate symbiotic bacterium, Rhodococcus rhodnii, which is required in the hindgut lumen of the insect for survival (Baines, 1956, Harrington, 1960).
In the paratransgenic model of Chagas disease prevention, the obligate midgut symbiont, R. rhodnii, is transformed with a “shuttle” DNA plasmid that expresses a protein product toxic to or capable of interfering with T. cruzi in the extracellular hindgut lumen of the R. prolixus vector. Delivery of the transformed bacteria can be accomplished and maintained by simulating coprophagic spread of symbionts (Durvasula, et al., 1999).
In earlier studies, transformed R. rhodnii that expressed the anti-microbial peptide (AMP) cecropin A were mixed with defibrinated rabbit blood and fed to aposymbiotic R. prolixus. These paratransgenic vectors were allowed to engorge on T. cruzi DM28 epimastigote-laden human blood over a ninety day period. At day 104, 65% of the insects examined had complete clearance of viable T. cruzi. The remaining 35% of the bugs had significantly reduced numbers of parasites (Durvasula, et al., 1997).
Cecropin A is an antimicrobial peptide (AMP) involved in the humoral innate immune response of many lepidopterans (Bulet, et al., 1999). These “AMP's” are amphipathic, highly basic molecules that differentially bind to host and bacterial membranes based on charge and composition. Most AMP's disrupt the membranes of non-host cells, causing stress in the lipid bilayer that ultimately leads to lysis of the cell wall and cell death, however, other modes of action by AMP's have been reported including interfering with host metabolism and targeting cytoplasmic components.
Over the past few years, many AMP's have been found and characterized. We have selected specific AMP's to be evaluated together for synergistic effects on T. cruzi and identified combinations that might be linked in a new paratransgenic system. This subset includes molecules from five distinct organisms that range in size from 18 to 90 amino acids, and have been shown to be toxic to specific bacterial populations. Apidaecin (Casteels, et al., 1989, Castle, et al., 1999) and melittin (Asthana, et al., 2004, Habermann, 1972) are produced by the honeybee, Apis mellifera, cecropin A by the silk moth, Hyalophora cecropia, magainin II (Zasloff, 1987) by African clawed frog Xenopus laevis, moricin by the silk worm, Bombyx mori (Hara and Yamakawa, 1995, Hemmi, et al., 2002), and penaeidin by the Tiger shrimp, Penaeus monodon (Chiou, et al., 2005). We evaluated these peptides in toxicity assays against Escherichia coli, R. rhodnii, and T. cruzi. Suitable candidate AMP's were selected based upon their differential activity against the parasites described by the ratio of T. cruzi LC100 to R. rhodnii minimum bactericidal concentration (MBC). From this group of peptides, pair-wise combinations were found that resulted in synergistic toxicity against T. cruzi.
2. Materials and Methods
2.1. Bacteria, protozoa, and Medias
Liquid and solid-agar bacterial media were prepared from powdered formulations at standard concentrations supplied by Becton Dickinson (LB, BHI, LIB, SOB, Tryptone, Yeast Extract, and Agar). Chemically competent E. coli DH5α cells were obtained from Invitrogen. Rhodococcus rhodnii was obtained from ATCC (NCIB 11279 [KCC A-0203, N445; B/O, NRRL B-16535]) and cultured in BHI media at 28°C. Trypanosoma cruzi (M/HOM/AR/74/CA-I CL72) was purchased from ATCC and cultured in Liver-Infusion Broth supplemented with 10% heat-inactivated FCS (“BenchMark,” Gemini Bio-Products), and 10 mg/l Haemin (Alfa Aesar). Parasite cultures were maintained at 25°C in BD-Falcon T-25 tissue culture flasks and passaged weekly 1:5 into fresh media.
2.2. Anti-Microbial Peptides
The AMP's were purchased or synthesized to >95% purity. Apidaecin 14 from the honeybee, A. mellifera (GNNRPVYIPQPRPPHPRL-NH2 (Casteels, et al., 1989, Castle, et al., 1999)) was synthesized by Alpha Diagnostic International. Moricin from the domestic silkworm, B. mori (AKIPIKAIKTVGKAVGKGLRAINIASTANDVFNFLKPKKRKH (Hara and Yamakawa, 1995, Hemmi, et al., 2002)) and penaeidin from the black tiger shrimp, P. monodon (QGYQGGYTRPFPRPPYGGGYHPVPVCTSCHRLSPLQARACCRQLGRCCDAKQTYG (Chiou, et al., 2005)) were synthesized by Bio-Synthesis, Inc. Cecropin A from the moth, H. cecropia (KWKLFKKIEKVGQNIRDGIIKAGPAVAVVGQATQIAK-NH2 (Bulet, et al., 1999)), was purchased from AnaSpec, Inc. Magainin II from the African clawed frog, X. laevis (GIGKFLHSAKKFGKAFVGEIMNS (Zasloff, 1987)), and melittin from A. mellifera venom (GIGAVLKVLTTGLPALISWIKRKRQQ-NH2 (Asthana, et al., 2004, Habermann, 1972)) were purchased from GenScript Corporation. All peptides were soluble in water and stock solutions of 10 mM were prepared in sterile phosphate buffered saline (137 mM NaCl, 2.7 mM KCl, 10 mM phosphate buffer, pH 7.3-7.5 at 25°C).
2.3. Minimal Inhibitory Concentration (MIC) Determinations
The MIC of a given peptide on either bacterium was defined as the lowest concentration in a treatment series of the antimicrobial resulting in a decrease in cell viability. Liquid cultures of stationary phase E. coli and R. rhodnii were prepared by overnight incubation with shaking at 37°C and 28°C, respectively. The optical densities of the cultures at 600 nm were adjusted to 0.3 by dilution into sterile media. Dilutions of each AMP from a 10 mM stock were made in sterile PBS to 100× treatment concentrations and 10 μl of each were added to sterile 1.5 ml microfuge tubes. To each treatment tube, 990 μl of diluted E. coli or R. rhodnii were added, mixed, and incubated with shaking at 37°C and 28°C, respectively. Growth inhibition was measured visually by loss of turbidity and spectrophotometrically by a decrease in absorbance at 600 nm compared to untreated controls.
2.4. Minimum Bactericidal Concentration (MBC) Determinations
The MBC for each species of bacteria was defined as the lowest concentration of a given peptide that resulted in both, elimination of all growth on nutrient agar Petri dishes after treatment, and liquid culture absorbencies at 600 nm equal to that of the 100% kill control (100 μg/ml Carbenicillin) in the 96-well dish assay. E. coli and R. rhodnii liquid cultures and the AMP dilutions were prepared as described above. One microliter of the peptide dilutions at 100× concentrations were added to the wells of sterile 96-well “Microtest” assay dishes (Becton Dickinson) in triplicate. To each well, 100 μl of diluted E. coli or R. rhodnii was added and mixed by swirling. The peptide and cell dilutions were also added to 1.5 ml sterile microfuge tubes and mixed by inversion. The 96-well assay dishes were incubated for either 48 hours at 28°C (R. rhodnii), or 24 hours at 37°C (E. coli) after which the O.D. at 600 nm for each well was determined. The microfuge tube treatments were incubated at the cell-appropriate temperatures for 3 hours and subsequently transferred to 15 ml Falcon “Snap-cap” tubes containing 3 ml of fresh media. After overnight incubations, the cells were pelleted by centrifugation at 3,000 rpm for 5-10 minutes, re-suspended in 100 μl fresh media, and plated onto LB or BHI-agar. These plates were incubated overnight and examined for colony growth. All compounds resulting in a decrease in culture growth by O.D.600 measurements were defined as bacteriostatic (capable of inhibiting the growth of bacteria), while those that eliminated growth completely from the agar plates were defined as bactericidal.
2.5. Determination of Peptide Toxicity (LC100) Against T. cruzi
The LC100 value for a given toxin is the concentration that causes death in an environmental medium following a certain period of exposure. To determine these values for the AMP's against T. cruzi, apidaecin, cecropin A, magainin II, and melittin were serially diluted by a factor of two from 1 mM to 1 μM into triplicate wells of duplicate 96-well assay plates. Continuous cultures of T. cruzi were maintained in LIT media and split 1:5 weekly for no more than 10 passages total. On day 1 post splitting, the cultures were washed, placed in 1/10 volume fresh media, counted 3 times on a “Brite-Line” hemacytometer (Sigma) at 40× magnification, and re-suspended to a titer of 1.1 × 106 T. cruzi/ml. Diluted parasites (90 μl) were added to each well containing 10 μl peptides or controls to give a final titer of 1 × 105 parasites/well in AMP concentrations from 10 to 0.01 μM. The assay plates were incubated at 25°C and read on the Gemini XPS spectrofluorometer (Molecular Devices, Corp) at 600 nm daily for 96 hours. Results were averaged for the triplicate wells. Pair-wise treatment of T. cruzi was carried out as described above with two AMPs per treatment. Concentration values represent the final amount of each peptide in a given treatment. Each treatment was performed three times on triplicate samples. Synergistic, additive, or antagonist interactions between the AMP's were determined by examination of the Fractional Inhibitory Concentration (FIC) index calculated for each set at the IC50 (the concentration of paired AMP's resulting in a 50% reduction in untreated-culture growth after 96 hours) or MIC (the first concentration in a paired-AMP series resulting in a decrease in culture growth) concentrations. FIC indices are determined by the equation; FIC Index = FICA + FICB = A/ICA + B/ICB, where A and B = MIC or IC50 of peptides in combination and is defined as an interaction coefficient indicating whether the combined inhibitory effect of drugs is additive, antagonistic, or synergistic (White, et al., 1996).
2.6. Recovery of T. cruzi from 1.0 μM and 10.0 μM AMP Treatments
After 96 hour treatments, media from T. cruzi cultures treated with 1 μM and 10 μM concentrations of AMP's (singly or in pair-wise combinations described above) was replaced with fresh media and incubations continued for 96 hours further in the absence of AMP's. Growth in these cultures was analyzed on the spectrofluorometer as described above.
3. Results
3.1. Minimum Inhibitory Concentration Determinations
MIC values of apidaecin, cecropin A and magainin II were less for E. coli than R. rhodnii (Table 1). Penaeidin did not have an effect on either bacterium. Moricin had the same MIC value (1 μM) in both bacteria and melittin showed inhibition of R. rhodnii at a lower concentration than that required for E. coli. The MIC for these peptides against E. coli ranged from 10 nM for magainin II to 10 μM for melittin, and against R. rhodnii ranged from 1 μM for moricin to greater than 100 μM for cecropin A. Due to its apparent lack of activity in these assays, penaeidin was not used in subsequent studies.
Table 1.
Minimal Inhibitory Concentration (MIC) and Minimal Bactericidal Concentration (MBC) values for antimicrobial peptides determined after 24 hour single AMP treatments. All samples were plated in triplicate and values were averaged from three independent trials. Comparison of the toxicity profiles for Rhodococcus rhodnii and Trypanosoma cruzi. MBC/LD100 values less than 1.0 indicates that the peptide is more toxic to R rhodnii than to T.cruzi. Values greater than 1.0 indicated greater toxicity against T. cruzi. Equal values indicate that the peptide is as toxic to the R rhodnii as to T. cruzi.
| Peptide | MIC – R. rhodnii | MIC – E. coli | MBC - R. rhodnii | MBC – E. coli | LD(100) – T. cruzi | MBC/LD100 |
|---|---|---|---|---|---|---|
| Apidaecin | 100 uM | 100 nM | >320 uM | 10 uM | 199 uM | >1.6 |
| Cecropin A | >100 uM | 3 uM | >320 uM | 20 uM | 80 uM | >4.0 |
| Magainin | 10 uM | 10 nM | >320 uM | 30 uM | 33 uM | >10.7 |
| Melittin | 3 uM | 10 uM | 80 uM | 10 uM | 30 uM | 2.4 |
| Moricin | 1 uM | 1 uM | 10 uM | 2 uM | 10 uM | 1 |
3.2. Minimum Bactericidal Concentration Determinations
MBC values of the peptides for E. coli were lower than those for R. rhodnii (Table 1). Apidaecin, cecropin A, and magainin II were not bactericidal to Rhodococcus even at the highest concentration tested (320 μM) while Rhodococcus MBC values for melittin and moricin were at 80 μM and 10 μM, respectively. MBC values against E. coli fell within the range of 2 to 30 μM. Moricin showed the greatest toxicity against both organisms (2 μM MBC for E. coli, 10 μM MBC for R. rhodnii), while magainin II showed the least (30 μM MBC for E. coli, >320 μM MBC for R. rhodnii). The MIC and MBC values for melittin against E. coli were equal. This was not the case for R. rhodnii where melittin exhibited the more standard pattern of activity; inhibiting growth at 3 μM and killing it at 80 μM.
3.3. Determination of Peptide Toxicity (LC100) against T. cruzi
T. cruzi liquid cultures were treated with each peptide to determine lethal concentrations (LC100) for comparison to the MBC concentrations identified for E. coli and R. rhodnii (Table 1).
In single-peptide treatments only moricin was equally toxic to R. rhodnii and T. cruzi and was therefore eliminated from pair-wise testing. Comparison of the T. cruzi LC100 values to the R. rhodnii MBC values (Table 1) provides a measure to rank the peptides according to their differential toxicity against the parasite versus the symbiotic bacterium. Such differential toxicity is a crucial component of the paratransgenic approach. Apidaecin, cecropin A, magainin II, and melittin all exhibited greater toxicity against T. cruzi versus R. rhodnii and were thus considered suitable candidates for combination in paratransgenic studies.
3.4. Determination of T. cruzi killing by Dual Peptide Treatment
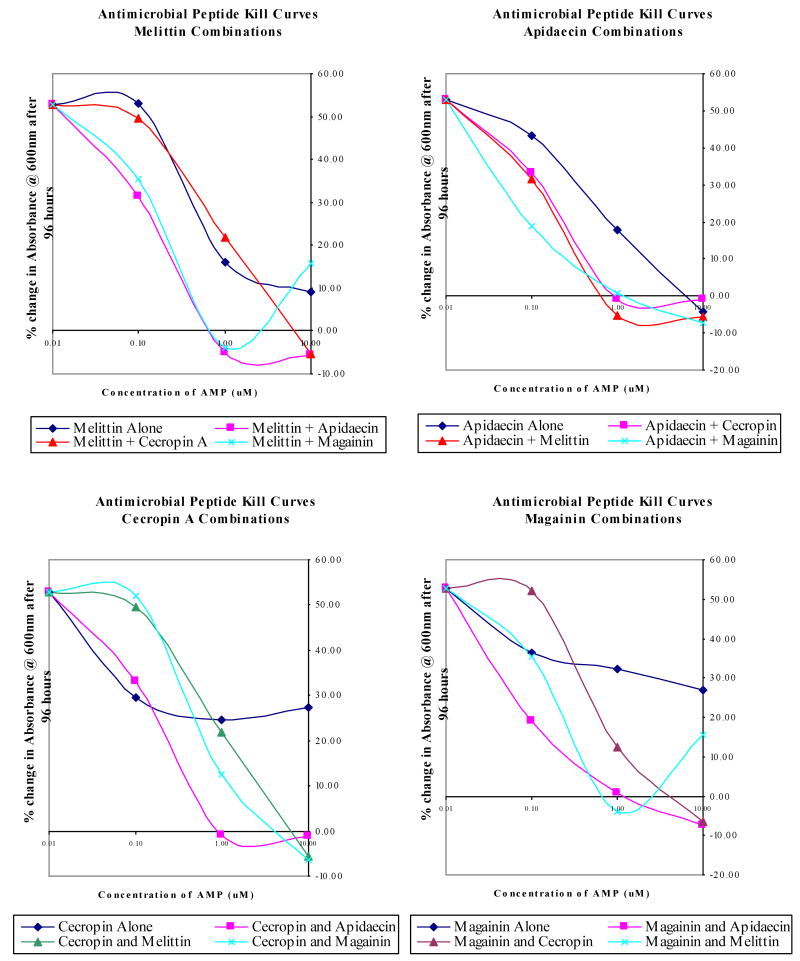
In dual peptide toxicity studies against T. cruzi, a variety of activity profiles was observed (Figure 1). Figure 1 depicts the activity of each peptide alone and in combination with the other AMP's. The data is presented as the percent change in turbidity of the T. cruzi cultures over the 96 hour incubation period vs. the concentration of added AMP's. Each of the peptides alone was capable of inhibiting the growth of T. cruzi significantly in the concentration range tested as evidenced by decreased levels of turbidity change. Addition of a second AMP to the treatment regimens resulted in additive, synergistic, or antagonistic effects on the level of growth inhibition. Most commonly, the effect of dual treatment was increased toxicity. Clearing of the cultures, indicated by a negative percent change in turbidity, was detected at the high concentrations of peptide and determined by microscopy to be the result of cell lysis. LC100 values (Table 1) were determined by a dual set of criteria, the first being a zero percent change in culture growth as measured spectrophotometrically during the 192 hour assay period (96 hour treatment followed by 96 hour incubation in fresh media without peptide). The second criterion was absence of visible parasites or parasite movement in the treated cultures by light microscopy at 100× magnification. All peptide combinations examined were trypanostatic, that is, capable of inhibiting the growth of trypanosomes. Peptide combinations that resulted in complete elimination of viable parasites were termed trypanocidal.
Fig. 1.
Antimicrobial peptide kill curves for dual-combination treatments of T. cruzi liquid cultures. Results were averaged from triplicate samples in three independent experiments. Results are displayed as the average percent change in absorbance at 600 nm minus the average percent change in untreated controls. Negative values represent lysis of the parasites by the AMPs with associated loss in turbidity.
Cecropin A, the peptide that was toxic to T. cruzi in the initial paratransgenic studies, failed to reach the LC100 value in the 0.1 to 10 μM range (Figure 1). However, cecropin A concentrations in the 1.0 to 10 μM range were completely trypanocidal to T. cruzi when coupled with either melittin or apidaecin at similar concentrations. These additive effects provide strong evidence that a paratransgenic system employing AMPs in combination, rather than singly, might result in more efficient parasite killing.
Apidaecin alone exhibits poor activity against T. cruzi, with an LC100 of 199 μM (Table 1). When used in pair wise treatments, apidaecin at concentrations of 1.0 to 10.0 μM achieved complete parasite killing in conjunction with similar concentrations of cecropin A, melittin, and magainin II (Figure 1). No antagonistic interactions were observed with apidaecin and synergy, as defined by the FIC equation, was noted between apidaecin and magainin II at the IC50 concentrations.
Single peptide treatments of melittin or magainin II, similar to cecropin A, were unable to reach the LC100 value at the 0 to 10 μM range. Melittin and magainin II interacted in multiple patterns when used in paired treatments, exhibiting synergy at lower concentrations and antagonism at the highest. T. cruzi kill assays of apidaecin in combination with cecropin A, melittin, or magainin II, display characteristics that predict improved parasite clearance.
3.5. Recovery of AMP-treated cells after transfer to non-AMP containing media
In all cultures treated for 96 hours with a single AMP between 0.1 and 10 μM then allowed to recover for 96 hours in AMP-free media, growth was resumed as evidenced by an increase in culture turbidity except for those treated with 10 μM melittin. This fact suggests that in this concentration range, single AMP treatments with apidaecin, cecropin A, and magainin II are trypanostatic, that is, inhibitory but not lethal. Growth after removal of the AMP was minimal in the cultures treated with the low concentrations of melittin compared to the other AMP's, reaching only 4% of non-treated control levels. In contrast to single AMP treatments, growth was not resumed in T. cruzi cultures treated with dual AMP's at trypanocidal concentrations for 96 hours and allowed 96 hour recovery in fresh media. The only exception to this was the melittin-magainin II combination that resulted in resumed growth at 10 μM, the concentration where antagonism between the molecules was observed.
4. Discussion
4.1. Evaluation of the AMP's for use in the paratransgenic system
The toxicity of anti-microbial peptides against E. coli and R. rhodnii is of particular importance towards development of a more efficient paratransgenic system for elimination of T. cruzi within its reduviid vector. In earlier studies with cecropin A both the E. coli and R. rhodnii strains expressing the AMP were able to survive, expand, and express measurable product. It is therefore reasonable to predict that other AMP's with similar MIC and MBC values will function efficiently without substantial lethality to E. coli cloning or R. rhodnii symbiotic strains. MIC and MBC values determined for three of the peptides tested; apidaecin, magainin II, and melittin, fell within a range suitable for future paratransgenic systems (Table 1).
The results for apidaecin suggest strongly that it would function more efficiently in T. cruzi clearance than cecropin A alone in the paratransgenic system. Its apparent inactivity against R. rhodnii, low LC100 concentration against the parasite (defined here as that concentration of peptide capable of eliminating culture growth and parasite activity during treatment and subsequent to removal of the AMP from the culture), and obvious additive or synergistic effects when in combination with the other AMP's, are the desired chemical characteristics. In addition, apidaecin did not exhibit antagonism with the other tested AMP's (Figure 1, Tables 2 & 3), further emphasizing the potential utility of apidaecin as an effector molecule to reduce the ability of paratransgenic R. prolixus to transmit T. cruzi.
Table 2.
IC50 and Fractional Inhibitory Concentration (FIC) Index values for 96 hour antimicrobial peptide treatments of T. cruzi. Treatment results were averaged from triplicate wells in three independent trials. Peptide interactions were determined from the equation; FIC Index = FICA + FICB = A/IC50A + B/IC50b, where A and B equal the IC50 of the peptides in combination. FIC index values less than 0.5 indicate synergistic interaction. FIC index values between 0.5 and 4.0 indicated additive interaction, and FIC index values greater than 4.0 indicate antagonism.
| Peptide A | Peptide B | IC50A | IC50B | A | B | FIC | Interaction |
|---|---|---|---|---|---|---|---|
| Apidaecin | Cecropin | 0.70 uM | 0.80 uM | 0.31 | 0.31 | 0.83 | Additive |
| Apidaecin | Magainin | 0.70 uM | 10.00 uM | 0.08 | 0.08 | 0.12 | Synergistic |
| Apidaecin | Melittin | 0.70 uM | 0.80 uM | 0.25 | 0.25 | 0.67 | Additive |
| Cecropin | Magainin | 0.80 uM | 10.00 uM | 0.70 | 0.70 | 0.95 | Additive |
| Cecropin | Melittin | 0.80 uM | 0.80 uM | 0.80 | 0.80 | 2.00 | Additive |
| Magainin | Melittin | 10.00 uM | 0.80 uM | 0.30 | 0.30 | 0.41 | Synergistic |
Table 3.
Minimal Inhibitory Concentration (MIC) and Fractional Inhibitory Concentration Index (FIC) values for 96 hour antimicrobial treatments of T. cruzi. Treatment results were averaged from triplicate wells in three independent trials. Peptide interactions were determined from the equation; FIC Index = FICA + FICB = A/MICA + B/MICB, where A and B equal the MIC of the peptides in combination. FIC index values less than 0.5 indicate synergistic interaction. FIC index values between 0.5 and 4.0 indicated additive interaction, and FIC index values greater than 4.0 indicate antagonism.
| Peptide A | Peptide B | MIC-A | MIC-B | A | B | FIC | Interaction |
|---|---|---|---|---|---|---|---|
| Apidaecin | Cecropin | 0.1 uM | 0.1 uM | 0.1 | 0.1 | 2.0 | Additive |
| Apidaecin | Magainin | 0.1 uM | 0.1 uM | 0.1 | 0.1 | 2.0 | Additive |
| Apidaecin | Melittin | 0.1 uM | 1.0 uM | 0.1 | 0.1 | 1.1 | Additive |
| Cecropin | Magainin | 0.1 uM | 0.1 uM | 1.0 | 1.0 | 20 | Antagonistic |
| Cecropin | Melittin | 0.1 uM | 1.0 uM | 1.0 | 1.0 | 11 | Antagonistic |
| Magainin | Melittin | 0.1 uM. | 1.0 uM | 0.1 | 0.1 | 1.41 | Additive |
In contrast to the results achieved with single AMP treatments, all pair-wise combination treatments in the range of 0.1 to 10 μM were able to achieve LC100 levels after 96 hours. This observation predicts that a paratransgenic system employing multiple AMP's would be far superior to those employing a single AMP.
While inhibition of T. cruzi growth was apparent after treatment with all peptides except penaeidin, loss of this inhibition resulted in the re-entry of a portion of the treated parasites to active vegetative growth, evident after removal of apidaecin, cecropin A, melittin, and magainin II from single AMP treatments. The ability of the dual AMP treatments with these peptides to achieve LC100 implies that the additive or synergistic effects of the inhibitory activities of multiple peptides can be lethal to T. cruzi. The sustained additive or synergistic effects of multiple peptides in the paratransgenic system would likely improve the 65% T. cruzi elimination rate seen in earlier studies with the single cecropin A peptide (Durvasula, et al., 1997).
Moricin was the most efficient anti-bacterial peptide tested, exhibiting the lowest MIC and MBC levels. This result was not unexpected as earlier studies clearly showed moricin to be highly toxic to multiple bacterial species: 0.31 μM MBC against E. coli and 0.19 μM against B. subtilis (Hara and Yamakawa, 1995). Moricin is also the most basic molecule examined here in agreement with the trend of higher basic character correlating with higher toxicity (Hemmi, et al., 2002). Interestingly, moricin was also more toxic to the bacteria than to the protozoa, a fact that rendered it unusable in the paratransgenic system.
Cecropin A, used as the toxic agent in the earlier paratransgenic studies, has some obvious limitations that the current studies suggest can be overcome by addition of a second AMP to the system. Cecropin A alone after 96 hour treatment resulted in no greater than 50% growth inhibition at the 0 to 10 μM concentration range. In combination with apidaecin, magainin II, or melittin, 100% lethality was achieved. Less favorably, cecropin A displayed antagonism against both magainin II and melittin at the lowest concentration tested. These results suggest that the concentration of certain AMP sets inside the insect vector may need to be within precise limits to avoid unwanted interactions between them. It is possible that cloning of the genes for different AMP's into the same expression vector may set the expression levels of one peptide compared to the other in ratios that can be evaluated for their efficacy against T. cruzi. These studies have been carried out utilizing a single strain of T.cruzi representing a human isolate and will be repeated on representative members of different strains including vector and animal reservoir isolates. By determination of strain-specific differences in AMP sensitivities, combinations of AMP's may be employed that result in improved efficiencies against geographically restricted strains of T. cruzi.
Finally, several new AMP's are available that could be studied for their applicability to our paratransgenic system. By examination and comparison of the physical characteristics of AMP's with toxicity against T. cruzi, it may be possible to optimize deployment of anti-parasitic molecules in the paratransgenic approach to control of Chagas disease transmission.
Acknowledgments
We would like to thank Dr. Nicole Klein, M.D. for her assistance with the toxicity assays. This work was funded by NIH/NIAID grant #AI-066045-01.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Asthana N, Yadav SP, Ghosh JK. Dissection of antibacterial and toxic activity of melittin: a leucine zipper motif plays a crucial role in determining its hemolytic activity but not antibacterial activity. Journal of Biological Chemistry. 2004;279:55042–55050. doi: 10.1074/jbc.M408881200. [DOI] [PubMed] [Google Scholar]
- Baines S. The role of the symbiotic bacteria in the nutrition of Rhodnius prolixus (Hemiptera) Journal of Experimental Biology. 1956;33:533–541. [Google Scholar]
- Bern C, Montgomery SP, Herwaldt BL, Rassi A, Jr, Marin-Neto JA, Dantas RO, Maguire JH, Acquatella H, Morillo C, Kirchhoff LV, Gilman RH, Reyes PA, Salvatella R, Moore AC. Evaluation and treatment of Chagas disease in the United States: A systematic review. Journal of the American Medical Association. 2007;298:2171–2181. doi: 10.1001/jama.298.18.2171. [DOI] [PubMed] [Google Scholar]
- Brener Z. Biology of Trypanosoma cruzi. Annual Review of Microbiology. 1973;27:347–382. doi: 10.1146/annurev.mi.27.100173.002023. [DOI] [PubMed] [Google Scholar]
- Bulet P, Hetru C, Dimarcq JL, Hoffmann D. Antimicrobial peptides in insects; structure and function. Developmental and Comparative Immunology. 1999;23:329–344. doi: 10.1016/s0145-305x(99)00015-4. [DOI] [PubMed] [Google Scholar]
- Casteels P, Ampe C, Jacobs F, Vaeck M, Tempst P. Apidaecins: Antibacterial peptides from honeybees. European Molecular Biology Organization Journal. 1989;8:2387–2391. doi: 10.1002/j.1460-2075.1989.tb08368.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castle M, Nazarian A, Yi SS, Tempst P. Lethal effects of apidaecin on Escherichia coli involve sequential molecular interactions with diverse targets. Journal of Biological Chemistry. 1999;274:32555–32564. doi: 10.1074/jbc.274.46.32555. [DOI] [PubMed] [Google Scholar]
- Chiou TT, Wu JL, Chen TT, JK L. Molecular cloning and characterization of cDNA of Penaeidin-like antimicrobial peptide from Tiger Shrimp (Penaeus monodon) Marine Biotechnology. 2005;7:119–127. doi: 10.1007/s10126-004-3164-4. [DOI] [PubMed] [Google Scholar]
- Costa FC, Vitor RW, Antunes CM, Carneiro M. Chagas Disease Control Programme in Brazil: A study of the effectiveness of 13 years of intervention. Bulletin of the World Health Organization. 1998;76:385–391. [PMC free article] [PubMed] [Google Scholar]
- Dias JC, Silveira AC, Schofield CJ. The impact of Chagas disease control in Latin America: A review. Memórias do Instituto Oswaldo Cruz. 2002;97:603–612. doi: 10.1590/s0074-02762002000500002. [DOI] [PubMed] [Google Scholar]
- Durvasula RV, Gumbs A, Panackal A, Kruglov O, Aksoy S, Merrifield RB, Richards FF, Beard CB. Prevention of insect-borne disease: An approach using transgenic symbiotic bacteria. Proceedings of the National Academy of Science U S A. 1997;94:3274–3278. doi: 10.1073/pnas.94.7.3274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durvasula RV, Gumbs A, Panackal A, Kruglov O, Taneja J, Kang AS, Cordon-Rosales C, Richards FF, Whitham RG, Beard CB. Expression of a functional antibody fragment in the gut of Rhodnius prolixus via transgenic bacterial symbiont Rhodococcus rhodnii. Medical and Veterinary Entomology. 1999;13:115–119. doi: 10.1046/j.1365-2915.1999.00175.x. [DOI] [PubMed] [Google Scholar]
- Habermann E. Bee and wasp venoms. Science. 1972;177:314–322. doi: 10.1126/science.177.4046.314. [DOI] [PubMed] [Google Scholar]
- Hara S, Yamakawa M. Moricin, a novel type of antibacterial peptide isolated from the silkworm, Bombyx mori. Journal of Biological Chemistry. 1995;270:29923–29927. doi: 10.1074/jbc.270.50.29923. [DOI] [PubMed] [Google Scholar]
- Harrington JS. Studies on Rhodnius prolixus: Growth and development of normal and sterile bugs, and the symbiotic relationship. Parasitology. 1960;50:279–286. doi: 10.1017/s0031182000025373. [DOI] [PubMed] [Google Scholar]
- Hemmi H, Ishibashi J, Hara S, Yamakawa M. Solution structure of moricin, an antibacterial peptide, isolated from the silkworm Bombyx mori. Federation of European Biochemical Societies Letters. 2002;518:33–38. doi: 10.1016/s0014-5793(02)02637-6. [DOI] [PubMed] [Google Scholar]
- Schofield CJ. Control of Chagas' disease vectors. British Medical Bulletin. 1985;41:187–194. doi: 10.1093/oxfordjournals.bmb.a072048. [DOI] [PubMed] [Google Scholar]
- White RL, Burgess DS, Manduru M, Bosso JA. Comparison of three different in vitro methods of detecting synergy: time-kill, checkerboard, and E test. Antimicrobial Agents and Chemotherapy. 1996;40:1914–1918. doi: 10.1128/aac.40.8.1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. Vector Resistance to Pesticides 1992 [Google Scholar]
- Zasloff M. Magainins, a class of antimicrobial peptides from Xenopus skin: isolation, characterization of two active forms, and partial cDNA sequence of a precursor. Proceedings of the National Academy of Science USA. 1987;84:5449–5453. doi: 10.1073/pnas.84.15.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]