Abstract
Objective
African-American (AA) women with breast cancer are more likely to have advanced disease at diagnosis, higher risk of recurrence and poorer prognosis than Caucasian (CA) women. We have recently shown higher insulin-like growth factor II (IGF-II) expression in paired breast tissue samples from AA women as compared to CA women. IGF-II is a potent mitogen that induces cell proliferation and survival signals through activation of the IGF-I and Insulin receptors (IGF-IR, IR) while IGF-II circulating levels are regulated by cellular uptake through the IGF2 receptor. We hypothesize that differential expression of the IGFIR and IGF2R among AA and CA women potentiates IGF-II mitogenic effects, thus contributing to the health disparity observed between these ethnic groups.
Design
We examined IGF-IR and IGF2R mRNA, protein expression and IGF1R phosphorylation in paired breast tissue samples from AA and CA women by Real Time-PCR, western blot analysis, immunohistochemistry and ELISA techniques.
Results
Our results showed significantly increased expression of IGF1R in AA normal tissues as compared to CA normal tissues. IGF1R expression was similar between AA normal and malignant tissues, while IGF1R, IRS-1 and Shc phosphorylation was significantly higher in AA tumor samples. Significantly higher levels of IGF2R were found in CA tumor samples as compared to AA tumor samples.
Conclusions
We conclude that IGFIR and IGF2R differential expression may contribute to the increased risk of malignant transformation in young AA women and to the more aggressive breast cancer phenotype observed among AA breast cancer patients and represent, along with IGF-II, potential therapeutic targets in breast cancer.
Keywords: IGF-II, IGF2R, IGF-IR, breast cancer, health disparities
Introduction
Survival disparities among the African-American (AA) population constitute a major challenge that encompasses leading causes of morbidity and mortality such as breast cancer. Breast cancer is a multifactorial disease that affects all populations and represents the second leading cause of mortality among women. Differences in breast cancer incidence and mortality rates among AA and Caucasian (CA) populations suggest that etiologic factors differ in their biologic expression and impact on disease outcome; poorer outcomes in AA women reflect, in part, the fact that breast cancer tends to be a more biologically aggressive disease in AA than in CA women [1]. The insulin-like growth factor-II (IGF-II) is a potent mitogen that plays an essential role not only in normal growth and development, but also in breast cancer susceptibility, growth and progression by signaling through the IGF1 and insulin receptors [2–6]. Moreover, we have recently shown that AA tumor samples express significantly higher levels of IGF-II as compared to CA tumors [7]. Furthermore, Pinheiro et al [8] showed significant differences between circulating levels of estradiol, IGF-1 and IGFBP-3 consistent with breast cancer risk between Caucasians and African-American women by using cross-sectional studies.
The IGF1R has been implicated in the initiation and progression of breast cancer [12]. This receptor is developmentally regulated, with highest levels of IGF1R mRNA found at embryonic stages [9, 10]. Transcription of the IGF1R gene is negatively regulated by breast cancer gene-1 (BRCA1), p53 and the Wilms’ tumor protein-1 (WT-1), all tumor suppressor proteins implicated in breast cancer development and progression. Control of cell surface receptor number is another way to regulate receptor activity through internalization and/or degradation. In fact, alternative splicing of the IGF1R mRNA results in the synthesis of two receptor proteins that appear to be internalized and/or degraded at different rates [11]. Previous studies have shown that fibroblasts derived from mice embryos lacking the IGF1R were resistant to transformation by most oncogenes, indicating that the presence of the IGF1R is important for acquisition of the malignant phenotype [13]. On the contrary, metastatic stages are usually associated with decrease in IGF1R levels [14]. In breast cancer, high IGF1R levels are also associated with development of resistance to Herceptin, a monoclonal antibody against the extracellular domain of HER2/NEU used in the treatment of ERBB2-overexpressing breast cancer [15, 16].
The IGF2 receptor (IGF2R) is a widely expressed, single transmembrane domain, multifunctional protein that is involved in lysosomal enzyme trafficking, peptide internalization, and degradation of IGF-II [18]. By targeting IGF-II to lysosomal degradation, this receptor plays a role in the maintenance of IGF-II levels in the circulation and in target tissues. Overexpression of IGF-II was shown to increase the lysosomal enzyme cathepsin D secretion in MCF-7 breast cancer cells [18]. Cathepsin D is secreted as an inactive 52 kDa protein that is processed extracellularly to different active forms ranging from 51 to 42 kDa [19, 20]. Its secretion has been correlated with a poor prognosis, increased metastatic potential and decreased disease free survival periods [21] for breast cancer patients. The IGF2R gene is developmentally regulated and its expression is maximal during fetal development and organogenesis [22]. Many human tumors (including breast carcinomas) show loss or mutation of one copy of the IGF2R gene, in some cases accompanied by loss or mutation of the single remaining copy [23–25]. Loss of IGF2R function is associated with tumor progression; therefore, the IGF2R is often referred to as a tumor suppressor [23]. The IGF2R has been implicated in some IGF-II stimulated actions, and studies in cell lines over-expressing the IGF2R have strengthened the conclusions drawn from earlier in vitro studies that suggested that the IGF2R may modulate G protein-regulated signal transduction by virtue of a pleckstrin homology domain-like motif in its cytoplasmic tail [26].
Therefore, the present study focuses on the differential expression of the IGF1 and IGF2 receptors as part of the mechanism(s) leading to: 1) An increase risk of malignant transformation in young AA women and 2) The overall increased mortality observed in AA women with breast cancer as compared to CA women.
Materials and Methods
Breast cancer tissue specimens
Paired frozen breast cancer (M) and normal tissue (N) specimens were obtained from the Cooperative Human Tissue Network (CHTN) from AA and CA women with ages ranging from 20–90 years (median age per group was AAM=60.2 years, CAM=60.1 years, AAN=51.9 years and CAN=62.7 years). All malignant samples were stage II or III (Bloom and Richardson’s) infiltrating ductal carcinomas (IDC) or papillary carcinomas (AAM stage II n=10, stage III n=17; CAM stage II n=12, stage III n=12). Total number of samples (n) per group is as follows: AAN= 23, CAN= 20, AAM= 27 and CAM= 24. Non-stained slides were also obtained with the tissue specimens as well as a pathology report containing information about patient age, macroscopic and microscopic characteristics of normal and tumor tissues, estrogen/progesterone/Her2 receptors status, and tumor grade/stage and metastases sites.
Western blot analysis
Total protein (30 μg) of tissue cell lysates were prepared in RIPA buffer [1XTBS, 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% SDS, 0.004% sodium azide, 10 μl/ml PMSF (stock concentration of 10 mg/ml), 10 μl/ml protease inhibitor cocktail (stock concentration of 50 KIU/ml RIPA), 10 μl/ml sodium orthovanadate (100mM)] and used to load polyacrylamide-SDS gradient gels (4–12%), transferred to a PVDF membrane (Invitrogen, Carlsbad, CA) using a X-Cell SureLockR electrophoretic Transfer module (Invitrogen, Carlsbad, CA). Protein concentration was measured using the Coomassie Plus Protein Assay Reagent™ (Pierce Biotechnology, Rockford, IL). PVDF membranes were blocked with 1% BSA IgG free (Sigma Chemical Co., St. Louis, MO) in PBS/0.05% Tween (for IGF-1R) or 5% milk powder (for hIGF2R). Membranes were then incubated with anti-IGF-1R monoclonal antibody which detects the receptor α-subunit (Santa Cruz Biotechnology, CA), rabbit anti-human M6P/IGF-II receptor antiserum (hIGF-2R) kindly provided by Dr. Carolyn Scott (Kolling Institute of Medical Research, Sydney, Australia), anti-phosphoIGF1R/INSR (Y1135/Y1136, Y1162/1163 respectively) (R&D Systems), anti-phospho IRS-1 (Y632), anti-phosphoShc (Y239/Y240) (Cell Signaling Technology), and total anti-IRS 1and Shc (Santa Cruz Biotechnology). Membranes were incubated at 4°C overnight. The blots were also probed with cytokeratin 18 and Pan-cytokeratin monoclonal antibodies (Santa Cruz Biotechnology, CA), used as epithelial cell markers. After 3 × 10 min washes in PBS/0.05% Tween, the corresponding biotinylated secondary antibodies (1:1000, Amersham, Arlington Heights, IL) were added to the membranes (1 hr at RT), followed by 3 ×10 min washes and incubation with HRP complexes (1:1000 Amersham, Arlington Heights, IL). Protein visualization was achieved by using enhanced chemiluminescence (ECL) and autoradiography with Hyperfilm ECL film (Amersham, Arlington Heights, IL). The signals on the x-ray films were quantified using ChemiImager™ 4000 (Alpha Innotech Corporation).
RNA extraction
Total RNA was extracted using Tri reagent (Molecular Research Center, Cincinnati, OH) according to manufacturer’s protocol. 40–80 mg of frozen tissue per sample was homogenized in the Tri reagent by using a hand-held homogenizer (Kontes, Thomas Scientific, NJ). Total RNA was kept at −80° C until assayed.
Real Time PCR
One Step SYBR real-time RT-PCR was performed to assess IGF1R mRNA expression (For- 5′-TTA AAA TGG CCA GAA CCT GAG -3′ and Rev- 5′-ATT ATA ACC AAG CCT CCC AC -3′) IGF2R (For-5′-TAC AAC TTC CGG TGG TAC ACC A -3′ and Rev- 5′-CAT GGC ATA CCA GTT TCC TCC A -3′). GAPDH was used as an internal control (For-5′-ACA ACT TTG GTA TCG TGG AAG GAC-3′ and Rev- 5′-AG GGA TGA TGT TCT GGA GAG C-3′). PCR amplifications were performed using the iCycler (BIO-RAD). Reactions were performed in a mixture consisting of a 50 μL volume solution containing 1X SYBR Green supermix PCR buffer (BIO-RAD), (100mM KCL, 6 mM MgCl2, 40mM Tris-HCL, PH 8.4, 0.4mM of each dNTP [dATP, dCTP, dGTP and dTTP], iTaq DNA Polymerase 50 U/mL, SYBR Green I, 20mM Fluorescein) 300 nM of each primer, 0.25U/mL MultiScribe Reverse Transcriptase (Promega) and 0.4U/mL Rnase Inhibitor (Promega). The RT-PCR protocol starts with 30 min at 42°C for the RT. Prior to the PCR step iTaq DNA polymerase activation at 95 °C for 10 min was performed. Followed by 30 sec denaturation at 95 °C, 15 sec annealing at 57 °C, and 1.5 min elongation at 72 °C for 40 cycles. Fluorescence was detected at the end of every 72°C extension phase. To exclude the contamination of non-specific PCR products such as primer dimers, melting curve analysis was applied to all final PCR products after the cycling protocol.
Immunohistochemistry
IGF-II, IGF1R and IGF2R immunohistochemical analysis was performed using purified rabbit polyclonal anti-human IGF-II antibodies (Y819, YenZym antibodies CA), anti-IGF-1R monoclonal antibody (Santa Cruz Biotechnology, CA), and rabbit anti-human M6P/IGF-II receptor antiserum (hIGF-2R) kindly provided by Dr. Carolyn Scott (Kolling Institute of Medical Research, Sydney, Australia).
5 μm tick paraffin block sections were de-waxed, rehydrated and treated with 1X antigen retrieval solution (Reveal, Biocare Medical). After endogenous peroxidase blocking (H2O2 3%), slides were incubated in corresponding primary antibodies (1:10) with blocking serum (mouse or rabbit ABC staining Systems, Santa Cruz Biotechnology) overnight at 4°C. The antibodies were revealed with the corresponding anti-mouse or anti-rabbit biotinylated antibodies for 30 min at room temperature, followed by streptavidin/peroxidase label (30 min at room temperature) using diaminobenzidine (DAB) chromogen as a substrate. The samples were counterstained with hematoxylin, dehydrated and mounted. Tissue sections were washed with 1X PBS between each immunostaining step.
Phosphorylation studies
IGF1R phosphorylation was assessed using the PathScan® phospho-IGF1 receptor β (tyr 1131) sandwich ELISA kit (Cell Signaling). Of note, according to the manufacturer, cross-reactivity studies using CHO-IR/IRS cell lysates showed that theIGF1R ELISA kit does not cross-react with the insulin receptor. Tissue lysates were prepared using 50–80 mg of frozen tissue per sample and homogenized in 1X lysis buffer (20 mM Tris-HCL pH 7.5, 150 mM NaCl, 1 mM Na2EDTA, 1 mM EGTA, 1% Triton, 2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate, 1 mM Na3VO4, 1 μg/ml leupeptin) by using a hand-held homogenizer (Kontes, Thomas Scientific, NJ). Total tissue lysates were kept at −80° C until assayed.
100 μl of tissue lysate (125 μg total protein concentration) were diluted in 100 μl of sample diluent and transferred to the IGF1R-coated microwells for overnight incubation at 4°C, followed by incubation in detection antibody. HRP-linked secondary antibody was added for 30 min. The reaction was visualized by addition of TMB substrate. Spectrophotometric determination was read at 450 nm. Wells were washed 4 times with 1X wash buffer in between steps.
Statistical analysis
Statistical differences between mean values were determined by using one-way ANOVA (for protein expression analysis between all groups), paired T test (for comparison between paired normal and tumor samples) and independent T test (for comparison between AA-CA samples) by using the SPSS 17.0 software (SPSS, Inc., Chicago, IL). *Values are expressed as the mean ± SEM of three or more replicate experiments. A level of P < 0.05 was considered significant.
Results
IGF1R and IGF2R mRNA expression in AA and CA breast cancer and normal tissue samples
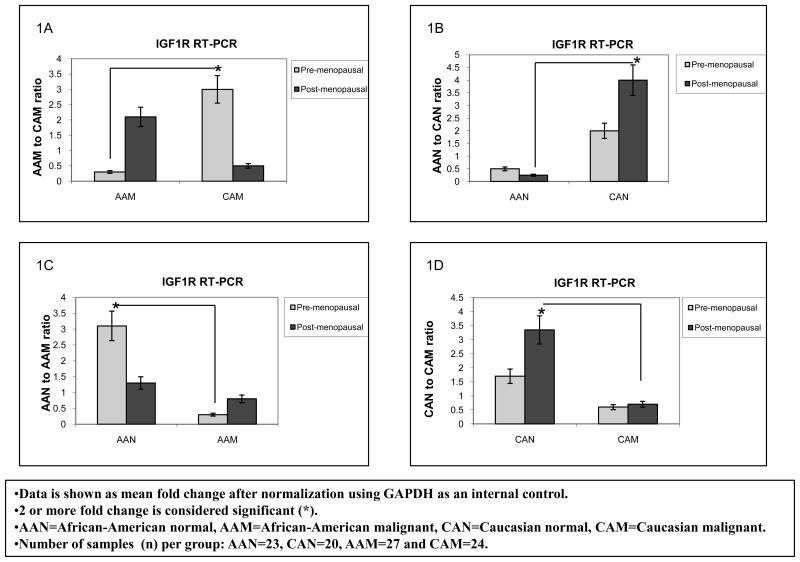
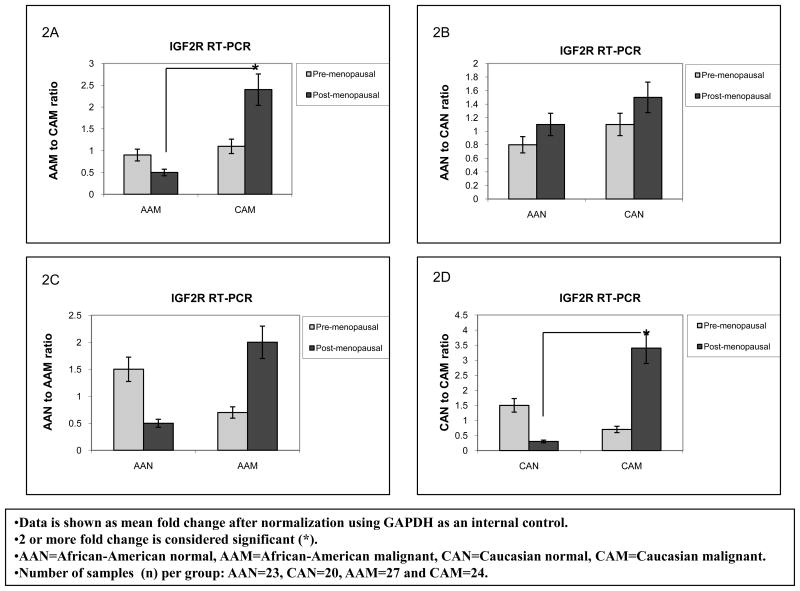
Overall breast cancer mortality rates are higher among AA as compared to CA women for all ages. In this respect, we tested our hypothesis that differential expression of the IGFIR and IGF2R among AA and CA women potentiates IGF-II mitogenic effects, thus contributing to the health disparity observed between these ethnic groups. We started by analyzing IGF1R and IGF2R gene expression using Real Time-PCR in paired breast tumor and normal tissue samples from AA and CA women. Furthermore, since there is a slightly higher incidence of breast cancer in pre-menopausal (<50 years) AA females than in CA women according to the Surveillance, Epidemiology, and End Results program Stat Database (SEER), we also compared IGF1R and IGF2R mRNA expression between AAN and CAN for this specific group. Figures 1A–B show IGF1R RT-PCR data seen as mean fold change after normalization using GAPDH as an internal control. Our results showed significantly increased levels of IGF1R mRNA (3 fold) in CA breast cancer tissue samples (CAM) as compared to AA tumor samples in pre-menopausal women (AAM) (Figure 1A). IGF1R mRNA levels comparison between normal samples from AA and CA women showed that post-menopausal CAN tissue samples expressed a 3.5 fold increase in IGF1R mRNA when compared to AAN in the same age group (figure 1B). These differences reached statistical significance for pre-menopausal CAM and post-menopausal CAN, correlating with an increased incidence of breast cancer risk in older CA women as compared to AA women. Figures 2A–B show IGF2R RT-PCR data seen as mean fold change after normalization using GAPDH as an internal control. The IGF2R plays an important role in the maintenance of circulating IGF-II levels, thus a decrease in IGF2R levels in cancer is associated with increased tumor cell growth and progression through IGF-II mediated activation of the IGF1 and insulin receptors. In the present study, post-menopausal CAM samples expressed a 2 fold increase in the IGF2R mRNA as compared to AAM samples (figure 2A). No significant changes were seen between AAN when compared to CAN tissue samples (figures 2B).
Figure 1.
IGF-1R gene expression in African-American and Caucasian paired breast tissue samples assessed by Real Time-PCR (RT-PCR). Figure 1(A–B) shows IGF-1R gene expression represented as fold change after normalization using GAPDH as an internal control. Two (2) or more fold change was considered significant (*). Figure 1A shows IGF-1R mRNA fold change between AAM and CAM samples divided into pre-menopausal and post-menopausal women. Figure 1B shows IGF-1R mRNA expression shown as fold change between AAN and CAN.
AAN=African-American normal tissue, AAM=African-American malignant tissue, CAN=Caucasian normal tissue and CAM=Caucasian malignant tissue. Total number of patients (n) analyzed per group was as follows: AAN= 23, AAM=27, CAN=20 and CAM=24.
Figure 2.
IGF2R gene expression in African-American and Caucasian paired breast tissue samples assessed by Real Time-PCR (RT-PCR). Figure 2(A–B) shows IGF2R gene expression represented as fold change after normalization using GAPDH as an internal control. Two (2) or more fold change was considered significant (*). Figure 2A shows IGF2R mRNA fold change between AAM and CAM samples (pre-menopausal and post-menopausal women). Figure 2B shows IGF2R mRNA comparison between AAN and CAN. AAN=African-American normal tissue, AAM=African-American malignant tissue, CAN=Caucasian normal tissue and CAM=Caucasian malignant tissue. Total number of patients (n) analyzed per group was as follows: AAN= 23, AAM=27, CAN=20 and CAM=24.
IGF1R and IGF2R protein expression in AA and CA normal and breast cancer tissues
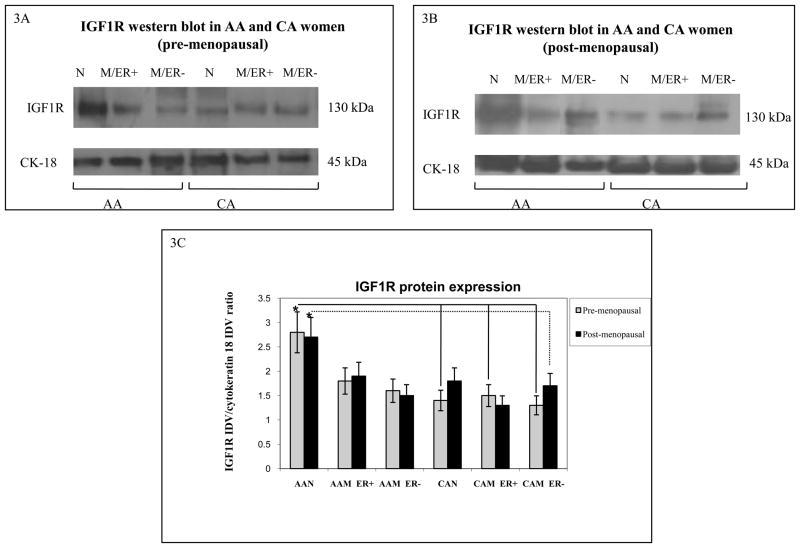
Next, we used Western blot analysis to study the correlation between IGF1R and IGF2R mRNA and protein expression in the breast tissue samples. Figures 3A–B show representative Western blots of IGF1R in AA and CA breast tissue samples. Figures 3C and 4C shows bar graphs of densitometric analysis of IGF1R and IGF2R densitometry units [integrated density units (IDVs)] normalized to cytokeratin 18 densitometry units. As seen on figure 3C, our results show that AAN tissue samples expressed significantly higher IGF1R protein levels than CAN (p*=0.03) and CAM (p*=0.04) samples in pre-menopausal women. A statistically significant difference continued to be seen among older women samples from AAN and CAM samples (p*=0.02). The discrepancy observed between IGF1R mRNA and protein expression between breast tissues samples from AA and CA women could be the result of different splice variants expression, which has been shown to have distinct internalization and/or degradation rates. No significant changes were found in IGF1R protein levels between tumor tissues from AA and CA women (figures 3A–C).
Figure 3.
Representative Western blot analyses of IGF-1R in paired tissue samples from African-American (AA) and Caucasian (CA) women. The samples were separated into pre-menopausal (figure 3A) and post-menopausal (figure 3B) groups based on the higher incidence of breast cancer in AA women younger than 45 years (but not after 45 years). Immunoreactive bands for IGF-1R and cytokeratin 18 were identified using ECL, scanned by densitometry and normalized to cytokeratin 18. The 130 kDa band represents mature IGF-1R. Cytokeratin 18 was used as an epithelial cell marker (45 kDa). Lower panel (3C) show bar graphs of IGF-1R data normalized to cytokeratin 18 and presented as the mean ± SE of all samples per group. Asterisks indicate values statistically different (*p<0.05). Total number of patients (n) analyzed per group was as follows: AAN= 23, AAM=27, CAN=20 and CAM=24.
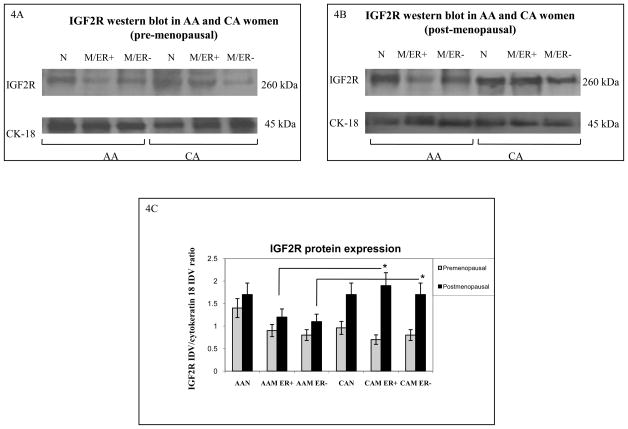
Figure 4.
Representative Western blot analyses of IGF2R in paired tissue samples from African-American (AA) and Caucasian (CA) women. The samples were separated into pre-menopausal (figure 4A) and post-menopausal (figure 4B) groups. Immunoreactive bands for IGF-1R and cytokeratin 18 were identified using ECL, scanned by densitometry and normalized to cytokeratin 18. The 270 kDa band represents pro-IGF2R. Cytokeratin 18 was used as an epithelial cell marker (45 kDa). Lower panel (4C) show bar graphs of IGF2R data normalized to cytokeratin 18 and presented as the mean ± SE of all samples per group. Asterisks indicate values statistically different (*p<0.05). Total number of patients (n) analyzed per group was as follows: AAN= 23, AAM=27, CAN=20 and CAM=24.
Figures 4A–B show representative Western blots of IGF2R in AA and CA breast tissue samples. No significant changes in IGF2R protein expression were seen when comparing AA to CA normal tissues (pre-menopausal women). Similarly, no differences were observed between AA and CA tumor samples (pre-menopausal women), figure 4A. Noteworthy, CAM samples from post-menopausal expressed significantly higher IGF2R protein levels (p*=0.03) than AAM samples (figure 4C). Higher levels of the IGF2R would translate into less circulating IGF-II proteins, leading to decreased activation of the IGF1R, insulin receptor isoform A and their hybrid receptors.
IGF1R phosphorylation in AA and CA normal and breast cancer tissues
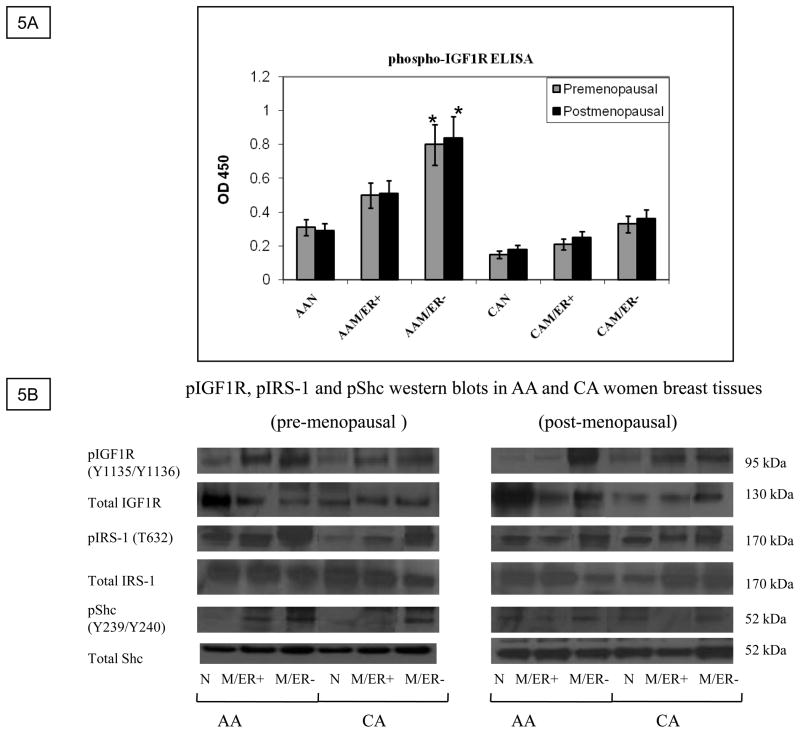
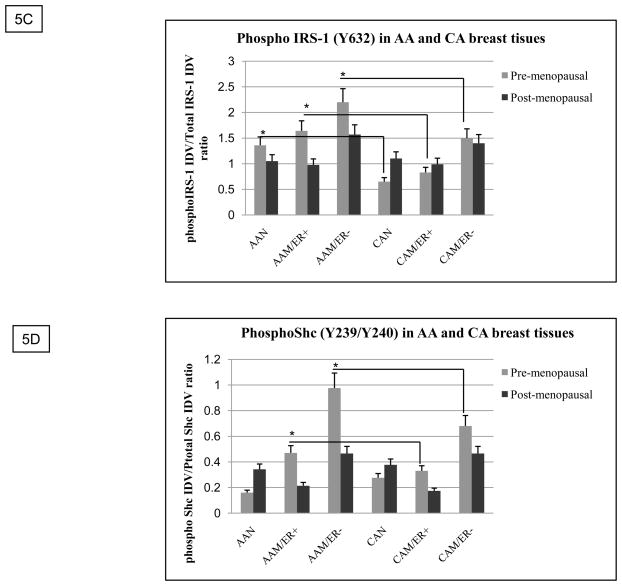
A reduction in IGF1R expression per se may not always reflect and/or translate into a decline in IGF-II signaling. Previous studies have suggested that the levels of phosphorylated IGF1R may be a more reliable indicator of IGFs function and its contribution to disease progression, thus we proceeded to analyze IGF1R phosphorylation levels in paired tissues from AA and CA women by using the PathScan® phospho-IGF1 receptor β (tyr 1131) sandwich ELISA kit (Cell Signaling). As seen on figure 5A, IGF1R phosphorylation (tyr 1131) was significantly increased in AA tumor samples as compared to CA tumor samples (p*=0.03), AAN (p*=0.03) and CAN (p*=0.02). Interestingly, although IGF1R phosphorylation was increased in AAM (ER+) samples, only AAM (ER−) samples reached statistically significance. No significant difference was observed between CAN and CAM tissue samples. To further confirm IGF1R phosphorylation studies, western blot analysis using an anti-phosphoIGF1R/INSR (Y1135/Y1136, Y1162/Y1163, which stimulates intrinsic kinase activity) was done. Figure 5B shows higher levels of phosphorylation in ER (−) AAM samples as compared to CA samples (pre- and post-menopausal women). Interestingly, post-menopausal ER (+) CAM samples showed higher levels of IGF1R phosphorylation than ER (+) post-menopausal AAM samples. These results are in agreement with statistical data showing increased incidence of breast cancer in older CA women. Furthermore, we also analyzed two downstream signaling molecules IRS-1(T632) and Shc (T239/240) phosphorylation (figure 5B, lower panels). Pre-menopausal AA samples (normal and malignant) showed higher IRS-1 phosphorylation levels at T632, which is a site predicted to bind SH2 domains in the p85 regulatory subunit of PI3K, resulting in activation of p110 catalytic subunit. Phosphorylation of Shc (T239/Y240) was significantly increased in premenopausal ER (+) AAM samples as compared to ER (+) CAM samples. In response to extracellular signals, the SH2 and PTB domains of Shc interact with the activated IGF1R, leading to Shc phosphorylation, binding to GRB2/Sos and activation of the Ras/Raf/MAPK pathway. Figures 5C–D show bar graphs of densitometry analysis of phosphorylated IRS-1 and phosphorylated Shc densitometry units [integrated density units (IDVs)] normalized to total IRS-1 and Shc densitometry units. Significantly increased levels of IRS-1 phosphorylation (*p< 0.05) were seen in normal and tumor pre-menopausal breast tissue samples as compared to CAN and CAM samples. Phosphorylated Shc protein was also significantly increased in pre-menopausal AAM women as compared to CAM.
Figure 5.
IGF-1R phosphorylation study using the PathScan® phospho-IGF1 receptor β (tyr 1131) ELISA kit. Figure 5A shows a bar graph representation of IGF-1R phosphorylation measured as OD 450 for all samples per group (AAN= 23, AAM=27, CAN=20 and CAM=24). Asterisks indicate values statistically different (*p<0.05). Figure 5B shows representative western blot analyses of total and phosphorylated IGF1R, IRS-1 and Shc proteins. Figure 5C–D show bar graphs of phosphorylated IRS-1 and Shc normalized to total IRS-1 and Shc proteins and presented as the mean ± SE of all samples per group. Asterisks indicate values statistically different (*p< 0.05).
IGF1R and IGF2R immunohistochemistry
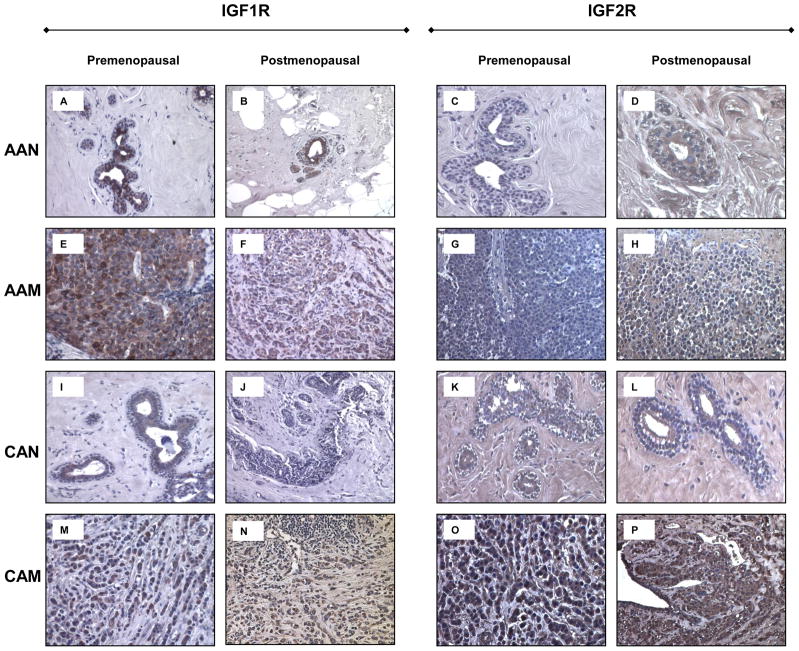
To further confirm IGF1R and IGF2R differential expression shown by Western blot analysis, we proceeded to study their expression by immunohistochemistry. As seen on figures 6A–B, normal tissue samples from AA women expressed higher levels of IGF1R as compared to their CA counterparts (figures 6I–J) for both, pre-menopausal and post-menopausal tissue samples. AAM (pre-menopausal) expressed higher levels of IGF1R than post-menopausal AAM samples. Increased IGF1R expression was seen in AAM as compared to CAM (pre-menopausal). No significant difference in staining was seen between post-menopausal AAM and CAM. IGF2R protein expression was higher in tumor samples from CA women (figures 6O–P) than in AA tumor samples for pre- and post-menopausal tissue samples (figures 6G–H). No significant difference in staining was observed among normal breast tissue samples from AA and CA women.
Figure 6.
Immunohistochemistry of IGF-1R and IGF2R in human normal and malignant breast tissue samples. Malignant AA and CA samples correspond to ER (+) invasive ductal carcinomas (IDC) Bloom and Richardson’s grade III. Panels A-D corresponds to IGF-1R and IGF2R immunostaining from AAN samples. Panels E-H corresponds to IGF-1R and IGF2R immunostaining from AAM samples. Panels I-L and M-P correspond to IGF-1R and IGF2R immunostaining, in CAN and CAM samples respectively. Original magnifications 20X (insert represent a 40X section). Total number of patients (n) analyzed per group was as follows: AAN= 15, AAM=15, CAN=13 and CAM=13.
Discussion
Considerable efforts are being made to identify factors that contribute to breast cancer disparities regarding incidence and mortality rates, and to translate this information into effective interventions for ameliorating disparities among different ethnic groups. Although racial disparities in breast cancer outcome can be attributed to many factors, including access to quality care, overall survival rates for AA vs. CA women were lower despite adjustments for uniform stage, treatment and follow-up in randomized phase III clinical trials [27, 28].
In this respect, the present study addresses the need of a more comprehensive molecular approach to better understand health disparities in breast cancer, by studying IGF1R and IGF2R expression in AA and CA paired breast tissue samples. Although there is a slightly higher incidence of breast cancer in pre-menopausal African-American (AA) women as compared to Caucasians (CA), post-menopausal CA women have a significantly higher incidence of this disease [1]. Several research groups have reported a correlation between survival disparities and high incidence of triple negative (ER, PR, HER2/NEU) tumors in AA women [27–29]. Of note, Albain et al showed a statistically significant decrease in survival for AA women with receptor positive tumors as well, suggesting that there are other factors involved in the survival disparity when the treatment type and ER status are similar by race/ethnic group. A study based on survival data from the Cancer and Leukemia Group B (CALGB) trial 8541, where patients with stage II breast cancer received chemotherapy, found that AA women were more likely to have higher rate of disease recurrence. This study done in patients with metastatic breast cancer demonstrated that AA patients have shorter survival despite achieving a similar response from chemotherapy [30]. These data highlights the importance of studying potential pathologic mechanisms of breast cancers in AA women in addition to demographic differences.
In the present study we have shown a significant increase in IGF1R protein levels in normal tissues from AA women as compared to AA and CA tumor samples and CA normal tissues. So far, the vast majority of studies describing overexpression of IGF1R in breast tumors has been based on mRNA analyses of tissue homogenates or established cancer cell lines for which appropriate normal controls do not exist. More focused studies of IGF1R expression in prostate tumors through use of immunohistochemistry or matched cell lines that correspond to normal and tumor tissue revealed that normal epithelium and early-stage tumors express abundant IGF1R, and that IGF1R expression is significantly reduced in advanced cancer [31]. Shin et al showed by using quantitative RT-PCR analysis that IGF1R mRNA expression levels in patients recruited from the Shangai Breast Cancer Study [32] were reduced in breast cancer tissues as compared to the adjacent normal tissues, and further reduced in more advanced tumors. An important role for the IGF signaling in mammary tumorigenesis has been demonstrated. Carboni et al [33] showed that overexpression of the IGF1R in transgenic mouse models resulted in the rapid appearance of mammary gland tumors; these tumors were transplantable into nude mice and their growth could be inhibited by a novel inhibitor of the IGF1R kinase. Jones et al [34] generated transgenic mice containing human IGF1R under a doxycycline-inducible MMTV promoter. Tumor formation was associated with increased levels of IGF1R signaling molecules (phosphorylated Akt, ERK1/2 and STAT3) and concluded that IGF1R overexpression is sufficient to induce mammary epithelial hyperplasia and tumor formation in vivo. Although these studies certainly illustrate the potential role of the IGF1R in mammary tumorigenesis, these in vitro experiments may not accurately reflect the in vivo environment found in human breast cancers. In addition, numerous BRCA mutations have been reported among AA breast cancer patients [35, 36]. Since AA have higher levels of mutations in the BRCA1 gene, and this protein inhibits the expression of the IGF1R, it is possible that higher levels of IGF1R are a result of lower BRCA inhibition or a gain of function of BRCA1. P53 is a tumor suppressor gene that inhibits the IGF1R and IGF-II; however mutations in p53 are associated with increased levels of both, IGF1R and IGF-II, caused by a gain of function in the mutated p53 protein. It has also been found that AA women tend to have more ER (−) tumors [37], p53 mutations [38], and c-met (stem cell factor/hepatocyte growth factor receptor) [39]. Maor et al [40] showed an association between higher IGF1R levels and BRCA1 mutation carriers. This could be a possible mechanistic explanation for the lower IGF1R levels observed in tumors derived from non-BRCA1 mutation carriers. The significantly increased IGF1R levels in normal AA tissues seen in our study may also be associated with the increased risk of malignant transformation in these women, since this receptor has been implicated in the initiation of breast carcinomas [12, 13].
Furthermore, the levels of phosphorylated/activated IGF1R or downstream signaling effectors represent another indicator of IGFs function and its contribution to disease progression along with the number of receptors. Studies in an animal model of leiomyoma (the Erk rat) revealed that the progression of normal uterine cells to leiomyomas was accompanied by a reduction in IGF1R expression levels with a concomitant significant increase in IGFs and phosphorylated IRS-1 [41]. The expression of the endogenous IGF1R gene in most cells and tissues tends to be low, and the up-regulation seen in many tumors and transformed cell lines is not dramatic. A reduction in IGF1R expression per se may not always translate into a decline in IGF-II signaling, for example Knowlden et al [42] observed that in MCF-7 and T47D breast cancer cell lines, comparable levels of IGF1R phosphorylation could be seen despite significantly higher receptor levels in the former. Noteworthy, AA tumor samples (despite lower IGF1R levels compared to normal AA samples) showed significantly higher levels of IGF1R phosphorylation than CA tumor samples, although no significant difference was found in IGF1R protein expression. Moreover, recent studies in our laboratory have shown that tumor tissues from AA women express higher levels of IGF-II as compared to tumor samples from CA women [43]. Given the complexity of this disease, we propose that measuring activation of signaling pathways may more accurately reflect the aggressiveness of the tumor and maybe more informative in the assessment for treatment and follow up.
Insulin receptor substrate (IRS)-1 and Shc are adaptor proteins in the insulin-like growth factor II/IGF1R/INSR pathways that mediate cell proliferation and survival. In addition to their role as scaffolding proteins in the cytoplasm, they are able to translocate to the nucleus and regulate gene transcription [44]. Our results showed higher levels of IRS-1 phosphorylation in AAM as compared to CAM at all age groups, while Shc phosphorylation was only increased in ER (+) premenopausal AAM samples as compared to CAM samples. IRS-1 phosphorylation has been associated with transformation of mammary epithelial cells, and increased cell proliferation and survival of breast cancer cells through activation of the PI3K/Akt pathway [44], while higher levels of Shc phosphorylation have been associated with proliferation of transformed cells and c-myc activation through phosphorylation and activation of the MAPK signaling cascade [45]. Combined molecular analysis of receptor activation and effector signaling proteins are complementary, and more specifically identifies signaling pathways involved in breast cancer cell survival, proliferation and chemoresistance.
Another important protein involved in IGF-II regulation is the IGF2 receptor. The IGF2R has evolved in mammals to carry out multiple functions. IGF-II binding to the IGF2R results in internalization/degradation of the ligand, thereby down-regulating the levels of this mitogenic factor [46]. IGF2R also binds M6P-bearing ligands and targets them to lysosomes [19, 47]. Binding of the IGF2R to the latent form of TGF-β promotes this growth factor activation leading to growth inhibition [47]. The IGF2R also binds prorenin [48], proliferin [49], and leukemia inhibitor factor [50]. The multiple functions of the IGF2R suggest a role for this receptor as a growth inhibitor. Furthermore, loss of heterozygosity (LOH) at the IGF2R locus has been correlated with poorly differentiated states in early breast carcinomas [41]. In our study, CA tumor samples expressed significantly higher levels of IGF2R protein than AA tumor samples. The serious implications for this significant difference in the IGF2R expression between AA and CA women with breast cancer are that tumors from AA patients will have increased levels of available IGF-II. We and others have shown that increased levels of IGF-II will stimulate tumor proliferation, reduce apoptosis, decrease TGF-β activation, and increase Cathepsin D, all of which will contribute to more aggressive and more chemoresistant tumors. In summary, our study contributes to a better understanding of how alteration in the IGF1 and IGF2 receptors expression and increased IGF signaling pathway contribute to the survival disparities observed between African-American and Caucasian women affected with breast cancer and these receptors represent, along with IGF-II, potential therapeutic targets.
Table 1.
African-American and Caucasian tumor samples characteristics divided by pre- or post-menopausal stage, estrogen receptor (ER), progesterone receptor (PR), HER2 status, grade/stage and metastases. 86% of tumor samples histologic type was invasive ductal carcinoma (n=81), while 14% were invasive lobular carcinomas (n=13).
Tumor characteristics by race/ethnic group
| Ethnicity | Age | ER status | PR status | HER2 status | Grade/Stage | Metastases | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| (+) | (−) | (+) | (−) | (+) | (−) | (II) | (III) | (+) | (−) | ||
| African-American tumor samples | Pre-menopausal | N=9 | N=5 | N=9 | N=5 | N=6 | N=8 | N=5 | N=8 | N=8 | N=3 |
| Post-menopausal | N=6 | N=7 | N=5 | N=8 | N=6 | N=7 | N=5 | N=9 | N=11 | N=5 | |
| Caucasian tumor samples | Pre-menopausal | N=5 | N=6 | N=4 | N=7 | N=5 | N=4 | N=7 | N=5 | N=7 | N=5 |
| Post-menopausal | N=6 | N=7 | N=7 | N=6 | N=8 | N=7 | N=5 | N=7 | N=9 | N=3 | |
86% of tumor samples histologic type was invasive ductal carcinoma (n=81), while 14% were invasive lobular carcinomas (n=13).
Acknowledgments
This research was supported by 5P20 MD001632, and NIGMS 5R25GM060507
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.National Cancer Institute, DC-CPS, Surveillance Research Program, Cancer Statistics Branch; Surveillance, Epidemiology, and End Results program (SEER) SEER Stat Database: Mortality-All COD, Public-Use with State, Total U.S. for Expanded Races/Hispanics (1991–2001) Released April 2004. http://www.seer.cancer.gov. [Google Scholar]
- 2.De Myets P. The structural basis of insulin and insulin-like growth factor-1 receptor binding and negative cooperativity, and its relevance to mitogenic versus metabolic signaling. Diabetologia. 1994;37(Suppl 2):S135–S148. doi: 10.1007/BF00400837. [DOI] [PubMed] [Google Scholar]
- 3.Morrione A, Valentinis B, Xu SQ, Yumet G, Louvi A, Efstratiadis A, Baserga R. IGF-II stimulates cell proliferation through the insulin receptor. Proc Natl Acad Sci USA. 1997;94:3777–3782. doi: 10.1073/pnas.94.8.3777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sciacca L, Constantino A, Pandini G, Mineo R, Frasca F, Scalia P, Sbraccia P, Goldfine ID, Vigneri R, Belfiore A. Insulin receptor activation by IGF-II in breast cancers: evidence for a new autocrine/paracrine mechanism. Oncogene. 1999;18:2471–2479. doi: 10.1038/sj.onc.1202600. [DOI] [PubMed] [Google Scholar]
- 5.Voskuil D, Bosma A, Vrieling A, Rookus M, Veer L. IGF system mRNA quantities in normal and tumor breast tissue of women with sporadic and familial breast cancer risk. Breast Cancer Research and Treatment. 2004;84:225–233. doi: 10.1023/B:BREA.0000019954.59130.d3. [DOI] [PubMed] [Google Scholar]
- 6.Pacher M, Seewald M, Mikula M, Oehler S, Mogg M, Vinatzer U, Eger A, Schweifer N, Varecka R, Sommergruber W, Mikulits W, Schreiber M. Impact of constitutive IGF1/IGF2 stimulation on the transcriptional program of human breast cancer cells. Carcinogenesis. 2007:49–59. doi: 10.1093/carcin/bgl091. [DOI] [PubMed] [Google Scholar]
- 7.Kalla Singh S, Tan QW, De León M, De León D. Differential IGF-II expression: A potential role for breast cancer survival disparity. Growth Hormone and IGF Research. 2010 Jan 19; doi: 10.1016/j.ghir.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pinheiro SP, Holmes MD, Pollak MN, Barbieri RL, Hankinson SE. Racial differences in premenopausal endogenous hormones. Cancer Epidemiol Biomarkers Prev. 2005;14:2147–2153. doi: 10.1158/1055-9965.EPI-04-0944. [DOI] [PubMed] [Google Scholar]
- 9.Werner H, Woloschak M, Adamo M, Shen-Orr Z, Roberts CT, LeRoith D. Developmental regulation of the rat IGF1 receptor gene. Proc Natl Acad Sci USA. 1989;86:7451–7455. doi: 10.1073/pnas.86.19.7451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beitner-Johnson D, Werner H, Roberts CT, Jr, LeRoith D. Regulation of IGF1R gene expression by Sp1: physical and functional interactions of Sp1 at GC boxes and at a CT element. Mol Endocrinol. 1995;9:1147–1156. doi: 10.1210/mend.9.9.7491107. [DOI] [PubMed] [Google Scholar]
- 11.Condorelli G, Buenos R, Smith RJ. Two alternative spliced forms of the human IGF1R have distinct biological activities and internalization kinetics. The Journal of Biological Chemistry. 1994;269:8510–8516. [PubMed] [Google Scholar]
- 12.Sarfstein R, Maor S, Reisner N, Abramovitch S, Werner H. Transcriptional regulation of the IGF-1 receptor gene in breast cancer. Molecular and Cellular Endocrinology. 2006;252:241–246. doi: 10.1016/j.mce.2006.03.018. [DOI] [PubMed] [Google Scholar]
- 13.Sell C, Ubini M, Rubin R, Liu JP, Efstratiadis A, Baserga R. Simian virus 40 large tumor antigen is unable to transform mouse embryonic fibroblast lacking type 1 insulin-like growth factor receptor. Proc Natl Acad Sci USA. 1993;90:11217–11221. doi: 10.1073/pnas.90.23.11217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schnarr B, Strunz K, Ohsam J, Benner A, Wacker J, Mayer D. Down-regulation of IGF1R and IRs-1 expression in advanced human breast cancer. Int J Cancer. 2000;89:506–513. doi: 10.1002/1097-0215(20001120)89:6<506::aid-ijc7>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 15.Lu Y, Zi X, Zhao Y, Mascarenhas D, Pollak M. IGF1R signaling and resistance to trastuzumab (herceptin) J Natl Cancer Inst. 2001;93:1852–1857. doi: 10.1093/jnci/93.24.1852. [DOI] [PubMed] [Google Scholar]
- 16.Roberts CT., Jr Control of IGF action by regulation of IGF1R expression. Endocrine Journal. 1996;43:S49–S55. doi: 10.1507/endocrj.43.suppl_s49. [DOI] [PubMed] [Google Scholar]
- 17.Hawkes C, Amritraj A, MacDonald RG. Heterotrimeric G proteins and the single-transmembrane domain IGF-II/M6P receptor: Functional interaction and relevance to cell signaling. Mol Neurobiol. 2007;35:329–345. doi: 10.1007/s12035-007-0021-2. [DOI] [PubMed] [Google Scholar]
- 18.De Leon DD, Terry C, asmerom Y, Nissley P. IGF-II modulates the routing of Cathepsin D in MCF-7 breast cancer cells. 1996;137:1851–1859. doi: 10.1210/endo.137.5.8612524. [DOI] [PubMed] [Google Scholar]
- 19.Faridi JS, Mohan S, De Léon D. Modulation of Cathepsin D routing by IGF-II involves IGF-II binding to IGF-II/M6P receptor in MCF-7 breast cancer cells. Growth Factors. 2004;22:169–177. doi: 10.1080/08977190410001725531. [DOI] [PubMed] [Google Scholar]
- 20.Capony F, Rougeot C, Montcourrier P, Cavailles V, Salazar G, Rochefort H. Increased secretion, altered processing and glycosylation of pro-cathepsin D in human mammary cancer cells. Cancer Res. 1989;49:3904–3909. [PubMed] [Google Scholar]
- 21.Rochefort H, Cavailles V, Augereau P, Capony F, Maudelonde T, Touitou I, et al. Overexpression and hormonal regulation of procathepsin D in mammary and endometrial cancer. J Steroid Biochem. 1989;34:177–182. doi: 10.1016/0022-4731(89)90080-0. [DOI] [PubMed] [Google Scholar]
- 22.Funk B, Kessler U, Eisenmenger W, Hansmann A, Kolb HJ, Kiess W. Expression of the IGF-II/M6P receptor in multiple human tissues during fetal life and early infancy. J Clin Endocrinol Metab. 1992;75:424–431. doi: 10.1210/jcem.75.2.1379254. [DOI] [PubMed] [Google Scholar]
- 23.Hankins GR, De Souza AT, Bentley RC, et al. M6P/IGF2 receptor: a candidate breast tumor suppressor gene. Oncogene. 1996;12:2003–2009. [PubMed] [Google Scholar]
- 24.Piao Z, Choi Y, Park C, Lee WJ, Park JH, Kim H. Deletion of the M6P/IGF2R gene in primary hepatocellular carcinoma. Cancer Lett. 1997;120:39–43. doi: 10.1016/s0304-3835(97)00289-9. [DOI] [PubMed] [Google Scholar]
- 25.Yamada T, De Souza AT, Finkelstein S, Jirtle RL. Loss of the gene encoding M6P/IGF2R is an early event in liver carcinogenesis. Proc Natl Acad Sci USA. 1997;94:10351–10355. doi: 10.1073/pnas.94.19.10351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ikezu T, Okamoto T, Giambarella U, Yokota T, Nishimoto I. In vivo coupling of IGF-II/M6P receptor to heteromeric G proteins the Journal of Biological Chemistry. 1995;270:29224–29228. doi: 10.1074/jbc.270.49.29224. [DOI] [PubMed] [Google Scholar]
- 27.Albain KS, Unger JM, Crowley JJ, Coltman CA, Hershman DL. Racial disparities in cancer survival among randomized clinical trials patients of the Southwest Oncology Group. J Natl Cancer Inst. 2009;101:984–992. doi: 10.1093/jnci/djp175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carey LA, Perou CM, Livasy CA, Dressler LG, Cowan D, Conway K, Karaca G, Troester MA, Tse CK, Edmiston S, Deming SL, Geradts J, Cheang MC, Nielsen TO, Moorman PG, Earp HS, Millikan RC. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA. 2006;295:2492–2502. doi: 10.1001/jama.295.21.2492. [DOI] [PubMed] [Google Scholar]
- 29.Bauer KR, Brown M, Cress RD, Parise CA, Caggiano V. Descriptive analysis of ER (−), PR (−), and HER2 (−) invasive breast cancer, the so called triple negative phenotype: a population-based study from the California Cancer Registry. Cancer. 2007;109:1721–1728. doi: 10.1002/cncr.22618. [DOI] [PubMed] [Google Scholar]
- 30.Polite BN, Cirrincione C, Fleming GF, Berry DA, Seidman A, Muss H, Norton L, Shapiro C, Bakri K, Marcom K, Lake D, Schwartz JH, Hudis C, Winer EP. Racial differences in clinical outcomes from metastatic breast cancer: a pooled analysis of CALGB 9342 and 9840- Cancer and Leukemia group B. J Clin Oncol. 2008;26:2659–2665. doi: 10.1200/JCO.2007.13.9782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tennant MK, Thrasher JB, Twomey PA, Drivdahl RH, Birnbaum RS, Plymate SR. Protein and mRNA for the IGF1R is decreased and IGF-II mRNA is increased in human prostate carcinoma compared to the benign prostate epithelium. J Clin Endocrinol Metab. 1996;81:3774–3782. doi: 10.1210/jcem.81.10.8855837. [DOI] [PubMed] [Google Scholar]
- 32.Shin A, Ren Z, Shu X-O, Cai Q, Gao Y-T, Zheng W. Expression patterns of IGF-1 and its receptor in mammary tissues and their associations with breast cancer survival. Breast Cancer Res Treat. 2007;105:55–61. doi: 10.1007/s10549-006-9427-1. [DOI] [PubMed] [Google Scholar]
- 33.Carboni JM, Lee AV, Hadsell DL, Rowley BR, Lee FY, Bol DK, Camuso AE, Gottardis M, Greer AF, Ho CP, Hurlburt W, Li A, Saulnier M, Velaparthi U, Wang C, Wen ML, Westhouse RA, Wittman M, Zimmermann K, Rupnow BA, Wong TW. Tumor development by transgenic expression of a constitutively active IGF1 receptor. Cancer Res. 2005;65:3781–3787. doi: 10.1158/0008-5472.CAN-04-4602. [DOI] [PubMed] [Google Scholar]
- 34.Jones RA, Campbell CI, Gunther EJ, Chodosh LA, Petrik JJ, Khokha R, Moorehead RA. Transgenic overexpression of IGF1R disrupts mammary ductal morphogenesis and induces tumor formation. Oncogene. 2007;26:1636–1644. doi: 10.1038/sj.onc.1209955. [DOI] [PubMed] [Google Scholar]
- 35.Olopade OI, Fackenthal JD, Dunston G, Tainsky MA, Collins F, Whitfield-Broome C. Breast cancer genetics in African Americans. Cancer. 2003;97:236–245. doi: 10.1002/cncr.11019. [DOI] [PubMed] [Google Scholar]
- 36.Pal T, Permuth-Wey J, Holtje T, Sutphen R. BRCA1 and BRCA2 mutations in a study of African American breast cancer patients. Cancer Epidemiol Biomarkers Prev. 2004;13:1794–1799. [PubMed] [Google Scholar]
- 37.Joe AK, Hibshoosh H. African-American/White differences in breast carcinoma: p53 alterations and other tumor characteristics. Cancer. 2005;104:661–662. doi: 10.1002/cncr.21210. [DOI] [PubMed] [Google Scholar]
- 38.Newman LA. Breast carcinoma in African-American and White women. Application of molecular biology to understand outcome disparities. Cancer. 2004;101:1261–1263. doi: 10.1002/cncr.20501. [DOI] [PubMed] [Google Scholar]
- 39.Jones BA, Kasl SV, Howe CL, Lachman L, Dubrow R, Curnen MM, Soler-Vila H, Beeghly A, Duan F, Owens P. African-American/White differences in breast carcinoma: p53 alterations and other tumor characteristics. Cancer. 2004;101:1293–1301. doi: 10.1002/cncr.20500. [DOI] [PubMed] [Google Scholar]
- 40.Maor S, Yosepovich A, Papa MZ, Yarden RI, Mayer D, Friedman E, Werner H. Elevated IGF1R levels in primary breast tumors associated with BRCA1 mutations. Cancer Lett. 2007;257:236–243. doi: 10.1016/j.canlet.2007.07.019. [DOI] [PubMed] [Google Scholar]
- 41.Burroughs KD, Howe SR, Okubo Y, Fuchs-Young R, LeRoith D, Walker CL. Dysregulation of IGF-1 signaling in uterine leiomyoma. J Endocrinol. 2002;172:83–93. doi: 10.1677/joe.0.1720083. [DOI] [PubMed] [Google Scholar]
- 42.Knowlden JM, Hutcheson IR, Barrow D, Gee JM, Nicholson RI. IGF1R signaling in tamoxifen-resistant breast cancer: a supporting role to the epidermal growth factor receptor. Endocrinology. 2005;146:4609–4618. doi: 10.1210/en.2005-0247. [DOI] [PubMed] [Google Scholar]
- 43.Kalla Singh S, Tan QW, Brito C, De León M, Garberoglio C, De León D. Differential IGF-II expression: A potential role for breast cancer survival disparity. Growth Horm IGF Res. 2010 doi: 10.1016/j.ghir.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chan BT, Lee AV. Insulin receptor substrate and breast tumorogenesis. J Mamm Gland Biol And Neoplasia. 2008;13:415–422. doi: 10.1007/s10911-008-9101-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ravichandran KS. Signaling via Shc family adapter proteins. Oncogene. 2001;20:6322–6330. doi: 10.1038/sj.onc.1204776. [DOI] [PubMed] [Google Scholar]
- 46.Delaine C, Alvino CL, McNeil KA, Mulhen TD, Gauguin L, De Myets P, Jones EY, Brown J, Wallace JC, Forbes BE. A novel binding site for the human IGF-II/M6P receptor on IGF-II. The Journal of Biological Chemistry. 2007;282(26):18886–18894. doi: 10.1074/jbc.M700531200. [DOI] [PubMed] [Google Scholar]
- 47.Oka Y, Rozek LM, Czech MP. Direct demonstration of rapid IGF2R initialization and recycling in rat adipocytes. Insulin stimulates 125I-IGF-II degradation by modulating the IGF2R recycling process. J Biol Chem. 1985;260:9435–9442. [PubMed] [Google Scholar]
- 48.Faust PL, Chirgwin JM, Kornfeld S. Renin, a secretory glycoprotein, acquires phosphomannosyl residues. J Cell Biol. 1987;105:1947–1955. doi: 10.1083/jcb.105.5.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee S-J, Nathans D. Proliferin secreted by cultured cells binds to mannose-6-phosphate receptors. J Biol Chem. 1988;263:3521–3527. [PubMed] [Google Scholar]
- 50.Blanchard F, Duplomb L, Raher S, Vusio P, Hoflack B, Jacques Y, Godard A. M6P/IGF-II receptor is a nanomolar affinity receptor for glycosylated human leukemia inhibitor factor. J Biol Chem. 1988;273:20886–20893. doi: 10.1074/jbc.273.33.20886. [DOI] [PubMed] [Google Scholar]