Abstract
Altered cognitive control is implicated in the shaping of cocaine dependence. One of the key component processes of cognitive control is error monitoring. Our previous imaging work highlighted greater activity in distinct cortical and subcortical regions including the dorsal anterior cingulate cortex (dACC), thalamus and insula when participants committed an error during the stop signal task (Li et al., 2008b). Importantly, dACC, thalamic and insular activity has been associated with drug craving. One hypothesis is that the intense interoceptive activity during craving prevents these cerebral structures from adequately registering error and/or monitoring performance. Alternatively, the dACC, thalamus and insula show abnormally heightened responses to performance errors, suggesting that excessive responses to salient stimuli such as drug cues could precipitate craving. The two hypotheses would each predict decreased and increased activity during stop error (SE) as compared to stop success (SS) trials in the SST. Here we showed that cocaine dependent patients (PCD) experienced greater subjective feeling of loss of control and cocaine craving during early (average of day 6) compared to late (average of day 18) abstinence. Furthermore, compared to PCD during late abstinence, PCD scanned during early abstinence showed increased thalamic as well as insular but not dACC responses to errors (SE>SS). These findings support the hypothesis that heightened thalamic reactivity to salient stimuli co-occur with cocaine craving and loss of self control.
Keywords: thalamus, subcortical, imaging, neuropsychology, fMRI, self control, craving, cocaine abuse
1. Introduction
Error processing is an important component process of cognitive control (Taylor et al., 2007). By detecting errors we modify ongoing behavior and establish new contingencies. Failure to register errors has been implicated in a number of psychiatric conditions including substance misuse (Garavan and Stout, 2005). For instance, we observed that, compared to healthy control participants, patients with cocaine dependence (PCD) demonstrated decreased post-error behavioral adjustment during the stop signal task (SST), suggesting impaired performance monitoring (Li et al., 2006b). Other investigators have employed different behavioral tasks and highlighted various aspects of altered error-related measures in substance abusing individuals (Franken et al., 2007; Garavan and Hester, 2007; Hester et al., 2007; Paulus et al., 2008; Verdejo-García et al., 2007; Yeung et al., 2007). It has also been theorized in computational modeling that altered error processing contributes to compulsory drug use in addiction (Reddish, 2004; Reddish and Johnson, 2007).
In our functional magnetic resonance imaging (fMRI) studies of the SST, we described a distinct pattern of activation in the dorsal anterior cingulate cortex (dACC), thalamus, and insula during error processing (Li et al., 2008b). Importantly, these cerebral structures are also implicated in mediating drug craving (see Koob and Volkow, 2010; Li and Sinha, 2008 for a review). For instance, structures including the ventral tegmental area, thalamus, ACC, insula, and amygdala demonstrated greater activation during fMRI in response to marijuana as compared to neutral cue in abstinent marijuana users (Filbey et al., 2009). Following up on their earlier studies of mesolimbic responses to cigarette cues, Franklin and colleagues investigated the effects of a dopamine transporter polymorphism on cue elicited activation in brain regions including the ventral striatum/pallidum, anterior cingulate and insula (Franklin et al., 2009). With perfusion MRI, Wang and colleagues demonstrated an association between abstinence induced craving and increased cerebral blood flow in the orbitofrontal cortex (OFC), ACC, ventral striatum, thalamus, amygdala, and insula, in cigarette smokers (Wang et al., 2007). A positive association between the severity of nicotine dependence and cue reactivity in the OFC and ACC was also observed by McClernon et al., 2008. In a pharmacological fMRI study, Hermann and colleagues showed decreased cue – as compared to control – induced activation in the thalamus after administration of an atypical dopamine receptor blocker in abstinent alcohol dependent patients. Overall, these along with many other studies support the role of a network of cerebral structures in mediating drug and alcohol craving (Duncan et al., 2007; Feldstein et al., in press; Grüsser et al., 2004; Hermann et al., 2006; King et al., in press; McClernon et al., 2009; Risinger et al., 2005; Rose et al., 2007; Weinstein et al., in press).
We are particularly interested in the overlapping pattern of regional brain activations during cognitive control and drug craving. In the SST, medial cortical regions including the dACC and subcortical structures including the thalamus and insula showed greater activation during stop errors as compared to stop successes. The same brain regions are also extensively implicated in drug craving. This association is of potential importance to understanding the etiology of drug addiction as deficits in cognitive control and drug craving co-occur in patients with drug addiction (Volkow et al., in press; see also Baicy and London, 2007; Koob and Volkow, 2010 for a review). Two hypotheses are in place to explain how altered dACC, thalamic and insular activity may be associated with craving and changes in cognitive control. One hypothesis is that the intense interoceptive activity during withdrawal and craving is ill adaptive for these cerebral structures to register error and/or monitor performance. An alternative hypothesis is that the dACC, thalamus and insula shows abnormally heightened responses to performance errors, suggesting that excessive responses to salient stimuli such as drug cues could precipitate craving. The two hypotheses would each predict decreased and increased activity during stop error (SE) as compared to stop success (SS) trials in the SST.
We tested these two hypotheses by comparing error-related regional brain activation in cocaine dependent patients early and late during abstinence, with patients experiencing significantly greater craving and subjective loss of control early during their abstinence.
2. Materials and Methods
Subjects, informed consent and assessment of impulse control
Twenty-six abstinent patients with cocaine dependence (PCD) participated in the study (Table 1). PCD met criteria for current cocaine dependence, as diagnosed by the Structured Clinical Interview for DSM-IV (First et al., 1995). Recent cocaine use was confirmed by urine toxicology screens upon admission. They were drug-free while staying in an inpatient treatment unit prior to the current fMRI study. All subjects were physically healthy with no major medical illnesses or current use of prescription medications. None of them reported having a history of head injury or neurological illness. Other exclusion criteria included dependence on another psychoactive substance (except nicotine) and current or past history of psychotic disorders. Individuals with current depressive or anxiety symptoms requiring treatment or currently being treated for these symptoms were excluded as well. The Human Investigation committee at Yale University School of Medicine approved all study procedures, and all subjects signed an informed consent prior to study participation.
Table 1.
Demographics of the subjects
| subject characteristic | Early (n=9) | Late (n=17) | p value | |
|---|---|---|---|---|
| days of abstinence before fMRI | 5.8 ± 4.7 | 18.0 ± 2.2 | <0.0001b | |
| men/women | 5/4 | 8/9 | 0.43a | |
| age (years) | 36.6 ± 6.2 | 36.8 ± 6.0 | 0.93b | |
| ethnicity | African American | 4 (44.4%) | 7 (41.2%) | 0.54a |
| Caucasian | 5 (55.6%) | 10 (58.8%) | ||
| education (years) | 11.4 ± 2.2 | 12.4 ± 1.0 | 0.16b | |
| average number of days of cocaine use / month prior to admission | 19.1 ± 9.5 | 17.8 ± 8.4 | 0.72b | |
| average number of years of cocaine use | 9.0 ± 5.1 | 11.7 ± 6.7 | 0.30b | |
| Life time diagnosis of depression | 1 (11.1%) | 2 (11.8%) | 0.71a | |
| Life time diagnosis of PTSD | 3 (33.3%) | 5 (29.4%) | 0.51a | |
Note: values are mean s.d.
binomial test
two-tailed 2-sample test
Cocaine craving was assessed with the cocaine craving questionnaire, brief version (CCQ-Brief), for all participants on the same day or within days of the scan (Sussner et al., 2006). The CCQ-Brief is a 10 item questionnaire, abbreviated from the CCQ-Now (Tiffany et al., 1993). It is highly correlated with the CCQ-Now and other cocaine craving measures (Sussner et al., 2006).
In all except 3 PCD's, we also assessed subjective feeling of difficulties in emotion regulation and impulse control using the Difficulties in Emotion Regulation Scale in the same week or, in some cases, on the same day of the fMRI (DERS, Gratz and Roemer, 2004). The DERS is a 36-item self-report measure, with each item rated on a 5-point analog scale: 1=almost never; 2=sometimes; 3=about half of the time; 4=most of the time; 5=almost always. It has been shown to have high internal consistency, test-retest reliability and construct validity in the general population and in cocaine dependent patients (Fox et al., 2007; Gratz and Roemer, 2004). The total score of DERS ranges from 36 to 180, with higher score indicating greater emotion dysregulation and difficulties in impulse control. Abstinent cocaine patients were found to have persistent problems in impulse control compared to healthy individuals (Fox et al., 2007).
Behavioral task
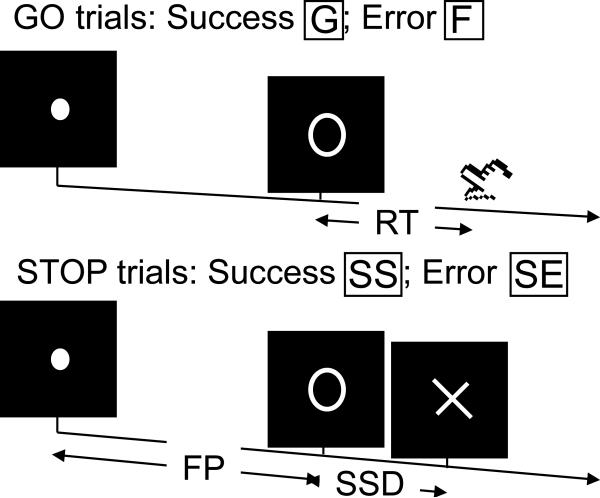
We employed a simple reaction time (RT) task in this stop-signal paradigm (Fig. 1). There were two trial types: “go” and “stop,” randomly intermixed. A small dot appeared on the screen to engage attention and eye fixation at the beginning of a go trial. After a randomized time interval (fore-period) anywhere between 1 and 5 s, the dot turned into a circle, prompting subjects to quickly press a button. The circle vanished at button press or after 1 s had elapsed, whichever came first, and the trial terminated. A premature button press prior to the appearance of the circle also terminated the trial. Three quarters of all trials were go trials. In the stop trials, an additional “X,” the “stop” signal, appeared after the go signal. The subjects were told to withhold button press upon seeing the stop signal. Likewise, a trial terminated at button press or when 1 s had elapsed since the appearance of the stop signal. The stop trials constituted the remaining one quarter of the trials. There was an inter-trial-interval of 2 s.
Figure 1.
(a) Stop signal paradigm. In “go” trials (75%) observers responded to the go signal (a circle) and in “stop” trials (25%) they had to withhold the response when they saw the stop signal (an X). In both trials the go signal appeared after a randomized time interval between 1 to 5 s (the fore-period or FP) following the appearance of the fixation point. The stop signal followed the go signal by a time delay – the stop signal delay (SSD). The SSD was updated according to a staircase procedure, whereby it increased and decreased by 64 ms following a stop success (SS) and stop error (SE) trial, respectively.
The time interval between the go and stop signals, or the stop signal delay (SSD), started at 200 ms and varied from one stop trial to the next according to a staircase procedure: if the subject succeeded in withholding the response, the SSD increased by 64 ms, making it more difficult for them to succeed again in the next stop trial; conversely, if they failed, SSD decreased by 64 ms, making it easier for the next stop trial. With the staircase procedure, a “critical” SSD could be computed that represents the time delay required for the subject to succeed in withholding a response half of the time in the stop trials (Levitt, 1970). One way to understand the stop signal task is in terms of a horse race model with a go process and a stop process racing toward a finishing line (Logan, 1994). The go process prepares and generates the movement while the stop process inhibits movement initiation: whichever process finishes first determines whether a response will be initiated or not. According to the race model, the time required for the stop signal to be processed so a response is withheld (i.e., stop signal reaction time or SSRT) can be computed by estimating the critical SSD at which a response can be correctly stopped in approximately 50% of the stop trials. The SSRT is computed in the current tracking stop signal task for each individual subject by subtracting the critical SSD from the median go trial RT. Generally speaking, the SSRT is the time required for a subject to cancel the movement after seeing the stop signal. A longer SSRT indicates poor response inhibition.
Subjects were instructed to respond to the go signal quickly while keeping in mind that a stop signal could come up in a small number of trials. Prior to the fMRI study each subject had a practice session outside the scanner. Each subject completed four 10-minute runs of the task with the SSD updated manually across runs. Depending on the actual stimulus timing (e.g., trials varied in fore-period duration) and speed of response, the total number of trials varied slightly across subjects in an experiment. With the staircase procedure we anticipated that the subjects would succeed in withholding their response in approximately 50% of the stop trials. This is thus an event-related fMRI study, with the go and stop trials randomly jittered to improve the efficiency of the study design. Additional details of the stop signal task were described previously (Duann et al., 2009; Li et al., 2006a; 2008b; 2008c; 2009a).
Imaging protocol
Conventional T1-weighted spin echo sagittal anatomical images were acquired for slice localization using a 3T scanner (Siemens Trio). Anatomical images of the functional slice locations were next obtained with spin echo imaging in the axial plane parallel to the AC-PC line with TR = 300 ms, TE = 2.5 ms, bandwidth = 300 Hz/pixel, flip angle = 60°, field of view = 220 × 220 mm, matrix = 256 × 256, 32 slices with slice thickness = 4mm and no gap. Functional, blood oxygenation level dependent (BOLD) signals were then acquired with a single-shot gradient echo echo-planar imaging (EPI) sequence. Thirty-two axial slices parallel to the AC-PC line covering the whole brain were acquired with TR = 2,000 ms, TE = 25 ms, bandwidth = 2004 Hz/pixel, flip angle = 85°, field of view = 220 × 220 mm, matrix = 64 × 64, 32 slices with slice thickness = 4mm and no gap. Three hundred images were acquired in each run for a total of 4 runs.
Data analysis and statistics
Data were analyzed with Statistical Parametric Mapping (SPM2, Wellcome Department of Imaging Neuroscience, University College London, U.K.). Images from the first five TRs at the beginning of each trial were discarded to enable the signal to achieve steady-state equilibrium between RF pulsing and relaxation. Images of each individual subject were first corrected for slice timing and realigned (motion-corrected). A mean functional image volume was constructed for each subject for each run from the realigned image volumes. These mean images were normalized to an MNI (Montreal Neurological Institute) EPI template with affine registration followed by nonlinear transformation (Ashburner and Friston, 1999; Friston et al., 1995a). The normalization parameters determined for the mean functional volume were then applied to the corresponding functional image volumes for each subject. Finally, images were smoothed with a Gaussian kernel of 10 mm at Full Width at Half Maximum.
Four main types of trial outcome were distinguished: go success (G), go error (F), stop success (SS), and stop error (SE) trial (Fig. 1). A statistical analytical design was constructed for each individual subject, using the general linear model (GLM) with the onsets of go signal in each of these trial types convolved with a canonical hemodynamic response function (HRF) and with the temporal derivative of the canonical HRF and entered as regressors in the model (Friston et al., 1995b). Additional regressors with the go trial RT and stop trial SSD were also included for parametric modulation. Realignment parameters in all 6 dimensions were also entered in the model. The data were high-pass filtered (1/128 Hz cutoff) to remove low-frequency signal drifts. Serial autocorrelation was corrected by a first-degree autoregressive or AR(1) model. The GLM estimated the component of variance that could be explained by each of the regressors.
We constructed for each individual subject a single statistical contrast: SE vs. SS. In group whole-brain analysis, we compared PCD who were scanned within 2 weeks of abstinence (n=9, 5.8 ± 4.7 days) and PCD who were scanned after 2 weeks of abstinence (n=17, 18.0 ± 2.2 days; p<0.0001, two tailed 2-sample t test) controlling for and without controlling for differences in general task performance. In region of interest analyses, to ascertain that the group differences specifically reflected error-related processing, we focused on a mask that included all brain regions that showed greater activation during SE as compared to SS across all PCD.
3. Results
Behavioral performance
All patients of cocaine dependence (PCD) succeeded in over 95% of go trials and approximately half of the stop trials, suggesting the success of the staircase tracking procedure (Table 2). Compared to the “late” abstinence group, the “early” abstinence group was significantly faster in go trial responses. This difference in go reaction time (RT) would be accounted for in group comparison of error-related activity. The two groups did not differ in other aspects of the stop signal performance.
Table 2.
General performance in the stop signal task
| group | Median go RT (ms) | %go | %stop | Critical SSD (ms) | SSRT (ms) | FP effect (effect size) | PES (effect size) |
|---|---|---|---|---|---|---|---|
| Early (n=9) | 486 ± 68 | 96.9 ± 1.6 | 50.9 ± 2.9 | 279 ± 62 | 207 ± 26 | 1.9 ± 1.2 | 2.4 ± 1.1 |
| Late (n=17) | 580 ± 85 | 96.3 ± 1.6 | 51.6 ± 2.1 | 364 ± 91 | 215 ± 52 | 2.2 ± 1.4 | 1.7 ± 1.6 |
| P value | 0.009* | 0.309 | 0.499 | 0.019* | 0.672 | 0.575 | 0.216 |
Note: %go and %stop: percentage of successful go and stop trials; SSRT: stop-signal reaction time; PES: post-error slowing; all numbers are mean ± standard deviation. P value based on 2-talied two sample t test.
<0.05.
Cocaine craving and subjective loss of control
Compared to the late abstinence group, the early abstinence group showed significant greater craving for cocaine, as assessed by the Cocaine Craving Questionnaire, Brief Version (32.9 ± 17.1vs. 15.8 ± 6.2, p<0.002). The early group also rated greater subjective feeling of loss of control on the DERS, compare to the late group (97.4 ± 22.8 vs. 74.3 ± 20.2, p<0.02).
Regional brain activation during early versus late abstinence: ROI and whole brain analyses
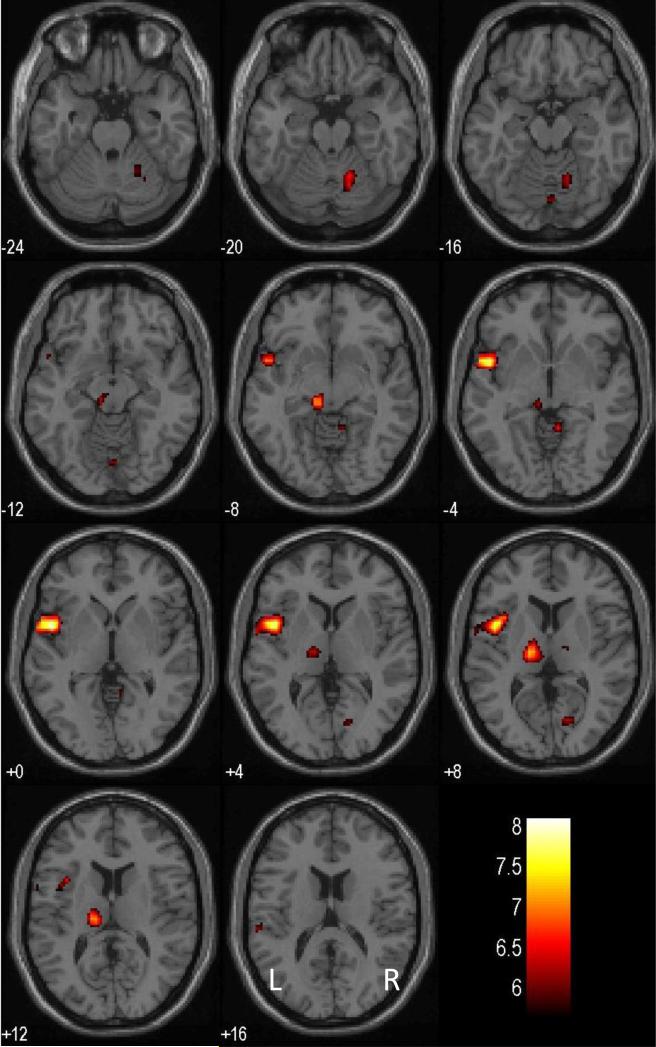
Across all 26 PCD, a number of brain regions showed greater activation during SE compared to SS (Fig. 2), at p<0.05, corrected for family-wise error (FWE) of multiple comparisons and 10 voxels in the extent of activation: left superior temporal gyrus and possibly insula (x=-48, y=4, z=0, voxel Z=5.63); left thalamus (x=-16, y=-16, z=8, voxel Z=5.28); a region in the left pretectal area of the midbrain (x=-8, y=-28, z=-8, voxel Z=5.18); and right cerebellar lobule (x=24, y=-48, z=-28; voxel Z=5.02). Thus, in region of interest (ROI) analysis focusing on this inclusive mask, the early abstinence group showed greater error-related thalamic activation (SE > SS), compared to late abstinence group (x=-8, y=-24, z=8, voxel Z=4.01, 1,984 mm3, p=0.001, FWE-corrected). No other regions within this mask showed significant differences between the two groups (all Z's<1.96, p's>0.05, FWE-corrected).
Figure 2.
Error-related regional brain activations of all 26 patients with cocaine dependence. BOLD contrasts were overlaid on a T1 structural image in axial sections. Images are in neurological orientation: R=R; Color bar represents voxel T value.
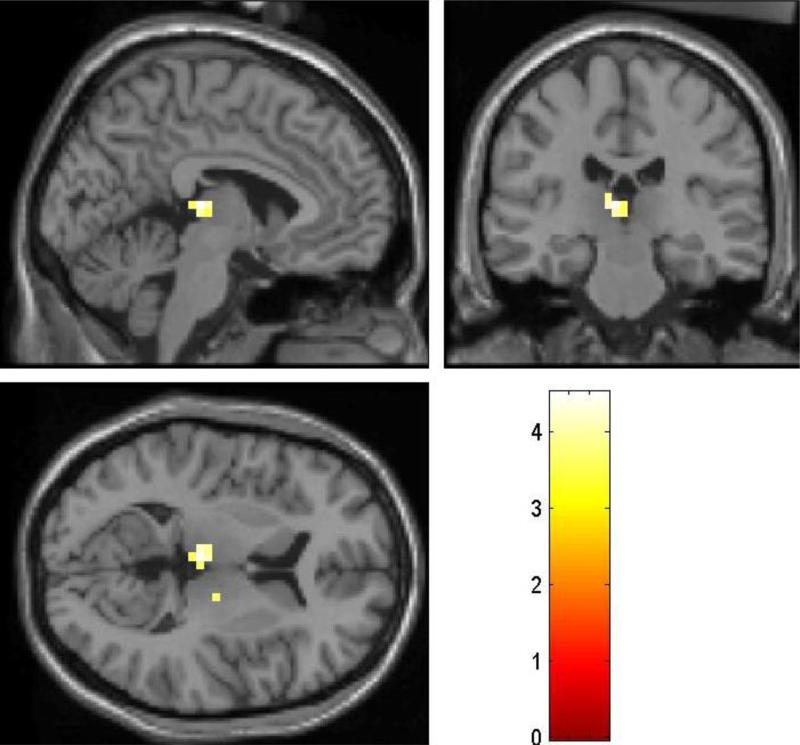
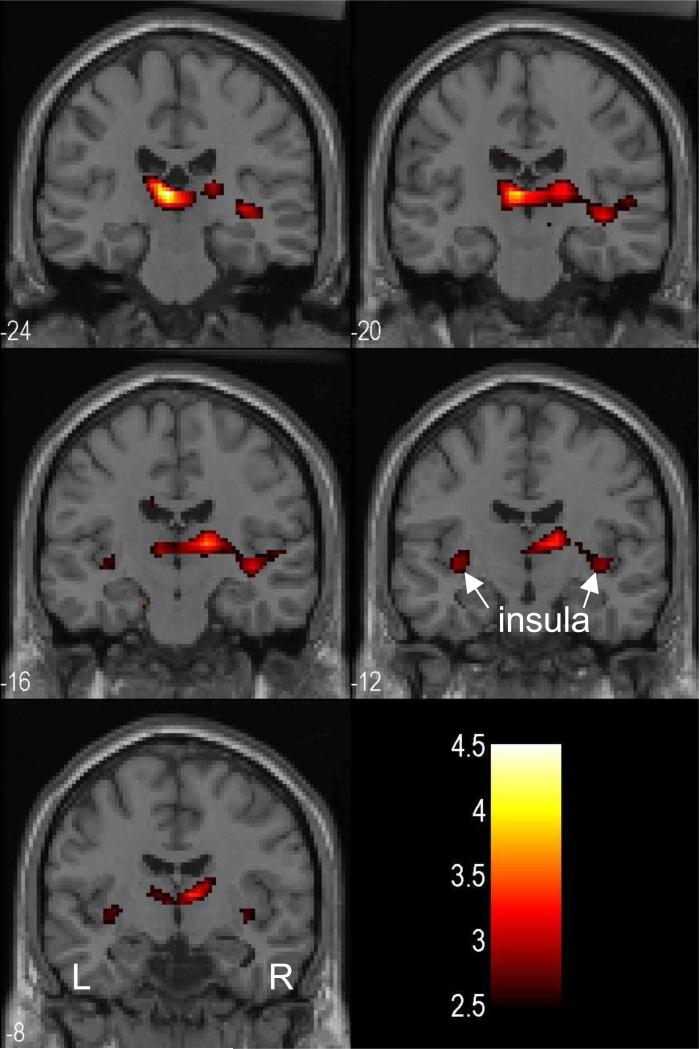
We also examined the group difference with whole brain analysis. Compared to PCD scanned during late abstinence, PCD scanned during early abstinence showed greater error-related activation in the area of the dorsomedial nucleus of the thalamus (x=-4, y=-24, z=8, voxel Z=3.80, 704mm3; x=16, y=-16, z=8, voxel Z=3.19, 64mm3) at a threshold of p<0.001, uncorrected (Fig. 3). At a more liberal threshold of p<0.005, uncorrected, bilateral posterior insula also showed greater activation (x=-36, y=-12, z=-4, voxel Z=2.62, 192mm3; x=40, y=-20, z=-4, voxel Z=2.90, 1,088mm3) in PCD during early compared to late abstinence. No voxels in the medial cortical regions including the cingulate cortex showed significantly greater activation during early as compared to late abstinence (p<0.01, uncorrected). Figure 4 shows the BOLD contrast overlaid on a structural image. Conversely, PCD scanned during late abstinence did not show any regional activation greater than PCD scanned during early abstinence for the same contrast, even when examined at a threshold of uncorrected p<0.01.
Figure 3.
Patients with cocaine dependence (PCD) who were scanned during early abstinence showed greater error-related activation in the thalamus (SE>SS), compared to PCD who were scanned during late abstinence. Color bar represents voxel T value; p<0.001, uncorrected.
Figure 4.
At a lowered threshold, p<0.005, uncorrected, bilateral posterior insula also showed greater activation in PCD scanned during early abstinence compared to PCD scanned during late abstinence. BOLD contrasts were superimposed on a T1 structural image in axial sections. Images are in neurological orientation: R=R; Color bar represents voxel T value.
Functional MRI results: alternative grouping of days of abstinence
To rule out the possibility that the grouping based on an arbitrary criterion of two weeks may influence the results, we performed alternative analyses on subjects grouped on the basis of a median split of their days of abstinence of fMRI. Compared to the late abstinence group (n=13, 8.5 ± 5.8 days), the early abstinence group (n=13, 19.0 ± 1.3 days; p<0.0001, two-sample t tests) showed greater cocaine craving as assessed by CCQ (28.0 ± 16.2 vs. 15.4 ± 6.5; p<0.02) but not subjective loss of control as assessed by DERS (90.9 ± 24.0 vs. 75.1 ± 21.4; p=0.101). In ROI analysis, the early abstinence group showed greater error-related activation at a similar locus of the thalamus (x=-12, y=-24, z=12; voxel Z=3.59, 1,856 mm3) but not any other regions within the mask. Likewise, in whole-brain comparison, the early abstinence group showed greater error-related activation in the left thalamus (x=-12, y=-24, z=12; voxel Z=3.59, 1,280 mm3), at a p<0.001, uncorrected. At a more liberal threshold of p<0.005, uncorrected, the right thalamus also greater activation in the early abstinence group (x=16, y=-20, z=12; voxel Z=3.18, 1,088 mm3). No brain regions showed greater error-related activation in the late as compared to early abstinence group.
Correlation of error-related thalamic activation with loss of control and drug craving
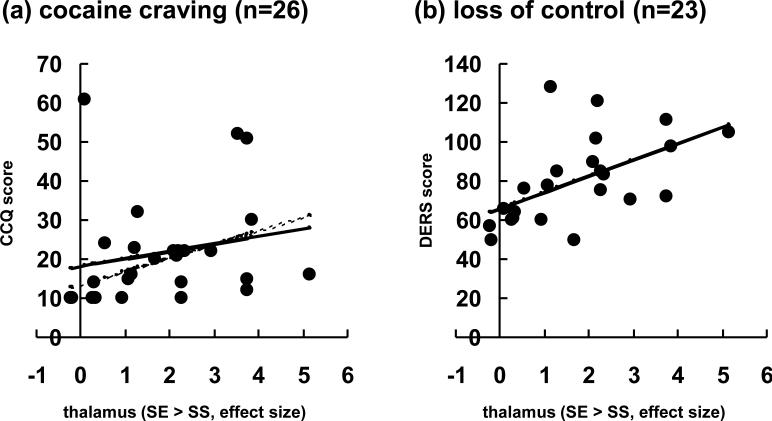
We correlated the error-related thalamic activation with cocaine craving report on the CCQ (n=26) and subjective report of loss of self control on the DERS (n=23; 3 subjects missing data) across subjects (Fig. 5). The results showed that there was a significant correlation between thalamic activation and loss of control (R=0.554, p=0.006). There was not a significant correlation between thalamic activation and cocaine craving (R=0.204, p=0.318), which appeared to be due to the presence of an outlier (> mean + 2 S.D.). With the outlier removed, there was a significant correlation between thalamic activation and cocaine craving (R=0.444, p=0.026).
Figure 5.
Linear correlation of error-related thalamic activation with (a) cocaine craving as assessed by the Cocaine Craving Questionnaire (CCQ) and with (b) loss of self control as assessed by the Difficulty in Emotion Regulation Scale (DERS). Note that DERS data were missing in three patients and that the correlation with CCQ was significant only after the outlier was removed (dashed line).
4. Discussion
Many preclinical and clinical studies suggested a role of the thalamus in performance monitoring, such as during matching sensory feedback with expected outcome of a motor response (Diamond and Ahissar, 2007; Urbain and Deschênes, 2007), re-evaluation of a reinforcer (Mitchell et al., 2007), task planning on the basis of external information (Wagner et al., 2006), processing corollary discharge of an eye movement (Bellebaum et al., 2005; Sommer and Wurtz, 2004), reception of negative feedback during the Wisconsin Card Sorting Task (Monchi et al., 2001), and self-generating actions in response to predictability of stimuli (Blakemore et al., 1998). The thalamus has also been suggested in earlier studies to mediate drug craving (Volkow et al., 1997). For instance, among cortical and other subcortical structures, the thalamus showed greater responses when individuals experience drug craving for nicotine/cigarettes (Franklin et al., 2007; Rose et al., 2007; Wang et al., 2007), cocaine and amphetamine (Caldecott-Hazard et al., 1988; Duncan et al., 2007), alcohol (George et al., 2001; Hermann et al., 2006), opiate (Krystal et al., 1995; Wooten et al., 1982; see also Hommer, 1999; Modell et al., 1990 for an overview of earlier literature). One plausible explanation is that the thalamic responses are driven by the intense somatosensory activities associated with craving. Thus, the intense somatosensory responses interfere with thalamic activity required for performance monitoring, when individuals experience drug craving and, as a result, are not able to exercise behavioral control (Hughes and Hatsukami, 1986). That is, drug craving obliterates the dynamic range required of thalamic activity to respond to errors.
We showed greater error-related thalamic activation in PCD during early abstinence when they experienced greater craving and loss of control, compared to PCD during late abstinence. Across subjects the extent of error-related thalamic activation was also positively correlated with subjective rating of loss of control and, though less significantly, with cocaine craving. The current results thus did not support the hypothesis that the intense somatosensory activity in the thalamus interferes its function in error processing. Instead, these findings are consistent with the alternative hypothesis that salient stimuli such as errors or drug cues would elicit greater thalamic activations in PCD when they experience cocaine craving. Thus, with the thalamus interconnected with the brain stem and prefrontal cortices that are pivotal in arousal, cognitive and affective regulation (see also Schiff, 2008; Sherman, 2007; Vertes, 2006 for a review), this thalamic activation may be associated with the precipitation of craving. In particular, our earlier study showed that, compared to stop success trials, stop errors evoked greater activation in a number of cortical and subcortical brain regions, including the dorsal anterior cingulate cortex extending to the supplementary motor area, thalamus and the epithalamus (Li et al., 2008b). These other brain regions did not differ between the two groups of subjects in error-related activation. Thus, increased thalamic activity to errors during early cocaine abstinence may suggest an important role of this structure in mediating self control and other aspects of the mental state specific to this early stage of cocaine abstinence.
Importantly, this stimulus-related activity differentiated between early and late stages of abstinence, suggesting that the excessive thalamic reactivity is malleable. For instance, in an fMRI study where participants attended to novel and habituated stimuli, improved performance speed was noted to co-occur with attenuation of thalamic responses in cocaine users (Goldstein et al., 2007). It was suggested that, reduced thalamic reactivity to salient stimuli, such as drug or drug-related cues, may underlie individuals’ ability to maintain abstinent. In another study, McClernon and colleagues examined smokers under an extinction-based smoking cessation paradigm in fMRI (McClernon et al., 2007). Dependent smokers were scanned at baseline, following 2-4 weeks of smoking cigarettes with reduced nicotine content while wearing a 21-mg nicotine patch, and 2-4 weeks following quitting smoking. The results showed that the extinction-based treatment attenuated responses to smoking as compared to control cues in amygdala, a subcortical structure directly interconnected with the thalamus. Furthermore, this “extinction” pattern of responses was also observed in the thalamus of future abstinent but not relapsing smokers (McClernon et al., 2007). Taken together, these studies suggest that thalamic activity is closely associated with craving and relapse to drug use, and is potentially amenable to psychological and pharmacological interventions.
At a relaxed threshold, our current findings also showed increased error-evoked activation of the posterior insula in PCD during early compared to late abstinence. The posterior insula is a higher order somatosensory structure receiving extensive somatosensory and autonomic inputs from the thalamus (Brooks et al., 2005; Craig, 2005). The posterior insula is situated at a beginning stage of information processing that serves to integrate many sensory inputs for interoception and emotional awareness (Craig, 2003; Wiens, 2005). Importantly, evidence is accumulating that implicates insula as an important structure in mediating drug craving (Franklin et al., 2007; Franklin et al., In Press; Wang et al., 2007; see also Naqvi and Bechara, in press, for an overview). In particular, both human and animals studies demonstrated that lesions to the insula abolished or significantly decreased drug craving (Contreras et al., 2007; Naqvi et al., 2007). The finding of greater insular activation in PCD at a time when they experience craving and loss of control thus is in keeping with our hypothesis of heightened arousal and reactivity to salient stimuli during an early stage of abstinence.
It is not entirely clear why the dACC did not exhibit greater error-related responses during early as compared to late abstinence, as with the thalamus and insula. One possible explanation is that the dACC is involved in a multitude of cognitive and affective processes in addition to its role in error processing. In particular, cocaine dependent patients showed hypoactivation in the ACC in a number of different behavioral tasks involving response inhibition, working memory and emotional including stress processing (Goldstein et al., 2009; Li et al., 2005; 2008a; Sinha et al., 2005; Tomasi et al., 2007). The hypoactivation of the ACC may reflect more of the effects of chronic use of cocaine, which does not evolve through the first few weeks of abstinence in PCD.
We also noted that the two groups of subjects did not differ in the stop signal reaction time (SSRT), an index of motor response inhibition in the stop signal task. Our previous studies showed that, compared to healthy controls subjects, cocaine dependent patients were prolonged in SSRT and impaired in inhibition-related regional brain activation (Li et al., 2006; 2008a). Thus, the current results appear to suggest that reported loss of control and cocaine craving and the associated changes in thalamic activation can occur independent of these altered processes in inhibitory control. More studies are required to understand how error-related thalamic responses interact with prefrontal mechanisms of inhibitory control to determine drug use behaviors in PCD, patients with other substance use disorders (SUD; Li et al., 2009b), and individuals vulnerable to developing SUD (Yan and Li, 2009). .
The current results are based on a contrast between stop error (SE) and stop success (SS) trials. One caveat thus is whether the results reflect greater error-related activation during SE > SS or less attention-related activation during SS > SE in the thalamus among PCD during early compared to late abstinence. Two lines of evidence could be considered. First, across subjects thalamus showed greater activation during SE > SS, not SS > SE (Li et al., 2008b; current results), suggesting that this alternative explanation is unlikely. However, one could still argue that the thalamus is “deactivated” during SS > SE and the extent of “deactivation” is greater in the early as compared to late abstinence group. That is, thalamus showed greater contrast between SE > SS not because of more activity during SE but because of less activity during SS. Thus, the greater thalamic “deactivation” during SS > SE may reflect more severe deficits in attention and/or response inhibition in the early abstinence group. We think that this scenario is inconsistent with the extant literature, which supports thalamic activation during attentional processing and deficits in attention in humans/animals with thalamic lesions (Christensen et al., 2008; Gur et al., 2007; Nikulin et al., 2008; Sadaghiani et al., 2009; Salmi et al., 2007; Snow et al., 2009).
In conclusion, this study presents preliminary findings further implicating thalamus and insula in mediating drug craving and loss of control in patients with cocaine dependence. Further studies with a greater sample size and tracking the evolution of withdrawal symptoms in conjunction with regional brain activation would further elucidate the thalamic processes of saliency processing and suggest how one may overcome this powerful psychological state to prevent relapse.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Ashburner J, Friston KJ. Nonlinear spatial normalization using basis functions. Hum Brain Mapp. 1999;7:254–266. doi: 10.1002/(SICI)1097-0193(1999)7:4<254::AID-HBM4>3.0.CO;2-G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baicy K, London ED. Corticolimbic dysregulation and chronic methamphetamine abuse. Addiction. 2007;102(Suppl 1):5–15. doi: 10.1111/j.1360-0443.2006.01777.x. [DOI] [PubMed] [Google Scholar]
- Bellebaum C, Daum I, Koch B, Schwarz M, Hoffmann KP. The role of the human thalamus in processing corollary discharge. Brain. 2005;128:1139–1154. doi: 10.1093/brain/awh474. [DOI] [PubMed] [Google Scholar]
- Blakemore SJ, Rees G, Frith CD. How do we predict the consequences of our actions? A functional imaging study. Neuropsychologia. 1998;36:521–529. doi: 10.1016/s0028-3932(97)00145-0. [DOI] [PubMed] [Google Scholar]
- Brooks JC, Zambreanu L, Godinez A, Craig AD, Tracey I. Somatotopic organisation of the human insula to painful heat studied with high resolution functional imaging. Neuroimage. 2005;27:201–209. doi: 10.1016/j.neuroimage.2005.03.041. [DOI] [PubMed] [Google Scholar]
- Caldecott-Hazard S, Mazziotta J, Phelps M. Cerebral correlates of depressed behavior in rats, visualized using 14C-2-deoxyglucose autoradiography. J Neurosci. 1988;8:1951–1961. doi: 10.1523/JNEUROSCI.08-06-01951.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen TA, Antonucci SM, Lockwood JL, Kittleson M, Plante E. Cortical and subcortical contributions to the attentive processing of speech. Neuroreport. 2008;19:1101–5. doi: 10.1097/WNR.0b013e3283060a9d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras M, Ceric F, Torrealba F. Inactivation of the interoceptive insula disrupts drug craving and malaise induced by lithium. Science. 2007;318:655–658. doi: 10.1126/science.1145590. [DOI] [PubMed] [Google Scholar]
- Craig AD. Forebrain emotional asymmetry: a neuroanatomical basis? Trends Cogn Sci. 2005;9:566–571. doi: 10.1016/j.tics.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Craig AD. Interoception: the sense of the physiological condition of the body. Curr Opin Neurobiol. 2003;13:500–505. doi: 10.1016/s0959-4388(03)00090-4. [DOI] [PubMed] [Google Scholar]
- Diamond ME, Ahissar E. When outgoing and incoming signals meet: new insights from the zona incerta. Neuron. 2007;56:578–579. doi: 10.1016/j.neuron.2007.11.006. [DOI] [PubMed] [Google Scholar]
- Duann J-R, Ide S, Luo X, Li C-SR. Functional connectivity delineates distinct roles of the inferior frontal cortex and pre-supplementary motor area during stop signal inhibition. J Neurosci. 2009;29:10171–10179. doi: 10.1523/JNEUROSCI.1300-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan E, Boshoven W, Harenski K, Fiallos A, Tracy H, Jovanovic T, Hu X, Drexler K, Kiets C. An fMRI study of the interaction of stress and cocaine cues on cocaine craving in cocaine-dependent men. Am J Addict. 2007;16:174–182. doi: 10.1080/10550490701375285. [DOI] [PubMed] [Google Scholar]
- Feldstein Ewing SW, Filbey FM, Chandler LD, Hutchison KE. Exploring the Relationship Between Depressive and Anxiety Symptoms and Neuronal Response to Alcohol Cues. Alcohol Clin Exp Res. doi: 10.1111/j.1530-0277.2009.01104.x. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filbey FM, Schacht JP, Myers US, Chavez RS, Hutchison KE. Marijuana craving in the brain. Proc Natl Acad Sci (U S A) 2009;106:13016–13021. doi: 10.1073/pnas.0903863106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Williams JBW, Gibbon M. Structured Clinical Interview for DSM-IV (SCID) American Psychiatric Association; Washington DC: 1995. [Google Scholar]
- Fox HC, Axelrod SR, Paliwal P, Sleeper J, Sinha R. Difficulties in emotion regulation and impulse control during cocaine abstinence. Drug Alcoh Depend. 2007;89:298–301. doi: 10.1016/j.drugalcdep.2006.12.026. [DOI] [PubMed] [Google Scholar]
- Franken IH, van Strien JW, Franzek EJ, van de Wetering BJ. Error-processing deficits in patients with cocaine dependence. Biol Psychol. 2007;75:45–51. doi: 10.1016/j.biopsycho.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Franklin TR, Lohoff FW, Wang Z, Sciortino N, Harper D, Li Y, Jens W, Cruz J, Kampman K, Ehrman R, Berrettini W, Detre JA, O'Brien CP, Childress AR. DAT genotype modulates brain and behavioral responses elicited by cigarette cues. Neuropsychopharmacology. 2009;34:717–728. doi: 10.1038/npp.2008.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin TR, Wang Z, Wang J, Sciortino N, Harper D, Li Y, Ehrman R, Kampman K, O'Brien CP, Detre JA, Childress AR. Limbic activation to cigarette smoking cues independent of nicotine withdrawal: a perfusion fMRI study. Neuropsychopharmacology. 2007;32:2301–2309. doi: 10.1038/sj.npp.1301371. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Ashburner J, Frith CD, Polone J-B, Heather JD, Frackowiak RSJ. Spatial registration and normalization of images. Hum Brain Mapp. 1995a;2:165–189. [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ, Poline J-B, Frith CD, Frackowiak RSJ. Statistical parametric maps in functional imaging: a general linear approach. Hum Brain Mapp. 1995b;2:189–210. [Google Scholar]
- Garavan H, Stout JC. Neurocognitive insights into substance abuse. Trends Cogn Sci. 2005;9:195–201. doi: 10.1016/j.tics.2005.02.008. [DOI] [PubMed] [Google Scholar]
- George MS, Anton RF, Bloomer C, Teneback C, Drobes DJ, Lorberbaum JP, Nahas Z, Vincent DJ. Activation of prefrontal cortex and anterior thalamus in alcoholic subjects on exposure to alcohol-specific cues. Arch Gen Psychiatry. 2001;58:345–352. doi: 10.1001/archpsyc.58.4.345. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Alia-Klein N, Tomasi D, Carrillo JH, Maloney T, Woicik PA, Wang R, Telang F, Volkow ND. Anterior cingulate cortex hypoactivations to an emotionally salient task in cocaine addiction. Proc Natl Acad Sci (USA) 2009;106:9453–9458. doi: 10.1073/pnas.0900491106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Tomasi D, Alia-Klein N, Zhang L, Telang F, Volkow ND. The effect of practice on a sustained attention task in cocaine abusers. Neuroimage. 2007;35:194–206. doi: 10.1016/j.neuroimage.2006.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratz KL, Roemer L. Multidimensional assessment of emotion regulation and dsyregulation: development, factor structure, and initial validation of the difficulties in emotion regulation scale. J Psychopathol Behav Assess. 2004;26:41–54. [Google Scholar]
- Grüsser SM, Wrase J, Klein S, Hermann D, Smolka MN, Ruf M, Weber-Fahr W, Flor H, Mann K, Braus DF, Heinz A. Cue-induced activation of the striatum and medial prefrontal cortex is associated with subsequent relapse in abstinent alcoholics. Psychopharmacology (Berl) 2004;175:296–302. doi: 10.1007/s00213-004-1828-4. [DOI] [PubMed] [Google Scholar]
- Gur RC, Turetsky BI, Loughead J, Waxman J, Snyder W, Ragland JD, Elliott MA, Bilker WB, Arnold SE, Gur RE. Hemodynamic responses in neural circuitries for detection of visual target and novelty: An event-related fMRI study. Hum Brain Mapp. 2007;28:263–74. doi: 10.1002/hbm.20319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann D, Smolka MN, Wrase J, Klein S, Nikitopoulos J, Georgi A, Braus DF, Flor H, Mann F, Heinz A. Blockade of cue-induced brain activation of abstinent alcoholics by a single administration of amisulpride as measured with fMRI. Alcohol Clin Exp Res. 2006;30:1349–1354. doi: 10.1111/j.1530-0277.2006.00174.x. [DOI] [PubMed] [Google Scholar]
- Hester R, Simões-Franklin C, Garavan H. Post-error behavior in active cocaine users: poor awareness of errors in the presence of intact performance adjustments. Neuropsychopharmacology. 2007;32:1974–1984. doi: 10.1038/sj.npp.1301326. [DOI] [PubMed] [Google Scholar]
- Hommer DW. Functional imaging of craving. Alcohol Res Health. 1999;23:187–96. [PMC free article] [PubMed] [Google Scholar]
- Hughes JR, Hatsukami D. Signs and symptoms of tobacco withdrawal. Arch Gen Psychiatry. 1986;43:289–294. doi: 10.1001/archpsyc.1986.01800030107013. [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krystal JH, Woods SW, Kosten TR, Rosen MI, Seibyl JP, van Dyck CC, Price LH, Zubal IG, Hoffer PB, Charney DS. Opiate dependence and withdrawal: preliminary assessment using single photon emission computerized tomography (SPECT). Am J Drug Alcohol Abuse. 1995;21:47–63. doi: 10.3109/00952999509095229. [DOI] [PubMed] [Google Scholar]
- Levitt H. Transformed up-down methods in psychoacoustics. J Acoust Soc Am. 1970;49:467–477. [PubMed] [Google Scholar]
- Li C-SR, Huang C, Constable RT, Sinha R. Imaging response inhibition in a stop signal task – neural correlates independent of signal monitoring and post-response processing. J Neurosci. 2006a;26:186–192. doi: 10.1523/JNEUROSCI.3741-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C-SR, Huang C, Yan P, Bhagawar Z, Milivojevic V, Sinha R. Neural correlates of impulse control during stop signal inhibition in cocaine dependent men. Neuropsychopharmacol. 2008a;33:1798–1807. doi: 10.1038/sj.npp.1301568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CS, Kosten TR, Sinha R. Sex differences in brain activation during stress imagery in abstinent cocaine users: a functional magnetic resonance imaging study. Biol Psychiatry. 2005;57:487–94. doi: 10.1016/j.biopsych.2004.11.048. [DOI] [PubMed] [Google Scholar]
- Li C-SR, Milivojevic V, Kemp KA, Hong K, Sinha R. Performance monitoring and stop signal inhibition in abstinent patients with cocaine dependence. Drug Alcoh Depend. 2006b;85:205–212. doi: 10.1016/j.drugalcdep.2006.04.008. [DOI] [PubMed] [Google Scholar]
- Li CS, Sinha R. Inhibitory control and emotional stress regulation: neuroimaging evidence for frontal-limbic dysfunction in psycho-stimulant addiction. Neurosci Biobehav Rev. 2008;32:581–597. doi: 10.1016/j.neubiorev.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C-SR, Yan P, Chao HH-A, Sinha R, Paliwal P, Constable RT, Zhang S, Lee TW. Error-specific medial cortical and subcortical activity during the stop signal task – a functional magnetic resonance imaging study. Neuroscience. 2008b;155:1142–1151. doi: 10.1016/j.neuroscience.2008.06.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C-SR, Chao HH-A, Lee TW. The neural correlates of speeded compared to delayed responses in a stop signal task: an indirect analogue of risk taking and association with an anxiety trait. Cerebral Cortex. 2009a;19:839–848. doi: 10.1093/cercor/bhn132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C-SR, Yan P, Sinha R, Lee TW. The subcortical processes of motor response inhibition during a stop signal task. NeuroImage. 2008c;41:1352–1363. doi: 10.1016/j.neuroimage.2008.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C-SR, Luo X, Yan P, Bergquist K, Sinha R. Altered Impulse Control in Alcohol Dependence: Neural Measures of Stop Signal Performance. Alcoholism: Clin Exp Res. 2009b;33:740–750. doi: 10.1111/j.1530-0277.2008.00891.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan GD. In: Inhibitory Processes in Attention, Memory and Language. Dagenbach D, Carr TH, editors. Academic Press; San Diego: 1994. pp. 189–239. [Google Scholar]
- McClernon FJ, Hiott FB, Liu J, Salley AN, Behm FM, Rose JE. Selectively reduced responses to smoking cues in amygdala following extinction-based smoking cessation: results of a preliminary functional magnetic resonance imaging study. Addict Biol. 2007;12:503–512. doi: 10.1111/j.1369-1600.2007.00075.x. [DOI] [PubMed] [Google Scholar]
- McClernon FJ, Kozink RV, Rose JE. Individual differences in nicotine dependence, withdrawal symptoms, and sex predict transient fMRI-BOLD responses to smoking cues. Neuropsychopharmacology. 2008;33:2148–2157. doi: 10.1038/sj.npp.1301618. [DOI] [PubMed] [Google Scholar]
- McClernon FJ, Kozink RV, Lutz AM, Rose JE. 24-h smoking abstinence potentiates fMRI-BOLD activation to smoking cues in cerebral cortex and dorsal striatum. Psychopharmacology (Berl) 2009;204:25–35. doi: 10.1007/s00213-008-1436-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell AS, Browning PG, Baxter MG. Neurotoxic lesions of the medial mediodorsal nucleus of the thalamus disrupt reinforcer devaluation effects in rhesus monkeys. J Neurosci. 2007;27:11289–11295. doi: 10.1523/JNEUROSCI.1914-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modell JG, Mountz JM, Beresford TP. Basal ganglia/limbic striatal and thalamocortical involvement in craving and loss of control in alcoholism. J Neuropsychiatry Clin Neurosci. 1990;2:123–144. doi: 10.1176/jnp.2.2.123. [DOI] [PubMed] [Google Scholar]
- Monchi O, Petrides M, Petre V, Worsley K, Dagher A. Wisconsin Card Sorting revisited: distinct neural circuits participating in different stages of the task identified by event-related functional magnetic resonance imaging. J Neurosci. 2001;21:7733–7741. doi: 10.1523/JNEUROSCI.21-19-07733.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naqvi NH, Bechara A. The hidden island of addiction: the insula. Trends Neurosci. doi: 10.1016/j.tins.2008.09.009. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naqvi NH, Rudrauf D, Damasio H, Bechara A. Damage to the insula disrupts addiction to cigarette smoking. Science. 2007;315:531–534. doi: 10.1126/science.1135926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikulin VV, Marzinzik F, Wahl M, Schneider GH, Kupsch A, Curio G, Klostermann F. Anticipatory activity in the human thalamus is predictive of reaction times. Neuroscience. 2008;155:1275–83. doi: 10.1016/j.neuroscience.2008.07.005. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Lovero KL, Wittmann M, Leland DS. Reduced behavioral and neural activation in stimulant users to different error rates during decision making. Biol Psychiatry. 2008;63:1054–1060. doi: 10.1016/j.biopsych.2007.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redish AD. Addiction as a computational process gone awry. Science. 2004;306:1944–1947. doi: 10.1126/science.1102384. [DOI] [PubMed] [Google Scholar]
- Redish AD, Johnson A. A computational model of craving and obsession. Ann N Y Acad Sci. 2007;1104:324–339. doi: 10.1196/annals.1390.014. [DOI] [PubMed] [Google Scholar]
- Risinger RC, Salmeron BJ, Ross TJ, Amen SL, Sanfilipo M, Hoffmann RG, Bloom AS, Garavan H, Stein EA. Neural correlates of high and craving during cocaine self-administration using BOLD fMRI. Neuroimage. 2005;26:1097–1108. doi: 10.1016/j.neuroimage.2005.03.030. [DOI] [PubMed] [Google Scholar]
- Rose JE, Behm FM, Salley AN, Bates JE, Coleman RE, Hawk TC, Turkington TG. Regional brain activity correlates of nicotine dependence. Neuropsychopharmacology. 2007;32:2441–2452. doi: 10.1038/sj.npp.1301379. [DOI] [PubMed] [Google Scholar]
- Sadaghiani S, Hesselmann G, Kleinschmidt A. Distributed and antagonistic contributions of ongoing activity fluctuations to auditory stimulus detection. J Neurosci. 2009;29:13410–7. doi: 10.1523/JNEUROSCI.2592-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmi J, Rinne T, Degerman A, Salonen O, Alho K. Orienting and maintenance of spatial attention in audition and vision: multimodal and modality-specific brain activations. Brain Struct Funct. 2007;212:181–94. doi: 10.1007/s00429-007-0152-2. [DOI] [PubMed] [Google Scholar]
- Schiff ND. Central thalamic contributions to arousal regulation and neurological disorders of consciousness. Ann N Y Acad Sci. 2008;1129:105–118. doi: 10.1196/annals.1417.029. [DOI] [PubMed] [Google Scholar]
- Sherman SM. The thalamus is more than just a relay. Curr Opin Neurobiol. 2007;17:417–422. doi: 10.1016/j.conb.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Lacadie C, Skudlarski P, Fulbright RK, Rounsaville BJ, Kosten TR, Wexler BE. Neural activity associated with stress-induced cocaine craving: a functional magnetic resonance imaging study. Psychopharmacology (Berl) 2005;183:171–180. doi: 10.1007/s00213-005-0147-8. [DOI] [PubMed] [Google Scholar]
- Snow JC, Allen HA, Rafal RD, Humphreys GW. Impaired attentional selection following lesions to human pulvinar: evidence for homology between human and monkey. Proc Natl Acad Sci USA. 2009;106:4054–9. doi: 10.1073/pnas.0810086106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer MA, Wurtz RH. What the brain stem tells the frontal cortex. II. Role of the SC-MD-FEF pathway in corollary discharge. J Neurophysiol. 2004;91:1403–1423. doi: 10.1152/jn.00740.2003. [DOI] [PubMed] [Google Scholar]
- Sussner BD, Smelson DA, Rodrigues S, Kline A, Losonczy M, Ziedonis D. The validity and reliability of a brief measure of cocaine craving. Drug Alcohol Depend. 2006;83:233–237. doi: 10.1016/j.drugalcdep.2005.11.022. [DOI] [PubMed] [Google Scholar]
- Taylor SF, Stern ER, Gehring WJ. Neural systems for error monitoring: recent findings and theoretical perspectives. Neuroscientist. 2007;13:160–172. doi: 10.1177/1073858406298184. [DOI] [PubMed] [Google Scholar]
- Tiffany ST, Singleton E, Haertzen CA, Henningfield JE. The development of a cocaine craving questionnaire. Drug Alcohol Depend. 1993;34:19–28. doi: 10.1016/0376-8716(93)90042-o. [DOI] [PubMed] [Google Scholar]
- Tomasi D, Goldstein RZ, Telang F, Maloney T, Alia-Klein N, Caparelli EC, Volkow ND. Widespread disruption in brain activation patterns to a working memory task during cocaine abstinence. Brain Res. 2007;1171:83–92. doi: 10.1016/j.brainres.2007.06.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbain N, Deschênes M. Motor cortex gates vibrissal responses in a thalamocortical projection pathway. Neuron. 2007;56:714–725. doi: 10.1016/j.neuron.2007.10.023. [DOI] [PubMed] [Google Scholar]
- Verdejo-García AJ, Perales JC, Pérez-García M. Cognitive impulsivity in cocaine and heroin polysubstance abusers. Addict Behav. 2007;32:950–966. doi: 10.1016/j.addbeh.2006.06.032. [DOI] [PubMed] [Google Scholar]
- Vertes RP. Interactions among the medial prefrontal cortex, hippocampus and midline thalamus in emotional and cognitive processing in the rat. Neuroscience. 2006;142:1–20. doi: 10.1016/j.neuroscience.2006.06.027. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Telang F, Logan J, Jayne M, Ma Y, Pradhan K, Wong C, Swanson JM. Cognitive control of drug craving inhibits brain reward regions in cocaine abusers. Neuroimage. doi: 10.1016/j.neuroimage.2009.10.088. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Logan J, Gatley SJ, Hitzemann R, Chen AD, Dewey SL, Pappas N. Decreased striatal dopaminergic responsiveness in detoxified cocaine-dependent subjects. Nature. 1997;386:830–833. doi: 10.1038/386830a0. [DOI] [PubMed] [Google Scholar]
- Wagner G, Koch K, Reichenbach JR, Sauer H, Schlösser RG. The special involvement of the rostrolateral prefrontal cortex in planning abilities: an event-related fMRI study with the Tower of London paradigm. Neuropsychologia. 2006;44:2337–2347. doi: 10.1016/j.neuropsychologia.2006.05.014. [DOI] [PubMed] [Google Scholar]
- Weinstein A, Greif J, Yemini Z, Lerman H, Weizman A, Even-Sapir E. Attenuation of cue-induced smoking urges and brain reward activity in smokers treated successfully with bupropion. J Psychopharmacol. doi: 10.1177/0269881109105456. In press. [DOI] [PubMed] [Google Scholar]
- Wang Z, Faith M, Patterson F, Tang K, Kerrin K, Wileyto EP, Detre JA, Lerman C. Neural substrates of abstinence-induced cigarette cravings in chronic smokers. J Neurosci. 2007;27:14035–14040. doi: 10.1523/JNEUROSCI.2966-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiens S. Interoception in emotional experience. Curr Opin Neurol. 2005;18:442–447. doi: 10.1097/01.wco.0000168079.92106.99. [DOI] [PubMed] [Google Scholar]
- Wooten GF, DiStefano P, Collins RC. Regional cerebral glucose utilization during morphine withdrawal in the rat. Proc Natl Acad Sci USA. 1982;79:3360–3364. doi: 10.1073/pnas.79.10.3360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan P, Li C-SR. Decreased amygdala activity during risk taking in non-dependent habitual alcohol users: a preliminary fMRI study of the stop signal task. Am J Drug Alcoh Abuse. 2009;35:284–289. doi: 10.1080/00952990902968569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung N, Ralph J, Nieuwenhuis S. Drink alcohol and dim the lights: the impact of cognitive deficits on medial frontal cortex function. Cogn Affect Behav Neurosci. 2007;7:347–355. doi: 10.3758/cabn.7.4.347. [DOI] [PubMed] [Google Scholar]