Abstract
Combination chemotherapy forms the backbone of cancer treatment. There is a need for new drug combinations for the treatment of mantle cell lymphoma (MCL). Herein, we show that gallium maltolate, a novel gallium compound, synergizes with bortezomib, a proteasome inhibitor, to induce cell death in MCL Granta cells. Cells exposed to either agent displayed caspase-3 activation, a loss of mitochondrial membrane potential, and a decrease in chymotrypsin-like activity. These effects were increased with both agents in combination. Our results show for the first time that the proteasome may be a target for gallium maltolate and suggest that the therapeutic potential of combination bortezomib and gallium maltolate warrants further investigation.
Keywords: Mantle cell lymphoma, bortezomib, gallium maltolate, chemotherapy, drug synergy, proteasome inhibition, apoptosis
1. INTRODUCTION
Despite advances in the treatment of non-Hodgkin’s lymphoma, a significant number of patients die from this disease each year. Hence, there is a great need to develop new drugs and strategies for the treatment of this malignancy and to explore novel drug combinations for use in the clinic. Several clinical trials have shown gallium nitrate, a metallodrug approved for the treatment of hypercalcemia of malignancy, to have significant activity in non-Hodgkin’s lymphoma [1]. Interestingly, mantle cell lymphomas (MCL) may be among the lymphoma subtypes more responsive to this drug [2].
The mechanisms of antineoplastic activity of gallium are only partly understood. Gallium shares similarity with iron in that it binds to transferrin, the iron transport protein present in the circulation, and may be taken up by cells via cell surface transferrin receptor-mediated endocytosis [3–5]. Prior studies have shown that gallium-induced cell death is, in part, related to gallium’s interference with iron-dependent processes, including cellular iron uptake and the activity of the iron-containing R2 subunit of ribonucleotide reductase [6]. Gallium nitrate also activates Bax and induces apoptosis through the mitochondrial pathway [7].
The development of gallium compounds with greater efficacy than gallium nitrate is of considerable interest as it may advance the use of gallium in the clinic. In recent preclinical studies, we showed that a novel compound, gallium maltolate, inhibits the growth of lymphoma cells resistant to gallium nitrate and has significantly greater antineoplastic activity than gallium nitrate against a panel of lymphoma cell lines [8].
The proteasome inhibitor bortezomib is used clinically for the treatment of MCL [9]. Because of the clinical sensitivity of this type of lymphoma to gallium nitrate and the need to develop novel therapeutic drug combinations, we examined the combined effects of gallium maltolate and bortezomib in MCL Granta cells. Our results reveal that these agents act synergistically to inhibit cell growth and induce cell death. Unexpectedly, we also discovered that gallium maltolate inhibits cellular proteasome activity, thus identifying a new pathway of action for this gallium compound.
2. Materials and Methods
2.1 Materials
Gallium maltolate was obtained from Titan Pharmaceuticals (South San Francisco, CA). Bortezomib was from Millenium Pharmaceuticals (Cambridge, MA). 3-(4,5-dimethlythiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) was purchased from Sigma Chemical Company (St. Louis, MO). JC-1 dye (5,5,6,6-tetrachloro-1,1,3,3-tetraehylbenzimidazolycarbocyanine iodide) was obtained from Cell Technology Inc. (Mountain View, CA).
2.2 Cells
The MCL Granta cell line was obtained from the British Columbia Cancer Agency and was maintained in RPMI 1640 medium with 10% fetal bovine serum in an atmosphere of 5% C02.
2.3 Cell proliferation assay
The proliferation of Granta cells in the presence of gallium maltolate and bortezomib singly and in combination was examined by MTT assay, as previously described [7].
2.3 Caspase-3 assay
The activity of effector caspases 3/7 was measured using an Apo-ONE Homogenous Caspase-3/7 assay, based on the enzymatic cleavage of the fluorogenic caspase substrate Z-DEVD-R110, as recommend by the manufacturer (Promega, Madison WI). Fluorescence was measured at 485/530 nm in a spectrofluorometer.
2.4 Mitochondrial Membrane Potential
The effect of gallium maltolate and bortezomib on mitochondrial permeability transition was examined using JC-1 dye as recommended by the manufacturer. Cells that had been incubated with these agents were washed, stained with JC-1 reagent, and analyzed by two parameter flow cytometry.
2.5 Proteosome activity
Cells were assayed for chymotrypsin-like activity of the proteasome as described by Daniel et al [10], using the fluorogenic peptide Suc-LLVY-AMC. Cells incubated with bortezomib, gallium maltolate, or both agents in combination for 24 h were disrupted in 50 mM Tris pH 8.0/150 mM NaCl, 0.5% NP-40/0.5% PMSF/0.5 mM DTT buffer and centrifuged to remove cellular debris. The supernatant was assayed for chymotrypsin-like activity over 60 min at 37°C in a reaction mixture containing 40 μg cytosolic protein, 20 μM Suc-LLVY-AMC, and 50 mM Tris pH 7.5 (total volume 100 μL) in a 96-well microplate. The fluorescence intensity in the wells was measured at excitation and emission wavelengths of 380 nm and 460 nm, respectively.
3. RESULTS AND DISCUSSION
3.1 Gallium maltolate acts synergistically with bortezomib to inhibit the proliferation of Granta cells, induce loss of mitochondrial membrane potential, and activate caspase-3
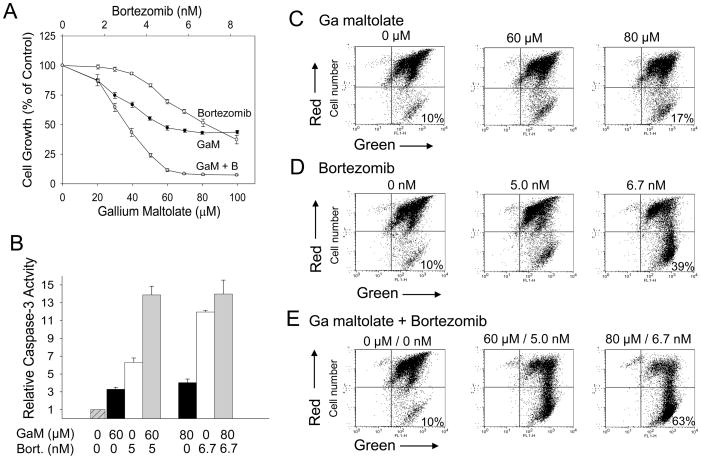
Whereas the individual antineoplastic activities of gallium maltolate and bortezomib in vitro and in vivo have previously been reported, their action in combination in MCL has not been examined. Such preclinical studies are important because they may provide clinically relevant information regarding the use of these agents in combination therapy to treat lymphoma and other hematologic malignancies. In the experiment shown in Figure 1A, as expected, both gallium maltolate and bortezomib as single agents inhibited the proliferation of Granta cells. Using the approach of Chou and Talalay for rigorous assessment of drug synergy [11], Granta cells were incubated with a combination of both gallium maltolate and bortezomib in a fixed molar ratio and the effect on cell proliferation was determined. As shown in Figure 1A, both drugs added together produced a far greater inhibition of cell growth than either drug alone; additional analysis of this drug interaction confirmed that the combined effect of gallium maltolate and bortezomib on Granta cell growth was truly synergistic (Table 1). Consistent with the induction of cell death by these agents, caspase-3 activity was increased in cells exposed to bortezomib and gallium maltolate alone; this was further increased when cells were incubated with both drugs in combination (Figure 1B).
Figure 1. Bortezomib synergistically enhances gallium maltolate-induced cell in mantle cell lymphoma cells.
A. Cell proliferation. Granta cells were plated in a 96-well plate (0.2 × 106 cells/ml) in the presence of increasing concentrations of gallium maltolate (GaM), bortezomib, or both drugs in combination at a fix molar ratio. Cell growth was measured by MTT assay after a 48-h incubation. Values shown represent means ± S.E. (n = 3). GaM, gallium maltolate; B, bortezomib. B. Caspase 3 activity. Granta cells incubated with increasing concentrations of gallium maltolate (GaM, black bar), bortezomib (Bort, white bar), or both drugs in combination (gray bar) were analyzed for caspase-3 activity after a 24 h incubation. Values shown are the means ± S.E. of a representative experiment performed in triplicate. C – E. Mitochondrial membrane permeability. Granta cells were analyzed by JC-1 staining after incubation with gallium maltolate (C), bortezomib (D), or both agents (E) for 24 h as described in Materials and Methods. A loss of mitochondrial membrane potential (increase in membrane permeability) results a decrease in mitochondrial red fluorescence (represented in the upper right quadrant on dot plot) and an increase in cytoplasmic green fluorescence (represented in the lower right quadrant on dot plot). The percentage of cells with green fluorescence is shown.
TABLE 1.
Combination Indices (CI) for Gallium Maltolate and Bortezomib in Combination
The CI was determined from the dose-response curves in Figure 1A. A CI of <1.0 is consistent with drug synergy.
| Growth inhibition | CI |
|---|---|
| 50% | 0.95 |
| 75% | 0.68 |
| 90% | 0.54 |
| 95% | 0.47 |
It is known that an early step in the induction of apoptosis via the intrinsic mitochondrial pathway involves a loss of mitochondrial membrane potential and the subsequent release of cytochrome c from the mitochondrion to the cytoplasm. This, in turn, results in the activation of apoptotic protease activating factor-1 and caspase-9 and the subsequent activation of caspase-3. [12]. To examine the effects of gallium maltolate and bortezomib on mitochondrial membrane potential, cells incubated with these agents were stained with JC-1 dye and examined for changes in red fluorescence in the mitochondria and the appearance of green fluorescence in the cytoplasm indicative of loss of mitochondrial membrane potential. As shown in Figure 1C and 1D, the fraction of cells displaying cytoplasmic green fluorescence increased with increasing concentrations of gallium maltolate or bortezomib. When both these agents were combined they produced greater loss of mitochondrial membrane potential than either agent alone (Figure 1E). Collectively, these experiments show that gallium maltolate, a novel gallium compound, acts synergistically with bortezomib to induce cell death through via action on the mitochondrion in MCL cells.
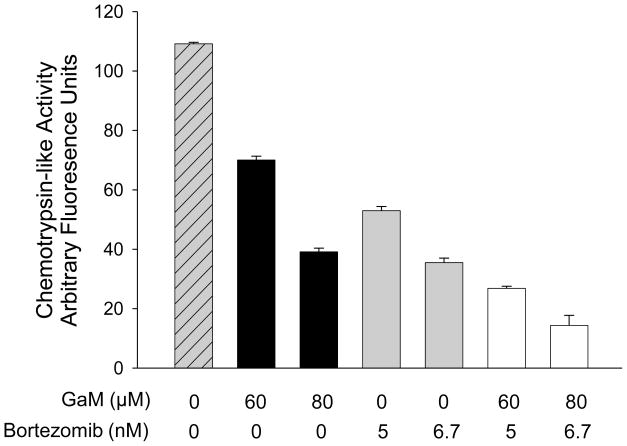
3.2 Inhibition of chymotrypsin-like activity by gallium maltolate and bortezomib
Consistent with its known inhibitory action on the proteasome, cells incubated with bortezomib displayed a decrease in chymotrypsin-like activity (Figure 2). Unexpectedly, exposure of cells to gallium maltolate alone also resulted in a decrease in chymotrypsin-like activity; the combination of both agents resulted in a further decrease in chymotrypsin-like activity (Figure 2). These studies provide new information regarding the mechanisms of action of gallium maltolate and show for the first time that this gallium compound may inhibit cell growth, in part, through an action on proteasomal activity. Accordingly, the synergistic effects of gallium maltolate and bortezomib on apoptosis-induction may be in part a result of their combined action on a common target, the proteasome. Whether the action of gallium maltolate is the result of a direct or indirect action of the metal complex on the proteasome remains to be determined; further studies are planned to address this question.
Figure 2. Inhibition of proteasome activity by gallium maltolate and bortezomib.
Granta cells were incubated with gallium maltolate, bortezomib, or both agents for 24 h. Chymotrypsin-like activity in the cell lysates was measured using the fluorogenic peptide Suc-LLVY-AMC substrate as described under Methods and Materials. Bars, means ± S.E. of a representative experiment performed in triplicate.
Gallium maltolate is among a next generation of gallium compounds in preclinical and early clinical development. Other gallium compounds in development include tris(8-quinolinolato) gallium (KP46), G4544, gallium thiosemicarbazone, and gallium methylpyridine and methylphenolate complexes [13–16]. Interestingly, one of the gallium complexes synthesized by using asymmetrical ligands containing pyridine and 2,6-substituted phenol moieties was recently shown to inhibit proteasome activity [16]. It is important to note that these various gallium compounds may have diverse biological actions. Indeed, our recent studies examining cross-resistance of lymphoma cells to gallium compounds suggest that mechanisms of antineoplastic action of gallium maltolate differ from that of gallium nitrate. This is illustrated by the finding that CCRF-CEM lymphoma cells with acquired resistance to gallium nitrate and p53 mutant lymphoma cells with endogenous resistance to gallium nitrate are still sensitive to the cytotoxicity of gallium maltolate [8]. Further investigation of the biologic actions of gallium maltolate will undoubtedly provide new insight into the handling of gallium compounds by lymphoma cells and may yield new information regarding cellular processes that could serve as therapeutic targets for metallodrugs in general. The ability of gallium maltolate to synergistically enhance the cytotoxicity of bortezomib in MCL cells has important clinical implications and warrants further study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Straus DJ. Gallium nitrate in the treatment of lymphoma. Semin Oncol. 2003;30:25–33. doi: 10.1016/s0093-7754(03)00173-8. [DOI] [PubMed] [Google Scholar]
- 2.Pro B, Bociek RG, Chitambar CR, Gregory SA, Leonard JP, Smith S, Novick S. Phase 2 multicenter trial of gallium nitrate in patients with advanced non-Hodgkin’s lymphoma (NHL) Blood. 2004;104:682A. [Google Scholar]
- 3.Larson SM, Rasey JS, Allen DR, Nelson NJ, Grunbaum Z, Harp GD, Williams DL. Common pathway for tumor cell uptake of Gallium-67 and Iron-59 via a transferrin receptor. J Natl Cancer Inst. 1980;64:41–53. [PubMed] [Google Scholar]
- 4.Chitambar CR, Seligman PA. Effects of different transferrin forms on transferrin receptor expression, iron uptake and cellular proliferation of human leukemic HL60 cells: Mechanisms responsible for the specific cytotoxicity of transferrin-gallium. J Clin Invest. 1986;78:1538–1546. doi: 10.1172/JCI112746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chitambar CR, Zivkovic Z. Uptake of gallium-67 by human leukemic cells: Demonstration of transferrin receptor-dependent and transferrin-independent mechanisms. Cancer Res. 1987;47:3929–3934. [PubMed] [Google Scholar]
- 6.Chitambar CR, Matthaeus WG, Antholine WE, Graff K, O’Brien WJ. Inhibition of leukemic HL60 cell growth by transferrin-gallium: Effects on ribonucleotide reductase and demonstration of drug synergy with hydroxyurea. Blood. 1988;72:1930–1936. [PubMed] [Google Scholar]
- 7.Chitambar CR, Wereley JP, Matsuyama S. Gallium-induced cell death in lymphoma: role of transferrin receptor cycling, involvement of Bax and the mitochondria, and effects of proteasome inhibition. Mol Cancer Ther. 2006;5:2834–2843. doi: 10.1158/1535-7163.MCT-06-0285. [DOI] [PubMed] [Google Scholar]
- 8.Chitambar CR, Purpi DP, Woodliff J, Yang M, Wereley JP. Development of gallium compounds for treatment of lymphoma: Gallium maltolate, a novel hydroxypyrone gallium compound induces apoptosis and circumvents lymphoma cell resistance to gallium nitrate. J Pharmacol Exp Ther. 2007;322:1228–1236. doi: 10.1124/jpet.107.126342. [DOI] [PubMed] [Google Scholar]
- 9.Kane RC, Dagher R, Farrell A, Ko CW, Sridhara R, Justice R, Pazdur R. Bortezomib for the treatment of mantle cell lymphoma. Clin Cancer Res. 2007;13:5291–5294. doi: 10.1158/1078-0432.CCR-07-0871. [DOI] [PubMed] [Google Scholar]
- 10.Daniel KG, Gupta P, Harbach RH, Guida WC, Dou QP. Organic copper complexes as a new class of proteasome inhibitors and apoptosis inducers in human cancer cells. Biochem Pharmacol. 2004;67:1139–1151. doi: 10.1016/j.bcp.2003.10.031. [DOI] [PubMed] [Google Scholar]
- 11.Chou T-C, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 12.Zimmermann KC, Bonzon C, Green DR. The machinery of programmed cell death. Pharmacol Ther. 2001;92:57–70. doi: 10.1016/s0163-7258(01)00159-0. [DOI] [PubMed] [Google Scholar]
- 13.Hofheinz RD, Dittrich C, Jakupec MA, Drescher A, Jaehde U, Gneist M, Graf von KN, Keppler BK, Hochhaus A. Early results from a phase I study on orally administered tris(8-quinolinolato)gallium(III) (FFC11, KP46) in patients with solid tumors--a CESAR study (Central European Society for Anticancer Drug Research--EWIV) Int J Clin Pharmacol Ther. 2005;43:590–591. doi: 10.5414/cpp43590. [DOI] [PubMed] [Google Scholar]
- 14.Novick SC, Julian TN, Majuru S, Mangelus M, Brown BD, Mehta B, Warrell RP. Initial phase I clinical and pharmacokinetic assessment of G4544, an oral gallium-containing compound. J Clin Oncol. 2008;26:8592. [Google Scholar]
- 15.Arion VB, Jakupec MA, Galanski M, Unfried P, Keppler BK. Synthesis, structure, spectroscopic and in vitro antitumour studies of a novel gallium(III) complex with 2-acetylpyridine 4N-dimethylthiosemicarbazone. J Inorg Biochem. 2002;91:298–305. doi: 10.1016/s0162-0134(02)00419-1. [DOI] [PubMed] [Google Scholar]
- 16.Chen D, Frezza M, Shakya R, Cui QC, Milacic V, Verani CN, Dou QP. Inhibition of the proteasome activity by gallium(III) complexes contributes to their anti prostate tumor effects. Cancer Res. 2007;67:9258–9265. doi: 10.1158/0008-5472.CAN-07-1813. [DOI] [PubMed] [Google Scholar]