Abstract
Objective
To evaluate bone mineral density (BMD) in children with Cushing disease before and after transphenoidal surgery (TSS).
Study design
Hologic dual-energy x-ray absorptiometry (DXA) scans of 35 children with Cushing disease were analyzed retrospectively. Sixteen of the 35 patients had follow up DXA scans 13–18 months after TSS. BMD and bone mineral apparent density (BMAD) for lumbar spine (LS) L1–L4 and femoral neck (FN) were calculated.
Results
Preoperatively, 38% and 23% of patients had osteopenia of the LS and FN, respectively. Both BMD and BMAD Z-scores of the LS were worse than those for the FN (−1.60 ± 1.37 vs. −1.04 ± 1.19, p=0.003), and (−1.90 ± 1.49 vs. −0.06 ± 1.90, p<.001); postoperative improvement in BMD and BMAD were more pronounced in LS as compared with the FN (0.84 ± 0.88 vs. 0.15 ± 0.62, p<.001) and (0.73 ± 1.13 vs −0.26 ± 1.21, p=0.015). Pubertal stage, cortisol levels, and length of disease had no effects on BMD.
Conclusions
In children with Cushing disease, vertebral BMD was more severely affected than femoral BMD and was independent of degree or duration of hypercortisolism. BMD for the LS improved significantly after TSS; osteopenia in this group may be reversible.
Keywords: Glucocorticoid, steroid excess, osteopenia
Cushing disease is rare in children (1). Osteopenia has been reported in adults with Cushing disease (2–7). Although exposure to excess glucocorticoids is a frequent cause of osteoporosis or osteopenia in both children and adults, limited data are available about bone mineral density (BMD) in children with Cushing disease (7–12). It is known that hypercortisolism is associated with loss of skeletal mass and can lead to increased vertebral fracture risk (13). Impaired BMD has been shown at the time of diagnosis in adults with a variety of causes of Cushing syndrome (CS) including Cushing disease (2–7).
Similarly impaired BMD at the time of diagnosis has been seen in three studies involving up to 14 patients with childhood-onset Cushing disease (7, 10, 11). BMD has been shown to improve following cure in adult patients; however limited follow-up data exist for children (2, 4, 5, 9, 14, 15). One longitudinal study in six children demonstrated that BMD impairment in childhood-onset Cushing disease could be partially reversed 2 years after normalization of cortisol levels (9). Another study looked at Hologic dual-energy x-ray absorptiometry (DXA) scans performed in 11 children at a mean of 4.5 years after cure of Cushing disease; however, information on BMD before surgery in these patients was not available (10). A prospective study of 14 patients with CS showed that the majority had major increases in bone mass after normalization of cortisol secretion; by the third year of remission BMD at the spine and hip had returned into the normal range in most patients compared with low pretreatment values (11).
Multiple factors contribute to decreased bone mineral density in Cushing disease, including a direct effect of glucocorticoids on osteoclasts and osteoblasts, both enhancing bone resorption and impairing bone formation (16, 17). Glucocorticoids also act to decrease gastrointestinal calcium absorption and renal calcium reabsorption (18). Children with Cushing disease often present with musculoskeletal weakness and can have decreased weight-bearing activity that may contribute to impaired BMD. In addition, patients with Cushing disease are at risk for other pituitary hormone deficiencies including central hypogonadism and growth hormone (GH) deficiency, both of which have the potential to contribute to osteopenia (1). As childhood and adolescence is a time of high bone mass accrual, one might expect that disordered bone formation and remodeling during this critical period may impair the achievement of peak bone mass (19). Whether poor peak bone mass accrual in childhood leads to an increased risk of osteoporosis later in life is under debate (20). In the present study, we analyzed BMD data at the spine and hip before and up to 18 months after transphenoidal surgery (TSS) in children with Cushing disease and examined whether BMD in these patients was associated with the degree of hypercortisolism.
METHODS
All patients were admitted to the NIH Warren Magnuson Clinical Center in the last 10 years. Studies were performed under clinical protocol 97-CH0076 that was approved by the NICHD Institutional Review Board. Informed consent from the patients’ parents (and assent from older children) was obtained for all patients. A total of 35 patients underwent TSS; 16 patients had follow up DXA scans performed 13–18 months following TSS. Cure of hypercortisolism was achieved in 34 of the patients by TSS alone; one patient required a second TSS, as well as radiation therapy. None of the patients presented with fractures.
Patients were evaluated for pubic hair, breast or testicular development and were grouped into Tanner stages 1,2 and 3 (pre-pubertal to mid-puberty) vs. Tanner 4 and 5 (late puberty).
Hologic, Inc (Bedford, MA) dual-energy x-ray absorptiometry (DXA) scans were obtained in 35 children with Cushing disease and were retrospectively analyzed (20 females, mean age 12.8 ± 3.1 yrs) (Table). DXA scans were performed on the Hologic densitometers QDR2000, QDR4500, and DelphiA. From measurement of an anthropomorphic spine phantom, the coefficient of variation for determination with the QDR2000 instrument was less than 0.5% and for the QDR4500 and DelphiA instruments the values were less than 0.4% for 6 months. Comparison of a spine phantom scanned on both the QDR2000 and QDR4500 instruments and another phantom on both the QDR4500 and Delphi instruments gave bone mineral density determinations that were within 1.0%. Femoral neck (FN) and lumbar spine (LS) Z-scores were calculated using the NICHD Children’s Reference Database, which provides age, race and sex specific reference curves (21). Low bone density for chronological age was defined as a Z-score ≤ −2.00 (22). Our cohort of patients with Cushing disease included those with short stature secondary to hypercortisolemia. As BMD calculations may be underestimated in shorter patients, we therefore normalized the BMD to a derived reference volume by correcting BMD values to adjust for differences in bone size by using bone mineral apparent density (BMAD), an estimation of volumetric bone mass (g/cm3) (23, 24). BMAD for FN and LS anterior posterior 2–4 were calculated as previously described (23). Z scores for BMAD were calculated using the Stanford University Bone Mineral Density website (http://www-stat-class.stanford.edu/pediatric-bones/#applet).
Table 1.
Demographic and clinical characteristics in patients with Cushing disease
| Cushing Disease | |
|---|---|
| Females (%)/Males (%) n=35 | 20 (57.14)/15 (42.86) |
| Age at surgery, years n=35 | 12.8 (3.07) |
| Race (%) n=35 | |
| Asian | 1 (2.86) |
| Black | 1 (2.86) |
| White | 25 (71.43) |
| Other/Unknown | 8 (22.86) |
| Ethnicity (%) n=35 | |
| Latino or Hispanic | 8 (22.86) |
| Not Latino or Hispanic | 27 (77.14) |
| Tanner Stage (%) n=35 | |
| 1, 2, or 3 | 23 (65.71) |
| 4 or 5 | 12 (34.29) |
| Initial Height SD score n=35 | −1.19 ± 1.12 |
| Height SD score at follow-up n=16 | −0.81 ± 1.17 |
| BMI Z-score n=35 | 2.02 ± 0.76 |
| Midnight cortisol, mcg/dL n=35 | 21. 7 ± 21.6 |
| Urinary free cortisol, mcg/m2 n =35 | 304.5 ± 325.4 |
| Length of disease, months n=35 | 27.2 ± 15.3 |
| Duration to follow-up n=16 (months) | 14.7 ± 1.5 |
Data are pre-operative values and are mean ± SD, unless otherwise specified.
Cortisol hypersecretion was demonstrated in all patients by 24-hour urinary free cortisol (UFC) measurements and diurnal cortisol measurements. Diurnal plasma cortisol was obtained by placing an IV at least 2 hours before the test; midnight cortisol levels were drawn at 11:30 PM and 12:00 AM while the patient was asleep. Plasma cortisol was measured by chemiluminescence immunoassay. UFC was averaged from two separate preoperative measurements, and was calculated per square meter of body-surface area, as previously described (25). Following TSS, 24-hour UFC was measured on postoperative days 5–9. Serum cortisol was measured beginning on postoperative day 5 and was repeated daily until postoperative day 10. During this postoperative period, dexamethasone was administered to patients who required replacement. Patients were defined as cured of disease by post-operative measurements of UFC < 10 micrograms/24 hours, plasma cortisol < 1 mcg/dL, and/or adrenocortical insufficiency for which they received replacement.
Statistical Analysis
Paired t-tests, or its non-parametric parallel (Wilcoxon signed-rank test only for the change in height SDS that was not normally distributed), were used to compare paired continuous data. A two-sample t-test was used for comparing continuous data from distinct groups. Dichotomous data from independent groups were compared by chi-square and Fisher exact tests, while paired data were compared by McNemar tests. Since the pre-operative LS and FN prevalences of osteopenia were from patients with and without follow-up, their comparison with the one-year post operative prevalences in patients with follow-up only were achieved by a composite exact test that took into consideration independent and paired status of data. The rationale and form of the test statistic for the composite test is analogous to that derived for a very similar problem, namely Birch generalization of Fisher exact test to a set of stratified 2×2 tables (26). Pearson correlation coefficients were used for correlation analyses. Data are presented as mean ± standard deviation (SD), unless otherwise indicated, and were analyzed using SAS system software version 9.1 (SAS Institute, Carey, North Carolina). A two-sided P-value of ≤ 0.05 was considered statistically significant.
RESULTS
BMD and BMAD at diagnosis
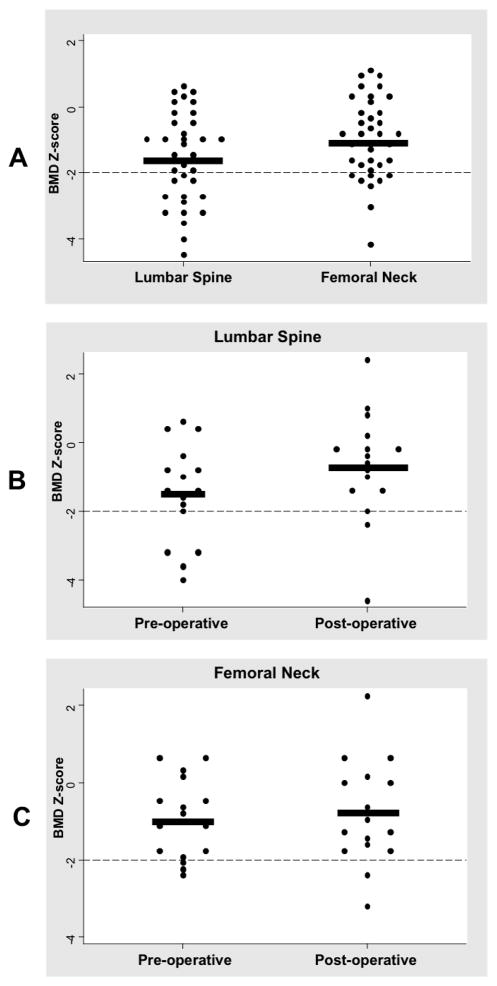
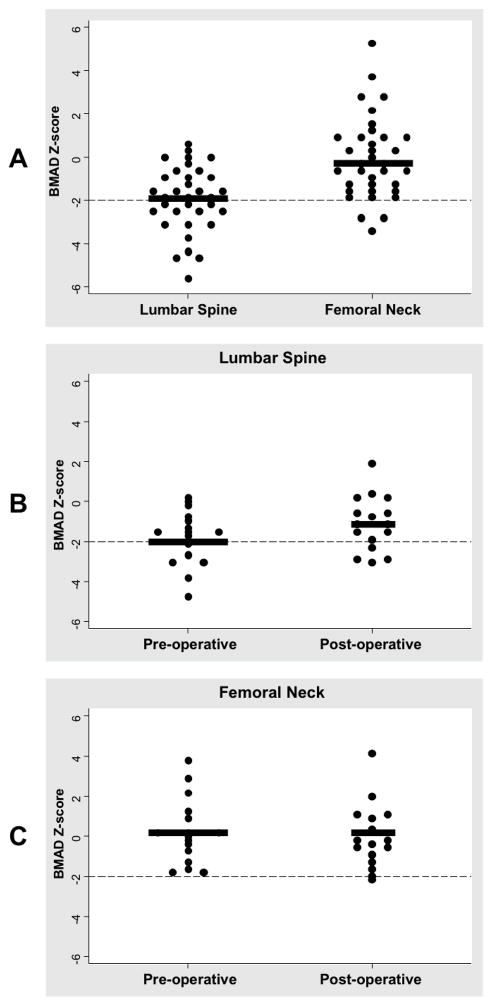
A total of 35 patients with Cushing disease had BMD measurements performed prior to their TSS (Table). Patients had an average height SD of −1.19 ± 1.12 secondary to growth arrest related to Cushing disease. As expected with Cushing disease, patients were overweight with an average BMI Z-score of 2.02 ± 0.76. The mean midnight serum cortisol and UFC were both elevated pre-operatively (Table). Preoperatively, the prevalence of osteopenia in the LS and FN was 38% and 23%, respectively. The average preoperative BMD Z-score of the LS was worse than that of the FN (−1.60 ± 1.37 vs. −1.04 ± 1.19, p=0.003) (Figure 1, A). Similar results were obtained when BMAD was analyzed; BMAD Z-score of the LS was worse than that of the FN (−1.90 ± 1.49 vs. −0.06 ± 1.90, p<.001) (Figure 2, A)
Figure 1.
A, Distributional plot for pre-operative lumbar spine and femoral neck BMD Z-scores. Dashed line is at the −2.00 level. Thick black line represents the means (−1.60 ± 1.37 vs. −1.04 ± 1.19, p=0.003). B, Distributional plot for lumbar spine BMD z-scores at baseline and follow up. Delta of 0.84 ± 0.88 (p=0.002). C, Distributional plot for femoral neck BMD z-scores at baseline and follow-up. Delta of 0.15 ± 0.62 (p=0.335).
Figure 2.
A, Distributional plot for pre-operative lumbar spine and femoral neck BMAD Z-scores. Dashed line is at the −2.00 level. Thick black line represent the means (−1.90 ± 1.49 vs. −0.06 ± 1.90, p<.001). B, Distributional plot for lumbar spine BMAD z-scores at baseline and follow up. Delta of 0.73 ± 1.13 (p=0.021). C, Distributional plot for femoral neck BMD z-scores at baseline and follow-up. Delta of −0.26 ± 1.21 (p=0.401).
BMD Z-scores were not significantly different comparing patients in pre-mid puberty (Tanner 1,2, and 3) to those in late puberty (Tanner 4 and 5). There also were no statistically significant correlations between 24-hour UFC, midnight plasma cortisol, or length of disease and LS or FN BMD or BMAD Z-scores.
BMD and BMAD at follow-up
BMD data was available for 16 patients with follow-up scans up to 18 months after surgical cure. There was a significant improvement in height at follow-up, with a mean difference in height SDS of 0.5 ± 0.99, p=0.025. At the follow-up visit, BMD Z-score of the LS was −0.66 ± 1.55, and the BMD Z-score of the FN was −0.79 ± 1.35. Improvement in LS BMD values from preoperative to follow-up was statistically significant, with a delta of 0.84 ± 0.88, p=0.002 (Figure 1, B). However, recovery of the FN BMD was not statistically significant (Figure 1, C). Improvement in BMD values from preoperative to follow-up was therefore more pronounced in the LS compared with the FN (0.84 ± 0.88 vs. 0.15 ± 0.62, p<.001). At 12.5%, the post-operative prevalence of osteopenia was significantly improved in both LS and FN sites (p<0.001 and p=0.008, respectively).
Using BMAD in the analysis, similar results to the LS BMD were seen, with significant improvement of Z-scores at follow-up, corresponding to a delta of 0.73 ± 1.13, p=0.021 (Figure 2, B). Recovery of the FN BMAD was not statistically significant, however this is not surprising as the BMAD analysis of the FN showed normal bone mineralization at baseline with a mean Z score of −0.06 (Figure 2, A and C). Greater improvement in BMAD values from preoperative to follow-up was seen in the LS as compared with the FN (0.73 ± 1.13 vs −0.26 ± 1.21, p=0.015).
DISCUSSION
Chronic glucocorticoid excess associated with Cushing disease has negative effects on bone turnover, leading to osteopenia in both adults and children. In our patients, the baseline BMD of the LS, composed predominantly of trabecular bone, was worse than that of the FN, comprised primarily of cortical bone. Treatment with pharmacological doses of glucocorticoids leads to more severe impairment of bone mass in trabecular as compared with cortical bone (17). This pattern of bone loss leading to more pronounced osteopenia in the lumbar spine has also seen previously in adult patients with Cushing disease (5, 27).
As maximal bone mass accumulation occurs during early-mid puberty, we hypothesized that the pubertal stage at the onset of hypercortisolism may have different effects on BMD (19). In our study population, there appeared to be no influence of pubertal stage on Z score of the LS or FN at the time of diagnosis of disease, as BMD Z-scores were not significantly different comparing patients in pre-mid puberty (Tanner 1, 2, and 3) with those in late puberty (Tanner 4 and 5). As a subset of our patients presented with growth arrest due to Cushing disease, and DXA scans inherently underestimate the bone density of patients with short stature, we used BMAD Z-scores to correct for differences in patient size. Using BMAD, we obtained similar results to the BMD Z-scores for the LS, however for the FN, the baseline mean BMAD was normal with a Z-score of −0.06 and the baseline BMD Z-score for the FN was low at −1.04.
GH deficiency (GHD) is associated with deficient bone mineral accrual (28). Children with Cushing disease have marked GH suppression at diagnosis and continued partial GHD one year post-operatively (29). Because of these data, we do not screen for GHD during the first year after TSS and do not routinely place our patients on GH treatment. We hypothesize that GH suppression and subsequent recovery of GH secretion in these patients may have contributed to the corresponding changes in their BMD. In addition, patients with Cushing disease may have musculoskeletal weakness which improves after cure of disease; increased weight bearing activity after recovery may serve as an additional cause for BMD partial resolution.
Previous studies in adults with Cushing disease have shown variable results with respect to a relationship between measures of hypercortisolemia and BMD Z-scores. In one study, BMD z scores were negatively correlated with 24 hr UFC, and plasma cortisol levels; however, two separate reports showed no significant correlations between serum cortisol or UFC concentrations and baseline BMD data in adults with Cushing disease (2, 5, 6). Our data in pediatric patients showed no correlations of degree of osteopenia with UFCs, plasma cortisol, or length of disease. Children with Cushing disease are more physically active one year after cure as compared with pre-operatively, and their improvement in muscle strength is another factor that is likely to contribute to the increase in BMD. Our group at the performed a prospective study of quality of life in 40 children with CS evaluated before and 1 year post treatment (30). The Child Health Questionnaire (CHQ) a validated survey that includes parent reported measures of physical well being of children compared with U.S. population normative data (31). In our 16 patients in whom we had both pre-and postoperative DXA scan and CHQ results (n=16) Prior to treatment, parents reported significant limitations in their child’s physical function, with a pre treatment physical function z score of −1.22 ± 1.77. Physical scores improved significantly from pre-treatment to one year after surgical cure, with a post treatment physical function score of −0.18 ± 1.40, (p=0.004).
Curative treatment of glucocorticoid excess appears to result in only partial reversal of BMD impairment in both adults and children with Cushing disease(2, 4, 5, 7–11, 14, 15). In our study, after resolution of hypercortisolism, Z-scores improved significantly for the LS, but the data for the FN did not reach statistical significance. From our group it was previously reported that persistent long-term effects of hypercortisolism on BMD could be seen in a 21-year-old identical twin who was successfully treated by TSS for Cushing disease at age 14 (12). An older cohort of patients from our institution was followed up to 7 years and confirmed partial reversal of BMD (11). However, this represented a mixed group of patients with both ACTH-dependent and ACTH-independent causes of CS.
We sought to clarify the effects of Cushing disease only on BMD. One smaller study has previously shown that bone impairment in childhood-onset Cushing disease can be partially reversed 2 years after normalization of cortisol levels (9). A cohort of 11 patients with Cushing disease cured by TSS showed near – normal BMD at a mean of 4.5 years after cure, however no information was available about the BMD in these patients at the time of diagnosis of Cushing disease (10). Our study shows that cortisol normalization after TSS leads to an improvement in bone density in the LS for children with Cushing disease at the 13–18 month follow-up visit. Complete reversal to normal BMD was not seen, however, and confirmation of restoration of normal bone mass requires longer follow up (14).
Acknowledgments
We are indebted to the patients and their families and our clinical staff that made this study possible.
Supported by the U.S. National Institutes of Health, National Institute of Child Health and Human Development intramural project (Z01-HD-000642-04).
Abbreviations
- BMD
bone mineral density
- TSS
transphenoidal surgery
- DXA
dual-energy x-ray absorptiometry
- BMAD
bone mineral apparent density
- LS
lumbar spine
- FN
femoral neck
- GH
growth hormone
- GHD
growth hormone deficiency
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflicts of interest.
References
- 1.Magiakou MA, Mastorakos G, Oldfield EH, Gomez MT, Doppman JL, Cutler GB, Jr, et al. Cushing’s syndrome in children and adolescents. Presentation, diagnosis, and therapy. N Engl J Med. 1994;331:629–636. doi: 10.1056/NEJM199409083311002. [DOI] [PubMed] [Google Scholar]
- 2.Futo L, Toke J, Patocs A, Szappanos A, Varga I, Glaz E, et al. Skeletal differences in bone mineral area and content before and after cure of endogenous Cushing’s syndrome. Osteoporos Int. 2008;19:941–949. doi: 10.1007/s00198-007-0514-x. [DOI] [PubMed] [Google Scholar]
- 3.Godang K, Ueland T, Bollerslev J. Decreased bone area, bone mineral content, formative markers, and increased bone resorptive markers in endogenous Cushing’s syndrome. Eur J Endocrinol. 1999;141:126–131. doi: 10.1530/eje.0.1410126. [DOI] [PubMed] [Google Scholar]
- 4.Hermus AR, Smals AG, Swinkels LM, Huysmans DA, Pieters GF, Sweep CF, et al. Bone mineral density and bone turnover before and after surgical cure of Cushing’s syndrome. J Clin Endocrinol Metab. 1995;80:2859–2865. doi: 10.1210/jcem.80.10.7559865. [DOI] [PubMed] [Google Scholar]
- 5.Kawamata A, Iihara M, Okamoto T, Obara T. Bone mineral density before and after surgical cure of Cushing’s syndrome due to adrenocortical adenoma: prospective study. World J Surg. 2008;32:890–896. doi: 10.1007/s00268-007-9394-7. [DOI] [PubMed] [Google Scholar]
- 6.van der Eerden AW, den Heijer M, Oyen WJ, Hermus AR. Cushing’s syndrome and bone mineral density: lowest Z scores in young patients. Neth J Med. 2007;65:137–141. [PubMed] [Google Scholar]
- 7.Di Somma C, Pivonello R, Loche S, Faggiano A, Marzullo P, Di Sarno A, et al. Severe impairment of bone mass and turnover in Cushing’s disease: comparison between childhood-onset and adulthood-onset disease. Clin Endocrinol. 2002;56:153–158. doi: 10.1046/j.0300-0664.2001.01454.doc.x. [DOI] [PubMed] [Google Scholar]
- 8.Devoe DJ, Miller WL, Conte FA, Kaplan SL, Grumbach MM, Rosenthal SM, et al. Long-term outcome in children and adolescents after transsphenoidal surgery for Cushing’s disease. J Clin Endocrinol Metab. 1997;82:3196–3202. doi: 10.1210/jcem.82.10.4290. [DOI] [PubMed] [Google Scholar]
- 9.Di Somma C, Pivonello R, Loche S, Faggiano A, Klain M, Salvatore M, et al. Effect of 2 years of cortisol normalization on the impaired bone mass and turnover in adolescent and adult patients with Cushing’s disease: a prospective study. Clin Endocrinol. 2003;58:302–308. doi: 10.1046/j.1365-2265.2003.01713.x. [DOI] [PubMed] [Google Scholar]
- 10.Scommegna S, Greening JP, Storr HL, Davies KM, Shaw NJ, Monson JP, et al. Bone mineral density at diagnosis and following successful treatment of pediatric Cushing’s disease. J Endocrinol Invest. 2005;28:231–235. doi: 10.1007/BF03345378. [DOI] [PubMed] [Google Scholar]
- 11.Leong GM, Abad V, Charmandari E, Reynolds JC, Hill S, Chrousos GP, et al. Effects of child- and adolescent-onset endogenous Cushing syndrome on bone mass, body composition, and growth: a 7-year prospective study into young adulthood. J Bone Miner Res. 2007;22:110–118. doi: 10.1359/jbmr.061010. [DOI] [PubMed] [Google Scholar]
- 12.Leong GM, Mercado-Asis LB, Reynolds JC, Hill SC, Oldfield EH, Chrousos GP. The effect of Cushing’s disease on bone mineral density, body composition, growth, and puberty: a report of an identical adolescent twin pair. J Clin Endocrinol Metab. 1996;81:1905–1911. doi: 10.1210/jcem.81.5.8626856. [DOI] [PubMed] [Google Scholar]
- 13.Khanine V, Fournier JJ, Requeda E, Luton JP, Simon F, Crouzet J. Osteoporotic fractures at presentation of Cushing’s disease: two case reports and a literature review. Joint Bone Spine. 2000;67:341–345. [PubMed] [Google Scholar]
- 14.Kristo C, Jemtland R, Ueland T, Godang K, Bollerslev J. Restoration of the coupling process and normalization of bone mass following successful treatment of endogenous Cushing’s syndrome: a prospective, long-term study. Eur J Endocrinol. 2006;154:109–118. doi: 10.1530/eje.1.02067. [DOI] [PubMed] [Google Scholar]
- 15.Manning PJ, Evans MC, Reid IR. Normal bone mineral density following cure of Cushing’s syndrome. Clin Endocrinol. 1992;36:229–234. doi: 10.1111/j.1365-2265.1992.tb01437.x. [DOI] [PubMed] [Google Scholar]
- 16.Weinstein RS, Jilka RL, Parfitt AM, Manolagas SC. Inhibition of osteoblastogenesis and promotion of apoptosis of osteoblasts and osteocytes by glucocorticoids. Potential mechanisms of their deleterious effects on bone. J Clin Invest. 1998;102:274–282. doi: 10.1172/JCI2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Canalis E. Clinical review 83: Mechanisms of glucocorticoid action in bone: implications to glucocorticoid-induced osteoporosis. J Clin Endocrinol Metab. 1996;81:3441–3447. doi: 10.1210/jcem.81.10.8855781. [DOI] [PubMed] [Google Scholar]
- 18.Lukert BP, Raisz LG. Glucocorticoid-induced osteoporosis: pathogenesis and management. Ann Intern Med. 1990;112:352–364. doi: 10.7326/0003-4819-112-5-352. [DOI] [PubMed] [Google Scholar]
- 19.Soyka LA, Fairfield WP, Klibanski A. Clinical review 117: Hormonal determinants and disorders of peak bone mass in children. J Clin Endocrinol Metab. 2000;85:3951–3963. doi: 10.1210/jcem.85.11.6994. [DOI] [PubMed] [Google Scholar]
- 20.Gafni RI, Baron J. Childhood bone mass acquisition and peak bone mass may not be important determinants of bone mass in late adulthood. Pediatrics. 2007;119 (Suppl 2):S131–136. doi: 10.1542/peds.2006-2023D. [DOI] [PubMed] [Google Scholar]
- 21.Kalkwarf HJ, Zemel BS, Gilsanz V, Lappe JM, Horlick M, Oberfield S, et al. The bone mineral density in childhood study: bone mineral content and density according to age, sex, and race. J Clin Endocrinol Metab. 2007;92:2087–2099. doi: 10.1210/jc.2006-2553. [DOI] [PubMed] [Google Scholar]
- 22.Gordon CM, Bachrach LK, Carpenter TO, Crabtree N, El-Hajj Fuleihan G, et al. Dual energy X-ray absorptiometry interpretation and reporting in children and adolescents: the 2007 ISCD Pediatric Official Positions. J Clin Densitom. 2008;11:43–58. doi: 10.1016/j.jocd.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 23.Katzman DK, Bachrach LK, Carter DR, Marcus R. Clinical and anthropometric correlates of bone mineral acquisition in healthy adolescent girls. J Clin Endocrinol Metab. 1991;73:1332–1339. doi: 10.1210/jcem-73-6-1332. [DOI] [PubMed] [Google Scholar]
- 24.Carter DR, Bouxsein ML, Marcus R. New approaches for interpreting projected bone densitometry data. J Bone Miner Res. 1992;7:137–145. doi: 10.1002/jbmr.5650070204. [DOI] [PubMed] [Google Scholar]
- 25.Lodish MB, Sinaii N, Patronas N, Batista DL, Keil M, Samuel J, et al. Blood pressure in pediatric patients with Cushing syndrome. J Clin Endocrinol Metab. 2009;94:2002–2008. doi: 10.1210/jc.2008-2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Agresti A. A Survey of Exact Inference for Contigency Tables. Stat Science. 1992;7:131–177. [Google Scholar]
- 27.Chiodini I, Carnevale V, Torlontano M, Fusilli S, Guglielmi G, Pileri M, et al. Alterations of bone turnover and bone mass at different skeletal sites due to pure glucocorticoid excess: study in eumenorrheic patients with Cushing’s syndrome. J Clin Endocrinol Metab. 1998;83:1863–1867. doi: 10.1210/jcem.83.6.4880. [DOI] [PubMed] [Google Scholar]
- 28.Monson JP, Drake WM, Carroll PV, Weaver JU, Rodriguez-Arnao J, Savage MO. Influence of growth hormone on accretion of bone mass. Horm Res. 2002;58 (Suppl 1):52–56. doi: 10.1159/000064765. [DOI] [PubMed] [Google Scholar]
- 29.Magiakou MA, Mastorakos G, Gomez MT, Rose SR, Chrousos GP. Suppressed spontaneous and stimulated growth hormone secretion in patients with Cushing’s disease before and after surgical cure. J Clin Endocrinol Metab. 1994;78:131–137. doi: 10.1210/jcem.78.1.7507118. [DOI] [PubMed] [Google Scholar]
- 30.Keil MF, Merke DP, Gandhi R, Wiggs EA, Obunse K, Stratakis CA. Quality of life in children and adolescents 1-year after cure of Cushing syndrome: a prospective study. Clin Endocrinol. 2009;71:326–333. doi: 10.1111/j.1365-2265.2008.03515.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Landgraf JAL, Ware J. Child Health Questionnaire (CHQ): A User’s Manual. Boston, MA: The Health Institute, New England Medical Center; 1999. [Google Scholar]