Abstract
Background
Variation at the human μ-opioid receptor has been associated with alcohol abuse. The A118G (N40D) polymorphism in humans is functionally mimicked by the C77G (P26R) polymorphism in rhesus monkeys; both show similar in vitro influences on ligand binding and in vivo correlations with physiological measures as well as behavioral measures including predilection towards alcohol consumption. Naltrexone, an antagonist at the receptor, has been used to treat alcoholism in humans and has been reported to show differences in effectiveness depending on genotype.
Methods
Here we describe a study in which we a priori selected rhesus monkeys based on genotype at the OPRM1 C77G single nucleotide polymorphism, trained them to self-administer alcohol, and assessed naltrexone responsiveness.
Results
Alcohol intake in rhesus monkeys varied with genotype across a range of alcohol concentrations (0.5 – 4% w/v) such that animals with the G/G genotype drank consistently more alcohol than those animals with the C/C genotype. Additionally, naltrexone attenuated alcohol drinking in a dose- and genotype-dependent manner. Animals harboring the G/G genotype were more sensitive to the effects of naltrexone and showed greater reductions in alcohol consumption at lower naltrexone doses compared to animals with a C/G or C/C genotype.
Conclusions
This preliminary study demonstrates a pharmacogenomic response to naltrexone in rhesus monkeys that parallels that seen in humans. This finding provides a basis for developing a pharmacogenetic animal model for naltrexone effect that can expand further our understanding of the causes and treatments of alcohol use disorders.
Keywords: naltrexone, opioid receptor mu, polymorphism, rhesus macaque, pharmacogenetic
1. Introduction
The human μ-opioid receptor gene (OPRM1) harbors a functional single nucleotide polymorphism (SNP), A118G, that has been associated with alcohol dependence (Bond et al., 1998; Bart et al., 2005; Kim et al., 2004; Ray and Hutchison, 2004; Rommelspacher et al., 2001; Smolka et al., 1999; Town et al., 1999). Humans with at least one copy of the G118 allele report a lower craving for alcohol and a higher alcohol-induced “high” across rising breath alcohol concentrations, as compared to individuals that harbor two A118 alleles (Ray and Hutchison, 2007). The G118 allele also has been associated with an increased risk of alcohol dependence (Bart et al., 2005).
The μ-opioid receptor is a primary target of naltrexone, an approved pharmacotherapy for the treatment of alcoholism (Blumberg and Dayton, 1973). Across several studies, A118G has been associated with naltrexone responsivity and favorable treatment outcomes in alcoholics (Anton et al., 2008). Naltrexone more effectively blunted the reported alcohol-induced high in individuals with the G118 allele (Ray and Hutchison, 2007) and alcoholics with the G118 allele showed significantly lower rates of relapse and a longer time to return to heavy drinking (Oslin et al., 2003). A Korean cohort also showed a greater therapeutic effect of naltrexone in individuals with at least one copy of the G118 variant genotype compared to A118 homozygotes (Kim et al., 2009). Other studies, however, fail to associate A118G and naltrexone treatment response in alcoholics (Arias et al., 2008; Gelernter et al., 2007; Mitchell et al., 2007; Tidey et al., 2008).
The rhesus monkey OPRM1 gene harbors a similar though distinct SNP, C77G, which similarly affects the ability of the receptor to bind the endogenous ligand β-endorphin (Bond et al., 1998; Miller et al., 2004). Because of the putative association between the human SNP and naltrexone efficacy, we undertook to examine whether we could detect differences in the effects of naltrexone between animals harboring different OPRM1 C77G genotypes.
2. Methods
2.1. Subjects
Six adult outbred and unrelated male rhesus monkeys (Macaca mulatta) of Indian origin, weighing 6.7–12.2 kg and unrelated a minimum of three generations, were selected by a priori genotyping. There is no evidence to suggest that variation not physically linked to the candidate polymorphism is not independently segregating among the subjects. Monkeys were housed in rooms with 12-h light/dark cycles. Monkeys had unrestricted access to water and were fed a diet of chow (Harlan Teklad Monkey Diet, Harlan Teklad, Madison, WI) and fresh fruit. All animals were maintained in accordance with the guidelines of the Committee on Animals of the Harvard Medical School and the “Guide for Care and Use of Laboratory Animals” of the Institute of Laboratory Animal Resources, National Research Council, Department of Health, Education and Welfare Publication No. (NIH) 85-23, revised 1996. Research protocols were approved by the Harvard Medical School Institutional Animal Care and Use Committee.
2.2. Genotyping
Animals were genotyped at OPRM1 C77G using FRET-based hybridization probe melting analysis performed on a LightCycler 2.0 (Roche Applied Science) using manufacturer protocols. Genotypes for 250+ animals were determined and frequencies presented (Vallender et al., 2008). From these animals representatives were chosen for each genotype. All animals in this study were resequenced for the complete transcribed sequence of the OPRM1 gene following previous methods (Vallender et al., 2008). C77G was in complete linkage disequilibrium with 5′ untranslated region polymorphisms so any associations found are with the extended haplotype rather than with C77G specifically.
2.3. Procedure
Monkeys were housed individually in stainless-steel cages with one side modified to form a drinking panel. The panel included two retractable sippers (Med Associates, Inc., Georgia, VT) equipped with solenoids to minimize dripping and connected with tubing to reservoirs mounted outside. A response lever was positioned below each sipper and lights mounted above the sippers were illuminated to serve as visual stimuli. In these experiments, only one side of the panel (e.g., right sipper/lever/stimulus lights) was active. Water was provided ad libitum prior to and during the self-administration session, and animals were provided with their daily complement of food several hours after the conclusion of the self-administration session.
Monkeys were trained under a fixed-ratio schedule of oral alcohol reinforcement. In the presence of a white light, every tenth response resulted in illumination of a red light and a 30s extension of the sipper. Depression of the sipper resulted in alcohol delivery. Between extensions of the sipper, all lights were off briefly, and responses had no consequences. Self-administration sessions lasted 3h.
The reinforcing effects of a range of alcohol concentrations (0.5 – 6% w/v), as well as water, were evaluated. Each solution was available for at least five sessions and until intake was stable (no upward or downward trend in mLs and/or g/kg consumed over three consecutive days). The effects of naltrexone were determined on drinking maintained by 2% alcohol. Following stable alcohol self-administration, a two-day testing procedure was initiated. On day 1, monkeys received a saline (vehicle) injection 30m before the self-administration session. On day 2, monkeys received a naltrexone (0.01 – 0.3 mg/kg) injection. The order of naltrexone concentrations were randomized for individual monkeys. Between each two-day test, monkeys returned to baseline self-administration. Finally, the procedure was repeated with a 4% alcohol maintenance solution.
Once intake was stabilized, monkeys were anesthetized with ketamine immediately following the session. Five mLs of blood was collected from the femoral vein, centrifuged at 3200 rpm for 8–12 minutes, the plasma drawn off and transferred to polypropylene tubes that were then frozen. Determinations of blood alcohol levels (BALs) were conducted using a rapid high performance plasma alcohol analysis using alcohol oxidase with AM1 series analyzer (Analox Instruments USA, Lunenberg, MA).
2.4. Data analysis
After each self-administration session, intake for individuals was determined in two ways: volume consumed and alcohol dose (g/kg) consumed. Dose was calculated as: (volume consumed in mLs * alcohol concentration in g/ml)/weight in kg. Because these measurements are correlated, we present only the data representing alcohol dose. To evaluate genotype effects on sensitivity to alcohol’s reinforcing effects, the mean intake across the last three sessions at each concentration was determined for each monkey. Mean intake (g/kg±SEM) was calculated for each genotype at each concentration. A two-way mixed factor ANOVA determined the effects of the between-group factor, genotype, and within-group factor, concentration, and their interaction. To evaluate genotype effects on naltrexone responsivity, intake after naltrexone was converted to percentage of intake after associated vehicle for each monkey. Mean intake (% vehicle±SEM) of alcohol was then calculated for each genotype at each naltrexone dose. Separate two-way mixed factor ANOVAs determined the effects of the between-group factor, genotype, and within-group factor, naltrexone dose, and their interaction, at each concentration. All significant effects were analyzed post-hoc with Tukey’s HSD test. All analyses were conducted with Systat12 statistical package. The alpha level for all tests was P≤0.05.
2.5. Drugs
Ethanol (95%; Pharmco Products, Brookfield, CT) was diluted to the appropriate concentrations with tap water. Naltrexone HCl was purchased from Sigma/RBI (St. Louis, MO) and dissolved in 0.9% saline solution. Intramuscular injection volumes were 0.02 mL/kg body weight.
3. Results
3.1. Alcohol self-administration
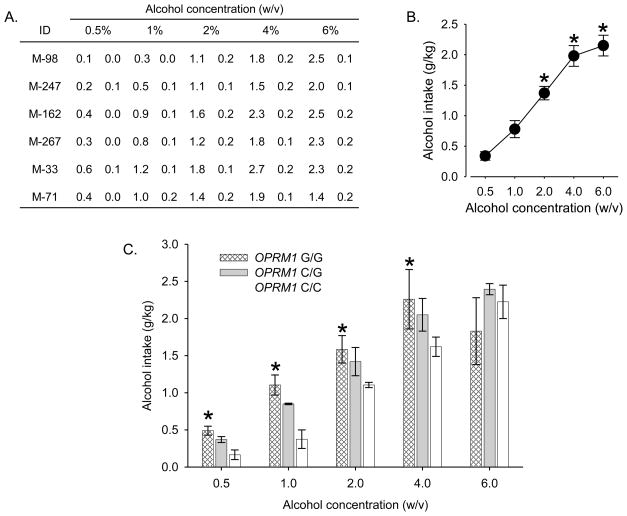
All monkeys acquired alcohol self-administration under the fixed-ratio schedule and intake was stable in each animal at all concentrations (Figure 1A). Variability between individuals was not associated with differences in age or weight (Supplemental Table 1). For the group, intake increased in a concentration-dependent manner (Figure 1B), and alcohol concentrations ≥2% maintained significantly higher intakes than concentrations of 0.5 or 1%. Intake appeared to reach asymptotic levels at concentrations of 4 and 6% alcohol. At these concentrations, monkeys drank approximately 2.0–2.2 g/kg of alcohol. Analysis of blood samples verified that the volumes consumed when concentrations ≥2% were available produced BALs in excess of 80 mg/dL in all monkeys (mean = 109.8 mg/dL; SEM±14.5). In humans, a BAL of 80 mg/dL is legal level for intoxicated driving in most jurisdictions. No significant differences in BALs were observed between individual monkeys.
Figure 1.
Alcohol self-administration in rhesus monkeys. A) Intake (mean g/kg±SD) of alcohol across the last three days of availability at each concentration in individual subjects. B) Mean intakes (±SEM) for the last three sessions at each concentration for the group of six monkeys. *: significant difference compared to 0.5% and 1% (w/v) alcohol solution (F[4,12]=92.5, p<0.0001; Tukey’s HSD test, p<0.05). C) Mean intakes (± range) for the last three sessions at each concentration for each genotype (N = 2 monkeys/genotype). *: significant increase compared to OPRM1 C/C (F[8,12]=2.9, p<0.05; Tukey’s HSD test, p<0.05).
Differences emerged when intake was examined as a function of genotype (Figure 1C). Monkeys with two G77 alleles (G/G) exhibited higher intakes than monkeys with two C77 alleles (C/C) and heterozygotes (C/G) were intermediate. For example, when 0.5% alcohol was available for self-administration, G/G drank the most alcohol (~0.49 g/kg), and C/C drank the least alcohol (~0.17 g/kg). C/G showed intermediate levels of intake (~0.37 g/kg). A similar pattern was apparent when 1 - 4% alcohol was available for self-administration. When a 6% concentration was available, no genotype-dependent effects were evident.
3.2. Naltrexone treatment
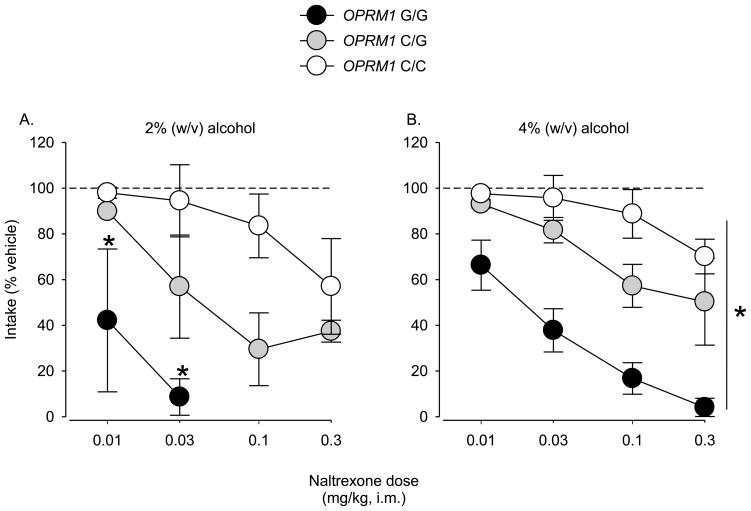
Naltrexone dose-dependently reduced self-administration of a 2% alcohol concentration in all monkeys (Figure 2A). However, low doses were more potent at reducing self-administration in G/G compared to C/C; again C/G effects were intermediate. The clearest example of this pattern was observed following administration of 0.03 mg/kg naltrexone. At this dose, intake markedly declined to 0.14 g/kg (91% reduction from vehicle levels ~1.52 g/kg) in G/G, to 0.91 g/kg (43% reduction from vehicle levels ~1.64 g/kg) in C/G, and did not decline in C/C (1.44 g/kg, 4% reduction from vehicle levels ~1.55 g/kg). At higher doses of naltrexone (0.3 mg/kg), intake in C/G was reduced to 38% of vehicle levels (0.61 g/kg; vehicle: ~1.68 g/kg) and in C/C to 57% of vehicle levels (0.86 g/kg; vehicle: ~1.53 g/kg).
Figure 2.
Reduction in self-administration of a A) 2% (w/v) alcohol solution following pretreatment with naltrexone in rhesus monkeys. *: significant reduction compared to OPRM1 C/C (F[2,3]=10.4, p<0.05; Tukey’s HSD test, p<0.05). Reduction in self-administration of a B) 4% (w/v) alcohol solution following pretreatment with naltrexone in rhesus monkeys. *: significant differences between all genotypes (F[2,3]=15.6, p<0.05; Tukey’s HSD test, p<0.05). Data are mean intakes (± range) expressed as a percentage of intake after vehicle administration for each genotype (N = 2 monkeys/genotype).
Similar genotype-dependent effects of naltrexone were evident when a higher concentration of alcohol was available for self-administration (Figure 2B). Naltrexone continued to be most potent at reducing self-administration in G/G. At this concentration, however, significant differences were also observed between C/G and C/C. In all genotypes, maximum reduction of alcohol self-administration occurred following pretreatment with the highest dose of naltrexone. At this dose, alcohol intake in G/G was reduced 96% from vehicle levels (0.09 g/kg; vehicle: ~2.30 g/kg). Intake in C/G was reduced 46% from vehicle levels (1.15 g/kg; vehicle: ~2.13 g/kg). Finally, in C/C intake was reduced 31% from vehicle levels (1.12 g/kg; vehicle: ~1.65 g/kg). Based on routine quantitative behavioral observations of the animals, Naltrexone, under our test conditions, did not induce changes in any species-typical behavior in any genotype.
4. Discussion
It has been demonstrated previously that rhesus monkeys harboring a G77 allele show an increased level of intake at a single alcohol concentration (Barr et al., 2007). However, the polymorphism minor allele frequency is sufficiently low across populations (20%, reported in (Vallender et al., 2008)) as to preclude the evaluation of G/G in this earlier study, despite assessing 82 individuals. Using an a priori genotyping approach we were able to balance genotypes (C/C, C/G, and G/G) and maximize our power to detect genotype-dependent effects and minimize animal usage. Using this approach we were able to not only recapitulate previous finding of increased alcohol consumption in monkeys with at least one G77 allele compared to C/C, but we also observed that G/G consume even larger amounts of alcohol C/G across a relatively wide range of alcohol concentrations (0.5 – 4% w/v). As a consequence of increased sensitivity, the G77 carrying individuals ultimately consume more alcohol compared to C/C, especially at lower alcohol concentrations (0.5 – 1% w/v). These data are in concordance with earlier studies that found G77 carrying rhesus macaques consumed greater volumes of alcohol and drink to intoxication (Barr et al., 2007). Association studies, both those presented here and others, do not necessitate that C77G be the causative SNP rather than some genetic variation with which it is in linkage disequilibrium; however, the former is highly likely given the results of previous in vitro studies (Bond et al., 1998; Miller et al., 2004) as well as homologies between effects across species.
When rhesus monkeys self-administered alcohol at either 2% or 4% concentrations, naltrexone dose-dependently attenuated alcohol intake. However, larger doses of naltrexone were required to attenuate intake of higher alcohol concentrations. Further, the effectiveness of naltrexone treatment was genotype-dependent. At both alcohol concentrations, an approximately 10- to 30-fold higher dose of naltrexone was required to reduce intake in C/C to the same level as that seen in the G/G. C/G display an intermediate level of responsivity. Viewed another way, a single dosing level of naltrexone will inhibit alcohol intake in G/G more than in C/C. This research is supported by recent complementary findings that while heterozygotes showed a significantly higher preference for alcohol than C77 homozygotes, this difference in preference effects was eliminated following naltrexone administration (Barr et al., 2009). While preliminary, this is one of the first demonstrations of a direct pharmacogenomic response in rhesus monkeys modeling a corresponding human response for a functionally similar, though genetically distinct, polymorphism.
Supplementary Material
Footnotes
Supplementary Materials are available with the online version of this article at doi:xxx/j.drugalcdep.xxx …
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anton RF, Oroszi G, O’Malley S, Couper D, Swift R, Pettinati H, Goldman D. An evaluation of mu-opioid receptor (OPRM1) as a predictor of naltrexone response in the treatment of alcohol dependence: results from the Combined Pharmacotherapies and Behavioral Interventions for Alcohol Dependence (COMBINE) study. Arch Gen Psychiatry. 2008;65:135–144. doi: 10.1001/archpsyc.65.2.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias AJ, Armeli S, Gelernter J, Covault J, Kallio A, Karhuvaara S, Koivisto T, Makela R, Kranzler HR. Effects of opioid receptor gene variation on targeted nalmefene treatment in heavy drinkers. Alcohol Clin Exp Res. 2008;32:1159–1166. doi: 10.1111/j.1530-0277.2008.00735.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr CS, Chen SA, Schwandt ML, Lindell SG, Sun H, Suomi SJ, Heilig M. Suppression of Alcohol Preference by Naltrexone in the Rhesus Macaque: A Critical Role of Genetic Variation at the mu-Opioid Receptor Gene Locus. Biol Psychiatry. 2009 doi: 10.1016/j.biopsych.2009.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr CS, Schwandt M, Lindell SG, Chen SA, Goldman D, Suomi SJ, Higley JD, Heilig M. Association of a functional polymorphism in the mu-opioid receptor gene with alcohol response and consumption in male rhesus macaques. Arch Gen Psychiatry. 2007;64:369–376. doi: 10.1001/archpsyc.64.3.369. [DOI] [PubMed] [Google Scholar]
- Bart G, Kreek MJ, Ott J, LaForge KS, Proudnikov D, Pollak L, Heilig M. Increased attributable risk related to a functional mu-opioid receptor gene polymorphism in association with alcohol dependence in central Sweden. Neuropsychopharmacology. 2005;30:417–422. doi: 10.1038/sj.npp.1300598. [DOI] [PubMed] [Google Scholar]
- Blumberg H, Dayton HB. Naloxone, naltrexone, and related noroxymorphones. Adv Biochem Psychopharmacol. 1973;8:33–43. [PubMed] [Google Scholar]
- Bond C, LaForge KS, Tian M, Melia D, Zhang S, Borg L, Gong J, Schluger J, Strong JA, Leal SM, Tischfield JA, Kreek MJ, Yu L. Single-nucleotide polymorphism in the human mu opioid receptor gene alters beta-endorphin binding and activity: possible implications for opiate addiction. Proc Natl Acad Sci U S A. 1998;95:9608–9613. doi: 10.1073/pnas.95.16.9608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelernter J, Gueorguieva R, Kranzler HR, Zhang H, Cramer J, Rosenheck R, Krystal JH. Opioid receptor gene (OPRM1, OPRK1, and OPRD1) variants and response to naltrexone treatment for alcohol dependence: results from the VA Cooperative Study. Alcohol Clin Exp Res. 2007;31:555–563. doi: 10.1111/j.1530-0277.2007.00339.x. [DOI] [PubMed] [Google Scholar]
- Kim SG, Kim CM, Choi SW, Jae YM, Lee HG, Son BK, Kim JG, Choi YS, Kim HO, Kim SY, Oslin DW. A micro opioid receptor gene polymorphism (A118G) and naltrexone treatment response in adherent Korean alcohol-dependent patients. Psychopharmacology (Berl) 2009;201:611–618. doi: 10.1007/s00213-008-1330-5. [DOI] [PubMed] [Google Scholar]
- Kim SG, Kim CM, Kang DH, Kim YJ, Byun WT, Kim SY, Park JM, Kim MJ, Oslin DW. Association of functional opioid receptor genotypes with alcohol dependence in Koreans. Alcohol Clin Exp Res. 2004;28:986–990. doi: 10.1097/01.alc.0000130803.62768.ab. [DOI] [PubMed] [Google Scholar]
- Miller GM, Bendor J, Tiefenbacher S, Yang H, Novak MA, Madras BK. A mu-opioid receptor single nucleotide polymorphism in rhesus monkey: association with stress response and aggression. Mol Psychiatry. 2004;9:99–108. doi: 10.1038/sj.mp.4001378. [DOI] [PubMed] [Google Scholar]
- Mitchell JM, Fields HL, White RL, Meadoff TM, Joslyn G, Rowbotham MC. The Asp40 mu-opioid receptor allele does not predict naltrexone treatment efficacy in heavy drinkers. J Clin Psychopharmacol. 2007;27:112–115. doi: 10.1097/JCP.0b013e31802e68b0. [DOI] [PubMed] [Google Scholar]
- Oslin DW, Berrettini W, Kranzler HR, Pettinati H, Gelernter J, Volpicelli JR, O’Brien CP. A functional polymorphism of the mu-opioid receptor gene is associated with naltrexone response in alcohol-dependent patients. Neuropsychopharmacology. 2003;28:1546–1552. doi: 10.1038/sj.npp.1300219. [DOI] [PubMed] [Google Scholar]
- Ray LA, Hutchison KE. A polymorphism of the mu-opioid receptor gene (OPRM1) and sensitivity to the effects of alcohol in humans. Alcohol Clin Exp Res. 2004;28:1789–1795. doi: 10.1097/01.alc.0000148114.34000.b9. [DOI] [PubMed] [Google Scholar]
- Ray LA, Hutchison KE. Effects of naltrexone on alcohol sensitivity and genetic moderators of medication response: a double-blind placebo-controlled study. Arch Gen Psychiatry. 2007;64:1069–1077. doi: 10.1001/archpsyc.64.9.1069. [DOI] [PubMed] [Google Scholar]
- Rommelspacher H, Smolka M, Schmidt LG, Samochowiec J, Hoehe MR. Genetic analysis of the mu-opioid receptor in alcohol-dependent individuals. Alcohol. 2001;24:129–135. doi: 10.1016/s0741-8329(01)00139-2. [DOI] [PubMed] [Google Scholar]
- Smolka M, Sander T, Schmidt LG, Samochowiec J, Rommelspacher H, Gscheidel N, Wendel B, Hoehe MR. mu-opioid receptor variants and dopaminergic sensitivity in alcohol withdrawal. Psychoneuroendocrinology. 1999;24:629–638. doi: 10.1016/s0306-4530(99)00017-7. [DOI] [PubMed] [Google Scholar]
- Tidey JW, Monti PM, Rohsenow DJ, Gwaltney CJ, Miranda R, Jr, McGeary JE, MacKillop J, Swift RM, Abrams DB, Shiffman S, Paty JA. Moderators of naltrexone’s effects on drinking, urge, and alcohol effects in non-treatment-seeking heavy drinkers in the natural environment. Alcohol Clin Exp Res. 2008;32:58–66. doi: 10.1111/j.1530-0277.2007.00545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Town T, Abdullah L, Crawford F, Schinka J, Ordorica PI, Francis E, Hughes P, Duara R, Mullan M. Association of a functional mu-opioid receptor allele (+118A) with alcohol dependency. Am J Med Genet. 1999;88:458–461. [PubMed] [Google Scholar]
- Vallender EJ, Priddy CM, Chen GL, Miller GM. Human expression variation in the mu-opioid receptor is paralleled in rhesus macaque. Behav Genet. 2008;38:390–395. doi: 10.1007/s10519-008-9207-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.