Summary
A prenatal hypothyroid state is associated with behavioral abnormalities in adulthood. Wistar–Kyoto (WKY) rats exhibit hypothyroidism and increased depressive and anxiety-like behaviors. Thus, the WKY could illuminate the mechanisms by which the reversal of developmental hypothyroidism in humans and animals results in adult behavioral improvement. We examined the outcome of maternal thyroxine (T4) treatment on thyroid hormone-regulated functions and adult behavior of the WKY offspring. Pregnant WKY dams completed gestation with and without T4 administration and their adult male offspring were tested. Measures included depressive and anxiety-like behaviors, and thyroid hormone (TH) concentrations in both plasma and specific brain regions. In addition, the expression of two proteins affecting thyroid hormone trafficking and metabolism, monocarboxylate transporter 8 (MCT-8) and iodothyronine deiodinase type III (Dio3), and of several behavior-altering molecules, glucocorticoid receptor (GR), prepro-thyrotropin releasing hormone (prepro-TRH) and corticotrophin releasing hormone (CRH), were determined in the hippocampus and amygdala of the offspring. Prenatal T4 treatment of WKYs did not affect adult depressive behavior but increased anxiety-like behavior and decreased plasma levels of THs. In the hippocampus of males treated with T4 in utero, Dio3 and MCT-8 protein levels were increased, while in the amygdala, there were increases of free T4, MCT-8, GR, prepro-TRH protein and CRH mRNA levels. These results show that T4 administration in utero programs adult peripheral and amygdalar thyroid hormone levels divergently, and that the resulting upregulation of anxiety-related genes in the amygdala could be responsible for the exacerbated anxiety-like behavior seen in WKYs after prenatal T4 treatment.
Keywords: Amygdala, anxiety, defensive burying, prenatal, thyroxine, Wistar Kyoto
Introduction
Early environmental disturbances are known to affect adult physiology and behavior as proposed in the “fetal origins of adult disease” concept of Barker (Barker et al. 2002). Prenatal thyroid function abnormalities can alter fetal brain development (Auso et al. 2004; Zoeller et al. 2004) leading to impairments in neuropsychological development and cognitive function of the adult offspring (Haddow et al. 1999; Obregon et al. 2007). The Wistar Kyoto (WKY) rat strain exhibits hypothyroidism and behavioral abnormalities including depressive behavior in the forced swim test (FST), and increased anxiety and passive coping in the defensive burying (DB) test (Ahmadiyeh et al. 2003; Ahmadiyeh et al. 2005; Ahmadiyeh et al. 2004; Baum et al. 2005; Nosek et al. 2008; Solberg et al. 2004). These physiological and behavioral phenotypes are determined by genetic and environmental influences, of which the genetic contribution has been explored extensively (Ahmadiyeh et al. 2003; Ahmadiyeh et al. 2005; Ahmadiyeh et al. 2004; Baum et al. 2005; Nosek et al. 2008; Solberg et al. 2004). In the current study we used the WKY to investigate the contribution of the hypothyroid milieu during prenatal development to adult behaviors.
Previously we have shown that depressive behavior in adulthood can result from prenatal exposure to alcohol-induced maternal hypothyroidism, since the depressive behavior can be relieved by administering T4 to the alcohol-consuming pregnant dam (Wilcoxon et al. 2005). Thus, administering T4 to pregnant hypothyroid WKYs could potentially ameliorate behavioral abnormalities in adult WKY offspring even though the behaviors depend on significant genetic contributions. In contrast, the WKY adult thyroid function should not be normalized because it is of genetic origin and because administration of T4 to the alcohol-consuming hypothyroid mother does not reverse adult hypothyroidism but instead suppresses adult thyroid function (Wilcoxon and Redei 2004).
Our previous results indicate that the WKY has differing sensitivity to thyroid hormones (TH) in the periphery vs. the brain. The WKY's peripheral response to exogenous TH compares to that of the Wistar rat strain, while WKY freezing behavior in the open field test of emotionality responds only to administration of high T3 dose, suggesting that WKY is centrally resistant to thyroid hormones (Redei et al. 2001). This observation points to brain-specific mechanisms, such as transporter systems and enzymatic control, which could be responsible for the divergence of brain and peripheral responses to TH. Because adult WKYs are centrally resistant to TH administration and because a large dose of T4 is needed to affect offspring behavior (Wilcoxon et al. 2005), we administered a supraphysiological dose of T4 to WKY dams.
Materials and Methods
Animals
All animal experimentation was carried out in accordance with the NIH guide for the care and use of laboratory animals and approved by the Northwestern Animal Use and Care Committee. Adult female WKY rats (Harlan, 14-16 weeks of age) were mated with WKY males overnight and gestational day 1 (G1) was assigned by the presence of sperm in vaginal smears. Thyroxine (T4, 20ug/ml) was given to pregnant dams via drinking water on gestation days G8-G20 (n=10, average body weight=202+/-5g on G8). Mean maternal intake of T4 was 1.1 +/-0.04 mg/day. Control dams (n=11) were given plain drinking water ad libitum.
Male offspring (n=8-12/group, one or two/litter/group) were weaned at 24 days of age, and tested in the DB and FST starting at 60 days of age between 0900h and 1200h. There was a two week rest period between the two tests and following the FST. Animals were then decapitated, trunk blood was collected into EDTA-coated tubes on ice, and plasma was obtained by centrifugation. Whole brains were stored in RNAlater at -80 °C and subsequently dissected as described previously (Wilcoxon et al. 2005).
Behavioral Testing
The DB test was originally developed as a behavioral test of anxiety (Treit et al. 1981). In the current study, the DB test was administered as described previously (Ahmadiyeh et al. 2004). Briefly, animals were habituated together with cagemates for 15 minutes in a Plexiglas chamber for 3 consecutive days. On day 4, animals were placed into the chamber individually with an electrified prod added. Upon contact, the prod delivered a 4.5mA shock from a generator (Lafayette Instruments, San Diego, California). Once the animal touched the prod, the 15 minute videotaped test period began. Behaviors, including latency to begin burying, total time spent burying (duration of burying), the number of approaches to the prod (defined as snout within 1.0cm of the prod), and time spent grooming, were scored.
The forced swim test (FST) of depressive behavior was administered 2 weeks after the DB test as previously described (Redei et al. 2001; Solberg et al. 2004). Briefly, animals were placed into a glass cylinder (30cm diameter, 45cm depth) containing 22-24°C tap water for 15 minutes. After 24 hours rats were again placed into the cylinder of water for 5 minutes. All testing took place between 1000h and 1400h. Activity during the second test was recorded for subsequent scoring using a technique in which behavior was scored as immobility, climbing, or swimming every 5s.
Radioimmunoassay
Plasma total T4, free T4, total T3, and free T3 were measured by RIA as previously described (Wilcoxon and Redei 2004). Corticosterone concentrations were measured as described previously (Wilcoxon and Redei 2004) in plasma using 125I-labeled Corticosterone RIA (MP Biochemicals, NY). The assay sensitivity was 1.2 ng/ml. All samples were analyzed in one assay for which the intra-assay CV was 2.4%.
T3 and T4 concentrations in hippocampus and amygdala
T3 and T4 were extracted as previously described (Campos-Barros et al. 1995) with some modifications as described below. Tissue concentrations were determined by RIA, using standard curves prepared from T4 and T3 (Sigma, St. Louis, MO). Tissue was homogenized in 3.5 volumes of 100% methanol containing 1mM propylthiouracil. The homogenates were extracted with chloroform-methanol (2:1), eluted with 0.5% CaCl2, evaporated, and taken up in 500ul RIA buffer. Samples were processed individually and each was assayed in duplicate. Recovery of T3 and T4 was determined by addition of [125I]-T4 or [125I]-T3 to each tissue sample during the initial extraction process. TH recovery averaged 80% for both groups. Concentrations are given as T4 or T3 ng/g wet tissue weight.
Western blots
Hippocampi and amygdalae were homogenized in ice-cold lysis buffer as described previously (Shukla et al. 2006). Samples containing 40ug protein were electrophoresed on 12% (w/v) SDS polyacrylamide gel and transferred onto polyvinylidene difluoride membranes for use with the following antibodies: anti-GR (1:1000, Santa Cruz Biotechnology, Santa Cruz, CA), anti-prepro-TRH (1:1000, Dr. E. Redei), anti-MCT-8 (1:1000, Upstate Biotechnology, Lake Placid, NY) and Dio3 (1:750, Novus Biologicals, Littleton, CO). β-actin protein levels were measured in the same membranes using a monoclonal antibody (1:10,000, Sigma, St. Louis, MO). The optical density of each protein was normalized to the corresponding β-actin signal using ImageJ software (NIH).
Quantitative real-time RT-PCR
Total RNA was isolated from individual amygdalae using Trizol reagent according the manufacturer's protocol (Life Technologies, Grand Island, NY, USA). Reverse transcription of 1ug total RNA was performed with reagents from TaqMan Reverse Transcription kit (Applied Biosystems, Branchburg, NJ). Resulting cDNA was used in quantitative real-time PCR with SYBR green chemistry (ABI 7300 Real-Time PCR System, Foster City, CA). Triplicate reactions were performed for each cDNA sample. Primers for CRH (IDT, Coralville, Iowa) were designed to span >1 exon: F: 5′ – CAGCCGTTGAATTTCTTGCA – 3′; R 5′ – AGCGGGACTTCTGTTGAGGTT – 3′. The 18s primer pair was obtained from ABI (Foster City, California). CRH mRNA expression was calculated relative to 18s using the 2−ΔΔCt method.
Statistical analysis
All data are expressed as mean +/- SEM. Student t-tests were conducted and correlations within the data were identified using Pearson r coefficients (Systat software, Chicago, IL). Some experiments used a subset of samples due to methodological differences among measures. Degrees of freedom for the different measures are indicated. p <0.05 was considered significant.
Results
Defensive burying test (DB)
Behaviors in the DB test, including latency to begin burying the prod, duration of burying, time spent grooming and number of approaches, were assessed in adult male offspring of WKY control and T4-treated mothers. In males treated with T4, we observed nonsignificant increases in the duration of burying time and in the number of approaches to the prod (data not shown). Latency to bury the prod after the initial shock was significantly lower (one-way ANOVA: F [1,14] = 11.0, p<.01, Figure 1A) and time spent grooming was significantly increased (one-way ANOVA: F[1,17]=4.8, p<.05, Figure 1B). Thus, prenatal T4 treatment resulted in behaviors that collectively indicate increased anxiety in adult WKY offspring.
Figure 1. Increased anxiety after prenatal T4 treatment.
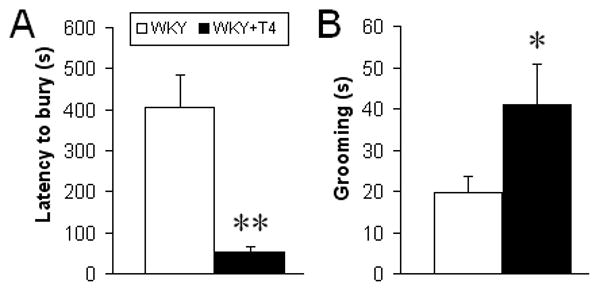
T4-treated males exhibited (A) decreased latency to bury the prod and (B) increased grooming in the DB test compared to controls (n=6-11 animals/group). No significant effect of prenatal T4 was observed in other DB measures: approaches, rears, or time spent burying the prod. One-way ANOVA, *p<0.05, **p<0.01.
Forced Swim Test (FST)
There were no differences in swimming, floating, or climbing behaviors between male offspring of control and T4-treated WKY dams (data not shown).
Corticosterone and thyroid hormone levels
There was no difference in plasma corticosterone levels between WKY and WKY+T4 offspring (WKY, 93.5 +/- 28.2 ng/ml; WKY+T4, 95.0 +/- 30.0 ng/ml, n=10/group). Prenatal T4 treatment resulted in nonsignificant suppression of total T4 (t(19) = 1.4, p=0.24) and free T4 (t(21) = 2.6, p=0.12) in the plasma, while total T3 (t(23) = 5.7, p<0.05) and free T3 (t(23) = 6.6, p<0.05) were significantly decreased (Table 1). In the brain, prenatal T4 treatment did not alter TH concentrations in the adult male hippocampus (Figure 2A), whereas the amygdala of prenatally T4-treated offspring showed a significant increase in free T4 concentration (t(11) = 15.9, p<0.01, Figure 2B) and a tendency of increased free T3 concentration (t(11) = 3.95, p=0.07, Figure 2B). While the active hormone T3 was not significantly increased in the amygdala of T4-treated WKYs, T3 levels in this region correlated significantly with latency to bury the prod in the DB test (r=-0.58, p<0.05).
Table 1. Plasma thyroid hormone levels in adult male WKY rats treated with T4 prenatally.
| Total | Free | |||
|---|---|---|---|---|
| WKY | WKY+T4 | WKY | WKY+T4 | |
| T4 | 73.16± 6.86 ug/dl | 68.52± 9.57 ug/dl | 1.85± 0.31 ng/dl | 1.64± 0.28 ng/dl |
| T3 | 81± 19.28 ng/dl | 66.41± 8.28* ng/dl | 8.3± 0.34 pg/ml | 7.1± 0.32* pg/ml |
Values are mean ± SEM. Student's t-test,
p<0.05.
n=10 animals/group.
Figure 2. Brain region-specific effects of prenatal T4 on thyroid hormone levels in adult offspring.
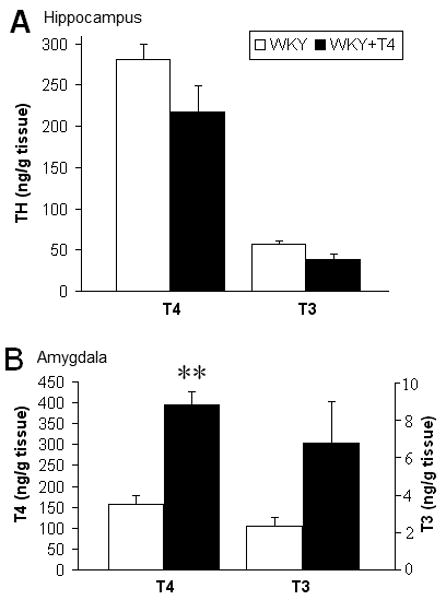
Free T4 and T3 concentrations were both non-significantly decreased in the hippocampus (A), but in the amygdala, T4 was significantly increased and T3 showed a similar trend (B). Student's t-test, n=6/group, **p<0.01.
Protein levels of MCT-8, Dio3, GR, and prepro-TRH
Protein levels of the MCT-8 TH transporter were significantly increased in both the hippocampus (t(7) = 9.4, p<0.05, Fig. 3A) and amygdala (t(7) = 6.8, p<0.05, Fig. 3B) of prenatally T4-treated offspring. The TH-inactivating enzyme Dio3 was also increased in the hippocampus of these offspring (t(7) = 9.2, p<0.05, Fig. 3A), but in the amygdala, Dio3 levels were very low as described elsewhere (Allen Brain Atlas, 2008; Lein et al., 2007), and were unchanged by prenatal T4 treatment (data not shown). In addition, there were increases of GR (t(7) = 9.6, p<0.05) and prepro-TRH (t(7) = 23.6, p<0.01) protein in the amygdala, while GR and prepro-TRH were unchanged in the hippocampus (data not shown).
Figure 3. Prenatal T4 treatment alters protein levels in the hippocampus and amygdala.
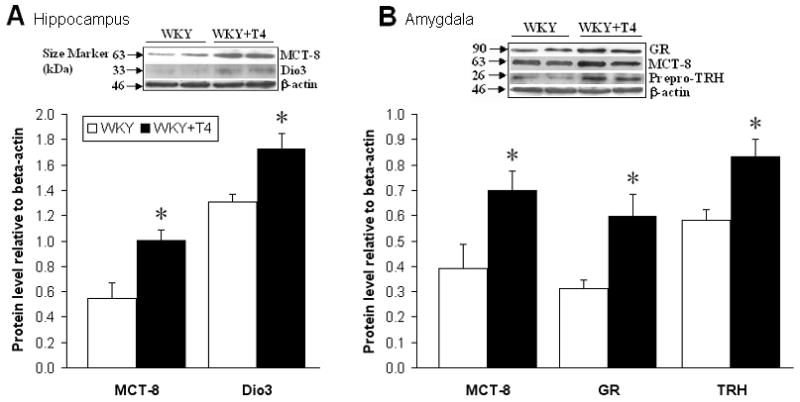
(A) Hippocampus: Levels of the TH transporter monocarboxylate transporter 8 (MCT-8) were increased and the TH-inactivating enzyme deiodinase-III (Dio3) was also elevated. (B) Amygdala: MCT-8 levels were increased along with glucocorticoid receptor (GR) and prepro-thyrotropin-releasing hormone (prepro-TRH) levels. Student's t-test, n=4/group, *p<0.05. Inserts are representative immunoblots.
CRH mRNA expression
Hippocampal CRH mRNA levels, as measured by quantitative real-time PCR, were approximately 50% of amygdala levels and barely detectable as found previously (Aguilar-Valles et al. 2005). Hippocampal CRH mRNA levels were not altered by prenatal T4 treatment (data not shown). In the amygdala, CRH mRNA levels were increased in offspring of T4 treated dams (WKY, 0.94 +/- 0.09; WKY+T4, 1.69 +/- 0.27; t(10)=6.5, n=6/group, p<0.05). This increase in CRH transcript expression was positively correlated with amygdala T4 (r=.76, p<.05) and T3 (r=.79, p<.05) levels.
Discussion
We intended to ameliorate adult depressive behavior with developmental T4 administration based on previous evidence that depressive behavior in adult offspring of alcohol consuming hypothyroid dams is reversed by supplementing maternal T4 (Wilcoxon et al. 2005). That we did not obtain this effect could be due to the genetic contribution to depressive behavior in this strain or to insufficiency of the T4 dose. Depressive-like behavior of adult WKYs can be ameliorated by large doses of T3 (Redei et al., 2001), suggesting that a behavioral change in the WKY requires supraphysiological thyroid hormone levels in the brain. The latter finding parallels human data wherein only supraphysiological doses of thyroid hormones have depression-ameliorating effects (Bauer et al. 1998).
Rather than affecting depressive behavior as expected, the prenatal T4 treatment effect pointed instead to increased anxiety. Decreased latency to bury the prod in the defensive burying paradigm is interpreted as increased anxiety (De Boer and Koolhaas 2003) as is increased grooming (Dunn et al. 1981) in a strain that is known for its low basal level of grooming (Nosek et al. 2008). In our study these behaviors could be an indirect consequence of the adult WKY offspring's exaggerated hypothyroid state, as subclinical and clinical hypothyroidism is known to increase anxiety (Larisch et al. 2004; Sait Gonen et al. 2004). However, hypothyroid adult WKYs do not show increased anxiety (Redei et al. 2001); thus, prenatal T4 administration is a likely cause for the increased anxiety-related behaviors observed in the adult WKY offspring.
Our findings demonstrate for the first time that prenatal T4 treatment has opposite effects on peripheral and brain thyroid hormone levels: elevation in the amygdala and suppression in the periphery. Our previous findings suggest that the WKY strain may have exaggerated differences in responsiveness between the periphery and brain to thyroid hormones (Redei et al. 2001), which we strongly corroborate here. Further, prenatal T4 treatment suppressed peripheral THs in the adult WKY similar to findings in another animal model (Wilcoxon and Redei 2004). In both cases this effect is probably attributable to elevated thyroid hormone levels at the perinatal critical period, during which they determine the set-point for adult thyroid function (Dussault and Fisher 1999; Pracyk et al. 1992; Walker and Courtin 1985).
It was long assumed that the brain maintains its own thyroid homeostasis that is rarely affected by peripheral thyroid dysfunction. Later, it was often assumed that peripheral T4 – but not T3 –enters the central nervous system. Our current finding of brain region-specific alterations of TH levels in the WKY is novel. Local conversion of T4 to T3 by deiodinase II (Dio2) in astrocytes is estimated to produce as much as 80% of the T3 in the adult rat brain (Bianco et al. 2002). However, Dio2-deficient mice lacking local brain T3 production show proper brain development and function (Galton et al. 2007), showing that T3 taken up from the periphery can support the brain T3 need. The nonessential nature of Dio2 also suggests that the primary regulators of T3 action are the molecules MCT-8 and Dio3, which transport T3 into the neuron, and metabolize it there (Heuer et al. 2005).
Our finding of increased T3 levels in the amygdala despite decreased peripheral TH levels raises the question of how local thyroid hormone levels are controlled in a brain region-specific manner. The amygdala-specific “hyperthyroid” TH milieu in T4-treated offspring is likely due to the increased TH transport via MCT-8 into amygdalar neurons combined with the low level of Dio3 expression in this region (Lein et al. 2007). The mechanism by which prenatal T4 treatment augments adult MCT-8 expression has yet to be elucidated, but the increase of MCT-8 expression is of particular interest as MCT-8 mutation in humans results in neurodevelopmental and global neurological impairment greater than that observed in cases of congenital hypothyroidism (Dumitrescu et al. 2004; Friesema et al. 2006; Schwartz and Stevenson 2007).
In T4-treated offspring, the “hyperthyroid” milieu of the amygdala correlates with a shorter latency to bury the prod, an indicator of anxiety. An intermediary in this relationship could be increased GR protein levels. We have shown previously that amygdalar GR is decreased in the offspring of hypothyroid dams, and prenatal T4 treatment reversed this decrease in GR expression (Wilcoxon et al. 2005). GR overexpression in mice causes increased anxiety-like behavior in several behavioral tests of anxiety, and targeted reduction of GR function in the nervous system impairs stress responses and reduces anxiety behavior (Tronche et al. 1999; Wei et al. 2004). These studies and our own suggest that the in utero environment programs adult expression of GR – and in the adult, increased amygdalar GR is anxiogenic. In addition, GR-bound glucocorticoids induce CRH expression in the amygdala (Kolber et al. 2008), which can lead to increased anxiety (Yilmazer-Hanke et al. 2004). Elevated CRH mRNA levels are found in the central amygdala of the WKY compared to F344 and Wistar rats in association with increased anxiety behavior in the elevated plus maze (Shepard and Myers 2008). In the current study, amygdalar CRH mRNA levels were further elevated in WKY offspring of T4-treated dams concomitant with increased anxiety measures, with no change in peripheral glucocorticoid levels.
In addition to elevated GR protein levels and CRH mRNA expression, prepro-TRH protein levels were also higher in the amygdala of T4-treated offspring. The WKY has higher basal amygdala TRH peptide levels compared to the Wistar rat and TRHergic neurons in the amygdala are active in response to environmental stress including exposure to the DB paradigm (Gutierrez-Mariscal et al. 2008). TRH peptide levels are not subject to classical hormonal feedback control in the amygdala, and T3 administration to adult rats did not alter TRH mRNA levels in limbic brain regions (Kim et al. 1996). Thus, the increase in amygdalar prepro-TRH levels we observed may be a unique effect of in utero T4 treatment. Further investigations are needed to fully characterize the processing of prepro-TRH in the amygdala and therefore the amount of TRH peptide that is present to influence anxiety-related behavior. Specialized prepro-TRH processing may also relate to paradoxical evidence that administration of exogenous TRH intracerebroventricularly is proposed to have anxiolytic actions (Gutierrez-Mariscal et al. 2008).
Our study establishes that prenatal T4 treatment can induce increased anxiety by altering brain region-specific expression of genes whose function is to modulate localized thyroid hormone availability. Thyroid hormones have a remarkable range of actions in the development and function of the nervous system, and the spectrum of consequences of prenatal thyroid dysfunction is becoming more recognized. In turn, there is a need to characterize brain-region specific effects of thyroid hormone excess or deficit in utero. The behavioral consequences of prenatal T4 treatment uncovered in this study in offspring of genetically hypothyroid mothers may differ from prenatal T4 effects in offspring of mothers with hypothyroidism of non-genetic etiology—specifically, that induced worldwide by iodine deficiency. Recognizing this complexity, we propose that the present study is the first step toward understanding the intricate neurodevelopmental effects of aberrant thyroid function during pregnancy.
Acknowledgments
We thank Dr. Jelena Radulovic for thoughtful discussions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allen Brain Atlas. Allen Institute for Brain Science; 2008. Internet. [Google Scholar]
- Aguilar-Valles A, Sanchez E, de Gortari P, Balderas I, Ramirez-Amaya V, Bermudez-Rattoni F, Joseph-Bravo P. Analysis of the stress response in rats trained in the water-maze: differential expression of corticotropin-releasing hormone, CRH-R1, glucocorticoid receptors and brain-derived neurotrophic factor in limbic regions. Neuroendocrinology. 2005;82(5-6):306–319. doi: 10.1159/000093129. [DOI] [PubMed] [Google Scholar]
- Ahmadiyeh N, Churchill GA, Shimomura K, Solberg LC, Takahashi JS, Redei EE. X-linked and lineage-dependent inheritance of coping responses to stress. Mamm Genome. 2003;14(11):748–757. doi: 10.1007/s00335-003-2292-x. [DOI] [PubMed] [Google Scholar]
- Ahmadiyeh N, Churchill GA, Solberg LC, Baum AE, Shimomura K, Takahashi JS, Redei EE. Lineage is an epigenetic modifier of QTL influencing behavioral coping with stress. Behav Genet. 2005;35(2):189–198. doi: 10.1007/s10519-004-1018-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmadiyeh N, Slone-Wilcoxon JL, Takahashi JS, Redei EE. Maternal behavior modulates X-linked inheritance of behavioral coping in the defensive burying test. Biol Psychiatry. 2004;55(11):1069–1074. doi: 10.1016/j.biopsych.2004.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auso E, Lavado-Autric R, Cuevas E, Del Rey FE, Morreale De Escobar G, Berbel P. A moderate and transient deficiency of maternal thyroid function at the beginning of fetal neocorticogenesis alters neuronal migration. Endocrinology. 2004;145(9):4037–4047. doi: 10.1210/en.2004-0274. [DOI] [PubMed] [Google Scholar]
- Barker DJ, Eriksson JG, Forsen T, Osmond C. Fetal origins of adult disease: strength of effects and biological basis. Int J Epidemiol. 2002;31(6):1235–1239. doi: 10.1093/ije/31.6.1235. [DOI] [PubMed] [Google Scholar]
- Bauer M, Hellweg R, Graf KJ, Baumgartner A. Treatment of refractory depression with high-dose thyroxine. Neuropsychopharmacology. 1998;18(6):444–455. doi: 10.1016/S0893-133X(97)00181-4. [DOI] [PubMed] [Google Scholar]
- Baum AE, Solberg LC, Kopp P, Ahmadiyeh N, Churchill G, Takahashi JS, Jameson JL, Redei EE. Quantitative trait Loci associated with elevated thyroid-stimulating hormone in the Wistar Kyoto rat. Endocrinology. 2005;146(2):870–878. doi: 10.1210/en.2004-0948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianco AC, Salvatore D, Gereben B, Berry MJ, Larsen PR. Biochemistry, cellular and molecular biology, and physiological roles of the iodothyronine selenodeiodinases. Endocr Rev. 2002;23(1):38–89. doi: 10.1210/edrv.23.1.0455. [DOI] [PubMed] [Google Scholar]
- Campos-Barros A, Meinhold H, Kohler R, Muller F, Eravci M, Baumgartner A. The effects of desipramine on thyroid hormone concentrations in rat brain. Naunyn Schmiedebergs Arch Pharmacol. 1995;351(5):469–474. doi: 10.1007/BF00171037. [DOI] [PubMed] [Google Scholar]
- De Boer SF, Koolhaas JM. Defensive burying in rodents: ethology, neurobiology and psychopharmacology. Eur J Pharmacol. 2003;463(1-3):145–161. doi: 10.1016/s0014-2999(03)01278-0. [DOI] [PubMed] [Google Scholar]
- Dumitrescu AM, Liao XH, Best TB, Brockmann K, Refetoff S. A novel syndrome combining thyroid and neurological abnormalities is associated with mutations in a monocarboxylate transporter gene. Am J Hum Genet. 2004;74(1):168–175. doi: 10.1086/380999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn AJ, Guild AL, Kramarcy NR, Ware MD. Benzodiazepines decrease grooming in response to novelty but not ACTH or beta-endorphin. Pharmacol Biochem Behav. 1981;15(4):605–608. doi: 10.1016/0091-3057(81)90217-3. [DOI] [PubMed] [Google Scholar]
- Dussault JH, Fisher DA. Thyroid function in mothers of hypothyroid newborns. Obstetrics and Gynecology. 1999;93(1):15–20. doi: 10.1016/s0029-7844(98)00369-x. [DOI] [PubMed] [Google Scholar]
- Friesema EC, Jansen J, Heuer H, Trajkovic M, Bauer K, Visser TJ. Mechanisms of disease: psychomotor retardation and high T3 levels caused by mutations in monocarboxylate transporter 8. Nat Clin Pract Endocrinol Metab. 2006;2(9):512–523. doi: 10.1038/ncpendmet0262. [DOI] [PubMed] [Google Scholar]
- Galton VA, Wood ET, St Germain EA, Withrow CA, Aldrich G, St Germain GM, Clark AS, St Germain DL. Thyroid hormone homeostasis and action in the type 2 deiodinase-deficient rodent brain during development. Endocrinology. 2007;148(7):3080–3088. doi: 10.1210/en.2006-1727. [DOI] [PubMed] [Google Scholar]
- Gutierrez-Mariscal M, de Gortari P, Lopez-Rubalcava C, Martinez A, Joseph-Bravo P. Analysis of the anxiolytic-like effect of TRH and the response of amygdalar TRHergic neurons in anxiety. Psychoneuroendocrinology. 2008;33(2):198–213. doi: 10.1016/j.psyneuen.2007.11.002. [DOI] [PubMed] [Google Scholar]
- Haddow JE, Palomaki GE, Allan WC, Williams JR, Knight GJ, Gagnon J, O'Heir CE, Mitchell ML, Hermos RJ, Waisbren SE, Faix JD, Klein RZ. Maternal thyroid deficiency during pregnancy and subsequent neuropsychological development of the child. N Engl J Med. 1999;341(8):549–555. doi: 10.1056/NEJM199908193410801. [DOI] [PubMed] [Google Scholar]
- Heuer H, Maier MK, Iden S, Mittag J, Friesema EC, Visser TJ, Bauer K. The monocarboxylate transporter 8 linked to human psychomotor retardation is highly expressed in thyroid hormone-sensitive neuron populations. Endocrinology. 2005;146(4):1701–1706. doi: 10.1210/en.2004-1179. [DOI] [PubMed] [Google Scholar]
- Kim SY, Post RM, Rosen JB. Differential regulation of basal and kindling-induced TRH mRNA expression by thyroid hormone in the hypothalamic and limbic structures. Neuroendocrinology. 1996;63(3):297–304. doi: 10.1159/000126969. [DOI] [PubMed] [Google Scholar]
- Kolber BJ, Roberts MS, Howell MP, Wozniak DF, Sands MS, Muglia LJ. Central amygdala glucocorticoid receptor action promotes fear-associated CRH activation and conditioning. Proc Natl Acad Sci U S A. 2008;105(33):12004–12009. doi: 10.1073/pnas.0803216105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larisch R, Kley K, Nikolaus S, Sitte W, Franz M, Hautzel H, Tress W, Muller HW. Depression and anxiety in different thyroid function states. Horm Metab Res. 2004;36(9):650–653. doi: 10.1055/s-2004-825925. [DOI] [PubMed] [Google Scholar]
- Lein ES, Hawrylycz MJ, Ao N, Ayres M, Bensinger A, Bernard A, Boe AF, Boguski MS, Brockway KS, Byrnes EJ, Chen L, Chen TM, Chin MC, Chong J, Crook BE, Czaplinska A, Dang CN, Datta S, Dee NR, Desaki AL, Desta T, Diep E, Dolbeare TA, Donelan MJ, Dong HW, Dougherty JG, Duncan BJ, Ebbert AJ, Eichele G, Estin LK, Faber C, Facer BA, Fields R, Fischer SR, Fliss TP, Frensley C, Gates SN, Glattfelder KJ, Halverson KR, Hart MR, Hohmann JG, Howell MP, Jeung DP, Johnson RA, Karr PT, Kawal R, Kidney JM, Knapik RH, Kuan CL, Lake JH, Laramee AR, Larsen KD, Lau C, Lemon TA, Liang AJ, Liu Y, Luong LT, Michaels J, Morgan JJ, Morgan RJ, Mortrud MT, Mosqueda NF, Ng LL, Ng R, Orta GJ, Overly CC, Pak TH, Parry SE, Pathak SD, Pearson OC, Puchalski RB, Riley ZL, Rockett HR, Rowland SA, Royall JJ, Ruiz MJ, Sarno NR, Schaffnit K, Shapovalova NV, Sivisay T, Slaughterbeck CR, Smith SC, Smith KA, Smith BI, Sodt AJ, Stewart NN, Stumpf KR, Sunkin SM, Sutram M, Tam A, Teemer CD, Thaller C, Thompson CL, Varnam LR, Visel A, Whitlock RM, Wohnoutka PE, Wolkey CK, Wong VY, Wood M, Yaylaoglu MB, Young RC, Youngstrom BL, Yuan XF, Zhang B, Zwingman TA, Jones AR. Genome-wide atlas of gene expression in the adult mouse brain. Nature. 2007;445(7124):168–176. doi: 10.1038/nature05453. [DOI] [PubMed] [Google Scholar]
- Nosek K, Dennis K, Andrus BM, Ahmadiyeh N, Baum AE, Woods LC, Redei EE. Context and strain-dependent behavioral response to stress. Behav Brain Funct. 2008;4:23. doi: 10.1186/1744-9081-4-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obregon MJ, Calvo RM, Del Rey FE, de Escobar GM. Ontogenesis of thyroid function and interactions with maternal function. Endocr Dev. 2007;10:86–98. doi: 10.1159/000106821. [DOI] [PubMed] [Google Scholar]
- Pracyk JB, Seidler FJ, McCook EC, Slotkin TA. Pituitary-thyroid axis reactivity to hyper- and hypothyroidism in the perinatal period: ontogeny of regulation of regulation and long-term programming of responses. J Dev Physiol. 1992;18(3):105–109. [PubMed] [Google Scholar]
- Redei EE, Solberg LC, Kluczynski JM, Pare WP. Paradoxical hormonal and behavioral responses to hypothyroid and hyperthyroid states in the Wistar-Kyoto rat. Neuropsychopharmacology. 2001;24(6):632–639. doi: 10.1016/S0893-133X(00)00229-3. [DOI] [PubMed] [Google Scholar]
- Sait Gonen M, Kisakol G, Savas Cilli A, Dikbas O, Gungor K, Inal A, Kaya A. Assessment of anxiety in subclinical thyroid disorders. Endocr J. 2004;51(3):311–315. doi: 10.1507/endocrj.51.311. [DOI] [PubMed] [Google Scholar]
- Schwartz CE, Stevenson RE. The MCT8 thyroid hormone transporter and Allan-Herndon-Dudley syndrome. Best Pract Res Clin Endocrinol Metab. 2007;21(2):307–321. doi: 10.1016/j.beem.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepard JD, Myers DA. Strain differences in anxiety-like behavior: association with corticotropin-releasing factor. Behav Brain Res. 2008;186(2):239–245. doi: 10.1016/j.bbr.2007.08.013. [DOI] [PubMed] [Google Scholar]
- Shukla PK, Tang L, Wang ZJ. Phosphorylation of neurogranin, protein kinase C, and Ca2+/calmodulin dependent protein kinase II in opioid tolerance and dependence. Neurosci Lett. 2006;404(3):266–269. doi: 10.1016/j.neulet.2006.06.013. [DOI] [PubMed] [Google Scholar]
- Solberg LC, Baum AE, Ahmadiyeh N, Shimomura K, Li R, Turek FW, Churchill GA, Takahashi JS, Redei EE. Sex- and lineage-specific inheritance of depression-like behavior in the rat. Mamm Genome. 2004;15(8):648–662. doi: 10.1007/s00335-004-2326-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treit D, Pinel JP, Fibiger HC. Conditioned defensive burying: a new paradigm for the study of anxiolytic agents. Pharmacol Biochem Behav. 1981;15(4):619–626. doi: 10.1016/0091-3057(81)90219-7. [DOI] [PubMed] [Google Scholar]
- Tronche F, Kellendonk C, Kretz O, Gass P, Anlag K, Orban PC, Bock R, Klein R, Schutz G. Disruption of the glucocorticoid receptor gene in the nervous system results in reduced anxiety. Nat Genet. 1999;23(1):99–103. doi: 10.1038/12703. [DOI] [PubMed] [Google Scholar]
- Walker P, Courtin F. Transient neonatal hyperthyroidism results in hypothyroidism in the adult rat. Endocrinology. 1985;116(6):2246–2250. doi: 10.1210/endo-116-6-2246. [DOI] [PubMed] [Google Scholar]
- Wei Q, Lu XY, Liu L, Schafer G, Shieh KR, Burke S, Robinson TE, Watson SJ, Seasholtz AF, Akil H. Glucocorticoid receptor overexpression in forebrain: a mouse model of increased emotional lability. Proc Natl Acad Sci U S A. 2004;101(32):11851–11856. doi: 10.1073/pnas.0402208101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcoxon JS, Kuo AG, Disterhoft JF, Redei EE. Behavioral deficits associated with fetal alcohol exposure are reversed by prenatal thyroid hormone treatment: a role for maternal thyroid hormone deficiency in FAE. Mol Psychiatry. 2005;10(10):961–971. doi: 10.1038/sj.mp.4001694. [DOI] [PubMed] [Google Scholar]
- Wilcoxon JS, Redei EE. Prenatal programming of adult thyroid function by alcohol and thyroid hormones. Am J Physiol Endocrinol Metab. 2004;287(2):E318–326. doi: 10.1152/ajpendo.00022.2004. [DOI] [PubMed] [Google Scholar]
- Yilmazer-Hanke DM, Hantsch M, Hanke J, Schulz C, Faber-Zuschratter H, Schwegler H. Neonatal thyroxine treatment: changes in the number of corticotropin-releasing-factor (CRF) and neuropeptide Y (NPY) containing neurons and density of tyrosine hydroxylase positive fibers (TH) in the amygdala correlate with anxiety-related behavior of wistar rats. Neuroscience. 2004;124(2):283–297. doi: 10.1016/j.neuroscience.2003.12.004. [DOI] [PubMed] [Google Scholar]
- Zoeller RT, Bigelow C, Rovet J. Lack of a relation between human neonatal thyroxine and pediatric neurobehavioral disorders: neonatal total thyroxine is not a good proxy measure of maternal thyroid hormone insufficiency. Thyroid. 2004;14(3):239–241. doi: 10.1089/105072504773297939. author reply 241-233. [DOI] [PubMed] [Google Scholar]


