Abstract
Anticipating a stressor elicits robust cardiovascular and affective responses. Despite the possibility that recovery from these responses may have implications for physical and mental well-being, little research has examined this issue. In this study, participants either gave a public speech or anticipated giving a speech. Compared with speech-givers, participants who anticipated giving a speech, on average, exhibited similar cardiovascular recovery (decreased heart rate [HR] and increased respiratory sinus arrhythmia [RSA]), and reported lower negative affect during recovery. Only in the anticipation condition, however, were cardiovascular recovery and affective recovery associated: poor affective recovery predicted incomplete HR recovery and decreased RSA. These are the first data to compare explicitly recovery from anticipation of a stressor with recovery from the stressor itself. These findings suggest that failing to recover from anticipation has unique physiological costs that, in turn, may contribute to mental and physical illness.
Keywords: anticipation, recovery, stress, affect, cardiovascular, HR, RSA
Alfred Hitchcock, the master of suspense, once said, “There is no terror in a bang, only in the anticipation of it.” In describing his theory of heightening suspense in films, Hitchcock touched on a topic that has long interested researchers: that anticipating a stressful event is itself stressful. Indeed, investigators have demonstrated that anticipating certain types of stressful events reliably elicits negative thoughts and emotions (Feldman, Cohen, Hamrick, & Lepore, 2004; Spacapan & Cohen, 1983), cardiovascular engagement (Epstein, 1970; Feldman, et al., 2004; Fredrickson, Mancuso, Branigan, & Tugade, 2000), cortisol reactivity (C. Kirschbaum, Wust, & Hellhammer, 1992; Mikolajczak, Roy, Luminet, & de Timary, 2008), and even immunological changes (Breznitz, et al., 1998). In fact, for some people, anticipating a stressful event is so aversive that, if possible, they will choose to shorten the anticipation period by experiencing the stressful event sooner rather than later (Berns, et al., 2006; Loewenstein, 1987).
Certainly, the various cognitive, emotional, and physiological effects associated with anticipating a stressful experience can be adaptive. For example, the negative affect associated with anticipating a stressful event can motivate people to take measures to try to avoid the impending stressful event (Aspinwall & Taylor, 1997). Similarly, the increased physiological response associated with anticipation can help people prepare their bodies for the stressor by increasing the metabolic resources available for responding to the event (Obrist, 1981). Less clear is what happens if people fail to recover after the anticipated stressor is no longer imminent. Successful physiological and affective recovery from stress, denoted as a relatively quick and/or complete return to baseline level from some previous activation level, has been postulated to be one of the most important factors in preventing stress from adversely influencing mental and physical health (Brosschot, Gerin, & Thayer, 2006; McEwen, 1998). To date, however, research examining this formulation has focused almost exclusively on recovery from the actual occurrence of stressful events, ranging from public speaking (Clemens Kirschbaum, Pirke, & Hellhammer, 1993) to terrorist attacks (Fredrickson, Tugade, Waugh, & Larkin, 2003). Relatively unexplored are the many times in people s lives when they must recover from the anticipation of a stressful event that does not transpire. It is clear that these frequent anticipatory experiences can be stressful regardless of whether the events occur or not. For example, persistent anticipatory negative thoughts and associated physiological arousal are a feature of both the heightened worry central to generalized anxiety disorder (GAD; DSM IV), trait anxiety (Gonzalez-Bono, et al., 2002; Hofmann, et al., 2005), and the pessimism associated with depression (Andersen, Spielman, & Bargh, 1992; Miranda, Fontes, & Marroquin, 2008). Unsuccessful recovery from anticipatory stress (i.e., relatively slow or incomplete return to baseline levels), therefore, may be an important pathway through which stress influences mental and physical health (Waugh, Tugade, & Fredrickson, 2008).
One potential difference in the mechanisms underlying recovery from anticipation and recovery from the stressful event itself is the interaction between affect and cardiovascular responding (CV). Unsuccessful HR recovery and poor parasympathetic control (as indexed in our study by respiratory sinus arrhythmia [RSA]; Berntson, et al., 1997) are robust predictors of cardiovascular disease and all-cause mortality (Lauer & Froelicher, 2002; Thayer & Lane, 2007). The relation between CV and state affect in stressful situations, however, is weak (Burns, 1995, Cohen, et al., 2000), in part because CV responses during stressors are driven primarily by the effort required to meet an external challenge (Peters, et al., 1998) and less by individual differences in affective responses to that challenge. Because CV responses during anticipation are due mainly to the perceived effort required to meet the challenge (Obrist, 1981), and not the actual effort, there may be a tighter coupling between CV recovery (decreases in HR and increases in RSA) and the affective states associated with these perceptions. Indeed, there is indirect evidence that when recovering from the anticipation of a negative event, individual differences in affective recovery are associated with cardiovascular recovery. Low trait resilience – the inability to successfully adapt to stressful situations (Block & Kremen, 1996) – was found to predict both slower cardiovascular recovery (Tugade & Fredrickson, 2004) and incomplete affective recovery (Waugh, Fredrickson, & Taylor, 2008) from anticipatory threat.
In the present study, we examined whether the interaction of affective recovery and CV recovery is a mechanism that differentiates recovery from anticipation of a stressor from recovery from the stressor itself. Participants were randomly assigned to either give a speech or only anticipate having to give a speech. We predicted that affective recovery would be associated with CV recovery, but only for those participants who were recovering from the anticipation of giving a speech.
Methods
Participants
Participants were recruited through advertisements on local classifieds websites (e.g. http://www.craigslist.com). Participation was limited to individuals who did not have any cardiovascular problems, were not taking medication to address cardiovascular problems, were between the ages of 18 and 55, had a body mass index less than 30, and were not pregnant. Sixty-one individuals participated in this study (33 females; Mean age = 33.6 years, SD = 12.7 years).
Self-report measures
Affect
At various points in the experimental session (see Procedure), participants rated “how much you feel right now” on each of 20 different emotion terms from 1 (“not at all”) to 5 (“a great deal”) using the Positive and Negative Affect Schedule (PANAS; Watson, Clark, & Tellegen, 1988). The positive affect (PA) subscale consisted of seven emotion terms (proud, excited, strong, enthusiastic, determined, attentive, and active) with reliability αs = .84 to .91 (for each scale in the session). The negative affect (NA) subscale consisted of ten emotion terms (distressed, upset, guilty, scared, hostile, irritable, ashamed, nervous, jittery, and afraid) with reliability αs = .81 to .86. We excluded three emotion terms (inspired, alert, and interested) because they did not load highly with either PA or NA.
Physiological measures
Acquisition
Physiological responses were recorded at a sampling rate of 1 kHz with an integrated system and software package (Biopac MP150, AcqKnowledge; Biopac Systems, Goleta, CA). Cardiovascular responses were recorded with the electrocardiogram (ECG) amplifier module and disposable snap ECG electrodes using a standard or modified lead II configuration.1 Respiration was measured with a respiratory belt placed around the participants upper chest.
Signal processing
Physiological data were scored in 1-minute intervals using Mindware software (HRV 2.51; Mindware, Westerville, OH). We inspected the cardiovascular data for artifacts and missing R-peaks (based on improbable inter-beat intervals). For each minute, if one R-peak was missing, an R-peak was inserted at a time-point halfway in between the two neighboring R-peaks. If more than one R-peak was missing, that minute was not scored. After correcting for artifacts and missing R-peaks, the data were submitted to Fast Fourier Transformation. RSA was calculated as the natural log of the high frequency power (.15 – .40 Hz), an acceptable method for determining cardiac vagal control (Berntson, et al., 1997). The HRV module also calculated HR in beats per minute (BPM)2 and respiratory rate (RR) in breaths per minute.
Procedure
Pre-task (10 minutes) and baseline (5 minutes)
After participants signed the consent forms, the experimenter attached the ECG sensors. After a 10-minute habituation period, a 5-minute baseline period was recorded during which participants rested quietly (Figure 1).
Figure 1.
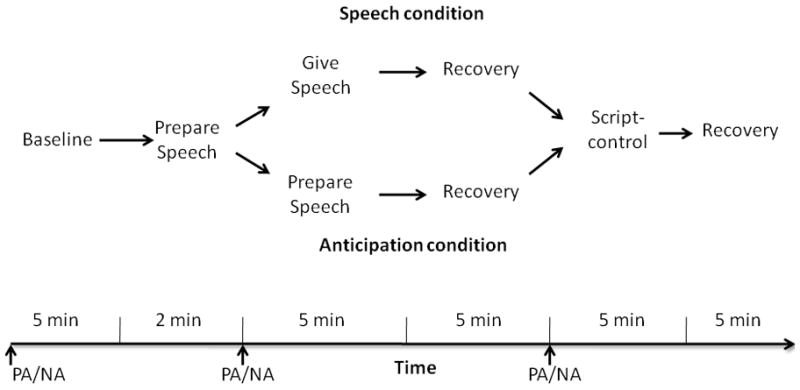
Schematic of the task. PA/NA = times at which positive affect (PA) and negative affect (NA) measurements were taken.
Speech Preparation (2 minutes)
After baseline, the experimenter explained to the participants that they would have two minutes to prepare a five-minute speech that they would then give to an evaluator, who would be judging their speech on clarity, coherence, and persuasiveness. They were then told that there would be two separate coin flips. After two minutes of preparation, the first coin flip would determine whether they had to give the speech immediately or wait another five minutes for the second coin flip, which would determine whether they gave the speech then or not at all. The experimenter then told participants the speech topic was, “Why are you a good friend?”- a topic used successfully in previous studies to induce anticipatory cardiovascular arousal (Fredrickson, et al., 2000) - and left them alone to prepare the speech for two minutes.
Stress period (5 minutes)
After two minutes of speech preparation, participants rated their current affect. The experimenter then flipped a real coin to randomly assign participants into either the Speech (n = 26) or the Anticipation (n = 35) condition. Speech condition. If the coin landed heads, participants gave their prepared speech to a trained stoic evaluator for five minutes. Anticipation condition. If the coin landed tails, the experimenter told participants that they had five more minutes to wait to find out if they would have to give the speech. After five minutes, the experimenter flipped a double-tailed coin to ensure that the participants in the anticipation condition would not have to give the speech. No participants reported suspicion about this fixed coin flip.
Recovery period (5 minutes)
After giving the speech (speech condition), or just anticipating giving the speech (anticipation condition), participants in both conditions sat and rested for five minutes. After this recovery period, participants again rated their current affect.
Script-control (10 minutes of reading plus recovery)
After the recovery period, to isolate the physiological activity due mainly to psychological states and not to the physical demand associated with speaking (Brown, Szabo, & Seraganian, 1988), all participants underwent a speech-control session in which they read a neutral script aloud3. After five minutes of reading aloud, participants were told to relax and sit quietly for five minutes.
Post-task
At the end of the experimental session, participants were debriefed and paid.
Statistical Strategy
Affective responses
For all repeated-measures analyses of variance (ANOVAs), the degrees of freedom were subjected to Greenhouse-Geisser correction and the alphas were subjected to bonferroni corrections at each level of analysis.
Psychophysiology
Following previous research (Kristjansson, Kircher, & Webb, 2007), we used hierarchical linear modeling (HLM6; Raudenbush, Bryk, & Congdon, 2008) to analyze the physiological data. For HR and RSA, we specified a 2-level HLM model. Level 1 of the model consisted of data points for each of the 17 minutes within the experimental session. Level 2 of the model consisted of changes in slopes and intercepts at Level 1 for each participant.
We took the following steps to build each of the HLM models. First, we partialled out possible confounds between the conditions due to speaking (Brown, et al., 1988). For the participants in the speech condition, we regressed HR and RSA responses for each minute of the stress and recovery periods on the HR and RSA responses, respectively, in the corresponding minute of the script-control periods and subtracted this regression intercept. This created psychological indices of physiological activity; to avoid confusion with the raw measures, we will call these variables pHR and pRSA.
Next, at Level 1 we fit a series of dummy-coded variables that corresponded to theorized patterns in the data, and patterns that we observed when graphing the data. To do this, we used a piecewise regression approach (Llabre, Spitzer, Saab, & Schneiderman, 2001) in which we fit different regression lines to different task periods (baseline, preparation, stress, and recovery) within one continuous time-series. Each regression line corresponded to one of three possible patterns in the data: magnitude change (1 s during period, 0 s everywhere else), linear slope (centered to the middle of the period), and quadratic curve (also centered to the middle of the period).
Next, we added condition at Level 2 of the model predicting each of the Level 1 intercepts and slopes. We dummy-coded condition as 1 (speech) and 2 (anticipation) and then standardized this variable so that the intercepts represent the mean of all participants. To assess the relation between affective and cardiovascular recovery, at Level 2 we added positive and negative affect recovery variables. To calculate affective recovery relative to baseline affect, we regressed post-recovery positive/negative affect on baseline positive/negative affect (each affect variable regressed separately) and created standardized residuals. Finally, to examine whether the relation between affective and cardiovascular recovery was moderated by stress condition, we multiplied the standardized condition variable with each affective recovery variable and added these interaction terms to Level 2.4
Level 2 predictors were treated as random effects: that is, error terms were estimated at each Level 2 equation to allow for randomly varying slopes (Bryk & Raudenbush, 1992). We report robust standard errors because negative affective recovery did not follow a normal distribution, S-W(61) = .75, p < .001. Finally, we used restricted maximum likelihood to estimate the coefficients.
Results
Affective recovery
First, we compared participants affective responses after recovery and compared these with their affective responses during baseline and after speech preparation (Figure 2). Separate Stressor (Speech, Anticipation) repeated over Period (Baseline, Prep, Recovery) ANOVAs conducted on negative and positive affect yielded significant main effects (αcorr = .025) of Period for both negative affect, F(2[1.9], 118[112.6]) = 20.19, p < .001, and positive affect, F(2[1.9], 118[112.6]) = 5.46, p = .005, both εs = .95. Negative affect followed a quadratic pattern, F(1, 59) = 35.25, p < .001, characterized (αcorr = .0125) by an increase from baseline to speech preparation, t(60) = 4.41, p < .001, d = .65, followed by a decrease from speech preparation to post-recovery, t(60) = 6.22, p < .001, d = .82. Participants also marginally decreased in positive affect from baseline to speech preparation, t(60) = 2.53, p =.014, d = .25, but unlike negative affect, there was no post-recovery rebound, t(60) = .76, p > .0125, d = .06. This pattern of results indicates that our task was successful as a stress induction.
Figure 2.
Positive and negative affect throughout the task. Participants in the speech and anticipation conditions only differ in their negative affect during recovery. Error bars are standard error of the mean. Base = baseline; Prep = speech preparation; Rec = recovery.
The main effect of period for negative affect was qualified (αcorr = .025) by an interaction of period and stressor, F(2[1.9], 118[112.6]) = 3.76, p = .028, ε = .95 (Figure 2). Whereas participants in the anticipation condition reported significantly lower negative affect after recovery than during baseline, t(34) = 2.84, p = .007, d = .45, suggesting a relief effect, participants in the speech condition did not differ in the level of negative affect they reported at baseline and after recovery, t(25) = 1.34, p > .025, d = .30. Thus, on average, participants recovered successfully both from anticipation and from the stressful event itself, with a slight affective benefit (decreased NA) for participants recovering from anticipation.
pHR model
We first examined pHR as an index of CV recovery to test the hypothesis that affective recovery would predict CV recovery, but only for those participants recovering from the anticipation of a speech. Based on a priori reasoning and on visual inspection of the data, we examined the magnitude of changes during speech preparation, stress, and recovery, as well as linear and quadratic effects during the stress period (Table 1; Figure 3a). At Level 2, we added PA and NA recovery as well as the interaction between PA/NA recovery and condition. This is the resulting model:
Table 1.
Hierarchical Linear Modeling of Heart Rate
| Predictors | Coefficient | SE | t | p |
|---|---|---|---|---|
| Intercept: baseline HR | ||||
| Intercept, γ00 | 70.314 | 1.439 | 48.861 | < .001 |
| Condition, γ01 | −0.676 | 1.494 | −0.453 | .652 |
| PA change, γ02 | 1.332 | 1.625 | 0.82 | .416 |
| PA by Condition, γ03 | 0.106 | 1.537 | 0.069 | .946 |
| NA change, γ04 | −3.266 | 1.184 | −2.759 | .008 |
| NA by Condition, γ05 | −1.554 | 1.157 | −1.343 | .185 |
| Preparation Magnitude | ||||
| Intercept, γ10 | 8.025 | 1.056 | 7.602 | < .001 |
| Condition, γ11 | 0.363 | 1.098 | 0.331 | .742 |
| PA change, γ12 | 1.501 | 1.235 | 1.216 | .230 |
| PA by Condition, γ13 | 0.112 | 1.309 | 0.086 | .932 |
| NA change, γ14 | 0.474 | 1.026 | 0.462 | .646 |
| NA by Condition, γ15 | 0.007 | 1.028 | 0.007 | .995 |
| Preparation Slope | ||||
| Intercept, γ20 | −1.566 | 0.298 | −5.263 | < .001 |
| Condition, γ21 | 0.181 | 0.319 | 0.57 | .571 |
| PA change, γ22 | 0.331 | 0.306 | 1.082 | .285 |
| PA by Condition, γ23 | 0.103 | 0.329 | 0.313 | .755 |
| NA change, γ24 | 0.121 | 0.215 | 0.564 | .574 |
| NA by Condition, γ25 | −0.184 | 0.207 | −0.888 | .379 |
| ‘Stress’ Magnitude | ||||
| Intercept, γ30 | 5.817 | 1.136 | 5.121 | < .001 |
| Condition, γ31 | −0.626 | 1.258 | −0.498 | .620 |
| PA change, γ32 | 0.408 | 1.008 | 0.404 | .687 |
| PA by Condition, γ33 | 0.763 | 1.113 | 0.685 | .496 |
| NA change, γ34 | 1.005 | 0.797 | 1.261 | .213 |
| NA by Condition, γ35 | 0.709 | 0.814 | 0.871 | .388 |
| ‘Stress’ Slope | ||||
| Intercept, γ40 | −0.902 | 0.267 | −3.378 | .002 |
| Condition, γ41 | 1.025 | 0.293 | 3.501 | .001 |
| PA change, γ42 | −0.169 | 0.235 | −0.718 | .476 |
| PA by Condition, γ43 | 0.287 | 0.260 | 1.105 | .274 |
| NA change, γ44 | 0.329 | 0.261 | 1.262 | .213 |
| NA by Condition, γ45 | −0.138 | 0.257 | −0.536 | .594 |
| ‘Stress’ Quadratic | ||||
| Intercept, γ50 | 0.444 | 0.165 | 2.686 | .010 |
| Condition, γ51 | −0.342 | 0.183 | −1.871 | .066 |
| PA change, γ52 | −0.091 | 0.158 | −0.573 | .568 |
| PA by Condition, γ53 | −0.206 | 0.170 | −1.21 | .232 |
| NA change, γ54 | −0.149 | 0.093 | −1.598 | .115 |
| NA by Condition, γ55 | 0.056 | 0.104 | 0.534 | .595 |
| Recovery Magnitude | ||||
| Intercept, γ60 | 0.518 | 0.319 | 1.624 | .110 |
| Condition, γ61 | 0.200 | 0.296 | 0.675 | .502 |
| PA change, γ62 | −0.296 | 0.303 | −0.977 | .333 |
| PA by Condition, γ63 | −0.443 | 0.275 | −1.609 | .113 |
| NA change, γ64 | 0.629 | 0.306 | 2.059 | .044 |
| NA by Condition, γ65 | 1.146 | 0.286 | 4.013 | < .001 |
Note. n = 61, df = 55. Each bolded subtitle indicates the level 1 predictor. PA = Positive Affect, NA = Negative Affect. Condition refers to stressor type (anticipation, speech) and is standardized, so coefficients need to be multiplied by 2 to calculate the estimated difference between conditions.
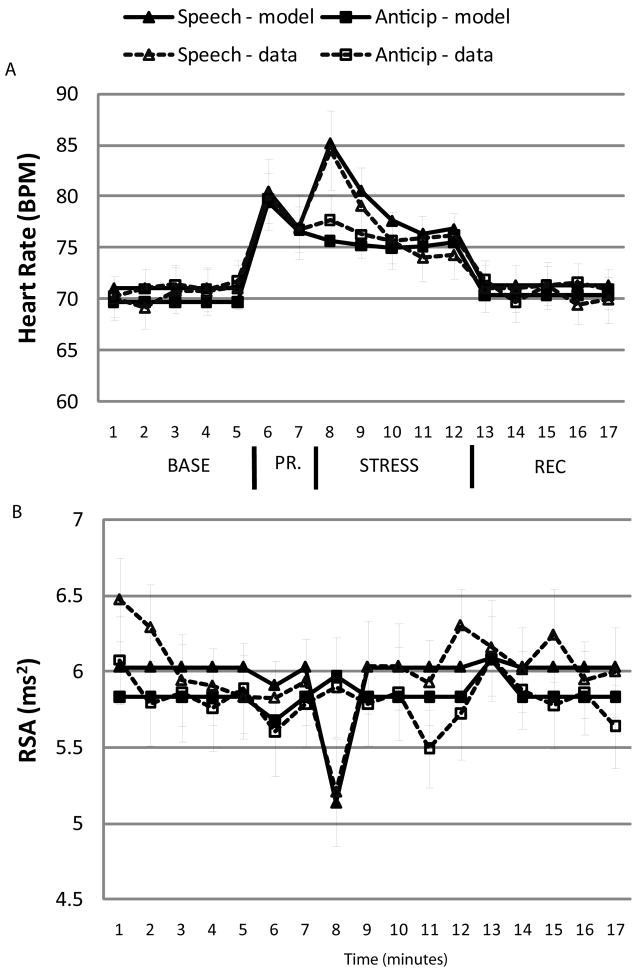
Figure 3.
Cardiovascular data. Grey dotted lines represent the A. pHR and B. pRSA data (raw data transformed by partialling out HR and RSA activity in script-reading condition) for the speech and anticipation conditions. Black lines represent the fitted Level 1 and Level 2 parameters from the full HLM models. BASE = baseline; PR. = speech preparation; REC = recovery; RSA = respiratory sinus arrhythmia; BPM = beats per minute. Error bars are standard error of the mean.
Level 1:
Level 2:
The subscript i corresponds to each parameter at Level 1. Prep and Rec refer to the speech preparation and recovery periods, respectively. PARec and NARec refer to the positive and negative affective recovery variables, respectively. M is magnitude change, L is linear slope, and Q is quadratic curve.
Preparation and Stress periods
Overall, relative to baseline, participants experienced an increase in pHR when preparing the speech (γ10 = 8.03 bpm) and during the stress period (γ30 = 5.82 bpm; Table 1). Although there was no effect of condition on overall stress magnitude5, there was a significant effect of condition on the linear slope and marginal effect of condition on the quadratic curve during the stress period. Simple-slopes analyses revealed that for participants in the speech condition, there was a significant decrement in HR of 2.08 bpm for each successive minute of the stress period, t(55) = 3.89, p < .001, and a quadratic trend across the stress period of about .84 bpm per minute, t(55) = 2.46, p =.017. There were no linear or quadratic trends in HR for participants in the anticipation condition, both ts < 1.1, ps > .05. Considered together with visual inspection of the data, these results indicate that participants in the speech condition experienced an initial spike in pHR for the first few minutes of the speech that declined to similar pHR levels exhibited by participants in the anticipation condition for the last half of the stress period (as reflected in the non-significant difference in stress magnitude). Importantly, this similarity in pHR levels in the two stress conditions in the final minutes of the stress period facilitates the interpretation of differences in the recovery responses. There were no effects of PA and NA recovery on HR responses during the preparation and stress periods, and no interactions between these affective recovery variables and stress condition.
Recovery period
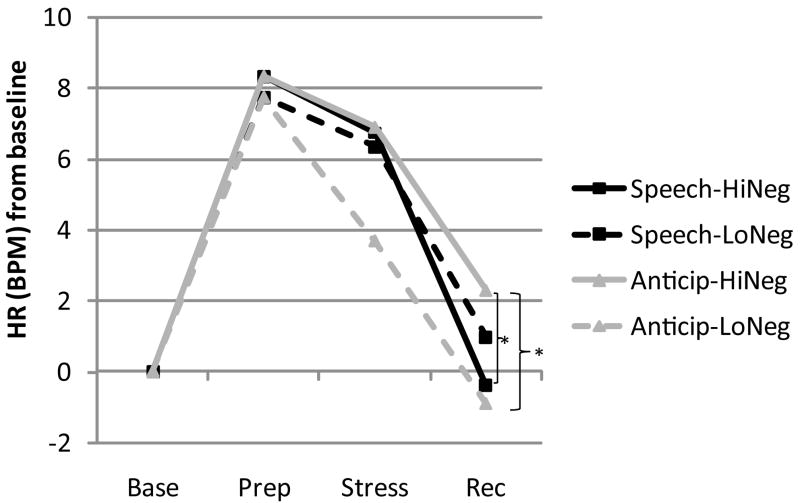
Overall, relative to baseline, there was no significant change in pHR during the recovery period and there was no interaction with stress condition, indicating that on average, pHR for participants in both the anticipation and speech conditions returned to baseline levels. There was, however, the predicted interaction between stress condition and NA recovery on pHR recovery (Figure 4). Simple slopes analyses reveal that for participants in the anticipation condition, increased negative affect during the recovery period predicted increased pHR (1.61 bpm) during recovery, t(55) = 3.36, p = .002. There was no significant relation between negative affective recovery and pHR recovery for participants in the speech condition, t(55) = −1.49, p > .05. Follow-up analyses reveal that those participants who experienced high negative affect during recovery (+1 SD) in the anticipation condition also exhibited significantly higher pHR during recovery, both compared with their own baseline (2.30 bpm), t(55) = 3.30, p = .002, and compared with participants in the speech condition who exhibited high negative affect during recovery, t(55) = 3.41, p = .002.
Figure 4.
Interaction between stress condition and negative affect recovery on heart rate recovery. Values are derived from fitted parameters from full HR HLM model. HiNeg and LoNeg represent participants at + 1 SD and −1 SD of negative affect during the recovery period (controlling for baseline negative affect). Participants in the anticipation condition who exhibited greater negative affect during recovery exhibited greater heart rate during recovery compared with: a) their own baseline levels of heart rate; b) participants in the anticipation condition who exhibited less negative affect during recovery; and c) participants in the speech condition who also exhibited increased negative affect during recovery.
* p < .05.
pRSA model
We next examined pRSA as an index of CV recovery to test the hypothesis that affective recovery would predict CV recovery, but only for those participants recovering from the anticipation of a speech. RSA tends to exhibit a phasic response during the first few moments of stress and recovery from stress (Mezzacappa, Kelsey, Katkin, & Sloan, 2001). This a priori reasoning, in conjunction with visual inspection of the data, led us to include parameters characterizing the first minute of each period (preparation, stress, recovery) instead of parameters characterizing linear slopes and quadratic curves. Moreover, to control for effects of breathing on pRSA, we added respiration rate as a covariate. As with pHR, we also included PA recovery, NA recovery, condition, and the interaction between condition and affective recovery as predictors at Level 2. This is the resulting model (Table 2; Figure 3b):
Table 2.
Hierarchical Linear Modeling of Respiratory Sinus Arrhythmia (RSA)
| Predictors | Coefficient | SE | t | p |
|---|---|---|---|---|
| Intercept: baseline RSA | ||||
| Intercept, γ00 | 5.918 | 0.168 | 35.188 | < .001 |
| Condition, γ01 | −0.095 | 0.172 | −0.552 | .583 |
| PA change, γ02 | −0.396 | 0.174 | −2.277 | .027 |
| PA by Condition, γ03 | −0.264 | 0.166 | −1.586 | .118 |
| NA change, γ04 | 0.212 | 0.128 | 1.65 | .104 |
| NA by Condition, γ05 | 0.145 | 0.122 | 1.188 | .240 |
| Respiration Rate | ||||
| Intercept, γ10 | −0.036 | 0.008 | −4.683 | < .001 |
| Condition, γ11 | −0.014 | 0.007 | −1.924 | .059 |
| PA change, γ12 | 0.006 | 0.006 | 0.974 | .335 |
| PA by Condition, γ13 | 0.002 | 0.006 | 0.373 | .710 |
| NA change, γ14 | −0.003 | 0.007 | −0.366 | .715 |
| NA by Condition, γ15 | 0.007 | 0.007 | 1.004 | .320 |
| ‘Prep 1st minute | ||||
| Intercept, γ20 | −0.132 | 0.119 | −1.105 | .274 |
| Condition, γ21 | −0.014 | 0.124 | −0.111 | .912 |
| PA change, γ22 | −0.049 | 0.103 | −0.477 | .635 |
| PA by Condition, γ23 | −0.013 | 0.104 | −0.12 | .905 |
| NA change, γ24 | 0.064 | 0.111 | 0.577 | .566 |
| NA by Condition, γ25 | 0.170 | 0.109 | 1.556 | .125 |
| ‘Stress’ 1st minute | ||||
| Intercept, γ30 | −0.298 | 0.145 | −2.048 | .045 |
| Condition, γ31 | 0.517 | 0.157 | 3.288 | .002 |
| PA change, γ32 | 0.116 | 0.110 | 1.05 | .299 |
| PA by Condition, γ33 | 0.043 | 0.123 | 0.353 | .725 |
| NA change, γ34 | 0.000 | 0.132 | 0.001 | .999 |
| NA by Condition, γ35 | −0.192 | 0.129 | −1.488 | .142 |
| Recovery 1st minute | ||||
| Intercept, γ40 | 0.163 | 0.080 | 2.032 | .047 |
| Condition, γ41 | 0.090 | 0.087 | 1.032 | .307 |
| PA change, γ42 | −0.160 | 0.064 | −2.502 | .016 |
| PA by Condition, γ43 | 0.132 | 0.066 | 1.994 | .051 |
| NA change, γ44 | 0.039 | 0.061 | 0.633 | .529 |
| NA by Condition, γ45 | −0.268 | 0.065 | −4.1 | < .001 |
Note. n = 61, df = 55. Each bolded subtitle indicates the level 1 predictor. PA = Positive Affect, NA = Negative Affect. Condition refers to stressor type (anticipation, speech) and is standardized, so coefficients need to be multiplied by 2 to calculate the estimated difference between conditions.
Level 1:
Level 2:
Preparation and Stress period
There was a general decrease in pRSA during the first minute of the stress period (γ30 = −.298 ms2); this effect was significantly moderated, however, by stress condition. Simple slopes analyses revealed that whereas participants in the speech condition exhibited a significant drop (−.89 ms2) in pRSA during the first minute of the stress period, t(55) = −3.19, p = .003, participants in the anticipation condition did not (.14 ms2), t(55) = .99, p > .05, suggesting that the initial spike in pHR activation for participants who gave the speech was due to a withdrawal of parasympathetic influence on the heart. There was no significant change in pRSA during the preparation period, nor did NA or PA recovery predict pRSA during the preparation and stress periods.
Recovery period
Consistent with previous research (Mezzacappa, et al., 2001), there was significantly increased pRSA during the first minute of the recovery period (γ40 = .17 ms2), and this effect was not moderated by stress condition, suggesting that on average, recovery from anticipation and recovery from a stressor are similarly parasympathetically mediated. This effect, however, was moderated by a main effect of PA recovery and by an interaction of NA recovery and stress condition. For the main effect, greater PA during recovery predicted decreased pRSA (γ42 = −.16 ms2) during the first minute of recovery, t(55) = −2.50, p = .016. For the interaction of NA recovery and stress condition, simple slopes analyses reveal that for participants in the anticipation condition, increased NA during the recovery period predicted decreased pRSA response during recovery (−.19 ms2), t(55) = −2.93, p = .005. These results mirror the pHR findings and suggest that the persistent pHR found for high NA during recovery from anticipation may be partially due to the lack of a parasympathetic response during recovery. In contrast, for participants in the speech condition, increased NA during the recovery period predicted increased pRSA response during recovery (.35 ms2), t(55) = 3.05, p = .004. Taken together with the findings that NA recovery did not predict pHR recovery for participants in the speech condition, this pattern of results suggests that engagement of the parasympathetic system protected high NA participants in the speech condition from similarly high pHR levels during recovery.
Discussion
In this study, we formally compared recovery from anticipatory stress to recovery from the stressful event itself. After an initial spike in HR and dip in RSA for speech-givers, most likely due to the increased task engagement and/or to the effort involved with giving the speech (Obrist, Webb, Sutterer, & Howard, 1970), anticipating a speech and giving a speech induced similar sustained levels of HR, followed by an increase in RSA and the return of HR to baseline after the offset of the stress period. This pattern of findings suggests that on average, recovery from the anticipation of a stressor involves a similar cardiovascular profile as recovering from the stressful event itself. As hypothesized, however, the affective mechanisms underlying these cardiovascular recovery profiles were quite different. On average, participants who only anticipated giving a speech exhibited decreased NA during recovery compared both with their own baseline and with participants who gave a speech. Consistent with our hypothesis, however, there was a physiological cost for those in the anticipation condition who did not show this NA recovery: persistent NA from baseline to the recovery period predicted increased HR and decreased RSA during recovery.
This finding elucidates the results of studies showing little to no relation between NA and cardiovascular responses during actual stressors. Experiencing a stressful event, like a public speech, conflates physiological responses due to both psychological states and physical engagement with the environment. Just anticipating a stressful event, however, eliminates this conflation, thus revealing the relation between psychological stress (NA) and cardiovascular recovery. The design of the present study does not allow us to determine whether emotional recovery influenced peripheral physiology or vice-versa, or whether there was a third variable (e.g. persistent negative cognitions; Brosschot, et al., 2006) that influenced both. Importantly, though, these data are the first pieces of evidence that the mechanisms involved with recovering from anticipation of a stressor may be different than those involved in recovering from the stressor itself. These findings also highlight the importance of examining recovery from anticipation, given that there is a physiological cost (increased HR and decreased RSA) for failing to recover affectively, which in turn may have implications for physical health (Lauer & Froelicher, 2002).
One of the remarkable findings from this study was that after the first two minutes, anticipating a speech and giving a speech elicited similar levels of HR. This finding, however, comes with two caveats. First, to isolate HR activity due to psychological influences, we partialled out HR activity due to speaking (Brown, et al., 1988) as measured during script-reading. Indeed, without controlling for the effects of speaking, giving a speech did elicit greater levels of HR activity than did anticipating giving a speech (see Footnote 5). The main benefit of controlling for the physiological demands of speaking to create a more psychological measure of HR is that it reduces possible non-psychological confounds between anticipating giving a speech and actually giving a speech (Feldman, et al., 2004). One limitation of partialling out the HR due to speaking, however, is that it statistically treats the physiological demands of speaking and the psychological demands of giving a speech as additive. It is unclear whether these two sources of physiological demand are indeed additive, or if they interact in a different manner. The second caveat is that we operationalized anticipation as the active preparation of a public speech and other forms of anticipation involve different physiological profiles. For example, passive anticipation more reliably activates the vascular system (e.g. increased systolic blood pressure) than the myocardial system (e.g. HR; Gregg, James, Matyas, & Thorsteinsson, 1999). Future investigations of recovery from anticipatory stress should broaden our operationalization of anticipation by addressing these caveats.
In sum, this study is the first to compare directly cardiovascular and affective recovery from the anticipation of a stressor with recovery from the stressor itself. On average, recovering from anticipation and recovering from a stressor exhibited strikingly similar cardiovascular profiles – a decrease in HR to baseline levels. These two situations were differentiated, however, by affective recovery. On average, participants who anticipated the speech reported lower NA during recovery compared both with their own baseline and with speech-givers NA affect during recovery. Failure to show this NA recovery, however, came with a cardiovascular cost – persistently raised HR during recovery. These results suggest that investigators who are interested in stress-related physical and mental health outcomes should also examine recovery from anticipatory stress, paying particular attention to the potential deleterious effects associated with poor affective recovery following anticipation of a stressor.
Acknowledgments
This research was supported by Grant MH074849 from the National Institute of Mental Health to Ian H. Gotlib and Grant HL079383from the National Heart Lung and Blood Institute to Wendy B. Mendes. The authors thank Brian Dunmire with his help running participants and processing physiology data. The authors report no conflicts of interest, either financial or scholarly.
- HR
heart rate
- CV
cardiovascular
- PA
positive affect
- NA
negative affect
- RSA
respiratory sinus arrhythmia
Footnotes
The distribution of the lead configurations was similar for both the speech and anticipation conditions, χ2 = .321, p > .05, and adding lead configuration as a factor in the models did not affect the results. We also measured electromyographic activity to assess startle eye-blinks in response to auditory startle probes at various points in the task. Because of insufficient blink data, these data are not presented here.
We recognize that IBI is the preferred metric over HR. Using IBI as the dependent variable did not alter any of the patterns or significance levels in the data. We chose to use HR for ease of interpretability, particularly given our emphasis on cardiovascular activation and recovery from activation.
Participants reported less positive (M = 2.93, SE = .16) and less negative (M = 1.17, SE = .05) affect to reading the script than they did during baseline (Ms = 3.26, 1.34 for positive and negative affect, respectively), ts(37) = 2.92, 2.22, respectively, both ps < .05.
The anticipation and speech groups did not differ in their gender distribution, χ2(1,61) = .32, p > .05, or mean age, t(54) = 1.19, p > .05. Moreover, including gender or age at level 2 of the HLM models did not affect any of the results.
When using the raw HR as the dependent variable instead of pHR, the only effect that changed was for stress magnitude: participants who gave a speech exhibited greater HR (6.10 bpm) than did participants who anticipated giving a speech, t(55) = 2.02, p = .048. This raw HR difference between the stress conditions is 4.9 bpm greater than when using pHR (1.23 bpm), and is roughly equivalent to the average HR response to reading the script (4.49 bpm). This further supports our reasoning that stress level differences between giving a speech and anticipating giving a speech are due to the demands of speaking and justifies our use of pHR instead of raw HR. Nevertheless, in the discussion section we present the benefits and limitations of this approach.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Christian E. Waugh, Stanford University.
Sommer Panage, Stanford University
Wendy Berry Mendes, Harvard University.
Ian H. Gotlib, Stanford University.
References
- Andersen SM, Spielman LA, Bargh JA. Future-event schemas and certainty about the future: Automaticity in depressives’ future-event predictions. Journal of Personality and Social Psychology. 1992;63(5):711–723. doi: 10.1037//0022-3514.63.5.711. [DOI] [PubMed] [Google Scholar]
- Aspinwall LG, Taylor SE. A stitch in time: Self-regulation and proactive coping. Psychological Bulletin. 1997;121(3):417–436. doi: 10.1037/0033-2909.121.3.417. [DOI] [PubMed] [Google Scholar]
- Berns GS, Chappelow J, Cekic M, Zink CF, Pagnoni G, Martin-Skurski ME. Neurobiological substrates of dread. Science. 2006;312:754–758. doi: 10.1126/science.1123721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berntson GG, Bigger JT, Jr, Eckberg DL, Grossman P, Kaufmann PG, Malik M, et al. Heart rate variability: Origins, methods, and interpretive caveats. Psychophysiology. 1997;34(6):623–648. doi: 10.1111/j.1469-8986.1997.tb02140.x. [DOI] [PubMed] [Google Scholar]
- Block J, Kremen AM. IQ and ego-resiliency: Conceptual and empirical connections and separateness. Journal of Personality and Social Psychology. 1996;70(2):349–361. doi: 10.1037//0022-3514.70.2.349. [DOI] [PubMed] [Google Scholar]
- Breznitz S, Ben-Zur H, Berzon Y, Weiss DW, Levitan G, Tarcic N, et al. Experimental induction and termination of acute psychological stress in human volunteers: Effects on immunological, neuroendocrine, cardiovascular, and psychological parameters. Brain, Behavior, and Immunity. 1998;12:34–52. doi: 10.1006/brbi.1997.0511. [DOI] [PubMed] [Google Scholar]
- Brosschot JF, Gerin W, Thayer JF. The perseverative cognition hypothesis: A review of worry, prolonged stress-related physiological activation, and health. Journal of Psychosomatic Research. 2006;60:113–224. doi: 10.1016/j.jpsychores.2005.06.074. [DOI] [PubMed] [Google Scholar]
- Brown TG, Szabo A, Seraganian P. Physical versus psychological determinants of heart rate reactivity to mental arithmetic. Psychophysiology. 1988;25(5):532–537. doi: 10.1111/j.1469-8986.1988.tb01888.x. [DOI] [PubMed] [Google Scholar]
- Bryk A, Raudenbush S. Hierarchical linear models: Applications and data analysis methods. Newbury Park: Sage Publications; 1992. [Google Scholar]
- Burns JW. Interactive effects of traits, states, and gender on cardiovascular reactivity during different situations. Journal of Behavioral Medicine. 1995;18(3):279–303. doi: 10.1007/BF01857874. [DOI] [PubMed] [Google Scholar]
- Cohen S, Hamrick N, Rodriguez MS, Feldman PJ, Rabin BS, Manuck SB. The stability of and intercorrelations among cardiovascular, immune, endocrine, and psychological reactivity. Annals of Behavioral Medicine. 2000;22(3):171–179. doi: 10.1007/BF02895111. [DOI] [PubMed] [Google Scholar]
- Epstein SS. Heart rate and skin conductance during experimentally induced anxiety: Effects of anticipated intensity of noxious stimulation and experience. Journal of experimental psychology. 1970;84(1):105–112. doi: 10.1037/h0028929. [DOI] [PubMed] [Google Scholar]
- Feldman PJ, Cohen S, Hamrick N, Lepore SJ. Psychological stress, appraisal, emotion, and cardiovascular response in a public speaking task. Psychology and Health. 2004;19(3):353–368. [Google Scholar]
- Fredrickson BL, Mancuso RA, Branigan C, Tugade MM. The undoing effect of positive emotions. Motivation and Emotion. 2000;24(4):237–258. doi: 10.1023/a:1010796329158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredrickson BL, Tugade MM, Waugh CE, Larkin GR. What good are positive emotions in crisis? A prospective study of resilience and emotions following the terrorist attacks on the United States on September 11th, 2001. Journal of Personality and Social Psychology. 2003;84(2):365–376. doi: 10.1037/0022-3514.84.2.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Bono E, Moya-Albiol L, Salvador A, Carrillo E, Ricarte J, Gomez-Amor J. Anticipatory autonomic responses to a public speaking task in women: the role of trait anxiety. Biological Psychology. 2002;60(1):37–49. doi: 10.1016/s0301-0511(02)00008-x. [DOI] [PubMed] [Google Scholar]
- Gregg ME, James JE, Matyas TA, Thorsteinsson EB. Hemodynamic profile of stress-induced anticipation and recovery. International Journal of Psychophysiology. 1999;34:147–162. doi: 10.1016/s0167-8760(99)00074-4. [DOI] [PubMed] [Google Scholar]
- Hofmann SG, Moscovitch DA, Litz BT, Kim HJ, Davis LL, Pizzagalli DA. The Worried Mind: Autonomic and Prefrontal Activation During Worrying. Emotion. 2005;5(4):464–475. doi: 10.1037/1528-3542.5.4.464. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Pirke KM, Hellhammer DH. The “Trier Social Stress Test”: A tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28(1–2):76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Wust S, Hellhammer D. Consistent sex differences in cortisol responses to psychological stress. Psychosom Med. 1992;54(6):648–657. doi: 10.1097/00006842-199211000-00004. [DOI] [PubMed] [Google Scholar]
- Kristjansson SD, Kircher JC, Webb AK. Multilevel models for repeated measures research designs in psychophysiology: An introduction to growth curve modeling. Psychophysiology. 2007;44:728–736. doi: 10.1111/j.1469-8986.2007.00544.x. [DOI] [PubMed] [Google Scholar]
- Lauer MS, Froelicher V. Abnormal heart-rate recovery after exercise. Lancet. 2002;360(9340):1176–1177. doi: 10.1016/S0140-6736(02)11224-4. [DOI] [PubMed] [Google Scholar]
- Llabre MM, Spitzer SB, Saab PG, Schneiderman N. Piecewise latent growth curve modeling of systolic blood pressure reactivity and recovery from the cold pressor test. Psychophysiology. 2001;38:951–960. doi: 10.1111/1469-8986.3860951. [DOI] [PubMed] [Google Scholar]
- Loewenstein G. Anticipation and the Valuation of Delayed Consumption. Economic Journal. 1987;97(387):666–684. [Google Scholar]
- McEwen BS. Stress, adaptation, and disease. Allostasis and allostatic load. Annals of the New York Academy of Sciences. 1998;840:33–44. doi: 10.1111/j.1749-6632.1998.tb09546.x. [DOI] [PubMed] [Google Scholar]
- Mezzacappa ES, Kelsey RM, Katkin ES, Sloan RP. Vagal rebound and recovery from psychological stress. Psychosomatic Medicine. 2001;63(4):650–657. doi: 10.1097/00006842-200107000-00018. [DOI] [PubMed] [Google Scholar]
- Mikolajczak M, Roy E, Luminet O, de Timary P. Resilience and hypothalamic-pituitary-adrenal axis reactivity under acute stress in young men. Stress. 2008;11(6):477–482. doi: 10.1080/10253890701850262. [DOI] [PubMed] [Google Scholar]
- Miranda R, Fontes M, Marroquin B. Cognitive content-specificity in future expectancies: role of hopelessness and intolerance of uncertainty in depression and GAD symptoms. Behaviour Research and Therapy. 2008;46(10):1151–1159. doi: 10.1016/j.brat.2008.05.009. S0005-7967(08)00134-4 [pii] [DOI] [PubMed] [Google Scholar]
- Obrist PA. Cardiovascular psychophysiology: A perspective. New York: Plenum; 1981. [Google Scholar]
- Obrist PA, Webb RA, Sutterer JR, Howard JL. The cardiac-somatic relationship: Some reformations. Psychophysiology. 1970;5:569–587. doi: 10.1111/j.1469-8986.1970.tb02246.x. [DOI] [PubMed] [Google Scholar]
- Peters ML, Godaert GLR, Ballieux RE, van Vliet M, Willemsen JJ, Sweep FCGJ, et al. Cardiovascular and endocrine responses to experimental stress: Effects of mental effort and controllability. Psychoneuroendocrinology. 1998;23(1):1–17. doi: 10.1016/s0306-4530(97)00082-6. [DOI] [PubMed] [Google Scholar]
- Raudenbush S, Bryk A, Congdon R. HLM6. Chicago: Scientific Software International; 2008. [Google Scholar]
- Spacapan S, Cohen S. Effects and aftereffects of stressor expectations. Journal of Personality and Social Psychology. 1983;45(6):1243–1254. doi: 10.1037//0022-3514.45.6.1243. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Lane RD. The role of vagal function in the risk for cardiovascular disease and mortality. Biological Psychology. 2007;74:224–242. doi: 10.1016/j.biopsycho.2005.11.013. [DOI] [PubMed] [Google Scholar]
- Tugade MM, Fredrickson BL. Journal of Personality and Social Psychology. 2. Vol. 86. 2004. Resilient individuals use positive emotions to bounce back from negative emotional experiences; pp. 320–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS scales. Journal of Personality and Social Psychology. 1988;54(6):1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Waugh CE, Fredrickson BL, Taylor SF. Adapting to life s slings and arrows: Individual differences in resilience when recovering from an anticipated threat. Journal of Research in Personality. 2008;42:1031–1046. doi: 10.1016/j.jrp.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waugh CE, Tugade MM, Fredrickson BL. Psychophysiology of resilience to stress. In: Tepe V, editor. SOAR: Biobehavioral resilience to stress. Taylor & Francis; 2008. pp. 117–138. [Google Scholar]