Abstract
A fast and simple model which uses lower animals on the evolutionary scale is beneficial for developing procedures for the reversal of neurobehavioral teratogenicity with neural stem cells. Here, we established a procedure for the derivation of chick neural stem cells, establishing embryonic day (E) 10 as optimal for progression to neuronal phenotypes. Cells were obtained from the embryonic cerebral hemispheres and incubated for 5–7 days in enriched medium containing epidermal growth factor (EGF) and basic fibroblast growth factor (FGF2) according to a procedure originally developed for mice. A small percentage of the cells survived, proliferated and formed nestin-positive neurospheres. After removal of the growth factors to allow differentiation (5 days), 74% of the cells differentiated into all major lineages of the nervous system, including neurons (Beta III tubulin-positive, 54% of the total number of differentiated cells), astrocytes (GFAP-positive, 26%), and oligodendrocytes (O4-positive, 20%). These findings demonstrate that the cells were indeed neural stem cells. Next, the cells were transplanted in two allograft chick models; (1) direct cerebral transplantation to 24-hours-old chicks, followed by post-transplantation cell tracking at 24 hours, 6 days and 14 days, and (2) intravenous transplantation to chick embryos on E13, followed by cell tracking on E19. With both methods, transplanted cells were found in the brain. The chick embryo provides a convenient, precisely-timed and unlimited supply of neural progenitors for therapy by transplantation, as well as constituting a fast and simple model in which to evaluate the ability of neural stem cell transplantation to repair neural damage, steps that are critical for progress toward therapeutic applications.
Keywords: Chick, Neural stem cell derivation, Intra-cerebral transplantation, Intra-venous transplantation
Introduction
Neural stem cell therapy has proven successful in animals as a means to reverse the consequences of neurobehavioral defects and neurodegenerative diseases [2,12,24,31], including models of Parkinson's disease, Alzheimer's disease, Huntington's disease [16,50] and multiple sclerosis [4]. In our work, we have shown how the neurobehavioral abnormalities evoked by prenatal exposure of mice to drugs of abuse (heroin, [6,21]) or neuroactive pesticides [7] can similarly be reversed with neural stem cell therapy.
In the current study, we designed a model system for neural stem cell derivation and transplantation in the chick, which has the following advantages: 1) availability of an unlimited number of subjects, as timed incubated eggs are cheap and easily obtained; 2) teratogen injection into the egg, directly exposing the embryo to a defined concentration, similar to the zebrafish and related models [28]; 3) absence of litter effects, so that every individual offspring represents an independent subject [48]; 4) no maternal influence on the outcome of teratogen exposure [47]; 5) an ability to test neurobehavioral deficits in the absence of confounding influences on self-sufficiency[3], since imprinting behaviors can be evaluated immediately after hatching, before the chicks commence consumption of food or water; 6) since the chick hatches at a more advanced neurodevelopmental stage than the mouse or rat, neurobehavioral evaluations can be conducted immediately, rather than requiring a protracted postnatal period of development; and 7) the chick model facilitates the fast screening of a series of compounds, enabling dose-response determinations, a focus on critical periods of exposure, and likely endpoints to be studied [17]. A chick model, therefore, would complement and significantly enhance the evaluation of neurobehavioral teratogenicity and the potential for neural stem cell reversal strategies.
We modeled the chick studies after our earlier work in mice, where we focused on "region-specific behaviors" and their related synaptic alterations, mainly learning in the Morris maze, which in rodents is highly dependent on the integrity of septohippocampal acetylcholine (ACh) innervation [33–34,42]. In our mouse studies, animals exposed prenatally to a variety of neuroteratogens that target ACh neurotransmission showed deficits in ACh receptor function, centered around impaired ability of receptor activation to elicit translocation/activation of specific PKC isoforms. This was accompanied by reactive upregulation of elements regulating presynaptic activity and postsynaptic receptor expression that nevertheless failed to compensate for the signaling deficit, so that animals displayed impaired Morris maze performance. Because we identified the synaptic signaling defect underlying the behavioral abnormalities, we were able to reverse both synaptic and performance deficits using interventions that replaced defective ACh function, including neural stem cell therapy [6,21]. In the chick, the intermedial part of the hyperstriatum ventrale (IMHV) parallels the function of the rodent hippocampus, with imprinting as the corresponding dependent behavior. Prehatch exposure of the chick to chlorpyrifos, nicotine or heroin, agents which all produce ACh functional deficits in the rodent hippocampus [44,52,54], elicited corresponding impairment of imprinting and Ach-receptor mediated activation/translocation of PKC γ and β [17]. Here, we present the first steps in formulating a parallel chick model of neural stem cell therapy for reversal of neurobehavioral teratogenicity, starting with the derivation of chick neural progenitor cells and characterization of those cells. We transplanted the cells into both posthatch chicks and chick embryos via two distinct approaches, in order to demonstrate their ability to migrate to the site of damage and thus to potentially repair synaptic function. In the first approach, chicks received neural stem cell transplants posthatch. In the second method, intravenous (IV) transplantation was conducted on E13. In both instances, our goal was to develop a simple, cheap model capable of evaluations in large numbers of animals, thus expediting progress in neural progenitor therapy of neurobehavioral teratogenicity.
Materials and Methods
General
All animal studies described here were approved by the Hebrew University Institutional Animal Care and Use Committee. Cells were derived from chick embryo cerebral hemispheres and shown by immunocytochemistry studies to contain nestin prior to differentiation, and β III tubulin, GFAP and O4 post-differentiation, proving that the cells were neural stem cells. Cells were transplanted via both methods: intra-cerebral transplantation to posthatched chicks, and IV injection to E13 chick embryos. In both cases, the cells were tracked in the animals' brains up to 14 days post transplantation.
Derivation of neural stem cells
The procedure was based on those described previously in rodents [5,37]. Fertile gallus gallus domesticus eggs were incubated at 37.8°C and 50–60% humidity and on E10, the brains of the embryos were removed and the tissues of the cerebral hemispheres were isolated and minced, and the cells were digested in undiluted accutase for 5 min. at 37 °C, dissociated with a 5 ml pipette into a single-cell suspension, and incubated at 37°C and 5% CO2 for up to 7 days by established procedures [5–6,21]. The cells were supplemented daily with growth factors; 10 ng/ml basic fibroblast growth factor (FGF2) and 20 ng/ml epidermal growth factor (EGF) (both from Peprotech Asia, Israel). The cells that proliferated formed clusters, which grew into neurospheres. On the third day post-derivation, cells were sedimented at 68 × g for 8 min at 26°C. The pellet was resuspended in 5 ml of enriched medium and the cells were re-plated in a T-25 uncoated flask with the same growth factors.
Primary characterization of the cells
Aliquots (5 µl) were examined daily with a phase-bright microscope (Nikon, Tokyo, Japan), using trypan blue exclusion to characterize viable cells/spheres. Preparations were tested for their dependence on growth factors by incubation with or without EGF and FGF2, separately and together, according to previously established protocols [5,37]
Immunocytochemistry
Plating cells
35 mm Petri dishes were precoated first with poly-L-ornithine, and then fibronectin (both from Sigma). Then cell preparations were plated and incubated at 37°C and 5% CO2 for 3–4 h, allowing the cells to attach. Plates were washed with sterile DMEM (Biological Industries) after incubation. Spheres were dissociated into single cells using Accutase (see Preparation of cells for transplantation below) and 100,000 cells were plated onto each dish [37].
Determining the presence of neural stem cells
For nestin labeling, 400 spheres were plated in each dish, whereas for evaluation of differentiation potential, 10–15 spheres were plated. Cells were fixed with 5% acid alcohol (5% acetic acid in ethanol absolute) for 8 min at −20°C, then washed thoroughly with sterile DMEM. Blocking was performed with 1% normal goat serum (Biological industries) for 15 min, and was followed by a 45 min incubation with primary antibody, 1:50 rabbit anti-nestin (Abcam, Cambridge, UK) diluted in blocking solution. After washings, cells were incubated for 30 min in the dark with the secondary antibody, 1:100 donkey anti-rabbit-alexa fluor 488 conjugated (Molecular Probes, Oregon, USA), diluted in blocking solution. After several washes, cells were mounted with DAPI-containing mounting solution (Vector, Peterborough, UK) and covered with cover slips. Plates were kept at 4°C and taken for immunofluorescent evaluation (Olympus, NY, USA) at least 24 h after the staining procedure.
Neural stem cell phenotypes
For quantitation of nervous system cell phenotypes, we prepared dissociated cells derived from embryonic days E10, E11, E12, E13 and E14. After cell attachment, we added 2 ml of enriched medium (contents described above) without growth factors and the plates were returned to the incubator for 5 days, allowing the cells to differentiate. Since all the primary antibodies (for neurons, astrocytes, and oligodendrocytes) were from the mouse, it was important to stain for each individually, and not concurrently, in an effort to avoid complications of interfering primary and secondary antibodies.
Identifying neurons
To identify neuronal phenotypes, cells were fixed using methanol for 15 min then washed in phosphate buffered saline (PBS) and permeabilized with 0.25% triton-containing PBS (PBST) for 10 min. Cells were washed and blocked with 1% BSA in PBST for 30 min. Primary antibody was then administered, 1:1000 mouse IgG anti-β III tubulin (Abcam) diluted in blocking solution, for 1 h at room temperature or overnight in a humid chamber at 4°C. This was followed by washings and administration of the secondary antibody, 1:1000 goat anti-mouse IgG FITC conjugated (Abcam) diluted in 1% BSA-containing PBS, for 1 h at room temperature in the dark. After washings, cells were mounted with DAPI-containing mounting solution and coverslipped. Plates were kept at 4°C and taken for immunofluorescent evaluation at least 24 h after staining.
Identifying astrocytes
For astrocyte staining, cells were fixed with methanol for 15 min, washed in PBS and permeabilized with PBST for 10 min. Cells were then washed and blocked using 1% BSA in PBST for 30 min. The antibody was then administered, 1:400 mouse anti-GFAP-cy3 conjugated (Abcam) diluted in blocking solution, for 1 h at room temperature or overnight in a humid chamber in 4°C. The remaining procedures were the same as for neuronal staining. Because mouse anti-GFAP antibody is Cy3-conjugated, not requiring secondary antibody, we employed this procedure in concert with anti-β III tubulin antibody staining, effectively double labeling the cells.
Identifying oligodendrocytes
For oligodendrocyte staining, cells were fixed with 4% formaldehyde (Gadot, Netanya, Israel) for 10 min, then washed in PBS and blocked with 1% BSA and 10% normal goat serum in PBS. (In cases where oligodendrocyte staining was carried out on the same sample as neuronal and/or astrocyte staining, 4% formaldehyde and not methanol was used for fixation.) Cells were then washed and primary antibody was administered, 1:50 mouse IgM anti-O4 (Chemicon, Temecula, CA, USA) diluted in PBS, for 1 h in a humid chamber at 37°C. After washings, secondary antibody was administered, 1:500 goat anti-mouse IgM-rhodamine conjugated (Abcam) diluted in PBS, followed by the same procedures as already described. The mouse anti-O4 antibody is IgM and the corresponding secondary antibody is highly specific to IgM, allowing double labeling with the neuronal marker, mouse IgG anti-β III tubulin.
Cell quantitation
The stained cells were mounted with DAPI-containing mounting solution. Quantitation was done by a blinded observer, counting the differentiated cells in random fields; the random fields were taken to be representative samples, and the number of cells counted was used as the basis for calculating how many cells were on each entire plate. Each plate was determined to have contained at least 300.
Preparation of cells for transplantation
In preparation for transplantation, 5–7 days-old cells in growth medium were sedimented as already described. The medium was removed and the remaining pellet of cells was triturated with 200 µl accutase (Sigma) and incubated for 10 min at 37 °C in 5% CO2 to dissociate the spheres into single cells while minimizing cell death [51]. The cells were then triturated and washed with 5 ml PBS, and sedimented as before. The pellet was resuspended in 1 ml of 2 µM CM-Dil cell tracker (Invitrogen) in PBS and the cells were incubated for 5 min at 37 °C, and then for 15 min at 4 °C [25]. The cells were triturated and washed with PBS and counted.
Posthatch intracerebral transplantation
For posthatch transplantation, chicks were immobilized in a wooden box and the head plumage was wet in order to see the brain through the semi-transparent skin and uncalcified skull. Xylazine was used for analgesia. The survival rate was 100%. The injection was performed using a customized 10 µl syringe (Hamilton, Bonaduz, Switzerland) fitted with a fine needle (26G) to which a stop was added so as to limit penetration to a maximum depth of 4 mm[19]. The needle was held in place for at least 3 sec after the injection [19,38]. Each chick received a single injection of 50,000 cells in 5 µl PBS.
IV transplantation in E13 chick embryos
Eggs containing E13 chick embryos were briefly removed from the incubator and carefully cut from the blunt end in order to reveal the air cell and the blood vessels attached to the chorio-allantoic membrane. Each embryo received a single IV injection of 400,000 cells in 100 µl PBS using a 30G needle, according to a previously described procedure [49]. After transplantation, the cut was covered with transparent adhesive tape and the eggs were returned into the incubator until E19.
Immunohistochemistry
Chicks were perfused 24 h, 6 days and 14 days post-transplantation using an established protocol [1]; each chick was anesthetized with an intraperitoneal injection of 80 mg/kg pentobarbital and perfused transcardially with 4% formaldehyde. The brain was removed and placed in 4% formaldehyde for 24 h, then transferred to 30% sucrose in PBS for 48–72 h. Each brain was then frozen in isopentane (Sigma) and liquid nitrogen and stored at −80°C. For E19 chicks receiving IV transplants, brains were immersion-fixed in 4% formaldehyde and subjected to the same subsequent procedures.
Frozen, fixed brains were sunk in OCT Optimal Cutting Temperature compound (Sakura Finetek, CA, USA) and returned to −80°C for at least 1 h. The relevant sections of the brains were then cut serially in the cryostat (Leica, Wetzlar, Germany) into 10 µm thick slices; three sections out of every 10 were mounted on slides (Fisher Scientific, Schwerte, Germany). Slides were kept at −80°C until examined.
Slides were defrosted for 1 h at −20°C followed by 30 min at room temperature, and then were mounted with DAPI-containing mounting solution to allow for recognition of the brain regions to which the transplanted cells migrated. Slides were observed (blind test) under a fluorescent microscope to detect CM-Dil-labeled cells.
Results
E10 derived cells form nestin-positive neurospheres
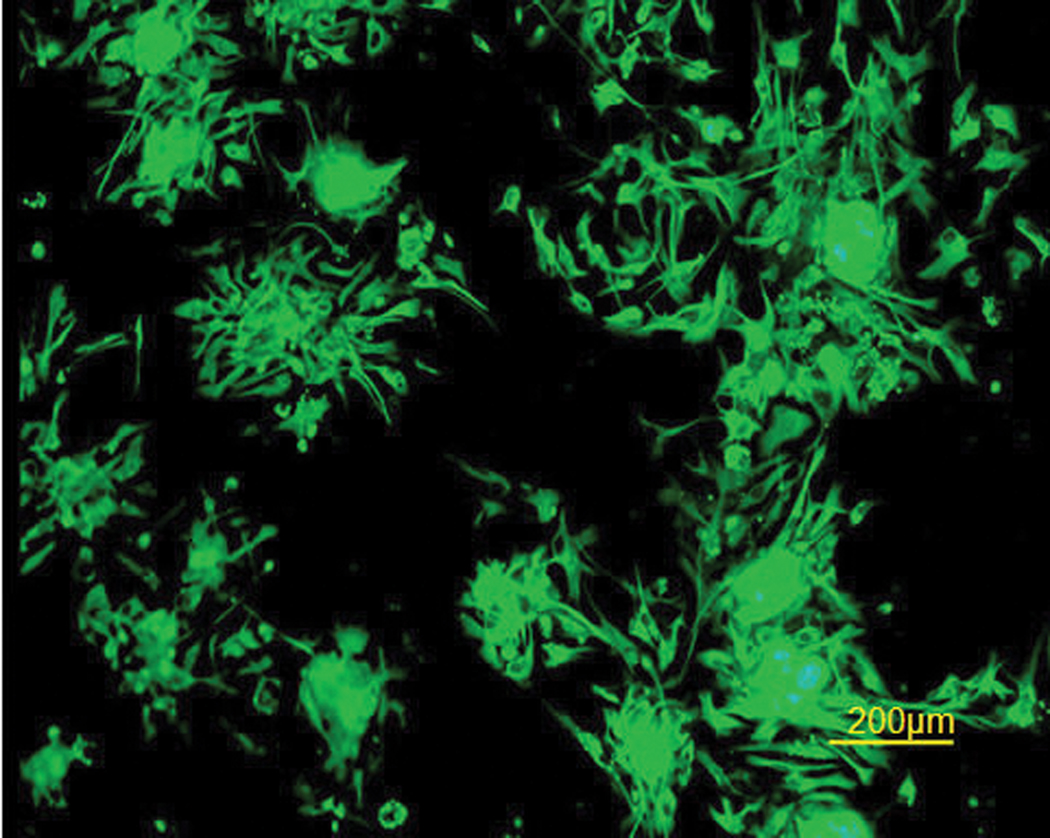
To characterize cell growth and proliferation, cells derived from cerebral hemispheres of E10 chick embryos were grown for 5–7 days with EGF and FGF2. The cells stained positively for nestin, a neural progenitor and neural stem cell marker. This demonstrated that these cells indeed have the potential for developing into more than one phenotype (Figure 1).
Figure 1. Undifferentiated E10 neurospheres are nestin+.
Immunofluorescent staining for nestin as a neural stem cell marker. E10 cells formed neurospheres that were attached to Petri dishes on day 5.
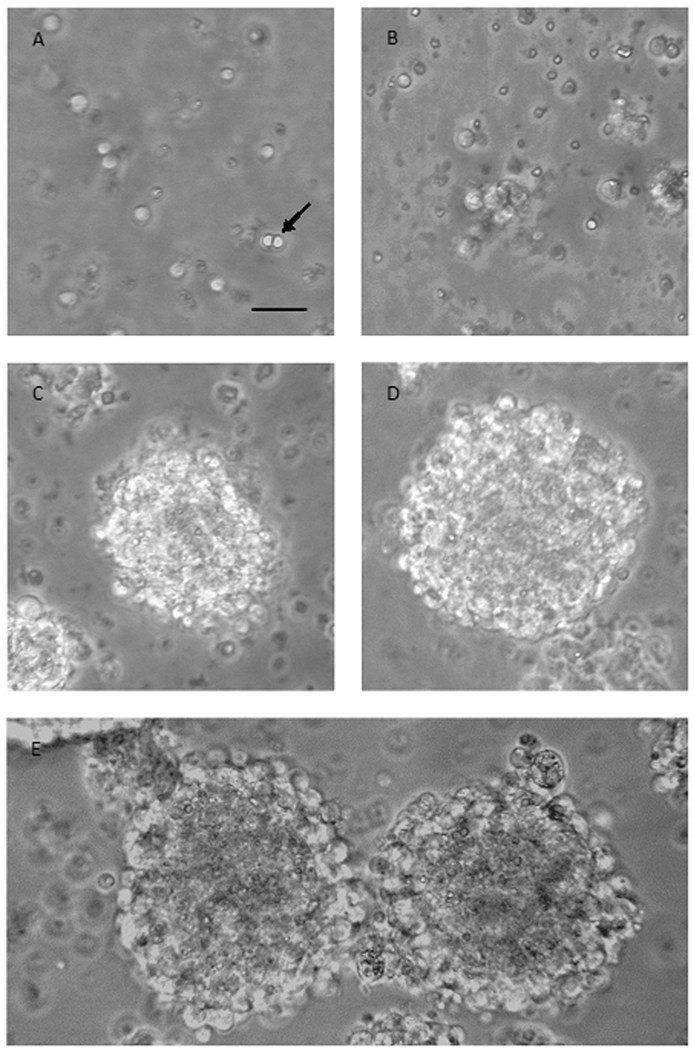
Of the plated cells, 0.5% survived, divided and formed clusters by day 2, which then grew into neurospheres by days 3 and 4 (Figure 2).
Figure 2. E10 cells gradually form neurospheres.
Phase-bright micrographs showing progressive growth of cells into clusters during the first days in culture with EGF and FGF2. (A) E10 cells at plating day (day 1) are separated into single cells. (B) E10 cells on day 2 have formed clusters of 5–15 cells. (C) E10 cells at day 3 show the formation of bigger, non-rounded clusters. (D) E10 cells at day 4, after being re-plated on day 3, form bigger and rounder clusters. (E) E10 cells at day 5 have grown into round neurospheres. Scale bar 50µm; applies A–E.
Optimizing the derivation day
In order to establish the preferred embryonic derivation day, cells were obtained at different embryonic developmental stages. E10, E11, E12 and E13 cells formed neurospheres and differentiated into the three basic cell types, neurons, astrocytes and oligodendrocytes, with the majority of cells showing differentiated phenotypes: 74% for E10, 76% for E11, 95% for E12 and 79% for E13. With the progression from E10 to E13, increasing proportions of the cells differentiated into glial cells rather than neurons (Figure 3). E14 cells did not form neurospheres, and showed little cell survival or cell attachment to the surface of the flask. Some of the surviving cells grew processes within 3–4 days. Accordingly, E14 cells were not examined further, since these characteristics do not match a common neural stem cell form. Although significance levels could not be calculated, due to the number of brains used, the earliest stage (E10) appeared optimal for survival and differentiation into neurons and was therefore chosen for most of the other evaluations.
Figure 3. Cultures derived from different embryonic days display diverse differentiation patterns.
E10, E11, E12 and E13 cells were grown in the presence of EGF and FGF2, dissociated into single cells, attached to Petri dishes and allowed to differentiate for 5 days without growth factors. Each plate was stained for a different cell marker; β III tubulin for neurons, GFAP for astrocytes and O4 for oligodendrocytes. The number of differentiated cells on each plate was blind counted as percentage out of the total number of cells counted on that plate, marked by DAPI nuclear stain. The sum of the percentages from each plate was used to calculate the total differentiation rate, and the presented data are percentage out of the differentiated cells. Data are mean of 3–5 plates. As derivation day progressed, fewer neurons were formed and more astrocytes emerged. Beta3T, β III tubulin; GFAP, glial fibrillary acidic protein.
Every embryonic day (E) represents one derivation, 3–5 Petri dishes per derivation.
Both EGF and FGF2 are required for optimal growth
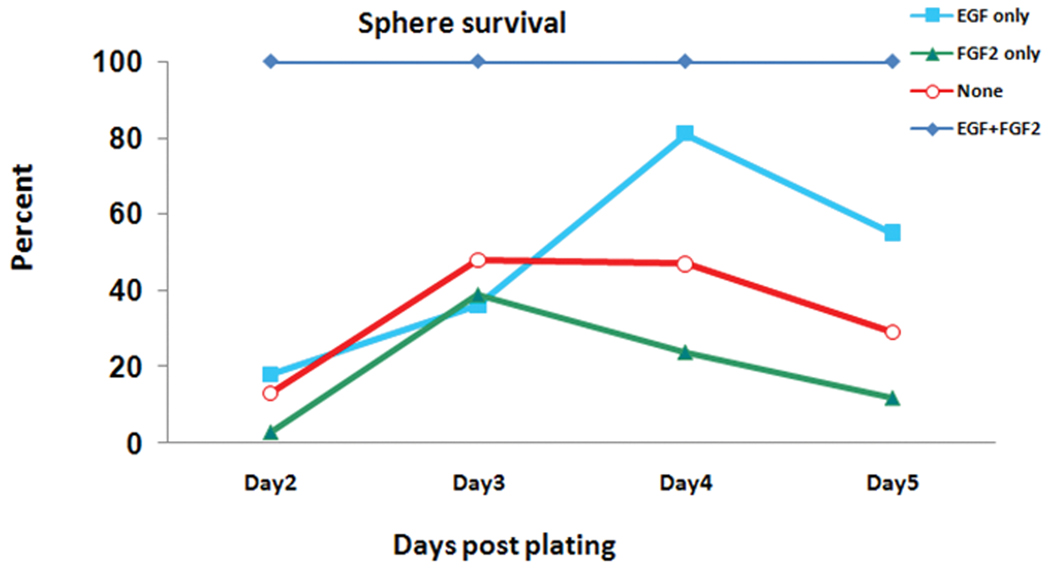
In order to determine the optimal environment for cell survival and differentiation, E10 cells were incubated with or without EGF and/or FGF2. Daily counts of viable cells indicated that the presence of both EGF and FGF2 was required for maximal cell growth as represented by the 100%-line in Figure 4. By itself, neither growth factor was as effective in promoting cell survival as the combination; EGF alone had a small promotive effect, while FGF2 alone did not improve on, and even appeared to decrease survival compared to the control level.
Figure 4. E10 cells grow best in the presence of both EGF and FGF2.
Daily counts of viable cells using trypan blue in the presence of either EGF or FGF2, both or none. 107 E10 cells were plated on day 1. Exposure of cells to EGF, FGF2 or none resulted in lower number of spheres compared with the group exposed to both growth factors. Data are presented as percent of living spheres compared to both EGF and FGF2 (the 100% line). EGF, epidermal growth factor; FGF2, basic fibroblast growth factor. The line representing EGF+FGF is an average of three derivations; all other lines represent percentage survival of only one derivation (not an average).
Neurospheres differentiate in vitro into all three types of neural cells
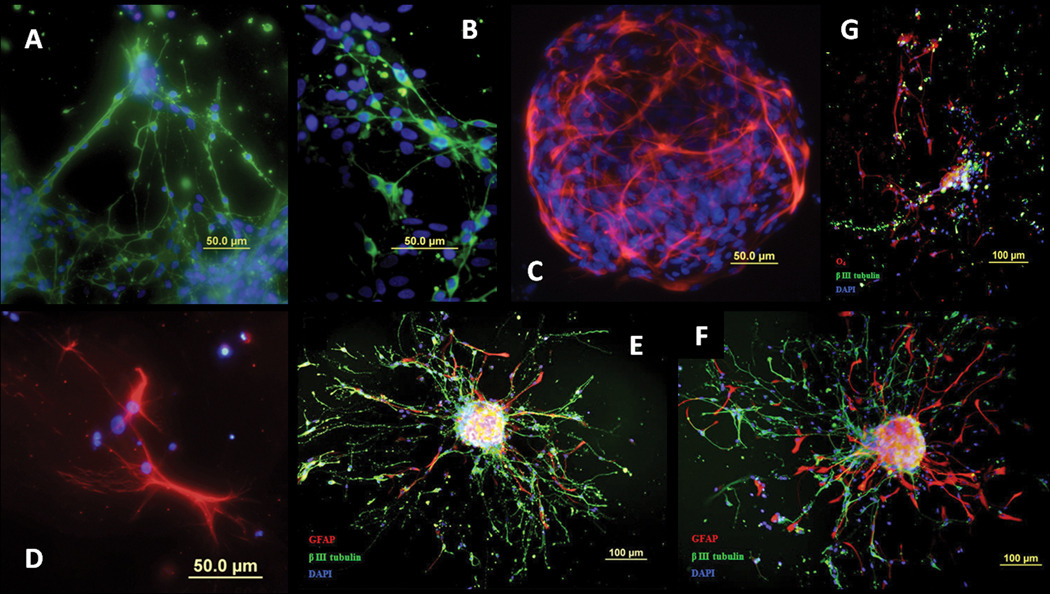
E10 cells were attached to Petri dishes and allowed to differentiate for 5 days without growth factors. Quantitation of the dissociated spheres revealed that 74% of E10 cells differentiated in 5 days and then stained positively for neurons (β III tubulin) (Figure 5A,B,E–G), astrocytes (GFAP) (Figure 5C,E,F) and oligodendrocytes (O4) (Figure 5D,G). Cells migrated out of the neurospheres, grew processes as they differentiated and connected with other cells and neurospheres. Differentiated cells were extremely branched, especially neurons and astrocytes. All plates were additionally stained with DAPI, which marks cell nuclei, showing that the phenotype staining indeed highlighted elements of cell bodies.
Figure 5. E10 neurospheres differentiate into neurons, astrocytes and oligodendrocytes.
E10 neurospheres were grown for 5 days with EGF and FGF2, then attached to Petri dishes and allowed to differentiate for 5 days without growth factors. Cells differentiated into (A,B) neurons (β III tubulin, green), (C) astrocytes (GFAP, red) and (D) oligodendrocytes (O4, red). Nuclei were stained using DAPI (blue). (E,F) Triple labeling of β III tubulin, GFAP and DAPI. (G) Triple labeling of β III tubulin, O4 and DAPI. GFAP, glial fibrillary acidic protein; DAPI, 4',6-diamidino-2-phenylindole.
Cerebral transplantation of neural stem cells in posthatch chicks
In order for neural stem cell cultures to be useful for transplantation, they have to survive in vivo [4,6,12,16,24,45,50], as well as finding the right target zone, and then once there, they need to proliferate and replace damaged cells [8]; alternatively they can release cytokines and/or evoke cytokine release from the host tissue that leads to proliferation of endogenous neural precursors [6,9,14,18,32]. The administration of neural stem cells led to the presence of detectable, viable transplanted cells; At 24h post-transplantation, transplanted cells were seen along the left lateral cerebral cortex, around and in the left lateral ventricle, meaning that the cells survived and also migrated after the transplantation (Figure 6A–C). After 6 days, transplanted cells were seen mainly around the left lateral ventricle (Figure 6D,E). At 14 days post-transplantation, cells were not only located in the left cerebral cortex but also migrated to the right cerebral cortex (Figure 6F). It is clear, then, that the transplanted cells survive and migrate in the brains of chicks for at least 14 days after transplantation. Quantitation of the number of surviving cells showed about 8000 at 24h, declining to 2500–4000 at 6 to 14 days.
Figure 6. Intra-cerebrally transplanted Neural stem cells survive and migrate in the posthatched chick brain for at least 14 days.
CM-Dil labeled single cells were transplanted intra-cerebrally into 24h-old chicks and the cells were tracked 24h (A–C), 6 days (D,E) and 14 days (F) post transplantation. (A) Transplanted cells migrated and seen along the left cerebral cortex 24h post transplantation. (B) Transplanted cells migrated and seen around the left ventricle 24h post transplantation. (C) Transplanted cells migrated and seen inside the left ventricle 24h post transplantation. (D,E) Transplanted cells are seen around the left ventricle 6 days post transplantation. (F) Transplanted cells are seen along the left cerebral cortex 14 days post transplantation. (G) Quantification of in vivo cell survival 24h, 6 days and 14 days post transplantation. Transplanted cells – red (CM-Dil). Nuclei – blue (DAPI). h, hours Every bar represents two brains (total of 6). Error bars indicate SEM.
IV transplantation of neural stem cells in the chick embryo
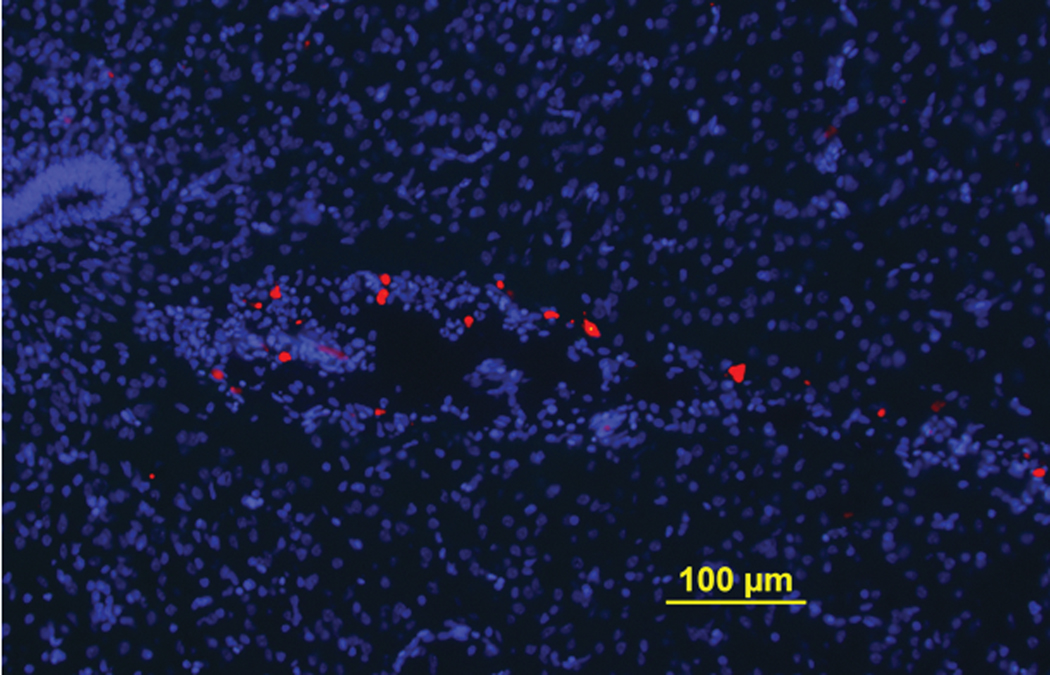
IV administration of neural stem cells is beginning to emerge as a strategy to make delivery of these cells less invasive [26,35–36,49]. Here, we injected cells directly into the blood vessels of the chorio-allantoic membrane on E13, a day shown as optimal after preliminary studies, with the objective of determining if the cells could indeed reach the brain. Evaluations on E19 showed definitively that the cells reached the third ventricle (Figure 7), with average of 1300 cells surviving in that brain area 6 days after IV administration.
Figure 7. IV transplanted neural stem cells in the chick embryo migrated to its brain and survived for at least 6 days.
CM-Dil labeled single cells were IV transplanted into E13 chick embryos and the cells were tracked in E19 chick embryos’ brains. Transplanted cells have migrated along the blood vessels and reached the third ventricle of the chick embryos’ brains.
Discussion
The current study presents an avian (chick) model for neural stem cell derivation and transplantation, the first step toward developing this model for the amelioration and reversal of the adverse effects of neuroteratogens or neurodegenerative disorders. We established a procedure for deriving neural stem cells from the chick embryo and confirmed their survival and growth in culture, their differentiation into neurons, astrocytes and oligodendrocytes, and the role of specific growth factors in proliferation, survival and differentiation. We further demonstrated the survival of neural stem cells and their migration in the brain after posthatch intracerebral administration, or even after IV administration. These findings thus encompass ages at which imprinting behaviors can be assessed to determine the reversal of neurobehavioral teratogenesis [17,29] as well as later behavioral indices [20,27]. It should be noted that the surviving cells did not promote tumor growth.
The undifferentiated cells that proliferated in vitro were nestin-positive, identifying them as neural stem cells [30] (Figure 1). It follows that undifferentiated E10-derived cells from chick cerebral hemispheres are not pluripotent; they are committed to a neural lineage, namely neurons, astrocytes and oligodendrocytes, as was demonstrated after removing growth factors from the culture. This specificity further supports them as good candidates for neural stem cell transplantation, since therapeutic actions are optimal when cells are not fully differentiated, but yet are differentiated enough to avoid forming tumors, as is the case with totipotent stem cells [53].
The cells were seeded 10,000,000 per flask, and while most cells differentiated and died, each surviving progenitor is to be assumed to have formed a single sphere. Once the spheres stabilized in number, though they continued to grow in size, an average of about 50,000 spheres were seen per flask – a 0.5% survival rate, indeed in accordance with earlier work [5] where we found that 0.5% of E10 cerebral hemispheres-derived cells survived and proliferated in the presence of growth factors. The neural stem cells proliferated best in the presence of both EGF and FGF2, which suppress differentiation in favor of maintaining mitotic activity [15]. Thus, as in rodent models [5,41], growth factors can be supplied to promote cell replication so as to optimize yield, and can then be removed to permit differentiation into neural phenotypes. Further, we found that deriving the cells at different stages led to preferential expression of specific phenotypes, with predominance of neurons at earlier stages and astrocytes at later stages; this means that the cultures can be manipulated so as to optimize the differentiation fate of the neural stem cells to target specific cell types as required to offset a given defect. Neurodegenerative disease models [16,50] may benefit more from E10-derived cells which show greater neuronal differentiation, whereas glia-related disease models [40] may benefit from cells derived on later days. However, E14-derived cells did not form neurospheres, but were seen attached to the surface of the flask, some of which grew processes within 3–4 days post plating. It follows that from E14 the embryonic CNS is almost entirely differentiated, in accord with earlier conclusions [13], and are thus not suitable for transplantation.
For our purposes, then, E10-derived cell cultures would seem to be optimal for repairing the defects elicited by prenatal exposure to neurotoxicants, since they should be able to replace damaged neuronal circuits and restore behavioral function [17].
Transplanted neural stem cells survived well in chicks’ brains, well into the posthatch period and spanning ages at which behavioral tests can reveal the efficacy of this treatment in restoring function after developmental injury [20,27]. Extrapolating from other models [6], it is likely that the survival of transplanted neural stem cells extends far beyond the posthatch period studied here, which would certainly be required if neural stem cells are to be used to replace neurons and restore damaged circuits in neurodegenerative disorders [8,11,46]. However, direct neuronal replacement is not the only way in which these transplants may be effective. Exogenously-administered neural stem cells also produce a trophic effect, enhancing the production of endogenous neural precursors in the damaged brain [6,14]. These indirect effects have been shown to account for the correlation between endogenous hippocampal cell proliferation and restoration of cognitive function [10,22]. Consequently, the fact that the neural stem cells transplanted into newly hatched chicks not only survived but also migrated to other areas in the host brain, raises the likelihood that trophic actions may be exerted even if the damage is diffuse rather than restricted to a single brain region. Indeed, transplanted CM-Dil labeled cells were found throughout the cortex and the lateral ventricle, some even reaching the contralateral hemisphere.
Minimizing immunological rejection is a major goal in neural stem cell transplantation. Inactive hematopoietic precursor cells are present in the chick embryo and yolk sac from E2, natural killer cells from E8, B cells from E12, macrophages from E15 and T cells from E16 [43]. The chick’s own antibodies begin to function by posthatch day 21, although there is limited activity derived from maternal antibodies for the first 3 weeks posthatch [55]. Therefore, there is a window of almost total immunologic passivity until E16 and relative immunologic privilege for 3 weeks posthatch, which provides an opportunity for transplantation; this is another important advantage of the chick model. Further, as seen here, chick-derived neural stem cells do not lead to tumor formation, another major disadvantage noted with many stem cell models. The final advantage of the avian model is the potential to administer the neural stem cells non-invasively, via IV injection; this certainly is a promising feature for potential clinical application of transplantation.
Although fewer cells were found in chicks' brains when using IV administration when compared to direct cerebral transplantation, they were still seen in large numbers, and there is clearly great potential for improving this method as was shown in the rodent [35–36]. Our results are paralleled in the rodent model, where IV transplanted cells similarly can reach the damaged CNS [35–36] and elicit physical and neurobehavioral improvements.
The transplanted cells were identified in the host brain using Dil cell tracker. Concern was raised regarding the validity of Dil staining, because transplanted cells that die in the host brain may transfer the stain to the host cells [23,39]. Conditions of these studies were entirely different from ours: the Dil concentration was 10–40 times higher than ours and the sources of the transplanted cells were human cord blood cells and cells used in bone tissue engineering. Nevertheless, to be sure that the Dil transfer shown in these studies is not relevant to our investigation, we added in a recent study [7] a control group transplanted with Dil-labeled adult cortical cells, destined to die in the host brain. No labeled cells were found in the host brains of this group, demonstrating that the marker does not transfer from dead cells post transplantation to host cells under the relevant parameters (our cell type and Dil concentration). The specificity of our Dil labeling is further enhanced by the fact that adult neural stem cells derived from the subventricular zone were easily visualized in the host brain [7].
In summary, we devised a chick model for the derivation of neural stem cells, their differentiation into specific neural phenotypes, and their transplantation into the brain, laying the groundwork for future testing of therapies to reverse defined neurobehavioral and neurodevelopmental defects [17]. Derivation of the cells is optimal on E10, a stage which fosters differentiation into neurons, and transplantation is effective even whether cells are delivered via IV or direct intracerebral administration. By delaying the embryonic age at which cells are derived, the model can potentially be applied to reversal of glia-related diseases as well. The model, which is simple and easily provides large numbers of subjects both for deriving neural stem cells and for testing their ability to reverse the consequences of injury, can be utilized readily for rapid screening of toxicants and reversal strategies, and can thus work in concert with established, but more cumbersome rodent models. The potential for this model to actually reverse neurobehavioral defects is the next step in our research.
Acknowledgements
The authors thank Dr. Ronald S. Goldstein (Mina and Everard Goodman Faculty of Life Sciences, Bar-Ilan University, Ramat-Gan, Israel) for his advice and guidance in some of the procedural aspects of this research. Supported by NIH grant ES014258, the United States-Israel Binational Science Foundation BSF2005003 and the Israeli Anti-Drug Authority.
Abbreviations
- ACh
acetylcholine
- BSA
bovine serum albumin
- CNS
central nervous system
- DAPI
4',6-diamidino-2-phenylindole
- E
embryonic day
- EGF
epidermal growth factor
- FGF2
basic fibroblast growth factor
- IMHV
intermedial part of the hyperstriatum ventrale
- IV
intravenous
- NSC
neural stem cells
- PBS
phosphate-buffered saline
- PBST
PBS containing 0.25% triton
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ambalavanar R, McCabe BJ, Potter KN, Horn G. Learning-related fos-like immunoreactivity in the chick brain: time-course and co-localization with GABA and parvalbumin. Neuroscience. 1999;93:1515–1524. doi: 10.1016/s0306-4522(99)00217-1. [DOI] [PubMed] [Google Scholar]
- 2.Barnabe-Heider F, Frisen J. Stem cells for spinal cord repair. Cell Stem Cell. 2008;3:16–24. doi: 10.1016/j.stem.2008.06.011. [DOI] [PubMed] [Google Scholar]
- 3.Barron S, Kelly SJ, Riley EP. Neonatal alcohol exposure alters suckling behavior in neonatal rat pups. Pharmacol Biochem Behav. 1991;39:423–427. doi: 10.1016/0091-3057(91)90202-d. [DOI] [PubMed] [Google Scholar]
- 4.Ben-Hur T. Immunomodulation by neural stem cells. J Neurol Sci. 2008;265:102–104. doi: 10.1016/j.jns.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 5.Ben-Hur T, Rogister B, Murray K, Rougon G, Dubois-Dalcq M. Growth and fate of PSA-NCAM+ precursors of the postnatal brain. J Neurosci. 1998;18:5777–5788. doi: 10.1523/JNEUROSCI.18-15-05777.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ben-Shaanan TL, Ben-Hur T, Yanai J. Transplantation of neural progenitors enhances production of endogenous cells in the impaired brain. Molecular Psychiatry. 2008;13:222–231. doi: 10.1038/sj.mp.4002084. [DOI] [PubMed] [Google Scholar]
- 7.Billauer-Haimovitch H, Slotkin TA, Dotan S, Langford R, Pinkas A, Yanai J. Reversal of chlorpyrifos neurobehavioral teratogenicity in mice by nicotine administration and neural stem cell transplantation. Behavioral Brain Research. 2009 doi: 10.1016/j.bbr.2009.08.006. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bjorklund A, Lindvall O. Cell replacement therapies for central nervous system disorders. Nat Neurosci. 2000;3:537–544. doi: 10.1038/75705. [DOI] [PubMed] [Google Scholar]
- 9.Butovsky O, Ziv Y, Schwartz A, Landa G, Talpalar AE, Pluchino S, Martino G, Schwartz M. Microglia activated by IL-4 or IFN-gamma differentially induce neurogenesis and oligodendrogenesis from adult stem/progenitor cells. Mol Cell Neurosci. 2006;31:149–160. doi: 10.1016/j.mcn.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 10.Drapeau E, Mayo W, Aurousseau C, Le Moal M, Piazza PV, Abrous DN. Spatial memory performances of aged rats in the water maze predict levels of hippocampal neurogenesis. Proc Natl Acad Sci U S A. 2003;100:14385–14390. doi: 10.1073/pnas.2334169100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Englund U, Bjorklund A, Wictorin K, Lindvall O, Kokaia M. Grafted neural stem cells develop into functional pyramidal neurons and integrate into host cortical circuitry. Proc Natl Acad Sci U S A. 2002;99:17089–17094. doi: 10.1073/pnas.252589099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haas S, Weidner N, Winkler J. Adult stem cell therapy in stroke. Curr Opin Neurol. 2005;18:59–64. doi: 10.1097/00019052-200502000-00012. [DOI] [PubMed] [Google Scholar]
- 13.V.a.H. Hamburger HL. A series of normal stages in the development of the chick embryo. J Morphol. 1951;88:49–92. [PubMed] [Google Scholar]
- 14.Hattiangady B, Shuai B, Cai J, Coksaygan T, Rao MS, Shetty AK. Increased dentate neurogenesis after grafting of glial restricted progenitors or neural stem cells in the aging hippocampus. Stem Cells. 2007;25:2104–2117. doi: 10.1634/stemcells.2006-0726. [DOI] [PubMed] [Google Scholar]
- 15.Herbst RS. Review of epidermal growth factor receptor biology. Int J Radiat Oncol Biol Phys. 2004;59:21–26. doi: 10.1016/j.ijrobp.2003.11.041. [DOI] [PubMed] [Google Scholar]
- 16.Hou L, Hong T. Stem cells and neurodegenerative diseases. Sci China C Life Sci. 2008;51:287–294. doi: 10.1007/s11427-008-0049-1. [DOI] [PubMed] [Google Scholar]
- 17.Izrael M, Van der Zee EA, Slotkin TA, Yanai J. Cholinergic synaptic signaling mechanisms underlying behavioral teratogenicity: effects of nicotine, chlorpyrifos, and heroin converge on protein kinase C translocation in the intermedial part of the hyperstriatum ventrale and on imprinting behavior in an avian model. J Neurosci Res. 2004:499–507. doi: 10.1002/jnr.20287. [DOI] [PubMed] [Google Scholar]
- 18.Johansson S, Price J, Modo M. Effect of Inflammatory Cytokines on MHC Expression and Differentiation of Human Neural Stem/Progenitor Cells. Stem Cells. 2008 doi: 10.1634/stemcells.2008-0116. [DOI] [PubMed] [Google Scholar]
- 19.Johnston AN, Rose SP. Memory consolidation in day-old chicks requires BDNF but not NGF or NT-3; an antisense study. Brain Res Mol Brain Res. 2001;88:26–36. doi: 10.1016/s0169-328x(01)00016-x. [DOI] [PubMed] [Google Scholar]
- 20.Jones RB, Gentle MJ. Olfaction and behavioral modification in domestic chicks (Gallus domesticus) Physiol Behav. 1985;34:917–924. doi: 10.1016/0031-9384(85)90014-9. [DOI] [PubMed] [Google Scholar]
- 21.Katz S, Ben-Hur T, Ben-Shaanan TL, Yanai J. Reversal of heroin neurobehavioral teratogenicity by grafting of neural progenitors. Journal of Neurochemistry. 2008;104:38–49. doi: 10.1111/j.1471-4159.2007.05004.x. [DOI] [PubMed] [Google Scholar]
- 22.Kleindienst A, McGinn MJ, Harvey HB, Colello RJ, Hamm RJ, Bullock MR. Enhanced hippocampal neurogenesis by intraventricular S100B infusion is associated with improved cognitive recovery after traumatic brain injury. J Neurotrauma. 2005;22:645–655. doi: 10.1089/neu.2005.22.645. [DOI] [PubMed] [Google Scholar]
- 23.Kruyt MC, De Bruijn J, Veenhof M, Oner FC, Van Blitterswijk CA, Verbout AJ, Dhert WJ. Application and limitations of chloromethyl-benzamidodialkylcarbocyanine for tracing cells used in bone Tissue engineering. Tissue Eng. 2003;9:105–115. doi: 10.1089/107632703762687582. [DOI] [PubMed] [Google Scholar]
- 24.Kulbatski I, Mothe AJ, Nomura H, Tator CH. Endogenous and exogenous CNS derived stem/progenitor cell approaches for neurotrauma. Curr Drug Targets. 2005;6:111–126. doi: 10.2174/1389450053345037. [DOI] [PubMed] [Google Scholar]
- 25.Lee G, Kim H, Elkabetz Y, Al Shamy G, Panagiotakos G, Barberi T, Tabar V, Studer L. Isolation and directed differentiation of neural crest stem cells derived from human embryonic stem cells. Nat Biotechnol. 2007;25:1468–1475. doi: 10.1038/nbt1365. [DOI] [PubMed] [Google Scholar]
- 26.Lee ST, Chu K, Jung KH, Kim SJ, Kim DH, Kang KM, Hong NH, Kim JH, Ban JJ, Park HK, et al. Anti-inflammatory mechanism of intravascular neural stem cell transplantation in haemorrhagic stroke. Brain. 2008;131:616–629. doi: 10.1093/brain/awm306. [DOI] [PubMed] [Google Scholar]
- 27.Lesley PZ, Rogers J, Giorgio Vallortigara. Advantages of having a lateralized brain., Proceedings of the Royal Society. Biological Sciences. 2004;271:S420–S422. doi: 10.1098/rsbl.2004.0200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levin ED, Chrysanthis E, Yacisin K, Linney E. Chlorpyrifos exposure of developing zebrafish: effects on survival and long-term effects on response latency and spatial discrimination. Neurotoxicol Teratol. 2003;25:51–57. doi: 10.1016/s0892-0362(02)00322-7. [DOI] [PubMed] [Google Scholar]
- 29.McCabe BJ, Horn G, Bateson PP. Effects of restricted lesions of the chick forebrain on the acquisition of filial preferences during imprinting. Brain Res. 1981;205:29–37. doi: 10.1016/0006-8993(81)90717-4. [DOI] [PubMed] [Google Scholar]
- 30.Michalczyk K, Ziman M. Nestin structure and predicted function in cellular cytoskeletal organisation. Histol Histopathol. 2005;20:665–671. doi: 10.14670/HH-20.665. [DOI] [PubMed] [Google Scholar]
- 31.Mochizuki N, Takagi N, Kurokawa K, Onozato C, Moriyama Y, Tanonaka K, Takeo S. Injection of neural progenitor cells improved learning and memory dysfunction after cerebral ischemia. Exp Neurol. 2008;211:194–202. doi: 10.1016/j.expneurol.2008.01.027. [DOI] [PubMed] [Google Scholar]
- 32.Munoz JR, Stoutenger BR, Robinson AP, Spees JL, Prockop DJ. Human stem/progenitor cells from bone marrow promote neurogenesis of endogenous neural stem cells in the hippocampus of mice. Proc Natl Acad Sci U S A. 2005;102:18171–18176. doi: 10.1073/pnas.0508945102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nilsson OG, Shapiro ML, Gage FH, Olton DS, Bjorklund A. Spatial learning and memory following fimbria-fornix transection and grafting of fetal septal neurons to the hippocampus. Exp Brain Res. 1987;67:195–215. doi: 10.1007/BF00269466. [DOI] [PubMed] [Google Scholar]
- 34.Olton DS, Walker JA, Gage FH. Hippocampal connections and spatial discrimination. Brain Res. 1978;139:295–308. doi: 10.1016/0006-8993(78)90930-7. [DOI] [PubMed] [Google Scholar]
- 35.Pluchino S, Quattrini A, Brambilla E, Gritti A, Salani G, Dina G, Galli R, Del Carro U, Amadio S, Bergami A, et al. Injection of adult neurospheres induces recovery in a chronic model of multiple sclerosis. Nature. 2003;422:688–694. doi: 10.1038/nature01552. [DOI] [PubMed] [Google Scholar]
- 36.Pluchino S, Zanotti L, Rossi B, Brambilla E, Ottoboni L, Salani G, Martinello M, Cattalini A, Bergami A, Furlan R, et al. Neurosphere-derived multipotent precursors promote neuroprotection by an immunomodulatory mechanism. Nature. 2005;436:266–271. doi: 10.1038/nature03889. [DOI] [PubMed] [Google Scholar]
- 37.Rietze RL, Reynolds BA. Neural stem cell isolation and characterization. Methods Enzymol. 2006;419:3–23. doi: 10.1016/S0076-6879(06)19001-1. [DOI] [PubMed] [Google Scholar]
- 38.Rose SP. God's organism? The chick as a model system for memory studies. Learn Mem. 2000;7:1–17. doi: 10.1101/lm.7.1.1. [DOI] [PubMed] [Google Scholar]
- 39.Schormann W, Hammersen FJ, Brulport M, Hermes M, Bauer A, Rudolph C, Schug M, Lehmann T, Nussler A, Ungefroren H, et al. Tracking of human cells in mice. Histochem Cell Biol. 2008;130:329–338. doi: 10.1007/s00418-008-0428-5. [DOI] [PubMed] [Google Scholar]
- 40.Shahani N, Nalini A, Gourie-Devi M, Raju TR. Reactive astrogliosis in neonatal rat spinal cord after exposure to cerebrospinal fluid from patients with amyotrophic lateral sclerosis. Exp Neurol. 1998;149:295–298. doi: 10.1006/exnr.1997.6651. [DOI] [PubMed] [Google Scholar]
- 41.Shi Y, Sun G, Zhao C, Stewart R. Neural stem cell self-renewal. Crit Rev Oncol Hematol. 2008;65:43–53. doi: 10.1016/j.critrevonc.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shim I, Ha Y, Chung JY, Lee HJ, Yang KH, Chang JW. Association of learning and memory impairments with changes in the septohippocampal cholinergic system in rats with kaolin-induced hydrocephalus. Neurosurgery. 2003;53:416–425. doi: 10.1227/01.neu.0000073989.07810.d8. discussion 425. [DOI] [PubMed] [Google Scholar]
- 43.Siatskas C, Boyd R. Regulation of chicken haemopoiesis by cytokines. Dev Comp Immunol. 2000;24:37–59. doi: 10.1016/s0145-305x(99)00051-8. [DOI] [PubMed] [Google Scholar]
- 44.Slotkin TA, Cousins MM, Tate CA, Seidler FJ. Persistent cholinergic presynaptic deficits after neonatal chlorpyrifos exposure. Brain Res. 2001;902:229–243. doi: 10.1016/s0006-8993(01)02387-3. [DOI] [PubMed] [Google Scholar]
- 45.Snyder EY, Daley GQ, Goodell M. Taking stock and planning for the next decade: realistic prospects for stem cell therapies for the nervous system. J Neurosci Res. 2004;76:157–168. doi: 10.1002/jnr.20033. [DOI] [PubMed] [Google Scholar]
- 46.Snyder EY, Loring JF. A role for stem cell biology in the physiological and pathological aspects of aging. J Am Geriatr Soc. 2005;53:S287–S291. doi: 10.1111/j.1532-5415.2005.53491.x. [DOI] [PubMed] [Google Scholar]
- 47.Sobrian SK, Jones BL, Varghese S. Prenatal maternal stress as a model of depression: II Porsolt swim test. Neurotoxicol Teratol. 1999;21:327. [Google Scholar]
- 48.Spear LP, File SE. Methodological considerations in neurobehavioral teratology. Pharmacol Biochem Behav. 1996;55:455–457. doi: 10.1016/s0091-3057(96)00272-9. [DOI] [PubMed] [Google Scholar]
- 49.Taizi M, Deutsch VR, Leitner A, Ohana A, Goldstein RS. A novel and rapid in vivo system for testing therapeutics on human leukemias. Exp Hematol. 2006;34:1698–1708. doi: 10.1016/j.exphem.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 50.Takahashi J. Stem cell therapy for Parkinson's disease. Ernst Schering Res Found Workshop. 2006:229–244. doi: 10.1007/3-540-31437-7_15. [DOI] [PubMed] [Google Scholar]
- 51.Wachs FP, Couillard-Despres S, Engelhardt M, Wilhelm D, Ploetz S, Vroemen M, Kaesbauer J, Uyanik G, Klucken J, Karl C, et al. High efficacy of clonal growth and expansion of adult neural stem cells. Lab Invest. 2003;83 doi: 10.1097/01.lab.0000075556.74231.a5. 949-262. [DOI] [PubMed] [Google Scholar]
- 52.Yanai J, Barg J, Vatury O, Slotkin TA. Cell signaling as a target and underlying mechanism for neurobehavioral teratogenesis. Ann N Y Acad Sci. 2002;965:473–478. doi: 10.1111/j.1749-6632.2002.tb04188.x. [DOI] [PubMed] [Google Scholar]
- 53.Yanai J, Doetchman T, Laufer N, Maslaton J, Mor-Yosef S, Safran A, Shani M, Sofer D. Embryonic cultures but not embryos transplanted to the mouse's brain grow rapidly without immunosuppression. Int J Neurosci. 1995;81:21–26. doi: 10.3109/00207459509015295. [DOI] [PubMed] [Google Scholar]
- 54.Yanai J, Pick CG, Rogel-Fuchs Y, Zahalka EA. Alterations in hippocampal cholinergic receptors and hippocampal behaviors after early exposure to nicotine. Brain Res Bull. 1992;29:363–368. doi: 10.1016/0361-9230(92)90069-a. [DOI] [PubMed] [Google Scholar]
- 55.Zhao C, Song C, Wang X, Li Z, Sha J, Han H, Zhang Y. Induction of immunological tolerance in chickens inoculated with xenogeneic antigens at an early stage of embryonic development. Dev Comp Immunol. 2006;30:431–440. doi: 10.1016/j.dci.2005.06.013. [DOI] [PubMed] [Google Scholar]