Abstract
Levels of the stress-sensitive hormone cortisol increase dramatically in the first 30-40 minutes after waking, an effect known as the cortisol awakening response (CAR). There is considerable cross-sectional evidence that psychosocial stress is associated with an increased CAR, and the CAR has been found to be altered in the presence of stress-related diseases, including Major Depressive Disorder (MDD). To date, no prospective longitudinal studies have examined whether individual differences in the CAR serve as a premorbid risk factor for MDD. In a sample of 230 late adolescents, clinical diagnoses of MDD were predicted from the CAR as well as other indicators of basal cortisol functioning gathered one year earlier, including: waking cortisol levels, bedtime cortisol levels, the size of the CAR, average cortisol, and the slope of the diurnal cortisol rhythm across the waking day. Age and gender, health and health behaviors, baseline neuroticism, exposure to stressful life events and past episodes of mood and anxiety disorders were included as covariates, to help ensure effects are attributable to the CAR rather than related variables. A higher baseline CAR was associated with a significantly increased risk of developing MDD by follow-up, even when excluding individuals with baseline MDD. No other baseline cortisol measures were significant prospective predictors of MDD. In summary, the CAR is a significant prospective risk factor for the development of MDD in young adults, providing some support for the possibility that a heightened CAR may play a role in the etiology of Major Depressive Disorder.
Keywords: Hypothalamic pituitary adrenal axis, cortisol awakening response, major depressive disorder, life events, prospective, diurnal cortisol rhythms
Introduction
Dysregulation of the hypothalamic-pituitary-adrenal axis (HPA), one of the body's major stress-sensitive physiological systems, is frequently implicated in the etiology of Major Depressive Disorder (MDD) (Ehlert et al., 2001; Gold and Chrousos, 2002; Holsboer, 2000). Robust cross-sectional associations have been found between the presence of MDD and a variety of alterations of the HPA-axis, including elevated cortisol levels, elevated corticotropin releasing hormone (CRH), and impaired negative feedback control of the HPA (Chrousos and Gold, 1992; Ehlert et al., 2001; Thase et al., 2002). It is not yet clearly established in human populations whether such alterations are correlates or consequences of the experience of depressive disorder and/or its treatments, or whether they reflect risk or vulnerability factors that are present prior to the onset of disorder, thus potentially playing a role in the pathophysiology of MDD (Bhagwagar and Cowen, 2007). In support of the latter hypothesis, HPA-axis alterations have been found among young people without a history of MDD but who are at risk for MDD due to having a biological parent with a history of disorder (Mannie et al., 2007). The gold standard approach to identifying a risk factor, however, involves measuring HPA-axis characteristics prior to the onset of MDD, and then following the same individuals longitudinally to identify whether, and which types of HPA-axis alterations are associated with higher rates of the development of MDD over the intervening time period.
The few existing studies taking this prospective longitudinal approach have converged upon similar findings. Although elevated cortisol levels across the full day and especially during the evening hours, are the most frequent cross-sectional correlates of MDD (Angold, 2003; Dahl et al., 1991), it is elevated morning cortisol that is prospectively predictive of depression (Goodyer et al., 2000; Halligan et al., 2007; Harris et al., 2000).
Cortisol diurnal rhythms vary according to individual sleep-wake schedules, being high upon waking, increasing to peak levels approximately 30-40 minutes after waking, and declining to a nadir around bedtime (Kirschbaum and Helhammer, 2000; Pruessner et al., 1997; Wilhelm et al., 2007). The increase after waking, or cortisol awakening response (CAR), has become the target of considerable interest over the past 10 years for a number of reasons. First, CAR increases are large (on average 50-60% above waking levels) and highly variable across individuals; as a result, individual differences in the size of this indicator can be easily detected and modeled statistically, and are of a magnitude that is likely to be physiologically meaningful (Adam et al., 2006; Clow et al., 2004). The substantial CAR increases occur on top of already elevated wakeup cortisol levels, and hence have the potential to have strong impacts on the relatively low affinity central glucorcorticoid receptor systems that have been implicated in the development of MDD (Holsboer, 2000; van Rossum et al., 2006). Second, there is evidence of both genetic and psychosocial contributions to the size of the CAR (Schulz et al., 1998; Wüst et al., 2000), making it a candidate mechanism for both genetic and environmental pathways to the development of depression. In particular, a larger CAR has been associated with higher levels of both acute and chronic life stress (Adam et al., 2006; Schlotz et al., 2004). It has been theorized that CAR increases are designed, in the short term, to help people meet the anticipated demands of their upcoming day, but that too large, too small, or frequently or chronically elevated CARs may be detrimental to health and functioning over the longer term (Adam et al., 2006).
Because all of the past prospective studies examined morning cortisol levels at fixed clock times (8 AM), rather than in relation to person-specific waketimes (Halligan et al., 2007), they were not in a position to identify whether the size of the cortisol awakening response serves as a risk factor for MDD. As a result, there are no existing prospective longitudinal studies examining whether an elevated CAR is associated with increased risk for future MDD. Studies examining cross-sectional associations between the CAR and depression have found mixed results: some have found significantly lower CARs in individuals with current MDD diagnoses (Huber et al., 2006; Stetler and Miller, 2005), whereas others have found current MDD or high depressive symptoms to be associated with higher CARs (Bhagwagar et al., 2005; Pruessner et al., 2003). As noted earlier, however, how the HPA axis looks in the presence of current MDD disorder, and the premorbid individual differences in the HPA axis that are predictive of the later onset of MDD may not be the same, especially if changes in the HPA axis occur with the development of, or in response to the experience of MDD. Indeed, theorists of the role of the HPA axis in the development of depression argue that depressive symptoms emerge by way of HPA axis changes (Ehlert et al., 2001) in response to extreme or chronic stress exposure. Thus, to identify HPA axis risk factors for MDD, it is important to assess which aspects of premorbid (pre-change) HPA axis function are associated with the later emergence of MDD.
In the current study, using prospective data from a large sample of older adolescents (aged 16 to 18 years), we examined whether baseline differences in CARs were predictive of new onsets of MDD over the subsequent year. The possible effects of baseline and past episodes of MDD were accounted for in our analyses, and a wide variety of health and lifestyle covariates known to influence cortisol and MDD were examined. We also compared the predictive value of the CAR with several other indicators of basal HPA axis functioning, examined whether the predictive value of the CAR varied by gender, and conducted a number of robustness tests, such as examining whether our results varied when we excluded rather than covaried individuals with baseline MDD, and predicting to a larger outcome group including individuals with subclinical MDD symptoms. Given that several known risk factors for MDD including high levels of life stress, sadness, and loneliness are associated with elevated, rather than blunted CARs (Adam et al., 2006; Chida and Steptoe, 2009; Clow et al., 2004; Schulz et al., 1998), we hypothesized that youth with higher baseline CARs would be at increased risk of developing MDD by follow-up.
Methods
Participants
Older adolescents (average age 17.0 years) from two diverse public high schools in suburban Chicago and Los Angeles were selected for a longitudinal study of risk factors for the development of emotional disorders (Youth Emotion Project). In each school, all high school Juniors were asked to complete a screening questionnaire, in which basic demographic characteristics, and levels of neuroticism were assessed, using the Neuroticism Scale of the Revised Eysenck Personality Questionnaire, or EPQ-R) (Eysenck et al., 1985). To increase the proportion of participants at risk for MDD, adolescents high on neuroticism (a personality trait involving a tendency to experience negative affective states such as anxiety, anger and sadness, and a known prospective risk factor for depression)(Costa et al., 2001; Kendler et al., 2004) were oversampled, such that 60% of the resulting sample scored in the top third of the neuroticism distribution. The full study sample involved 627 individuals selected based on the screener. The current analyses focus on a subsample that was randomly selected from the larger group and asked to provide diary and cortisol data. Individuals were excluded from the current analytic subsample if they: used of corticosteroid-based medications, had psychotic symptoms, provided insufficient cortisol data, or had more than 3 months delay between baseline psychopathology and cortisol measurements. This resulted in 230 participants (173 female, 57 male; 48% Caucasian, 10% African-American, 19% Hispanic, 5% Asian/Pacific Islander, 18% Multiracial/Other). The greater predominance of females over males in this sample is accounted for by the fact that individuals with high levels of neuroticism were oversampled, and females are, on average, higher on this personality trait (Costa et al., 2001; Lynn and Martin, 1997). In addition, females were disproportionately likely to agree to participate in the study if invited.
Procedure
The current analyses used three assessments: baseline questionnaires/interviews, a cortisol assessment (within three months after baseline, average = 39 days) and follow-up interviews (approximately one year after the cortisol assessment, average = 368 days). The cortisol assessment was lagged slightly after the baseline questionnaire and interview assessment because it was believed that participant burden would be too high if all of these assessments were conducted simultaneously.
Baseline Questionnaires and Interviews
Demographic data
Basic demographic data obtained at the screening phase for the study and included age, gender, and race/ethnicity.
Diagnostic assessment
The Structured Clinical Interview for DSM–IV (SCID)(First et al., 2002) was used to determine current and lifetime psychiatric diagnoses of Major Depressive Disorder (MDD). Both initial onsets (single episodes) and recurrent major depressive episodes are referred to here as MDD. Other unipolar mood disorders (Dysthymia, Minor Depressive disorder) and anxiety disorders were also assessed. Anxiety disorders assessed included Social Phobia, Generalized Anxiety Disorder, Panic Disorder, Posttraumatic Stress Disorder, Specific Phobia, Obsessive Compulsive Disorder, and Anxiety Disorder NOS (not otherwise specified).
Diagnostic status for clinical MDD required meeting DSM-IV symptom criteria for a Major Depressive Episode (MDE) with no history of manic, hypomanic or mixed episodes. Each diagnosis was assigned a Clinical Severity Rating (CSR) from 0 (no impairment/distress) to 8 (severe impairment/distress)(DiNardo and Barlow, 1988). Only cases with a CSR of four or greater were included in the primary analyses because this score corresponded to clinically significant distress or impairment (caseness). Secondary analyses also examined the same question predicting to a larger outcome group that also included subclinical cases, who either: a) met full symptom criteria for MDD but were not judged to have clinically significant levels of distress or impairment (i.e., they had a CSR of 1 to 3) or b) had significant levels of distress or impairment but did not have sufficient symptom number, or symptom duration (2 weeks) to qualify for a DSM-IV diagnosis of MDD. For 69 cases, a second interviewer observed the SCID and provided independent diagnoses, with Cohen's kappas of .72 and .94 for mood and anxiety disorders, and correlations between .74 and .97 for CSR severity ratings (Zinbarg et al., 2009).
Life Stress
Chronic and episodic stress experienced over the past year were assessed using the Life Stress Interview (Hammen et al., 1987; Hammen, 1991). For chronic stress, participants' reports of typical stress in 10 domains (including close friendships, social group relations, romantic relationships, relations with family members, academic, neighborhood conditions, job, finances, health of self, and health of family members) over the past year were rated by interviewers on a 5-point scale. The average score across all domains was calculated. For episodic stress, the severity of each specific stressful event occurring over the past year was rated on a 5 point scale by a team of independent coders. For events that were at least mild to moderately stressful (2.5 or higher), the average severity was multiplied by the total number of events to provide an average episodic stress score. Inter-rater reliability (intraclass correlations examining absolute agreement) was .88 for chronic stress and .79 for episodic stress. Chronic and episodic stress measures were standardized and average together to provide an aggregate indicator of the combined chronic and episodic life stress encountered over the past year.
Neuroticism
Trait neuroticism (N) was assessed using four personality questionnaires, each measuring N or one of its facets: The Revised Eysenck Personality Questionnaire (N Scale) (Eysenck et al., 1985); the International Personality Item Pool-NEO-PI-R (Goldberg, 1992); the Behavioral Inhibition Scale (Carver and White, 1994) and Big Five Mini-Markers N scale (Saucier, 1994). Measures were standardized and averaged to form a N composite, with a Cronbach's alpha of .85 (Griffith et al., 2007; Zinbarg et al., 2009).
Cortisol Assessment
Salivary cortisol was gathered six times per day over three consecutive typical weekdays during the school year: at wake-up, 40 minutes after waking, at approximately 3, 8, and 12 hours post-awakening (signaled by a programmed watch), and bedtime. Cortisol was gathered by passive drool: participants expressed their saliva through a small straw into a polypropylene tube, and labeled tubes with the time and date. Participants were instructed not to eat or drink or brush their teeth in the ½ hour before sampling. Samples were returned by mail or to a frequently-checked drop-box at participants' schools, refrigerated at -20 degrees Celsius, then sent by courier to Trier, Germany to be assayed for cortisol. Cortisol levels are stable at room temperature for several weeks and are unaffected by the conditions associated with a postal journey (Clements and Parker, 1998). Assays were conducted in duplicate using a time-resolved immunoassay with fluorometric detection (DELFIA) (Dressendorfer et al., 1992). Intra-assay coefficients of variation (CVs) were between 4.0% and 6.7%, and inter-assay CVs ranged from 7.1% to 9.0%. Due to a strong positive skew, a natural logarithmic transformation was performed on cortisol values prior to their use in analysis.
Measures of basal cortisol activity included: wake-up, wake-up plus 40 minute values, and bedtime values, size of the CAR (wake-up plus 40 minute minus wake-up cortisol level), slope of the diurnal cortisol rhythm from wake-up to bedtime (bedtime minus wake-up level divided by total time awake), and average cortisol (calculated by taking the area under the curve (AUC) defined by all cortisol data points across the day, divided by the total time awake). Each measure was averaged across the 3 collection days. It is important to note that this study was originally designed to examine multiple elements of the cortisol diurnal rhythm across the day, in addition to the CAR. As a result, in order to reduce participant burden, the protocol for the CAR is less intensive, and thus less precise, than the original CAR protocol, in which cortisol levels were obtained every 10 to 15 minutes after awakening (Pruessner et al., 1997).
Momentary mood reports
Just before completing each saliva sample, participants completed a short (5 min.) diary report regarding their location, activities, and 0-3 point ratings of 20 current mood states. Mood state data were factor analyzed using principal axis factoring with an oblimin rotation of the mood state items averaged across moments for each person. Factor analyses revealed two distinct negative mood state factors of potential relevance to cortisol and to prospective risk for depression: negative affect (nervous, lonely, frustrated, worried, irritable, stressed, sad; α = .91) and fatigue (sleepy, tired; α = .95).
Health Variables
Health variables known to be associated with cortisol or MDD were measured by questionnaire, including: presence of chronic health conditions such as asthma or allergies, medication use (including use of oral contraceptives and use of psychotropic medications), typical hours of sleep, time of waking on the cortisol testing days, and nicotine use (Adam, 2006; Kirschbaum, 1999; Kudielka and Kirschbaum, 2003). Note that because all adolescents were post-pubertal (age range of 16 to 18 years) by the time of cortisol testing, measures of pubertal status were not included.
Follow-up Interview
SCID and Life Stress Interviews were repeated one year (on average 368 days) after the cortisol assessment (1.1 years after Baseline). Procedures were identical to baseline except that disorders were assessed only for the time period since the last interview. Clinical MDD diagnoses were assigned when one or more new major depressive episodes had occurred in the time period since the baseline interview, with sufficient impairment (CSR 4 or greater) in the absence of past or current manic symptoms. Cases that came close to, but failed to meet MDD symptom or severity requirements were categorized as “subclinical’ for MDD according to the criteria described earlier. To measure life stress across the full study period, baseline levels of life stress as well as changes in life stress from baseline to follow-up were calculated.
Analysis Plan
The primary analysis involved a logistic regression, with the baseline cortisol measures of interest regressed on a dummy variable reflecting the occurrence of one or more clinical episodes of MDD since the baseline interview. A comprehensive set of covariates was included to ensure that associations between cortisol and later MDD were attributable to baseline cortisol, rather than to other risk factors for MDD or health confounds that may be related to cortisol. The full set of covariates included: a) baseline mental health status and history (ever having had a diagnosis of MDD, another mood disorder, or an anxiety disorder at the time of the baseline interview); b) baseline levels and changes in life stress from baseline to follow-up; c) baseline neuroticism and negative mood states on the days of cortisol testing and d) age, gender and health-related covariates. Health-related covariates included in the final models were use of psychotropic medication, presence of asthma, nicotine use, average hours of sleep, and time of waking on the cortisol testing days. Some covariates, such as nicotine use and time of waking, were included the model regardless of their level of statistical significance in predicting MDD, as they have been found in past research to have strong associations with diurnal cortisol patterns. Several other health and demographic variables were examined but not included in the final model in order to preserve degrees of freedom, and because they were unrelated to MDD onset over the followup period. These included oral contraceptive use and race/ethnicity indicators.
In a secondary analysis, individuals with current MDD at baseline were excluded rather than covaried in order to ensure that cortisol levels were predicting to new episodes of MDD, rather than the continuity of MDD from baseline to follow-up. Finally, to increase the sample size of the group considered “depressed” in the follow- up period, and to see whether our results held for less severe cases, we examined a larger outcome group including both the clinical MDD cases and the subclinical cases who, as described above, came close but did not quite meet either the symptom or severity criteria needed to qualify for MDD.
Results
Descriptive Data
Of the 230 participants, 56 (24%) were diagnosed with either past or current MDD at the time of the baseline assessment (16 youth or 7% were current); 41 (18%) were diagnosed with a past or current anxiety disorder, and 25 (11%) with another mood disorder. By follow-up, 18 (8%) youth met the symptom and severity criteria for a clinical MDD diagnsosis over the intervening time period (9 first onsets, 9 recurrences; 16 out of 18 were female), and an additional 20 (12 female, 8 male) met subclinical criteria, for a total of 38 individuals (28 female, 10 male) meeting criteria for either a clinical or a subclinical episode over the followup period. Due to our purposeful oversampling of youth high on neuroticism, MDD rates in this sample are higher than those typically observed in same-aged adolescent samples (Costello et al., 2003).
Table 1 presents descriptive statistics for baseline cortisol indicators and demographic, health, life stress and other covariates included in the models. Cortisol values in Table 1 reflect raw values for greater ease of interpretation (in nmol/L; to convert to μg/dL units, divide by 27.59), but a natural logarithmic transformation of these cortisol values was used in all analyses. On average, cortisol values followed the typical daily pattern: they were high upon waking (11.99 nmol/L), increased sharply immediately after waking (to 16.82 nmol/L at 40 min post-awakening), and then declined rapidly at first, and then more slowly thereafter, to reach a nadir at bedtime (2.85 nmol/L). There was, however, substantial variability in cortisol values at each sampling point, and in the parameters (average cortisol, diurnal cortisol slope, CAR) used to describe baseline diurnal cortisol patterns.
Table 1.
Descriptive Statistics for Cortisol and Other Predictor Variables (N=230)
| Variable | Mean | Std. Deviation | Minimum | Maximum |
|---|---|---|---|---|
| Gender (0=female, 1=male) | 0.25 | 0.43 | 0.00 | 1.00 |
| Risk Status | 1.42 | 0.75 | 0.00 | 2.00 |
| T1 Chronic Stress | 2.28 | 0.37 | 1.45 | 3.50 |
| T3 Chronic Stress | 2.19 | 0.32 | 1.50 | 3.15 |
| T1 Episodic Stress | 2.14 | 3.13 | 0.00 | 14.38 |
| T3 Episodic Stress | 1.31 | 2.33 | 0.00 | 16.50 |
| Neuroticism | 0.00 | 1.00 | -2.45 | 2.88 |
| Diary Reported Negative Affect | 0.00 | 1.00 | -1.52 | 2.84 |
| Diary Reported Fatigue | 0.00 | 1.00 | -2.50 | 2.51 |
| Age at Cortisol Testing | 17.04 | 0.36 | 16.07 | 18.16 |
| Caffeine Use (Cups or cans/day) | 0.68 | 1.20 | 0.00 | 12.00 |
| Tobacco Use (Cigarettes/day) | 0.14 | 0.86 | 0.00 | 10.00 |
| Psychotropic Medication (1=yes) | 0.04 | 0.19 | 0.00 | 1.00 |
| Has Asthma | 0.09 | 0.28 | 0.00 | 1.00 |
| Hours of Sleep | 7.13 | 0.95 | 4.00 | 10.00 |
| Time of Waking | 6.68 | 0.87 | 1.75 | 9.87 |
| S1 Wake-up | 11.99 | 6.33 | 1.19 | 37.05 |
| S2 W + 40 min | 16.82 | 7.86 | .81 | 37.96 |
| S3 Mid-morning | 6.66 | 4.44 | .48 | 31.07 |
| S4 Mid-afternoon | 4.75 | 4.11 | .64 | 42.42 |
| S5 Early Evening | 3.52 | 3.71 | .18 | 31.51 |
| S6 Bedtime | 2.85 | 3.71 | .12 | 30.24 |
| Average Cortisol Levels Across Waking Day (Area Under Curve) | 5.39 | 2.89 | .78 | 25.77 |
| Size of Cortisol Awakening Response (CAR) | 4.77 | 8.92 | -29.12 | 28.36 |
| Rate of Cortisol Decline from Wake-up to Bedtime (Slope) | -.56 | .38 | -2.18 | .74 |
Note: Cortisol values are presented here in original units (nmol/L) for ease of interpretation. In all analyses, natural logarithmic transformations of cortisol values were used.
Prospective prediction of MDD
Table 2 presents the results of the logistic regression analyses in which the development of a clinical episode of MDD over the followup period is predicted from the baseline cortisol parameters (CAR, diurnal cortisol slope and AUC), while covarying the effects of baseline and past diagnoses of MDD and other mood and anxiety disorders, and the set of health covariates. Interactions between the cortisol parameters and gender are also included, in order to examine gender differences in the associations between the baseline cortisol parameters and later MDD. All variables listed in Table 2 are entered simultaneously, such that the coefficients reflect the effects of each variable independent of each other variable in the model. As seen in Table 2, net of the other variables in the model, both baseline levels of life stress (OR = 4.0, p = .04), and increases in life stress from baseline to followup (OR = 16.7, p = .001) were significantly related to the development of MDD over followup. Adolescent age (OR = 3.8, p = .03), the presence of asthma at baseline (OR = 12.9, p = .02), and diary reports of fatigue (OR = 2.7, p = .04) at the time of cortisol testing were also significantly related to later MDD. Of the other covariates, there was a non-significant trend for male gender to predict reduced odds (OR = .02, p = .09) of developing MDD over the followup. There was also a trend for youth with a later average time of waking (6:50 AM for those who develop MDD by followup; 6:44 AM for those who do not) to be related to an increased odds (OR = 4.95, p = .06) of developing MDD. Not surprisingly, individuals with either past or baseline episodes of MDD were significantly more likely to have another episode by followup (OR = 6.9, p = .04).
Table 2.
Cortisol and Other Factors Predicting Clinical Major Depression Over Follow-up Period
| Exp(B) (Odds Ratio) | 95% C. I. Lower | 95% C. I. Upper | p-value | |
|---|---|---|---|---|
| Cortisol Variables | ||||
| Cortisol Awakening Response (CAR) | 2.96 | 1.06 | 8.26 | .04 |
| Diurnal Cortisol Slope (Slope) | .63 | .24 | 1.68 | .36 |
| Total Cortisol Across Day (AUC) | .63 | .24 | 1.66 | .35 |
| Cortisol x Gender Interactions | ||||
| CAR x Male Gender Interaction | .02 | .00 | .79 | .04 |
| Slope x Male Gender Interaction | .26 | .01 | 5.50 | .39 |
| AUC x Male Gender Interaction | 3.43 | .17 | 68.49 | .42 |
| Past Psychopathology | ||||
| Baseline or Past MDD | 6.88 | 1.10 | 42.98 | .04 |
| Baseline or Past Anxiety Disorder | 2.25 | .31 | 16.34 | .42 |
| Baseline or Past Other Mood Disorder | 5.92 | .73 | 48.19 | .10 |
| Baseline and Increases in Life Stress | ||||
| Baseline Life Stress | 4.00 | 1.04 | 15.35 | .04 |
| Increase in Life Stress | 16.72 | 3.18 | 88.05 | .001 |
| Negative Mood State on Cortisol Testing Days | ||||
| Stressed/Sad | .81 | .38 | 1.73 | .59 |
| Fatigued | 2.67 | 1.05 | 6.79 | .04 |
| Covariatesa | ||||
| Male Gender | .02 | .00 | 1.82 | .09 |
| Age at Time of Cortisol Testing | 3.80 | 1.17 | 12.31 | .03 |
Additional covariates included baseline neuroticism, psychotropic medication use, presence of asthma, nicotine use, average hours of sleep, and time of waking on the cortisol testing days.
Note: Continuous variables were standardized (Mean = 0, SD=1) to make it easier to compare effect sizes across variables. Dichotomous variables are left in their original form (0 = no, 1 = yes).
Beyond these effects, the size of the CAR significantly predicted later MDD, with a higher CAR associated with increased odds of having an episode of MDD over the follow-up period (OR = 3.0, p = .04). An examination of the gender interaction indicated that the association between the CAR and later MDD was significantly weaker for men than for women (OR = .02, p = .04). However, given that only two men developed clinical MDD over the followup period, this finding should be interpreted with caution, particularly in light of additional analyses (below) that do not show gender differences in the CAR-MDD association. Steeper diurnal cortisol slopes, and average cortisol across the day (AUC), were not significantly related to later MDD, nor were gender differences in the effects of these measures observed.
Due to multicollinearity between cortisol levels at specific time points (wakeup values, wakeup plus 40 minute values, and bedtime values) and the summary measures of the diurnal cortisol rhythm (CAR, average cortisol, and diurnal cortisol slope) calculated from them, the measures for wakeup, wakeup plus 40 minutes, and bedtime cortisol levels were entered together in a separate model without the CAR, AUC and slope measures, but containing an identical set of covariates. None of these variables (wakeup, wakeup plus 40 minute, and bedtime cortisol levels) were significant predictors of the development of MDD over the followup period (ORs = .93, p = .85; OR = 1.3, p = .59; and OR = .49, p = .10 for wakeup, wakeup plus 40 minute and bedtime cortisol levels, respectively).
Prediction of clinical MDD excluding baseline clinical MDD
There were no significant associations between either past or current MDD diagnoses and the size of the CAR at baseline [t(228) = -.62, p = .54 for past history of MDD; t(228) = -.26, p = .79 for current MDD at baseline). In a robustness test designed to further ensure that the individuals with baseline MDD were not influencing the results, the 16 individuals with current MDD at the time of the baseline assessment were excluded from the model, leaving a total sample size of 214, 15 of whom developed MDD. In this analysis, the size of the CAR was still strongly predictive of developing MDD over the followup period (OR = 4.0, p = .05). It is important to note that the ideal analysis would involve removing all the individuals who had a past history of MDD from the analysis, and thus predicting only to first onsets of clinical MDD. This approach is not possible due to the relatively small number of new onset clinical MDD cases occurring over the followup period (9 cases). Nonetheless, descriptive analyses are offered below that differentiate between recurrent episodes and first onsets of clinical MDD.
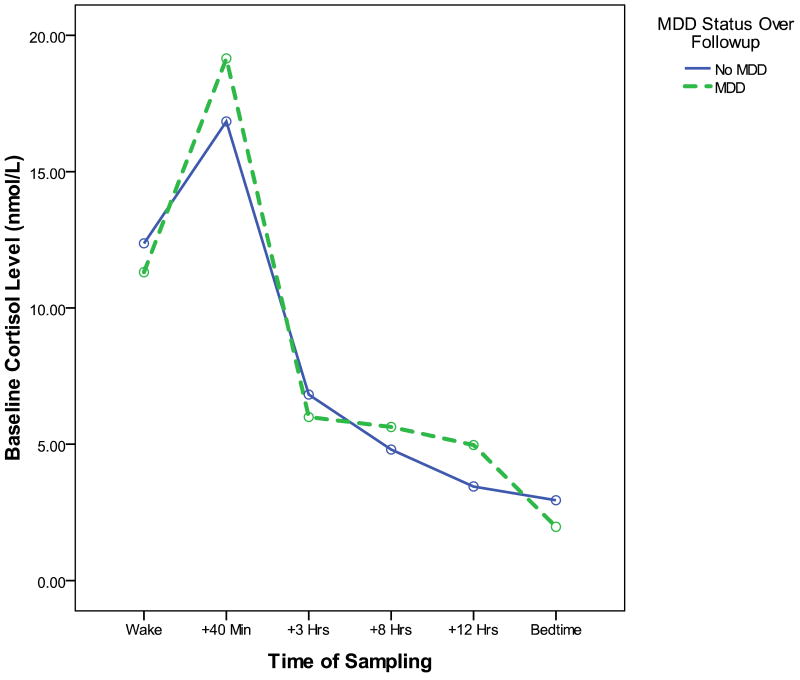
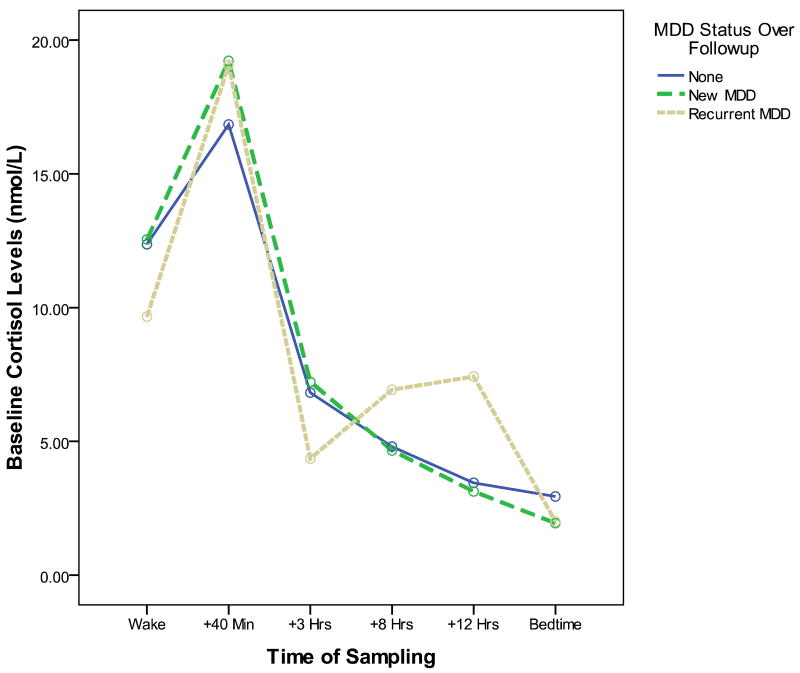
Figure 1 depicts the baseline diurnal cortisol patterns of participants without MDD at baseline who do (hatched line) and do not (solid line) go on to develop depression over the follow-up period. As suggested by our analyses, a larger baseline CAR to be the major characteristic distinguishing the cortisol profiles of these two groups. Although we do not have sufficient MDD cases at follow-up to examine separate statistical models predicting to first onsets of MDD versus recurrences, a visual inspection of Figure 2 suggests that both the new onset cases (dark hatched line) and the recurrent cases (light hatched line) are characterized the high baseline CAR pattern. An additional peak in cortisol values seems to be present at approximately 12 hours post-awakening in the soon-to-be-recurrent MDD group. This pattern requires replication and statistical confirmation in a larger group of recurrent cases; if replicated, further research should also examine whether this afternoon peak reflects post-prandial increases, or responses to stress experiences during the afternoon and evening hours.
Figure 1. Baseline diurnal cortisol profiles of participants who do (dashed line) and do not (solid line) go on to develop an episode of MDD over the following year.
Figure 2. Baseline diurnal cortisol profiles of participants who do (dashed lines) and do not (solid line) go on to develop an episode of MDD over the following year, differentiating between initial onsets of MDD (dark dashed line) and recurrences (light dashed line).
Prediction to a larger outcome group including both clinical and subclinical MDD
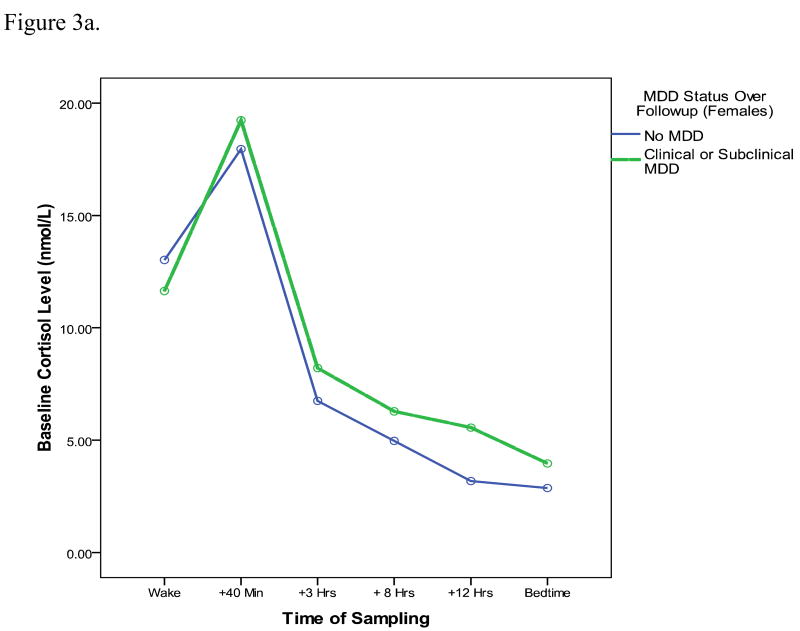
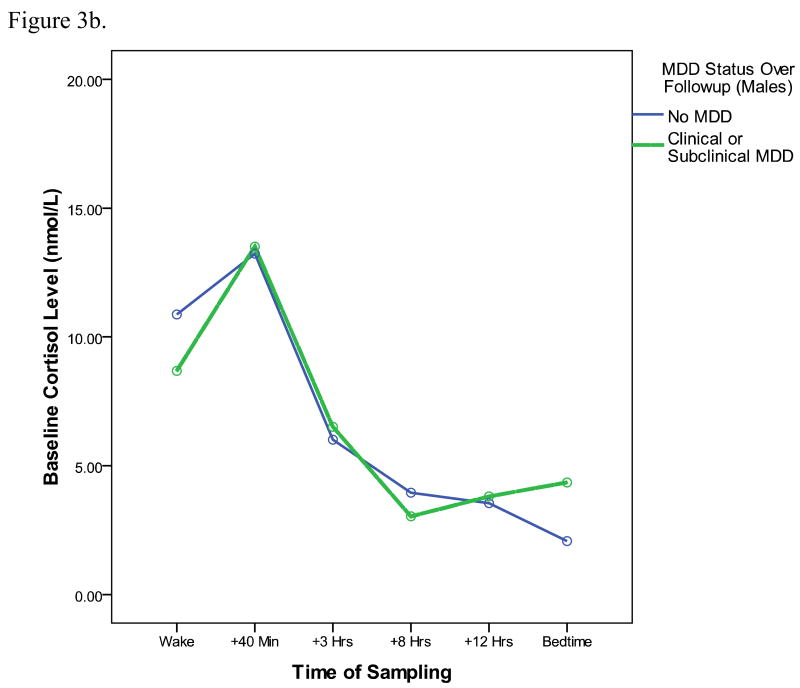
To examine whether results are true just for clinical levels of MDD, or generalize also to subclinical cases, we also conducted analyses predicting to a larger outcome group including clinical cases of MDD (those who met symptom and severity criteria) and also subclinical cases (individuals with MDD symptoms that did not meet full symptom and severity criteria). Of the 230 participants (173 female, 57 male), 38 (28 female, 10 male) had either a clinical or subclinical episode of MDD over the followup period. In an analysis predicting to this larger group of cases, and including the same set of covariates as prior models, the CAR is still a significant predictor of MDD at followup (OR = 2.5, p = .02). In contrast to the model predicting to clinical cases only, however, there are no significant interactions between gender and the CAR. Thus, results appear to generalize both to the prediction of subclinical MDD and appear to be similar for males and females. Figure 3 depicts the baseline cortisol profiles, separately for males and females, of individuals who develop either clinical or subclinical MDD over the followup period (with baseline MDD cases removed). Although the overall diurnal cortisol curves appear lower for males than for females, for both genders, the cortisol awakening responses is more pronounced for youth who go on to develop MDD by followup.
Figure 3.
Figure 3a. Baseline diurnal cortisol profiles of female participants who do (dashed lines) and do not (solid line) go on to develop either a clinical or subclinical MDD episode over the upcoming year.
Figure 3b. Baseline diurnal cortisol profiles of male participants who do (dashed lines) and do not (solid line) go on to develop either a clinical or subclinical MDD episode over the upcoming year.
Models including just the cortisol variables (no covariates)
Another robustness test involved models that included only the CAR, without the extensive set of health and lifestyle covariates (but excluding individuals with baseline MDD). In this analysis, the CAR is a still a significant predictor of clinical MDD (OR = 2.17, p = .02), and also to the larger outcome group including either clinical or subclinical MDD (OR = 2.0, p = .002) over the followup period. Thus, the associations between the CAR and later outcomes are present regardless of the inclusion or exclusion of covariates from the model. In addition, we also examined whether results were robust to removing, rather than covarying, youth who were using psychotropic medications (n = 10). The prospective prediction of MDD from the CAR remains significant regardless of whether psychotropic medication use is included as a covariate, or youth using psychotropic medication are removed from the model altogether.
Finally, with all covariates removed from the model, it is possible to predict to the larger outcome group of clinical and subclinical MDD from the baseline cortisol variables even after removing the 56 individuals with a lifetime history of MDD at baseline (N= 174 cases, and 19 first onsets of clinical or subclinical MDD remain in the sample after removing those with a lifetime history of MDD). With these individuals removed, the CAR is a significant predictor of new onsets of clinical or subclinical MDD by followup (OR = 2.22, p = .01).
Associations between life stress, the CAR and MDD
A reasonable hypothesis regarding the interrelations between life stress, the CAR and the later development of MDD is that an elevated CAR may be a biological pathway by which elevations in life stress contribute to the development of MDD. To test this possible meditational model, we examined whether the coefficients for baseline life stress and increases in life stress were affected by the introduction of the CAR and CAR by gender interactions into the model. The odds ratio for baseline life stress prior to the entry of the CAR variables was 3.25 (p = .05); the odds ratio for increase in life stress was 13.8 (p = .000). After the introduction of the CAR variables, the odds ratio for baseline life stress was 4.0 (p = .04); for increase in life stress it was 16.7 (p = .001). Thus, there is no sign that introduction of the CAR variables reduces the coefficients for life stress, suggesting that life stress (or at least life stress within the past year) and the CAR are independently associated with the development of MDD, rather than the CAR being a meditational pathway by which recent life stress influences the development of MDD.
Discussion
This study supports past research suggesting that morning cortisol levels are important prospective predictors of MDD (Goodyer et al., 2000; Halligan et al., 2007; Harris et al., 2000). It is however the first study to examine the cortisol awakening response specifically, and thus the first to demonstrate that the size of the CAR (the increase in cortisol after waking) is a significant prospective predictor of MDD. Notably, and somewhat in contrast to the past research, in the current study absolute levels of morning cortisol, such as cortisol levels at waking or cortisol levels 40 minutes after waking, did not significantly predict the onset of MDD. The past studies, however, measured cortisol levels at a fixed clock time (8 AM), rather than in relation to person-specific wake-times on each day of testing, making it challenging to compare their results with those of the current investigation. Additional research incorporating both an 8 AM clock time measure and CAR measures relative to person-specific waketimes would be helpful in clarifying the discrepancies between the current and prior studies regarding the importance of absolute morning cortisol levels.
Although other indicators of basal cortisol activity, such as elevated average levels, the slope of the diurnal cortisol rhythm, and elevated bedtime cortisol levels have been cross-sectionally related to the presence of current MDD (Dahl et al., 1991), these indicators were not found to be significant prospective predictors of later MDD. Thus, it appears that the aspects of HPA axis activity that are risk factors for MDD may be different than the HPA-axis correlates of a current disorder. This is perhaps not surprising, given that theoretical models of the role of the HPA axis in MDD suggest that stress-related changes in HPA-axis neurophysiology may be important in the etiology of this disorder (Ehlert et al., 2001).
Several strengths of the study increase our confidence in our finding that the size of the CAR is a prospective risk factor for MDD. We utilized a large sample size (N=230) and gathered multiple morning cortisol samples timed in relation to person-specific wake-times each day over the course of 3 days. We used diagnoses of MDD that were assessed by highly trained clinical interviewers rather than relying on self-reported depressive symptoms. Our results held while covarying the effects of past MDD, other mood disorders, and anxiety disorders, baseline neuroticism, baseline and increases in life stress, negative mood states on the cortisol testing days, and demographic and health-related covariates. Results were also robust to the removal of individuals with baseline MDD, the presence vs. absence of the covariates, and to the prediction of subclinical symptoms as well as clinical cases of MDD.
One disadvantage of our design was the time delay (on average just over one month) between the baseline interview and the cortisol sampling. A few individuals may have developed MDD during this time; in such cases, the presence of baseline MDD would not be appropriately covaried. To help account for this possibility, diary reports of negative affect on the exact days of cortisol testing were included as covariates. Although both baseline life events and increases in life events over the full followup period are included in the model, it is possible that life events occurring specifically during the time period between the baseline clinical interview and cortisol measurement may have contributed to elevated baseline CARs. However, given that the life events variables and CAR variables appear to be relatively independent in their prediction of later MDD, this seems unlikely to account for the CAR finding. Another limitation, as noted earlier, is that we employed a simplified, two-sample protocol for the assessment of the CAR, rather than a more intensive protocol in which measures are gathered every 10 to 15 minutes, or even a moderate intensity CAR protocol in which samples are gathered at 0, 30 and 60 minutes after waking. Although use of a two-sample CAR protocol has become relatively common, particularly in large-scale studies or studies examining cortisol rhythms across the full day rather than just the CAR (Adam and Kumari, In press; Chida and Steptoe, 2009), the two sample protocol is less precise, and did not allow us to measure the area under the curve of the CAR response, or to separate the CAR increase from the speed of recovery from the CAR peak. Another disadvantage is that we relied on noninvasive measures of HPA-axis function (i.e., daytime diurnal cortisol rhythm) rather than employing pharmacological challenges (such as the dexamethasone suppression test)(Young et al., 2006) that provide more nuanced insights regarding the integrity of central HPA-axis function.
In terms of the potential mechanisms for the CAR effect, the post-awakening cortisol increase occurs on top of the already elevated cortisol level at wake-up, such that the CAR often contains the highest cortisol level of the day, as well as cortisol's largest and most rapid increase. Circulating cortisol reaches several types of receptors in the brain. Glucocorticoid receptors (GRs) have a lower affinity for cortisol than mineralcorticoid receptors (MRs), and thus are extensively occupied only at very high cortisol levels, such as those that occur at the CAR peak (de Kloet, 1991). Perhaps elevated CARs contribute, over time, to changes over time in central GR populations involved in the negative feedback regulation of the HPA axis. As noted earlier, decreased HPA negative feedback efficacy and resulting elevations in peripheral cortisol levels are common correlates of MDD in adults (Thase et al., 2002). A variety of changes in the amygdala and hippocampus, both of which are rich in GRs, have been noted in MDD, including loss of hippocampal volume that is related both to the severity and length of exposure to MDD disorder (Bremner, 2000; Campbell and Macqueen, 2004). Given speculation that chronic exposure to high levels of glucocorticoids may play a role in these neurobiological changes associated with MDD, it is of great interest that elevated CAR responses occur prior to, and are predictive of the later emergence of MDD.
In terms of the potential origins of individual differences in the CAR, it has been found that individual differences in the CAR have a substantial genetic component (Wüst et al., 2000), thus large CARs may in part reflect a genetically-driven trait biological risk factor for MDD. However, the fact that there are no associations between the size of the CAR at baseline, and past or present diagnoses of MDD at baseline would argue somewhat against the notion of larger CARs being a stable, trait individual difference associated with MDD. CARs are also influenced by psychosocial experience, with higher CARs predicted by chronic stress (Pruessner et al., 1999; Schulz et al., 1998; Wüst et al., 2000), low levels of social contact (Stetler and Miller, 2005), and loneliness (Adam et al., 2006; Steptoe et al., 2004). Hence, CARs could also serve as a potential biological pathway by which negative psychosocial experience confers risk for MDD. Our measure of baseline life stress (which assessed life stress over the prior year), and our measure of increase in life stress over the year between baseline and followup were both significantly related to MDD at followup, as might be expected given extensive past research showing that life events are important prospective risk factors for the development of MDD. However, our analyses did not support the idea that an elevated CAR serves as a meditational pathway linking recent negative life events to later MDD. Rather, our life events measures and baseline CAR measures appeared to be independent predictors of MDD. It is important to note, however, that our life events measures only assessed the time period from one year prior to the baseline psychopathology assessment to the time of the followup interview, in a sample of older adolescents (on average, 17 years of age). Given that the HPA axis has been shown to be particularly malleable to adverse psychosocial experiences occurring in the first few years of life (Halligan et al., 2004; Weaver et al., 2004), and may undergo an additional period of plasticity and change during the early adolescent years (Stroud et al., 2009), it is entirely possible that the individual differences in the CAR we see reflect the influences of earlier experiences, or the interactions between earlier experience and recent life events. One of the few prior prospective studies of cortisol and depression in youth found that exposure to maternal depression in the first year of life accounted for the elevated early morning cortisol levels that were predictive of later MDD (Halligan et al., 2007). Future prospective studies should also assess the possible contributions of early and early adolescent life experience to individual differences in the CAR, and examine whether elevated CARs mediate associations between prior life events and MDD.
Future studies should also further investigate whether the cortisol awakening response prospectively predicts MDD for both male and female adolescents, given that the current study provided mixed results on that particular question. When predicting to clinical cases of MDD, we found that the CAR was a significant prospective predictor for females, but not males, but as noted earlier, these results should be interpreted with caution due to the very small number of males in the clinical MDD outcome group. When predicting to the larger outcome group including both clinical and subclinical MDD, there were no significant differences between males and females, and a visual inspection of the baseline cortisol profiles of the youth who would go on to develop clinical or subclinical MDD showed an elevated CAR for both males and females, when compared to their male and female peers who did not go on to develop these problems over the followup period. To resolve whether the association between baseline CARs and the development of clinical MDD is equally true for males and females, either a larger initial sample size or tracking of clinical MDD onsets over a longer followup period will be required.
In considering implications for prevention or intervention, the current results might be taken to indicate that pharmacological approaches to blocking elevated CARs in youth should be investigated. Such an idea, however, should be pursued with caution given that other physical and mental health disorders have been associated with hypocortisolemia, such as externalizing and risk taking behaviors (Shirtcliff et al., 2005), burnout (Pruessner et al., 1999) and chronic fatigue syndrome (Nater et al., 2007; Roberts et al., 2004). In addition, we have suggested elsewhere that psychosocial modulation of the CAR on a day-to-day basis is important for responding to the level of anticipated daily demands (Adam et al., 2006). Thus, additional research is needed to determine what constitutes a “healthy” CAR, including not only what level, but also what degree of variability in the CAR might be necessary for optimal functioning across multiple situations and multiple health conditions. Psychotherapeutic approaches that provide individuals with social and cognitive resources to better contain their affective and physiological responses to stress may also help to maintain CARs at appropriate levels, while still allowing them to be modulated by social demands when required (Gaab et al., 2006).
Descriptively, our results suggest that elevated CARs prospectively predict both new onsets and recurrences of depression over the year followup period, thus providing a potentially useful marker of increased proximal risk for major depressive episodes. Additional prospective longitudinal studies of youth are needed, with sample sizes large enough to formally compare results for new onsets versus recurrences of MDD. As noted earlier, in the current study, the CAR provided information regarding risk for depressive episodes that is independent of existing risk factors for MDD, such as gender and levels of chronic and episodic life stress. Future studies with larger sample sizes should also examine the extent to which these factors (gender, life events, CAR levels) interact in predicting future depressive episodes. In addition, future studies should follow youth for a longer period of time, to examine whether elevated CARs are predictive of MDD only in the near future (over the next year) or whether they predict elevated risk over a longer period of time. Perhaps most importantly, more work is also needed to understand the mechanisms by which elevated CARs confer risk for MDD, and to identify ways in which the potentially negative consequences of elevated CARs may be reduced, while still maintaining the adaptive functions of this important component of HPA axis activity.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adam EK. Transactions among adolescent trait and state emotion and diurnal and momentary cortisol activity in naturalistic settings. Psychoneuroendocrinology. 2006;31:664–679. doi: 10.1016/j.psyneuen.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Adam EK, et al. Day-to-day dynamics of experience-cortisol associations in a population-based sample of older adults. PNAS. 2006;103:17058–17063. doi: 10.1073/pnas.0605053103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adam EK, Kumari M. Assessing salivary cortisol in large-scale, epidemiological research. Psychoneuroendocrinolgy. doi: 10.1016/j.psyneuen.2009.06.011. In press. [DOI] [PubMed] [Google Scholar]
- Angold A. Adolescent depression, cortisol and DHEA. Psychological Medicine. 2003;33:573–581. doi: 10.1017/s003329170300775x. [DOI] [PubMed] [Google Scholar]
- Bhagwagar Z, Cowen PJ. It's not over when it's over: Persistent neurobiological abnomalities in recovered depressed patients. Psychological Medicine. 2007;38:307–313. doi: 10.1017/s0033291707001250. [DOI] [PubMed] [Google Scholar]
- Bhagwagar Z, et al. Increased salivary cortisol after waking in depression. Psychopharmacology. 2005;182:54–57. doi: 10.1007/s00213-005-0062-z. [DOI] [PubMed] [Google Scholar]
- Bremner JD. Hippocampal volume reduction in major depression. American Journal of Psychiatry. 2000;157:115–117. doi: 10.1176/ajp.157.1.115. [DOI] [PubMed] [Google Scholar]
- Campbell S, Macqueen G. The role of the hippocampus in the pathophysiology of major depression. Journal of Psychiatry & Neuroscience. 2004;29:417–426. [PMC free article] [PubMed] [Google Scholar]
- Carver CS, White TL. Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: The BIS/BAS scales. Journal of Personality and Social Psychology. 1994;67:319–333. [Google Scholar]
- Chida Y, Steptoe A. Cortisol awakening response and psychosocial factors: A systematic review and meta-analysis. Biological Psychology. 2009:265–278. doi: 10.1016/j.biopsycho.2008.10.004. [DOI] [PubMed] [Google Scholar]
- Chrousos GP, Gold PW. The concepts of stress and stress system disorders Overview of physical and behavioral homeostasis. Journal of American Medical Association. 1992;267:1244–1252. [PubMed] [Google Scholar]
- Clements AD, Parker CR. The relationship between salivary cortisol concentrations in frozen versus mailed samples. Psychoneuroendocrinology. 1998;23:613–616. doi: 10.1016/s0306-4530(98)00031-6. [DOI] [PubMed] [Google Scholar]
- Clow A, et al. The awakening cortisol response: Methodological issues and significance. Stress: The International Journal on the Biology of Stress. 2004;7:29–37. doi: 10.1080/10253890410001667205. [DOI] [PubMed] [Google Scholar]
- Costa PT, et al. Gender Differences in Personality Traits Across Cultures: Robust and Surprising Findings. Journal of Personality and Social Psychology. 2001;81:322–331. doi: 10.1037/0022-3514.81.2.322. [DOI] [PubMed] [Google Scholar]
- Costello EJ, et al. Prevalence and Development of Psychiatric Disorders in Childhood and Adolescence. Arch Gen Psychiatry. 2003;60:837–844. doi: 10.1001/archpsyc.60.8.837. [DOI] [PubMed] [Google Scholar]
- Dahl RE, et al. 24-Hour cortisol measures in adolescents with major depression: A controlled study. Biological Psychiatry. 1991;30:25–36. doi: 10.1016/0006-3223(91)90067-v. [DOI] [PubMed] [Google Scholar]
- de Kloet ER. Brain corticosteroid receptor balance and homeostatic control. Frontiers in Neuroendocrinology. 1991;12:95–164. doi: 10.1016/j.yfrne.2018.02.003. [DOI] [PubMed] [Google Scholar]
- DiNardo PA, Barlow DH. Anxiety Disorders Interview Schedule-Revised (ADIS-R) Graywind Publications; Albany, NY: 1988. [Google Scholar]
- Dressendorfer RA, et al. Synthesis of a cortisol–biotin conjugate and evaluation as a tracer in an immunoassay for salivary cortisol measurement. The Journal of Steroid Biochemistry and Molecular Biology. 1992;43:683–692. doi: 10.1016/0960-0760(92)90294-s. [DOI] [PubMed] [Google Scholar]
- Ehlert U, et al. Psychoneuroendocrinological contributions to the etiology of depression, posttraumatic stress disorder, and stress-related bodily disorders: The role of the hypothalamus-pituitary-adrenal axis. Biological Psychology. 2001;57:141–152. doi: 10.1016/s0301-0511(01)00092-8. [DOI] [PubMed] [Google Scholar]
- Eysenck SB, et al. A revised version of the Psychoticism scale. Personality and Individual Differences. 1985;6(1):21–29. [Google Scholar]
- First MB, et al. Biometrics Research. New York State Psychiatric Institute; New York: 2002. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Non-patient Edition (SCID-I/NP) [Google Scholar]
- Gaab J, et al. Psychoneuroendocrine effects of cognitive-behavioral stress management in a naturalistic setting--a randomized controlled trial. Psychoneuroendocrinology. 2006;31:428–438. doi: 10.1016/j.psyneuen.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Gold PW, Chrousos GP. Organization of the stress system and its dysregulation in melancholic and atypical depression: High vs low CRH/NE states. Molecular Psychiatry. 2002;7:254–275. doi: 10.1038/sj.mp.4001032. [DOI] [PubMed] [Google Scholar]
- Goldberg LR. The development of markers for the Big-Five factor structure. Psychological Assessment. 1992;4:26–42. [Google Scholar]
- Goodyer IM, et al. Recent life events, cortisol, dehydroepiandrosterone and the onset of major depression in high-risk adolescents. British Journal of Psychiatry. 2000;177:499–504. doi: 10.1192/bjp.177.6.499. [DOI] [PubMed] [Google Scholar]
- Griffith JW, et al. Neuroticism as a common dimension in the internalizing disorders. 2007 doi: 10.1017/S0033291709991449. Manuscript in preparation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halligan SL, et al. Disturbances in morning cortisol secretion in association with maternal postnatal depression predict subsequent depressive symptomatology in adolescents. Biological Psychiatry. 2007;62:40–46. doi: 10.1016/j.biopsych.2006.09.011. [DOI] [PubMed] [Google Scholar]
- Halligan SL, et al. Exposure to postnatal depression predicts elevated cortisol in adolescent offspring. Biological Psychiatry. 2004;55:376–381. doi: 10.1016/j.biopsych.2003.09.013. [DOI] [PubMed] [Google Scholar]
- Hammen C, et al. Children of depressed mothers: Maternal strain and symptom predictors of dysfunction. Journal of Abnormal Psychology. 1987;96:190–198. doi: 10.1037//0021-843x.96.3.190. [DOI] [PubMed] [Google Scholar]
- Hammen CL. Depression runs in families: The social context of risk and resilience in children of depressed mothers. Springer-Verlag; New York: 1991. [Google Scholar]
- Harris TO, et al. Morning cortisol as a risk factor for subsequent major depressive disorder in adult women. British Journal of Psychiatry. 2000;177:505–510. doi: 10.1192/bjp.177.6.505. [DOI] [PubMed] [Google Scholar]
- Holsboer F. The corticosteroid receptor hypothesis of depression. Neuropsychopharmacology. 2000;23:477–501. doi: 10.1016/S0893-133X(00)00159-7. [DOI] [PubMed] [Google Scholar]
- Huber TJ, et al. The cortisol awakening response is blunted in psychotherapy inpatients suffering from depression. Psychoneuroendocrinology. 2006;31:900–904. doi: 10.1016/j.psyneuen.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Kendler KS, et al. The Interrelationship of Neuroticism, Sex, and Stressful Life Events in the Prediction of Episodes of Major Depression. American Journal of Psychiatry. 2004;161:631–636. doi: 10.1176/appi.ajp.161.4.631. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Helhammer D. Salivary cortisol. In: Fink G, editor. Encyclopedia of Stress. Vol. 3. Academic Press; San Diego: 2000. [Google Scholar]
- Kirschbaum C, Kudielka B, Gaab J, Schommer NC, Helhammer DH. Impact of gender, menstrual cycle phase, and oral contraceptives on the activity of the hypothalamus-pituitary-adrenal axis. Psychosomatic Medicine. 1999;61:154–162. doi: 10.1097/00006842-199903000-00006. [DOI] [PubMed] [Google Scholar]
- Kudielka BM, Kirschbaum C. Awakening cortisol responses are influenced by health status and awakening time but not by menstrual cycle phase. Psychoneuroendocrinology. 2003;28:35–47. doi: 10.1016/s0306-4530(02)00008-2. [DOI] [PubMed] [Google Scholar]
- Lynn R, Martin T. Gender Differences in Extraversion, Neuroticism, and Psychoticism in 37 Nations. Journal of Social Psychology. 1997;137:369–373. doi: 10.1080/00224549709595447. [DOI] [PubMed] [Google Scholar]
- Mannie ZN, et al. Increased Waking Salivary Cortisol Levels in Young People at Familial Risk of Depression. Am J Psychiatry. 2007;164:617–621. doi: 10.1176/ajp.2007.164.4.617. [DOI] [PubMed] [Google Scholar]
- Nater UM, et al. Attenuated morning salivary cortisol concentrations in a population-based study of persons with chronic fatigue syndrome and well controls. Journal of Clinical Endocrinology and Metabolism. 2007;1747 doi: 10.1210/jc.2007-1747. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, et al. Burnout, perceived stress and cortisol responses to awakening. Psychosomatic Medicine. 1999;61:197–204. doi: 10.1097/00006842-199903000-00012. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, et al. Free cortisol levels after awakening: A reliable biological marker for the assessment of adrenocortical activity. Life Sciences. 1997;61:2539–2549. doi: 10.1016/s0024-3205(97)01008-4. [DOI] [PubMed] [Google Scholar]
- Pruessner M, et al. Self-Reported Depressive Symptoms and Stress Levels in Healthy Young Men: Associations With the Cortisol Response to Awakening. Psychosomatic Medicine. 2003;65:92–99. doi: 10.1097/01.psy.0000040950.22044.10. [DOI] [PubMed] [Google Scholar]
- Roberts ADL, et al. Salivary cortisol response to awakening in chronic fatigue syndrome. British Journal of Psychiatry. 2004;184:136–141. doi: 10.1192/bjp.184.2.136. [DOI] [PubMed] [Google Scholar]
- Saucier G. Mini-Markers: A brief version of Goldberg's unipolar Big-Five markers. Journal of Personality Assessment. 1994;63:506–516. doi: 10.1207/s15327752jpa6303_8. [DOI] [PubMed] [Google Scholar]
- Schlotz W, et al. Perceived Work Overload and Chronic Worrying Predict Weekend-Weekday Differences in the Cortisol Awakening Response. Psychosom Med. 2004;66:207–214. doi: 10.1097/01.psy.0000116715.78238.56. [DOI] [PubMed] [Google Scholar]
- Schulz P, et al. Increased free cortisol secretion after awakening in chronically stressed individuals due to work overload. Stress Medicine. 1998;14:91–97. [Google Scholar]
- Shirtcliff EA, et al. Low salivary cortisol levels and externalizing behavior problems in youth. Development and Psychopathology. 2005;17:167–184. doi: 10.1017/s0954579405050091. [DOI] [PubMed] [Google Scholar]
- Steptoe A, et al. Loneliness and neuroendocrine, cardiovascular, and inflammatory stress responses in middle-aged men and women. Psychoneuroendocrinology. 2004;29:593–611. doi: 10.1016/S0306-4530(03)00086-6. [DOI] [PubMed] [Google Scholar]
- Stetler C, Miller GE. Blunted cortisol response to awakening in mild to moderate depression: regulatory influences of sleep patterns and social contacts. Journal of Abnormal Psychology. 2005;114:697–705. doi: 10.1037/0021-843X.114.4.697. [DOI] [PubMed] [Google Scholar]
- Stroud LR, et al. Stress response and the adolescent transition: Performance versus peer rejection stressors. Development and Psychopathology. 2009;21:47–68. doi: 10.1017/S0954579409000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thase ME, et al. Biological aspects of depression. In: Gotlib IH, Hammen CL, editors. Handbook of depression Guilford. New York: 2002. [Google Scholar]
- van Rossum EFC, et al. Polymorphisms of the Glucocorticoid Receptor Gene and Major Depression. Biological Psychiatry. 2006;59:681–688. doi: 10.1016/j.biopsych.2006.02.007. [DOI] [PubMed] [Google Scholar]
- Weaver IC, et al. Epigenetic programming by maternal behavior. Nature Neuroscience. 2004;7:847–854. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- Wilhelm I, et al. Is the cortisol awakening rise a response to awakening? Psychoneuroendocrinology. 2007;32:358–366. doi: 10.1016/j.psyneuen.2007.01.008. [DOI] [PubMed] [Google Scholar]
- Wüst S, et al. Genetic factors, perceived chronic stress, and the free cortisol response to awakening. Psychoneuroendocrinology. 2000;25:707–20. doi: 10.1016/s0306-4530(00)00021-4. [DOI] [PubMed] [Google Scholar]
- Young EA, et al. Saliva Cortisol and Response to Dexamethasone in Children of Depressed Parents. Biological Psychiatry. 2006;60:831–836. doi: 10.1016/j.biopsych.2006.03.077. [DOI] [PubMed] [Google Scholar]
- Zinbarg RE, et al. The Northwestern-UCLA Youth Emotion Project: Associations of cognitive vulnerabilities, neuroticism and gender with past diagnoses of emotional disorders in adolescents. 2009 doi: 10.1016/j.brat.2009.12.008. Manuscript submitted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]