Abstract
Objective
There have been few comparisons of the effectiveness of collaborative depression care between older versus younger adults with co-morbid illness, particularly among low-income populations.
Design
Intent-to-treat analyses are conducted on pooled data from three randomized controlled trials that tested collaborative care aimed at improving depression, quality of life and treatment receipt.
Settings
Trials were conducted in oncology and primary care safety net clinics and diverse home health care programs.
Participants
1,081 patients with major depressive symptoms and cancer, diabetes or other co-morbid illness.
Intervention
Similar intervention protocols included patient, provider, socio-cultural and organizational adaptations.
Measurements
The PHQ-9 depression, SF-12/20 quality-of-life, self-reported hospitalization, ER, ICU utilization, and antidepressant, psychotherapy treatment receipt are assessed at baseline, 6, 12 months.
Results
There are no significant differences in reducing depression symptoms (P ranged 0.18-0.58), improving quality-of-life (t=1.86, df=669, P=0.07 for physical functioning at 12 months; and P ranged 0.23-0.99 for all others) between patients ≥60 versus 18-59. Both age group intervention patients have significantly higher rates of a 50% PHQ-9 reduction (older: Wald χ2[df=1]=4.82, p=0.03; younger: Wald χ2[df=1]=6.47, p=0.02), greater reduction in major depression rates (older: Wald χ2[df=1]=7.72, p=0.01; younger: Wald χ2[df=1]=4.0, p=0.05) than enhanced-usual-care patients at 6 months, and are no significant age group differences in treatment type or intensity.
Conclusion
Collaborative depression care in individuals with co-morbid illness is as effective in reducing depression in older patients as younger patients, including among low-income, minority patients. Patient, provider, and organizational adaptations of depression care management models may contribute to positive outcomes.
Keywords: collaborative multidisciplinary care, depression, comorbid illness, diabetes, cancer, home health
OBJECTIVE
Despite evidence that depression is treatable, disparities in care remain among older adults, particularly within low-income and diverse cultural groups.(1-8) However, few studies have compared collaborative multidisciplinary depression treatment outcomes or treatment participation between older and younger patients with co-morbid physical illness, racial/ethnic diversity and across diverse care systems. A review (9) of 21 studies comparing cohorts of elderly and middle-aged depressed patients found that treatment differences (antidepressants (AM) or ECT) remission rates were not clinically significant between older and younger patients; however, older patients were more likely to have comorbid physical illness which is a risk factor for poor treatment response.(9,10) The review included two studies of combined AM and interpersonal psychotherapy.(11,12)
Collaborative depression care, including psychotherapy and/or AM using a stepped care algorithm, long term maintenance/relapse prevention follow-up, and consideration of patient treatment preferences is effective for older adults in primary care,(3,13-17) patients with co-morbid illness,(18,19) and racial/ethnic minority patients in safety net care systems.(20,21) In this report, we pooled intent-to-treat data of 1,081 patients with co-morbidity from three similarly designed randomized clinical trials of multidisciplinary collaborative care to examine: depression treatment effectiveness, functional outcomes and treatment participation between older (≥ 60) and younger (18-59) patients with major depression and co-morbid illness at 6 and 12 months post-baseline.
Depression care models were adapted for diverse safety net oncology and primary care clinics and home health care populations and care systems. Adaptations were designed to address patient, provider and organizational system needs (e.g., bilingual psychotherapists and patient navigators in the community safety net systems; nurses, social workers or psychologist in home care system providing Problem-Solving Therapy (PST), telephone symptom monitoring/relapse prevention, and collaboration with physicians prescribing AM based on a stepped care algorithm.
METHODS
Study Sites, Sample Recruitment and Depression Screening
Trials were approved by the University of Southern California Health Sciences or University Park Institutional Review Board. The cancer (ADAPt-C) trial (21) (N=472; age 18 and older) and the diabetes (MDDP) trial (22) (N=387; 18 and older) recruited patients from oncology or primary care safety net clinics; the home care (HOPE-D) (23) trial recruited (N=311) patients ≥ 65 within a private Home Health Care agency, HMO or IPA program. Each trial used the Patient Health Questionnaire depression scale (PHQ-9) (24) for screening eligibility and outcome assessment. Criteria for major depressive disorder were based on a score of ≥ 2 for one of the two cardinal depression symptoms plus a PHQ-9 score of ≥10. The Cancer and Diabetes trials excluded patients with acute suicidality, a score of ≥8 on the AUDIT alcohol assessment, recent use of lithium or antipsychotic medication and in the cancer trial having advanced cancer that limited remaining life (25) expectancy to less than 6 months. Home care patients with significant cognitive impairment (Short Portable Mental Status Questionnaire scores of <5) were excluded. Study participants were randomly assigned to intervention (INT) or enhanced usual care (EUC) by selecting a sealed envelope naming a study group generated via computer algorithm. Independent blinded outcome assessments were telephone administered by trained research interviewers at 6 or 8 (for the HOPE-D trial) and 12 months.
Enhanced Usual Care
EUC patients received standard health system care and were given patient/family focused educational pamphlets on depression (in Spanish if preferred); cancer and diabetes trial patients were also given a listing of community, financial, social services, transportation, and child care resources. Within each trial, the treating oncologist or primary care or home health referring physician was informed of patients’ depression status and study participation. Treating physicians were free to prescribe EUC patients AM or to refer patients for mental health treatment, and patients were free to seek care in the community. Clinic oncologists or primary care physicians received two didactic sessions from the study psychiatrist in AM treatment and algorithm application; home health care referring physicians were provided a written description of the study and the algorithm. Outcome data were obtained via self-report.
Collaborative Depression Care Intervention
The collaborative care model in each trial provided a choice of first-line treatment: PST (16), antidepressant medication (AM), or combined treatment when clinically indicated (based on a stepped care algorithm); plus monthly telephone maintenance monitoring and relapse prevention follow-up over 12 months. (PST homework materials were linguistically and culturally adapted for the cancer and diabetes trials.) The Depression Clinical Specialist (DCS) (bilingual social workers in the diabetes and cancer safety net system trials) met weekly or biweekly via telephone or in-person for consultation with the study PI and study psychiatrist. In the HOPE-D trial, the DCS (a mental health nurse, social worker, or psychologist) met bi-monthly with a care system psychiatrist or in one system with a supervising mental health nurse. In the diabetes and HOPE-D trials, the primary care or referring physician prescribed AM; in the cancer trial, the study psychiatrist met with patients and prescribed AM. The initial DCS visit(s) included: a semi-structured psychiatric/psychosocial assessment; patient depression, PST and AM education; consideration of initial treatment choice; provision of respective care system and community resource navigation assistance; and included family members at patient request. Subsequent visits provided PST and/or AM monitoring and after completion of acute treatment, monthly follow-up maintenance telephone calls or home visits plus telephone calls. Didactic training was provided all DCS staff in PST, depression monitoring and AM use. In each trial, the DCS communicated with the primary care or referring physician or study psychiatrist in the oncology trial about patient’s depression status, co-morbid illness medications, medical and psychosocial status, and assessed need to prescribe or adjust AM dosage, consideration of an anti-anxiety agent or sedative-hypnotic. (Table 1) INT data were tracked via written or secure website in all trials.
Table 1.
Collaborative Depression Care Management Intervention
| Elements of the Collaborative Management | Cancer Trial | Diabetes Trial | Home Care Trial |
|---|---|---|---|
| Depression Treatment | PST and/or Antidepressant (AM)
|
||
| Provide PST by Depression Clinical Specialist (DCS) | Social worker, consultation with the study psychiatrist | Social worker, consultation with the study psychiatrist | Trained agency staff mental health nurse, social worker, or psychologist |
| Prescribe AM | Study psychiatrist | Clinic PCP applying study AM algorithm | Home health referring physician |
| Collaboration | The DCS communicates with AM prescriber about patient’s depression status, co-morbid illness medications, medical and psychosocial status, and assessed need to prescribe or adjust AM dosage consideration of an anti-anxiety agent or sedative-hypnotic. | ||
| PST Workplace | Clinic | Clinic | Residence |
| Maintenance Follow-Up | Telephone calls | Telephone calls | Tel/Home visits |
| Patient Navigator | Telephone/In-person | Telephone/In-person | DCS provider via /tel/home visit |
| Support Group | Open-ended optional PST support group | Open-ended optional PST support group | Not applicable |
Each trial provided personalized collaborative care based on the structured algorithm for stepped care in the IMPACT primary care trial (15-17) to ensure patients received care consistent with their preference, clinical presentations and depression treatment responses over time. For example, for a patient who has not had full response to treatment by 4-8 weeks, Step 2 of the algorithm is employed. The patient on AM may require AM change or augmentation, including PST. The patient on PST alone is again educated about the option of AM and based on discussion between the study psychiatrist and the DCS, AM is prescribed by the respective physician. The patient who has not had a full response by weeks 8-12 proceeds to algorithm Step 3. During the routine DCS clinical consultation meeting (or telephone discussion with the home care referring physician), the psychiatrist recommended either another AM or dosage change, or a combination of PST and AM if not tried at Step 2, or referral for additional treatment in a specialty mental health setting, which was facilitated by the DCS. Treatment at this step depended on clinical status, available community resources, and patients’ willingness to accept recommended care. Patients with improved depression (PHQ-9 score less than 10) were provided with ongoing monthly telephone monitoring and relapse-prevention behavioral activation support.
Organizational System and Patient Population Adaptations
Home Health Care
Organizational leaders were engaged in the implementation and conduct of the study, including decisions about study design and intervention adaptations that were deemed to best fit the specific needs of each organization. Collectively, staff of the respective organizations included home health care nurses, psychiatric nurses, social workers, a case manager and a master’s degreed psychologist. In each home care program, usual home care was initiated on receipt of treating physician referral for specified home care treatment. Routine depression screening via the PHQ-9 was mandated in each system to be carried out during the required home care admission visit or pre-admission telephone contact by a nurse.
Safety Net Care
In view of known barriers to participation in clinical trials and to depression treatment retention in low-income minority populations, efforts were made to facilitate recruitment and minimize attrition and acceptance of and adherence to PST or AM (26): 1) attention to cultural competence, eg., Spanish-speaking staff and intervention materials in Spanish adapted for literacy and idiomatic content, attention to family roles; 2) telephone outcome data collection; 3) optional evening/weekend telephone monitoring/relapse prevention and PST visits to coincide with oncology or diabetes appointments; and 4) patient navigation assistance with barriers to cancer, diabetes and depression treatment, including referral to community resources or services. Staff received self-administered training in cultural competency. Study participants were reimbursed for time in completing outcome interviews and in safety net clinics for transportation and co-pays for AM if indicated. PHQ-9 screening was conducted by a designated study recruiter in each clinic.
Data Collection
The PHQ-9 was used as both a screen and outcome measure because it provides a dichotomous diagnosis of major depression as well as a continuous severity score (27, 28), measures a common concept of depression across racial and ethnic groups (29) and is believed to be practically feasible and sustainable in real world oncology, primary care and home health systems of care. In recent years, the PHQ-9 has emerged as a reliable depression screening tool with a demonstrated ability to identify clinically important depression, to make accurate diagnoses of major depression, to monitor severity of depression over time, and to monitor significant improvement in response to therapy.(30,31) The Short-Form Health Survey(32) (SF-12 or SF-20 in HOPE-D)was used to assess physical, role, social and emotional functioning, general health, and pain (score ranged 0 to 100, high score indicated better functioning or greater pain). PHQ-9 and quality-of-life assessments were conducted at baseline, 6 or 8 and 12 months. Patient self-reported hospitalization, ICU admission and ER use.
Analyses
Demographic and baseline clinical characteristics are examined between age groups - age≥60 versus 18-59. No further stratification on age is attempted to avoid the issue of low cell frequency that potentially yields unreliable analytical results. To evaluate intervention effects, intent-to-treat analyses are conducted at each follow-up, analyzed separately by age group. Logistic regression models for dichotomous variables and linear regression models for continuous variables are fitted with adjustment of baseline scores and respective trial. Outcomes compared between INT and EUC include treatment response (a 50% reduction of PHQ-9 score from baseline that is considered a clinically meaningful improvement in depressive symptoms), major depression (PHQ-9 ≥10), composite scores of quality-of-life subscales, and health care utilization. All outcomes are also compared between age groups, controlling for intervention status as a covariate in the fitted model. In addition, among INT patients, treatment type and intensity are again compared between age groups. All analyses are conducted using SAS software, version 9.1.
Study retention rates among older patients are 65.5% at 6 months (cancer 53.8%, diabetes 78.6%, and HOPE-D 64.2%); with 57.7% at 12 months (41.8%, 71.8%, and 57.7%), respectively. Among younger patients, retention rates at 6 and 12 months are 74.3% (cancer 71.1%, diabetes 78.2%) and 65.1% (58.8% and 72.9%), respectively. The proportions of mortality and lost-to-follow-up are not significantly different between INT and EUC groups. Comparisons of baseline characteristics between patients who remained in the trial versus those lost find no significant differences in depression severity or functional measures. A relatively higher proportion of female and Hispanic patients remained in the trials (each χ2 test with df=1: female - 6 months, 82.6% vs. 76.7%, χ2=5.1, p=0.03; 12 months, 83.9% vs. 75.9%, χ2=10.65, p=0.001; Hispanic - 6 months, 76.8% vs. 65.9%, χ2=13.72, p=0.0002; 12 months, 77.9% vs. 66.6%, χ2=16.91, p<0.0001).
A sensitivity analysis was conducted comparing study results using all-available raw data and data imputed with the “Hot Deck Imputation” approach implemented in SOLARS software, version 3.2 (33) Intervention effects on depression and functional outcomes analyzed with imputed data are consistent with results analyzed with all-available raw data except role and emotional functioning, and pain at 12 months in older patients; and emotional functioning at 12 months in younger patients. In this paper, we present results with all-available raw data.
RESULTS
Pooled Sample
Patients with major depressive disorder (1,081) were pooled from three randomized controlled trials (ADAPt-C = 448, MDDP = 387 and HOPE-D = 246) (Figure 1). Demographic characteristics, baseline depression, receipt of antidepressants, and co-morbid medical illness are presented in Table 2, stratified by age groups and trials. Patients are predominantly Latino women in the Cancer and Diabetes trials and European White women in HOPE-D. Compared to younger patients, older patients have higher proportions of males (24.8% vs. 15.3%), non-Latino (51.4% vs. 9.2%), unmarried (61.6% vs. 56%), moderate to severe depression (PHQ-9≥15, 52.7% vs. 38.8%), and history of depression (65.2% vs. 57.7%). Older subjects in the pooled sample also have higher proportions of arthritis (39.5% vs. 21.7%), heart disease (27.1% vs. 3.6%), and lower proportions of diabetes (45.8% vs. 53%), and cancer (26.4% vs. 57.6%) than their counterparts. More older than younger patients reported having previously received AM at baseline (18.6% vs. 10.1%, χ2=16.03, df=1, p<0.0001). Ethnic minority patients are less likely to have previously received AM than European Whites (11.1% vs. 23.7%, χ2=23.4, df=1, p<0.0001).
Figure 1.
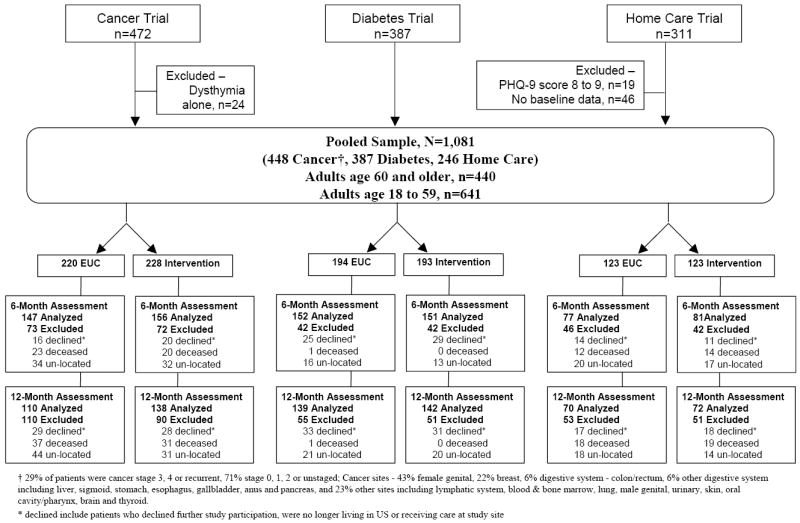
Pooled Data
Table 2.
Pooled Baseline Data*
|
Adults age 60 and older |
Adults age 18-59 |
|||||||
|---|---|---|---|---|---|---|---|---|
| Combined, N=440 | Cancer Trial, N=91 | Diabetes Trial, N=103 | Home Care Trial, N=246 | Combined, N=641 | Cancer Trial, N=357 | Diabetes Trial, N=284 | Age Group Difference | |
| Demographics | ||||||||
| Age, range | 60 - 97 | 60 - 90 | 60 - 79 | 65 - 97 | 18 - 59 | 18 - 59 | 28 - 59 | |
| Age, mean (SD) | 71.6 (8.9) | 65.4 (6.3) | 64.0 (3.9) | 77.1 (7.1) | 46.9 (9.5) | 44.2 (10.3) | 50.3 (7.0) | t=43.27, df=1079, P<.0001 |
| Female | 331 (75.2) | 67 (73.6) | 87 (84.5) | 177 (72.0) | 543 (84.7) | 312 (87.4) | 231 (81.3) | x2=15.16, df=1, P<.0001 |
| Ethnicity/Race | ||||||||
| • Latino | 214 (48.6) | 79 (86.8) | 100 (97.1) | 35 (14.2) | 582 (90.8) | 312 (87.4) | 270 (95.1) | x2=265.88, df=4, P<.0001 |
| • Black | 26 (5.9) | 4 (4.4) | 1 (1.0) | 21 (8.5) | 18 (2.8) | 16 (4.5) | 2 (0.7) | |
| • European White | 187 (42.5) | 5 (5.5) | 2 (1.9) | 180 (73.2) | 28 (4.4) | 18 (5.0) | 10 (3.5) | |
| • Asian | 7 (1.6) | 3 (3.3) | 0 | 4 (1.6) | 12 (1.9) | 11 (3.1) | 1 (0.4) | |
| • other | 6 (1.4) | 0 | 0 | 6 (2.4) | 1 (0.2) | 0 | 1 (0.4) | |
| Marital Status | ||||||||
| • Married | 169 (38.4) | 33 (36.3) | 44 (42.7) | 92 (37.4) | 282 (44.0) | 135 (37.8) | 147 (51.8) | x2=210.81, df=3, P<.0001 |
| • Divorced/Separated | 66 (15.0) | 18 (19.8) | 21 (20.4) | 27 (11.0) | 168 (26.2) | 94 (26.3) | 74 (26.1) | |
| • Widowed | 171 (38.9) | 23 (25.3) | 31 (30.1) | 117 (47.6) | 35 (5.5) | 12 (3.4) | 23 (8.1) | |
| • Single | 34 (7.7) | 17 (18.7) | 7 (6.8) | 10 (4.1) | 156 (24.3) | 116 (32.5) | 40 (14.1) | |
| Depression Status | ||||||||
| Depression Severity | ||||||||
| • Mild Major 10-14 | 208 (47.3) | 56 (61.5) | 52 (50.5) | 100 (40.7) | 392 (61.2) | 253 (70.9) | 139 (48.9) | x2=23.18, df=2, P<.0001 |
| • Moderate 15-19 | 180 (40.9) | 31 (34.1) | 49 (47.6) | 100 (40.7) | 208 (32.4) | 86 (24.1) | 122 (43.0) | |
| • Severe 20-27 | 52 (11.8) | 4 (4.4) | 2 (1.9) | 46 (18.7) | 41 (6.4) | 18 (5.0) | 23 (8.1) | |
| History of Depression | 287 (65.2) | 46 (50.5) | 62 (60.2) | 179 (72.8) | 370 (57.7) | 198 (55.5) | 172 (60.6) | x2=6.16, df=1, P=0.02 |
| Receipt of Antidepressants | 82 (18.6) | 5 (5.5) | 19 (18.4) | 58 (23.6) | 65 (10.1) | 24 (6.7) | 41 (14.4) | x2=16.03, df=1, P<.0001 |
| Co-morbid Medical Illness | ||||||||
| Diabetes | 196 (45.8) | 30 (33.0) | 103 (100.0) | 63 (26.9) | 340 (53.0) | 56 (15.7) | 284 (100.0) | All with df=1, x2=5.39, P=0.03 |
| Hypertension | 195 (45.6) | 41 (45.1) | 81 (78.6) | 73 (31.2) | 270 (42.1) | 80 (22.4) | 190 (66.9) | x2=1.24, P=0.27 |
| Arthritis | 169 (39.5) | 30 (33.0) | 57 (55.3) | 82 (35.0) | 139 (21.7) | 56 (15.7) | 83 (29.2) | x2=39.65, P<.0001 |
| Heart Disease | 116 (27.1) | 15 (16.5) | 10 (9.7) | 91 (38.9) | 23 (3.6) | 10 (2.8) | 13 (4.6) | x2=125.45, P<.0001 |
| Cancer | 113 (26.4) | 91 (100.0) | 4 (3.9) | 18 (7.7) | 369 (57.6) | 357 (100.0) | 12 (4.2) | x2=100.67, P<.0001 |
| Kidney Disease | 39 (9.1) | 13 (14.3) | 17 (16.5) | 9 (3.8) | 50 (7.8) | 28 (7.8) | 22 (7.7) | x2=0.58, P=0.45 |
Data are presented as No. (%) unless otherwise indicated. SE indicates standard error.
Depression Outcomes
Intervention effects on a 50% reduction of PHQ-9 scores, and persistent major depression (PHQ-9 score ≥10) at 6 and 12 months are presented in Table 3. There are no significant interactions between study arms or clinical trials with age groups on depression outcomes. Taking 50% PHQ-9 reduction from baseline to 6 months as an example, there is no significant interaction between study arm and age groups (Likelihood ratio test χ2=0.05, df=1, p=0.82), or between clinical trials and age groups (Likelihood ratio test χ2=0.30, df=1, p=0.58). Similar INT effects in older and younger age groups on 50% PHQ-9 reduction are observed over time. INT patients in both age groups had significantly greater improvement in 6-month 50% PHQ-9 reduction and a greater reduction in major depression rates. Combining INT and EUC groups, there are no significant age group differences in 50% PHQ-9 reduction or major depression.
Table 3.
Depression Outcomes
| Adjusted Analysis | |||||
|---|---|---|---|---|---|
| n (%) | OR (95% CI) | Wald χ2[df=1] | P | ||
| Intervention |
EUC |
Intervention versus EUC* |
|||
| Adults age 60 and older | |||||
| 50% PHQ-9 reduction | |||||
| • 6-month follow-up | 74 (52.5) | 59 (40.1) | 1.70 (1.06 - 2.74) | 4.82 | 0.03 |
| • 12-month follow-up | 70 (56.0) | 58 (45.0) | 1.65 (0.99 - 2.73) | 3.72 | 0.06 |
| Major depression PHQ-9 ≥10 | |||||
| • 6-month follow-up | 50 (35.5) | 73 (49.7) | 0.49 (0.30 - 0.81) | 7.72 | 0.01 |
| • 12-month follow-up | 41 (32.8) | 53 (41.1) | 0.61 (0.35 - 1.05) | 3.13 | 0.08 |
| Adults age 18-59 | |||||
| 50% PHQ-9 reduction | |||||
| • 6-month follow-up | 137 (55.5) | 100 (43.7) | 1.61 (1.12 - 2.32) | 6.47 | 0.02 |
| • 12-month follow-up | 136 (59.9) | 90 (47.4) | 1.65 (1.12 - 2.45) | 6.28 | 0.02 |
| Major depression PHQ-9 ≥10 | |||||
| • 6-month follow-up | 78 (31.6) | 87 (38.0) | 0.67 (0.45 - 0.99) | 4.00 | 0.05 |
| • 12-month follow-up | 65 (28.6) | 67 (35.3) | 0.66 (0.43 - 1.01) | 3.59 | 0.06 |
| Age 60+ | Age 18-59 | Old versus Young** | |||
| 50% PHQ-9 reduction | |||||
| • 6-month follow-up | 133 (46.2) | 237 (49.8) | 1.12 (0.75 - 1.67) | 0.32 | 0.58 |
| • 12-month follow-up | 128 (50.4) | 226 (54.2) | 1.34 (0.87 - 2.06) | 1.73 | 0.19 |
| Major depression PHQ-9 ≥10 | |||||
| • 6-month follow-up | 123 (42.7) | 165 (34.7) | 0.82 (0.54 - 1.27) | 0.78 | 0.38 |
| • 12-month follow-up | 94 (37.0) | 132 (31.7) | 0.72 (0.44 - 1.16) | 1.84 | 0.18 |
Logistic regression models are adjusted for baseline PHQ-9 score and type of trial.
Logistic regression models are adjusted for baseline PHQ-9 score, type of trial, and intervention status.
EUC indicates enhanced usual care; OR, odds ratio; CI, confidence interval. Number of participants in adults age 60 and older: 6-month n=288, 12-month n=254. Number of participants in adults age 18-59: 6-month n=476, 12-month n=417.
Functional Outcomes
There are no significant interactions between study arms or clinical trials with age groups on functional outcomes. Taking physical functioning at 6-month as an example, there is no significant interaction between study arms and age groups (F[1,757]=1.32, p=0.25) or between clinical trial and age groups (F[1,757]=1.50, p=0.22). At both follow-up times, INT patients have relatively better functional outcomes in both age groups, except physical functioning at both follow-ups in older adults and physical functioning at 6 months in younger patients. Significant (p values <0.05) intervention effects on social and emotional functioning at 6 months are consistent in older (mean±SD, social functioning: 54.29±38.51 in intervention vs. 28.98±37.69 in EUC; emotional: INT 62.20±24.20, EUC 55.71±23.69) and younger patients (social functioning: INT 71.56±31.37, EUC 65.94±33.32; emotional functioning: INT 59.72±24.23, EUC 55.13±24.36). In addition, older INT patients have significantly better role functioning at 6 and 12 months. Among younger adults, INT patients have significantly less pain at 6 and 12 months compared to EUC patients. Across INT and EUC groups, there is no significant difference at either 6- or 12-month follow-up in each functional outcome between younger and older adults (Table 4).
Table 4.
Comparisons of Quality of Life* Between Age groups
| Unadjusted Mean (SD) | Adjusted Analysis for Old versus Young | |||||
|---|---|---|---|---|---|---|
| Age 60+ |
Age 18-59 |
Mean Difference (95% CI) |
t |
df |
P |
|
| Physical Functioning | ||||||
| Baseline | 46.64 (44.51) | 81.83 (33.56) | 6.84 (2.00 - 11.68) | 2.77 | 1079 | 0.01 |
| 6-month follow-up | 41.38 (41.55) | 65.02 (43.04) | 2.34 (-5.21 - 9.89) | 0.61 | 762 | 0.55 |
| 12-month follow-up | 43.83 (41.56) | 59.83 (44.52) | 8.01 (-0.46 - 16.48) | 1.86 | 669 | 0.07 |
| Role Functioning | ||||||
| Baseline | 10.63 (26.83) | 15.52 (29.73) | -2.03 (-6.63 - 2.57) | -0.86 | 1079 | 0.39 |
| 6-month follow-up | 27.26 (36.91) | 45.12 (39.68) | -0.07 (-7.08 - 6.95) | -0.02 | 762 | 0.99 |
| 12-month follow-up | 28.75 (39.03) | 51.20 (40.34) | -3.77 (-11.74 - 4.20) | -0.93 | 668 | 0.36 |
| Bodily Pain | ||||||
| Baseline | 59.20 (36.94) | 42.00 (31.89) | 1.28 (-4.03 - 6.58) | 0.47 | 1079 | 0.64 |
| 6-month follow-up | 49.22 (36.41) | 36.71 (31.96) | -0.18 (-6.22 - 5.87) | -0.06 | 762 | 0.96 |
| 12-month follow-up | 53.35 (37.11) | 36.39 (32.79) | 2.28 (-4.44 - 9.00) | 0.67 | 669 | 0.51 |
| General Health | ||||||
| Baseline | 20.11 (19.16) | 23.05 (18.49) | -0.40 (-3.37 - 2.58) | -0.26 | 1079 | 0.80 |
| 6-month follow-up | 28.35 (23.79) | 31.57 (20.72) | -1.09 (-5.13 - 2.95) | -0.53 | 762 | 0.60 |
| 12-month follow-up | 30.39 (25.93) | 34.83 (21.49) | -2.59 (-7.29 - 2.10) | -1.08 | 669 | 0.28 |
| Social Functioning | ||||||
| Baseline | 38.69 (34.23) | 46.52 (32.54) | 1.73 (-3.44 - 6.90) | 0.66 | 1078 | 0.52 |
| 6-month follow-up | 51.57 (38.12) | 68.86 (32.41) | -3.91 (-10.25 - 2.43) | -1.21 | 761 | 0.23 |
| 12-month follow-up | 57.02 (38.09) | 69.18 (32.49) | 1.72 (-5.21 - 8.65) | 0.49 | 668 | 0.63 |
| Mental Health | ||||||
| Baseline | 43.29 (23.20) | 35.66 (20.01) | 0.54 (-2.85 - 3.93) | 0.31 | 1079 | 0.76 |
| 6-month follow-up | 58.89 (24.12) | 57.52 (24.38) | -0.19 (-4.81 - 4.43) | -0.08 | 761 | 0.94 |
| 12-month follow-up | 61.21 (24.83) | 59.16 (25.00) | 2.52 (-2.58 - 7.61) | 0.97 | 668 | 0.34 |
Short-Form Health Survey (SF) was used to assess quality of life scales (range 0 to 100, high score indicated better functioning and more pain). Home Care trial used SF 20 items survey (SF-20) and others used SF 12 items survey (SF-12). ANCOVA models were adjusted for baseline scores, type of trials and intervention status. EUC indicates enhanced usual care; SD, standard deviation; CI, confidence interval. Number of participants varied across waves and outcomes (old adults - baseline n=440, 6-month n=287-288, 12-month n=253-254; young adults - baseline n=640-641, 6-month n=475-476, 12-month n=417).
Health Care Utilization
There are no significant differences in patient reported hospitalization, ICU admission or, ER utilization in the past 6 months between intervention and EUC in either older or younger patients at either follow-up. Combining INT and EUC patients, the rates of hospitalization, ICU admission and ER use among older patients are 35%, 10%, 37% at 6 months; and 20%, 9% and 28% at 12 months, respectively; and in younger patients are 18%, 3% and 22% at 6 months and 15%, 3% and 22% at 12 months, respectively. Older patients have a higher adjusted rate of ICU admission than younger patients at 12-month follow-up (Wald χ2=4.58, df=1, p=0.04), however, otherwise there are no other significant age group differences in health care utilization at follow-up.
Treatment Receipt and Adherence
There is no significant interaction of age group with intervention status on receipt of depression treatment (Homogeneity χ2=1.73, df=1, p=0.19), indicating similar odds of receipt of depression treatment within older and younger age groups. Across age groups, INT patients are significantly more likely to use AM or psychotherapy than EUC patients (80.7% INT vs. 25.3% EUC; adjusted for trials, Wald χ2=286.12, df=1, p<0.0001). Of 94 older and 120 younger patients with persistent major depression (PHQ-9≥10) at 6 months, 39.4% older and 45% younger had PHQ-9 scores less than 10 at 12 months.
Intervention treatment participation (Table 5), 84% of older and 78% of younger patients were treated with PST and/or AM. Patient treatment refusal accounted for over 40% of untreated in both age groups; other reasons included, in older and younger groups respectively, death (15.2%, 16.7%), severe medical condition (6.1%, 1.4%), left care system (15.2%, 11.1%) and unable to locate (15.2%, 29.2%). The majority (85% older, 95% younger) of treated patients received PST (alone or in combination with AM); in both age groups, about 80% having at least 4 PST sessions. There were no significant differences in treatment type (Wald χ2=0.79, df=1, p=0.38), length of time on AM (t=-0.58, df=398, p=0.56), and number of PST sessions (t=-1.44, df=267, p=0.16) between older and younger groups with adjustment by trial. Treatment type or intensity did not vary in either age group between European White versus ethnic minorities.
Table 5.
Depression Treatment over 12 Months among Intervention Patients
| Unadjusted Mean (SD) or No. (%) | Adjusted Analysis for Old versus Young* | |||
|---|---|---|---|---|
| ALL |
Age 60+ |
Age 18-59 |
Statistic |
|
| Depression Treatment | Wald χ2=0.79, df=1, P=0.38 | |||
| • PST and AM | 230 (42.3%) | 97 (45.8%) | 133 (40.1%) | |
| • Only PST | 170 (31.3%) | 56 (26.4%) | 114 (34.3%) | |
| • Only AM | 39 (7.2%) | 26 (12.3%) | 13 (3.9%) | |
| • none | 105 (19.3%) | 33 (15.6%) | 72 (21.7%) | |
| Number of months on AM, Mean (SD) | 7.14 (4.33) | 6.28 (4.5) | 7.86 (4.06) | t=-1.44, df=267, P=0.16 |
| Of PST patients, PST sessions | Wald χ2=0.07, df=1, P=0.80 | |||
| • 1-3 sessions | 82 (20.5%) | 32 (20.9%) | 50 (20.2%) | |
| • 4 or more sessions | 318 (79.5%) | 121 (79.1%) | 197 (79.8%) | |
| Number of PST sessions, Mean (SD) | 8.26 (6.19) | 6.95 (4.59) | 9.07 (6.89) | t=-0.58, df=398, P=0.56 |
Regression model or logistic regression model adjusted for type of trial. PST indicates problem solving therapy; AM, antidepressant medication; SD, standard deviation.
CONCLUSION
In this pooled analysis, patient and care system specific collaborative multidisciplinary depression care is as effective in reducing depression in older as in younger adults with medical co-morbidity. Key study results include that this finding applied to patients with cancer, diabetes and other co-morbid illness as well as to low-income, minority patients being cared for in safety net care systems and for a significant number of patients, depression improvement continued for up to 12 months. Treatment participation was also similar between age groups, with an apparent preference for PST alone or in combination with antidepressants in both age and racial/ethnic sub-groups. Taken together, study results support the use of multidisciplinary collaborative care models that include treatment options, the application of a stepped care algorithm, maintenance and relapse-prevention telephone follow-up, and organizational and cultural adaptations in treating depression among patients with depression and co-morbid physical illnesses across the age spectrum.(7)
Patient, provider and organizational adaptations of multidisciplinary collaborative depression care in the study trials are likely to have contributed to depression symptom improvement as well as to depression treatment participation. Integrating mental health care within general health care that also improves communication across provider groups is increasingly recognized as an important pathway to improved patient care.(34) Perceived stigma may lead to reluctance to seek care in the mental health system, becoming a deterrent to receipt of care among older and minority populations.(35-37) Treatment preferences also affect treatment acceptance and adherence and older adults and minorities may prefer psycho-educational therapy formats.(16-17, 38) Effective organizational strategies (39-40) generally include multifaceted quality improvement disease management, such as the implementation of routine depression screening, systematic application of evidence-based practice guidelines, clinical decision-making protocols and algorithms, follow-up through remission and maintenance, enhanced roles of nurses or social workers as depression care managers as well as integration of mental health specialists.
This pooled analysis highlights the effectiveness of diverse multidisciplinary collaborative care models that are responsive to diverse organizational provider resources, practices and preferences. Across the pooled trials, the DCS role and discipline varied as did the discipline and role of the psychiatric consult. In both the cancer and diabetes trials, the DCS staff were bilingual and skilled in providing patient navigation assistance (eg., patient support and assistance with managing communication across different care providers and care systems and access to community resources). In contrast, home health care nurses provided these services with limited assistance from home health care social workers. Again, underscoring responsiveness to provider and care system preferences and needs, in the cancer trial oncologists preferred that the consulting psychiatrist prescribe antidepressant medication, whereas in the safety net clinics, primary care physicians were comfortable prescribing these medications and rarely consulted the psychiatrist via pager.
Study limitations include the high attrition rates in all three trials, reflecting a physically ill older population, relatively high cancer deaths, and loss to follow-up among low-income Hispanic patients. Screening was done at admission to home care, however, repeat screening at different home health visits might improve the validity of the screening process as nearly a third of a subset of patients screening positive at admission experienced significant improvement at a subsequent screen. (Screening cancer patients was done ≥90 days following diagnosis to rule out adjustment disorder.) Finally, a significant number of patients refused to participate in the RCT, primarily explained by the illness and disability of the HOPE-D population precipitating caregivers to pressed concern that participation might overtax the patient or cultural concerns about depression and stigma.
Collaborative multidisciplinary depression care among older adults with co-morbid illness can be effective across care systems and diverse patients and organizations. Two study sites represented in this pooled analysis are continuing the care model post-trial, thus supporting the potential utility of translating and (patient and organizationally) adapting collaborative depression care for older adults to enhance sustainable depression care for patients with comorbid illness.
Acknowledgments
The studies from which data were pooled were supported by grants from the National Institute of Mental Health (MDDP R01 MH068468; HOPE-D R 24 MH61700) and the National Cancer Institute (ADAPt-C R01CA105269) Principal Investigator, K Ell.
Footnotes
No Disclosures to Report
Trial Registration: ADAPT-C: Clinical Trials.gov, NCT00565110, MDDP: NCT00709150 clinicaltrials.gov/ct/gui
References
- 1.Alegría M, Chatterji P, Wells K, et al. Disparity in depression treatment among racial and ethnic minority populations in the United States. Psychiatr Serv. 2008;59:1264–1272. doi: 10.1176/appi.ps.59.11.1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Areán P, Alvidrez J, Barrera A, et al. Would older medical patients use psychological services? Gerontologist. 2002;42:392–398. doi: 10.1093/geront/42.3.392. [DOI] [PubMed] [Google Scholar]
- 3.Areán P, Gum AM, Tang L, et al. Service use and outcomes among elderly persons with low incomes being treated for depression. Psychiatr Serv. 2007;58:1057–1064. doi: 10.1176/ps.2007.58.8.1057. [DOI] [PubMed] [Google Scholar]
- 4.Givens J, Datto CJ, Ruckdeschel K, et al. Older patients’ aversion to antidepressants. A qualitative study. J Gen Intern Med. 2006;21:146–151. doi: 10.1111/j.1525-1497.2005.00296.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gum A, Areán PA, Hunkeler E, et al. Depression treatment preferences in older primary care patients. Gerontologist. 2008;46:14–22. doi: 10.1093/geront/46.1.14. [DOI] [PubMed] [Google Scholar]
- 6.Ayalon L, Areán PA, Linkins K, et al. Integration of mental health services into primary care overcomes ethnic disparities in access to mental health services between black and white elderly. Am J Geriatric Psychiatry. 2007;15:906–912. doi: 10.1097/JGP.0b013e318135113e. [DOI] [PubMed] [Google Scholar]
- 7.Kozel F, Andrew MD, Trivedi MH, et al. Treatment outcomes for older depressed patients with earlier versus late onset of first depressive episode: a sequenced treatment alternatives to relieve depression (STAR*D) report. Am J Geriatric Psychiatry. 2008;16:58–64. doi: 10.1097/JGP.0b013e31815a43d7. [DOI] [PubMed] [Google Scholar]
- 8.Cui X, Lyness J, Tang W, et al. Outcomes and predictors of late-life depression trajectories in older primary care patients. Am J Geriatric Psychiatry. 2008;16:406–415. doi: 10.1097/JGP.0b013e3181693264. [DOI] [PubMed] [Google Scholar]
- 9.Mitchell A, Subramaniam H. Prognosis of depression in old age compared to middle age: A systematic review of comparative studies. Am J Psychiatry. 2005;162:1588–1601. doi: 10.1176/appi.ajp.162.9.1588. [DOI] [PubMed] [Google Scholar]
- 10.Cohen A, Houck PR, Szanto K, et al. Reynolds CF. Collaborative care for depression: A cumulative meta-analysis and review of longer-term outcomes. Arch Intern Med. 2006;166:2314–2321. doi: 10.1001/archinte.166.21.2314. [DOI] [PubMed] [Google Scholar]
- 11.Reynolds CI, Dew MA, Frank E, et al. Effects of age at onset of first lifetime episode of recurrent major depression on treatment response and illness course in elderly patients. Amer J Psychiatry. 1999;155:795–799. doi: 10.1176/ajp.155.6.795. [DOI] [PubMed] [Google Scholar]
- 12.Reynolds CF, III, Frank E, Perel JM, et al. Nortriptyline and interpersonal psychotherapy as maintenance therapies for recurrent major depression: A randomized controlled trial in patients older than 59 years. JAMA. 1999;281:39–45. doi: 10.1001/jama.281.1.39. [DOI] [PubMed] [Google Scholar]
- 13.Bruce ML, Ten Have TR, Reynolds CF, III, et al. Reducing suicidal ideation and depressive symptoms in depressed older primary care patients: a randomized controlled trial. JAMA. 2004;291:1081–1091. doi: 10.1001/jama.291.9.1081. [DOI] [PubMed] [Google Scholar]
- 14.Areán P, Unützer J. Inequities in depression management in low-income, minority, and old-old adults: A matter of access to preferred treatments? J Amer Geriatr Soc. 2003;51:1808–1809. doi: 10.1046/j.1532-5415.2003.51569.x. [DOI] [PubMed] [Google Scholar]
- 15.Unützer J, Katon W, Callahan CM, et al. Collaborative care management of late-life depression in the primary care setting: A randomized controlled trial. JAMA. 2002;288:2836–2845. doi: 10.1001/jama.288.22.2836. [DOI] [PubMed] [Google Scholar]
- 16.Areán P, Hegel M, Vannoy S, et al. Effectiveness of problem-solving therapy for older, primary care patients with depression: Results from the IMPACT project. Gerontologist. 2008;49:311–323. doi: 10.1093/geront/48.3.311. [DOI] [PubMed] [Google Scholar]
- 17.Gellis ZC, McGinty J, Horowitz A, et al. Problem-Solving Therapy for late-life depression in home care: a randomized field trial. Am J Geriatric Psych. 2007;15:968–978. doi: 10.1097/JGP.0b013e3180cc2bd7. [DOI] [PubMed] [Google Scholar]
- 18.Katon W, Unützer J, Fan JW, et al. Cost-effectiveness and net benefit of enhanced treatment of depression for older adults with diabetes and depression. Diabetes Care. 2006;29:265–270. doi: 10.2337/diacare.29.02.06.dc05-1572. [DOI] [PubMed] [Google Scholar]
- 19.Katon W, Von Korff M, Lin EH, et al. The Pathways Study: A randomized trial of collaborative care in patients with diabetes and depression. Arch Gen Psychiatr. 2004;61:1042–1049. doi: 10.1001/archpsyc.61.10.1042. [DOI] [PubMed] [Google Scholar]
- 20.Dwight-Johnson M, Ell K, Lee PJ. Can collaborative care address the needs of low- income Latinas with comorbid depression and cancer? Psychosomatics. 2005;46:224–232. doi: 10.1176/appi.psy.46.3.224. [DOI] [PubMed] [Google Scholar]
- 21.Ell K, Xie B, Quon B, et al. Randomized controlled trial of collaborative care management of depression among low-income patients with cancer. J Clin Oncol. 2008;26:1–9. doi: 10.1200/JCO.2008.16.6371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ell K, Katon W, Cabassa L, et al. Depression and diabetes among low-income Hispanics: Design elements of a socio-culturally adapted collaborative care model randomized controlled trial. Int J Psych Med. 2009;39:113–132. doi: 10.2190/PM.39.2.a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ell K, Unützer J, Lee PJ, et al. Managing depression in home health care: A randomized clinical trial. Home Health Care Serv Q. 2007;26:81–104. doi: 10.1300/J027v26n03_05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spitzer R, Kroenke K, Williams JB. Validation and utility of a self-report version of PRIME-MD: The PHQ Primary Care Study. Primary care evaluation of mental disorders. JAMA. 1999;282:1737–1744. doi: 10.1001/jama.282.18.1737. [DOI] [PubMed] [Google Scholar]
- 25.Vinson D, Galliher JM, Reidinger C, et al. Comfortably engaging: Which approach to alcohol screening should we use? Ann Fam Med. 2004;2:398–404. doi: 10.1370/afm.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ell K, Quon B, Quinn D, et al. Improving treatment of depression among low-income patients with cancer: The design of the ADAPt-C study. Gen Hosp Psych. 2007;29:223–231. doi: 10.1016/j.genhosppsych.2007.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: Validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Löwe B, Gräfe K, Zipfel S, et al. Diagnosing ICD-10 depressive episodes: Superior criterion validity of the Patient Health Questionnaire. Psychother & Psychosomatics. 2005;73:386–390. doi: 10.1159/000080393. [DOI] [PubMed] [Google Scholar]
- 29.Huang F, Chung H, Kroenke K, et al. Using the Patient Health Questionnaire-9 to measure depression among racially and ethnically diverse primary care patients. J Gen Intern Med. 2006;21:547–552. doi: 10.1111/j.1525-1497.2006.00409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cameron I, Crawford JR, Lawton K, et al. Psychometric comparison of PHQ-9 and HADS for measuring depression severity in primary care. Br J Gen Prac. 2008;58:32–36. doi: 10.3399/bjgp08X263794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wittkampf KA, Naeije L, Schene AH, et al. Diagnostic accuracy of the mood module of the patient health questionnaire: A systematic review. Gen Hosp Psych. 2007;29:388–395. doi: 10.1016/j.genhosppsych.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 32.Ware J, Sherbourne CD. The MOS-36 Item Short-Form Health Survey (SF-36) Med Care. 1992;30:473–481. [PubMed] [Google Scholar]
- 33.Little RJA, Rubin DB. A Statistical analysis with missing data. 2. New York: Wiley - Statistical Solution Ltd, Saugus, MA; 2002. http://www.statsol.ie/html/solas/solasresources.html. [Google Scholar]
- 34.Kilbourne AI, Capobianco C, Reynolds J, et al. Improving integrated general medical and mental health services in community-based practices. Admin Policy Men Health. 2008;35:337–345. doi: 10.1007/s10488-008-0177-8. [DOI] [PubMed] [Google Scholar]
- 35.Sirey J, Bruce ML, Alexopoulos GS, et al. Perceived stigma and patient-rated severity of illness as predictors of antidepressant drug adherence. Psychiatr Services. 2001;52:1615–1620. doi: 10.1176/appi.ps.52.12.1615. [DOI] [PubMed] [Google Scholar]
- 36.Bartels S, Coakley EH, Zubritsky C, et al. Improving access to geriatric mental health services: A randomized trial comparing treatment engagement with integrated versus enhanced referral care for depression, anxiety, and at-risk alcohol use. Amer J Psychiatry. 2004;161:1455–1462. doi: 10.1176/appi.ajp.161.8.1455. [DOI] [PubMed] [Google Scholar]
- 37.Dwight-Johnson M, Sherbourne CD, Liao D, et al. Treatment preferences among depressed primary care patients. J Gen Intern Med. 2000;15:527–534. doi: 10.1046/j.1525-1497.2000.08035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cabassa L, Hansen MC, Palinkas LA, et al. Azúcar y nervios: Explanatory models and treatment experiences of Hispanics with diabetes and depression. Soc Sci Med. 2008;66:2413–2424. doi: 10.1016/j.socscimed.2008.01.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reuben D. Organizational interventions to improve health outcomes of older persons. Med Care. 2002;40:416–428. doi: 10.1097/00005650-200205000-00007. [DOI] [PubMed] [Google Scholar]
- 40.Henke R, McGuire TG, Zaslavsky AM, et al. Clinician- and organization-level factors in the adoption of evidence-based care for depression in primary care. Health Care Manage Rev. 2008;33:289–299. doi: 10.1097/01.HCM.0000318766.29277.49. [DOI] [PubMed] [Google Scholar]


