Abstract
Currently, few users of anabolic-androgenic steroids (AAS) seek substance-abuse treatment. But this picture may soon change substantially, because illicit AAS use did not become widespread until the 1980s, and consequently the older members of this AAS-using population—those who initiated AAS as youths in the 1980s—are only now reaching middle age. Members of this group, especially those who have developed AAS dependence, may therefore be entering the age of risk for cardiac and psychoneuroendocrine complications sufficient to motivate them for substance-abuse treatment. We suggest that this treatment should address at least three etiologic mechanisms by which AAS dependence might develop. First, individuals with body-image disorders such as “muscle dysmorphia” may become dependent on AAS for their anabolic effects; these body-image disorders may respond to psychological therapies or pharmacologic treatments. Second, AAS suppress the male hypothalamic-pituitary-gonadal axis via their androgenic effects, potentially causing hypogonadism during AAS withdrawal. Men experiencing prolonged dysphoric effects or frank major depression from hypogonadism may desire to resume AAS, thus contributing to AAS dependence. AAS-induced hypogonadism may require treatment with human chorionic gonadotropin or clomiphene to reactivate neuroendocrine function, and may necessitate antidepressant treatments in cases of depression inadequately responsive to endocrine therapies alone. Third, human and animal evidence indicates that AAS also possess hedonic effects, which likely promote dependence via mechanisms shared with classical addictive drugs, especially opioids. Indeed, the opioid antagonist naltrexone blocks AAS dependence in animals. By inference, pharmacological and psychosocial treatments for human opioid dependence might also benefit AAS-dependent individuals.
Keywords: Anabolic-androgenic steroids, Androgens, Testosterone, Substance dependence, Treatment, Men
1. Introduction
The anabolic-androgenic steroids (AAS) are a family of hormones that includes the natural hormone testosterone, together with numerous synthetic testosterone analogues (Pope and Brower, 2009). When taken in supraphysiologic doses and combined with strenuous exercise and proper nutrition, AAS cause users to gain muscle mass and lose body fat, even beyond naturally attainable limits (Kouri et al., 1995). AAS have been used by competitive athletes since the 1950s (Wade, 1972), but it was not until about 1980 that AAS use began to spread from elite athletics to the general population (Kanayama et al., 2008). Over the last 30 years AAS use has grown into a widespread form of substance misuse. The United States National Household Survey (Substance Abuse and Mental Health Services Administration, 1994) found that nearly a million Americans had already used AAS by 1994 (the most recent year that AAS were covered by this survey), and more recent annual anonymous surveys by the Monitoring the Future Study (Johnston et al., 2009) have found that 1.3%-1.9% of American young adults reported lifetime AAS use. Thus in the United States alone, the lifetime prevalence of AAS use has likely now surpassed 2 million. Surveys from British Commonwealth countries (Baker et al., 2006; Handelsman and Gupta, 1997), Scandinavia (Nilsson et al., 2001), other European countries (Kokkevi et al., 2008), and Brazil (Galduroz et al., 2005) have produced comparable or greater prevalence estimates, adding millions more to the total number of AAS users worldwide. The great majority of these users are male; AAS use is uncommon in women, because women rarely aspire to become extremely muscular, and are also subject to undesirable masculinizing effects of AAS (Kanayama et al., 2007b). Therefore, the following discussion is focused primarily on men.
Accumulating evidence shows that AAS can cause a dependence syndrome, where individuals may use these drugs almost continuously for years, often despite adverse effects (Kanayama et al., in press). Eight field studies using DSM criteria (American Psychiatric Association, 1987, 1994; Kanayama et al., 2009b) have now collectively assessed the prevalence of AAS dependence among 653 AAS users (641 men, 12 women); of these, 197 (194 men, 3 women; 30.1% of the total group) were judged to show dependence (Kanayama et al., 2009a). Using this 30% estimate, it would follow that there may be millions of AAS-dependent individuals worldwide, potentially at risk for complications of long-term AAS exposure. To date, little has been written about treatment of such individuals, but rapidly accumulating findings from human and animal studies have now begun to suggest theoretical models for treating AAS dependence. In this review, we first discuss why this topic is timely and important. We then present three principal mechanisms that may contribute to AAS dependence, together with their treatment implications.
2. Will the patients come to treatment?
Currently, few AAS users seek substance-abuse treatment, and indeed many are skeptical of physicians' knowledge about AAS (Pope et al., 2004). AAS users often do not consider their drug use pathological, and may even perceive AAS as a positive aspect of a healthy and athletic lifestyle (Cohen et al., 2007; Collins, 2002). However, secular trends may soon change this pattern, because illicit AAS use first became widespread only in the 1980s. Therefore, the demographic leading edge of the illicit AAS-using population—the group who initiated AAS as youths in the early 1980s—is only now reaching middle age. These aging AAS users may thus be at increasing risk for AAS-associated medical (Hall and Hall, 2005; Hartgens and Kuipers, 2004; Kanayama et al., 2008) and psychiatric effects (Hall et al., 2005; Pope and Brower, 2009), as well as other forms of substance abuse (Kanayama et al., 2009c). These other substance-abuse disorders may arise before or after the onset of AAS dependence itself, and may include opioid abuse and dependence (Kanayama et al., 2003a; McBride et al., 1996; Wines et al., 1999); abuse of other performance-enhancing drugs, such as human growth hormone, clenbuterol, and insulin (Hildebrandt et al., 2007; Holt and Sonksen, 2008; Nilsson et al., 2001); and sometimes multiple substance abuse, including alcohol (Skarberg et al., 2009).
Therefore, it will likely become increasingly important for clinicians to identify and reach out to individuals with these various problems who might be candidates for AAS-dependence treatment. AAS-related adverse effects may first bring individuals to the attention of general practitioners or other health professionals who see middle-aged men (Brower, 2009). Alternatively, such individuals might first be identified by cardiologists encountering AAS-induced cardiomyopathy (D'Andrea et al., 2007; Kasikcioglu et al., 2009; Krieg et al., 2007; Weiner et al., 2009) or possible atherosclerotic disease (Liu et al., 2003; Maravelias et al., 2005); endocrinologists encountering sexual dysfunction, infertility, or other consequences of AAS-induced hypogonadism (Swerdloff et al., 2002; Tan and Scally, 2009; Urhausen et al., 2003); nephrologists encountering renal injury associated with AAS use (Herlitz et al., 2009); mental health professionals encountering AAS-induced mood disorders (Brower et al., 1989; Malone and Dimeff, 1992; Malone et al., 1995; Pope and Katz, 2003); or addiction treatment practitioners encountering AAS use concomitant with other substance use disorders (Skarberg et al., 2009). In short, professionals in many disciplines should be sensitized to the possibility of AAS dependence and be prepared to pursue this issue with their patients.
3. Three possible mechanisms of AAS dependence
Growing evidence suggests that classical drugs of abuse cause addiction via common neural pathways (Kalivas and Volkow, 2005). Specifically, dopaminergic activity in the nucleus accumbens likely modulates the acute reward of drug intoxication, and these acute effects are followed by a more chronic process of cellular adaptation, characterized by diminished cognitive control and an increased drive in response to drug cues. This model, however, is based largely on studies of drugs with “hedonic” effects—drugs that deliver an immediate reward of intoxication when ingested. AAS, on the other hand, produce few immediate intoxicating effects (Kanayama et al., 2009b; Kanayama et al., in press), and thus probably induce dependence via a more complex process.
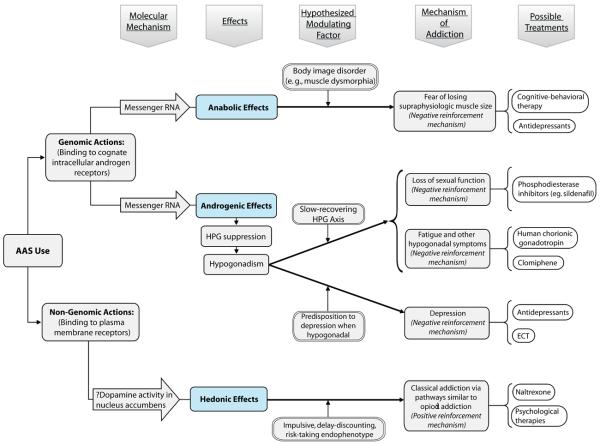
Specifically, we hypothesize that AAS dependence evolves in different individuals via any or all of three different mechanisms. As shown in Figure 1, two of these mechanisms involve the genomic effects of AAS, whereby these hormones bind to intranuclear androgen receptors, activating the production of messenger RNA transcripts that in turn encode a wide variety of structural, enzymatic, and receptor proteins (Li and Al-Azzawi, 2009). Among the actions of these proteins are anabolic (muscle-building) and androgenic (masculinizing) effects. The anabolic effects represent the principal motivation for most people to begin illicit AAS use (Kanayama et al., 2006; Kanayama et al., 2003b); once AAS use is underway, some users with pathological concerns about muscularity may continue AAS at increasing doses and for longer periods, thus contributing to AAS dependence, as discussed in section 4.
Figure 1.
A proposed theoretical model showing three hypothesized mechanisms by which anabolic-androgenic steroid (AAS) dependence may develop. Note that hypothesized predisposing factors are not assumed to be exclusively premorbid traits; as discussed in the text, evidence suggests that the association between these factors and AAS dependence may well be bidirectional in each case, in that AAS use may further exacerbate a premorbid trait. For example, the “impulsive delay-discounting risk-taking endophenotype” likely predisposes to AAS use as well as to conduct disorder and other forms of substance abuse. However, evidence suggests that AAS exposure may exacerbate impulsive and risk-taking symptoms by further increasing sensitivity to reward and decreasing sensitivity for punishment. These hypothesized bidirectional mechanisms are not shown in the figure for reasons of clarity.
The androgenic effects of exogenous AAS may cause suppression of the hypothalamic-pituitary-gonadal (HPG) axis, sometimes leading to hypogonadism that persists long after AAS are discontinued. Hypogonadism combines with other neuroendocrine factors to create a well-characterized AAS withdrawal syndrome (Hochberg et al., 2003), mediated by a variety of cortical neurotransmitter systems (Kashkin and Kleber, 1989; Wood, 2008), and long recognized as a potential factor in the development of AAS dependence (Kashkin and Kleber, 1989). Individuals prone to dysphoric withdrawal effects may repeatedly resume AAS to self-treat these effects—although this phenomenon remains understudied (Kanayama et al., 2009a; Tan and Scally, 2009). We discuss this mechanism in section 5.
Finally, as shown in Figure 1, AAS may also have direct rewarding or hedonic properties, mediated not so much by their genomic effects (although these may well contribute) but more directly by the effects of AAS and their metabolites on plasma membranes (Frye, 2007). Animal data suggest that this third hedonic mechanism of AAS dependence is biologically similar to the mechanism of addiction for classical intoxicating drugs described above, albeit developing on a slower time-course, and likely modulated by opioidergic mechanisms (Wood, 2008). We discuss this third mechanism in section 6.
4.0. The body-image mechanism
4.1. The evidence
Most people take AAS for their anabolic effects—to gain muscle and lose body fat (Pope and Brower, 2009). Although some individuals seek these effects purely for athletic purposes, many ingest AAS primarily to enhance body appearance (Kanayama et al., in press). Interestingly, AAS abuse is much more common in Western countries than in Asia, perhaps because Western cultures focus more on a muscular male body image (Cafri et al., 2005; Pope et al., 2000a; Pope et al., 2000b; Pope et al., 2001; Pope et al., 1999) then do Asian cultures (Gray et al., 2006; Yang et al., 2005).
AAS use may be particularly associated with disorders of body image, such as “muscle dysmorphia” (Cafri et al., 2005; Hildebrandt et al., 2006; Kanayama et al., 2006; Olivardia et al., 2000; Pope et al., 1997), sometimes also called “reverse anorexia nervosa” (Cole et al., 2003; Pope et al., 1993), wherein individuals perceive themselves as small and weak even though they are actually large and muscular. These body image concerns may be both a cause and a consequence of AAS use. On the one hand, concerns about body image are likely risk factors predisposing to initial AAS use (Blouin and Goldfield, 1995; Brower et al., 1994; Cafri et al., 2005; Dodge et al., 2008; Kanayama et al., 2006; Kanayama et al., in press; Kanayama et al., 2003b; Litt and Dodge, 2008)—but perhaps paradoxically, many users become even more preoccupied with their muscularity after starting AAS (Kanayama et al., 2006; Kanayama et al., in press). Thus, although there is as yet no longitudinal research, to our knowledge, on body image problems among long-term AAS users, it seems plausible that body image concerns may stimulate repeated and escalating AAS use in some individuals, possibly leading to dependence.
4.2. Treatment implications
Although we are not aware of specific treatment studies of muscle dysmorphia per se, many studies have addressed treatment of body dysmorphic disorder in general. In particular, cognitive-behavioral therapy has been shown effective in several studies (McKay, 1999; McKay et al., 1997; Rosen et al., 1995; Veale et al., 1996) and serotonergic antidepressants (for example, selective serotonin reuptake inhibitors and clomipramine) have been found efficacious in placebo-controlled (Hollander et al., 1999; Phillips et al., 2002; Phillips and Rasmussen, 2004) and open-label trials (Allen et al., 2008; Ipser et al., 2009; Phillips, 2006; Phillips and Najjar, 2003). Thus, serotonergic antidepressants might be helpful both for muscle dysmorphia in AAS users and for possible depressive symptoms associated with AAS withdrawal, as described in section 5 below.
5. The androgenic mechanism
5.1. The evidence
As noted above, AAS suppress the HPG axis, causing men to develop decreased production of testosterone and of spermatozoa (Reyes-Fuentes and Veldhuis, 1993; Swerdloff et al., 2002; Tan and Scally, 2009; Urhausen et al., 2003). Partially for this reason, illicit AAS users typically take the drugs in courses, known as “cycles,” lasting from a few weeks to a few months (Llewellyn, 2009; Pope and Brower, 2009; Pope and Katz, 1988). Upon stopping a cycle (particularly a long cycle), male AAS users will often become temporarily hypogonadal as a result of HPG suppression; during the ensuing drug-free interval, the HPG axis can recover, restoring normal testosterone production. Illicit users sometimes ingest human chorionic gonadotropin (HCG) or clomiphene at the end of a cycle to hasten the return of testosterone production, and underground guides for AAS users offer instructions about how to do this (Llewellyn, 2009).
Most AAS users will recover normal HPG function within a few weeks to months, even after prolonged cycles of highly supraphysiologic doses of AAS (Knuth et al., 1989). However, some users appear slow to recover HPG function and demonstrate prolonged hypogonadism, occasionally persisting more than a year after stopping AAS (Boyadjiev et al., 2000; Gazvani et al., 1997; Jarow and Lipshultz, 1990; Menon, 2003; Urhausen et al., 2003; van Breda et al., 2003). Hypogonadism may create a variety of adverse physiologic effects, including changes in body composition (loss of muscle and increased fat), loss of sexual drive and function, and fatigue (Almeida et al., 2004; Tan and Scally, 2009). A growing literature has also shown that AAS withdrawal may induce major depression (Brower, 2002; Malone et al., 1995; Pope and Katz, 2003). The predisposition to major depression may be idiosyncratic, as illustrated by a recent laboratory study of pharmacologically-induced hypogonadism in 31 healthy young men (Schmidt et al., 2004). Most of these men showed only modest subjective effects, such as decreased sexual interest, but three (10%) developed marked depressive symptoms, which were reversed by testosterone. By analogy, some illicit AAS users may show an idiosyncratic vulnerability to depression when hypogonadal during AAS withdrawal, and hence resume AAS to self-treat these symptoms.
5.2. Treatment implications
Like body image disorder, vulnerability to prolonged HPG suppression and associated depression may be both causes and consequences of AAS dependence. Regardless of the direction of causality, however, it seems important that clinicians know how to identify and promptly treat hypogonadal syndromes (Brower, 2009; Tan and Scally, 2009). Men may be reluctant to disclose both prior AAS use and current sexual impairment if not carefully and non-judgmentally asked (Brower, 2002), and they may be unaware that they are hypogonadal unless tested (Kanayama et al., 2007a). If hypogonadism is discovered, it may require evaluation and treatment by an endocrinologist, who might use HCG to accelerate testicular production of testosterone (Medras and Tworowska, 2001; Menon, 2003; Repcekova and Mikulaj, 1977; Turek et al., 1995), perhaps supplemented with clomiphene to stimulate pituitary function (Guay et al., 2003; Santen, 1981; Tan et al., 2009; Tan and Vasudevan, 2003; Winters et al., 1979; Winters and Troen, 1985). One group experienced in treating AAS-induced hypogonadism (Tan and Scally, 2009) has used HCG, clomiphene, and tamoxifen collectively for varying time courses to treat AAS-induced hypogonadism. Phosphodiesterase inhibitors such as sildenafil may help sexual function.
In patients exhibiting depressive symptoms that fail to respond promptly to restorative endocrine treatment, antidepressant treatment is likely indicated, and hospitalization may occasionally be required because of the risk of suicide (Brower et al., 1989; Papazisis et al., 2007; Petersson et al., 2006; Thiblin et al., 2000; Thiblin et al., 1999; Trenton and Currier, 2005). Data on treatment of AAS-associated depression are limited; one small case series reported success with fluoxetine in four cases (Malone and Dimeff, 1992), and another report described a patient hospitalized with AAS-induced depression who failed pharmacotherapy, but responded well to electroconvulsive therapy (Allnutt and Chaimowitz, 1994). Pending further studies, it seems reasonable to approach AAS-induced depression with the same pharmacological and psychosocial strategies as other endogenous depressions—but with careful attention to treating any hypogonadal component.
6. The hedonic mechanism
6.1. The evidence
Though not generally considered intoxicating drugs, AAS nevertheless may produce potentially reinforcing psychoactive effects such as increased self-confidence and aggressiveness (Pope et al., 2000c; Pope and Katz, 2003). These “hedonic” effects may vary across different AAS (Ballard and Wood, 2005), and may be modulated by synergistic effects of other drugs such as alcohol (Johansson et al., 2000; Lindqvist et al., 2002) and stimulants (Kurling et al., 2008). A hedonic mechanism of AAS dependence is supported by observations of the reinforcing effects of androgens in laboratory animals—since animals are presumably not motivated by body-image concerns or anticipation of hypogonadism. Specifically, conditioned place preference studies have shown that male mice (Arnedo et al., 2000; Arnedo et al., 2002) and rats (Alexander et al., 1994; de Beun et al., 1992) will prefer to spend time in a compartment or other environment where they have received subcutaneous injections of testosterone as opposed to compartments where they have received inert vehicle. Furthermore, male hamsters will self-administer oral solutions of testosterone (Johnson and Wood, 2001), and both male rats and male hamsters will self-administer testosterone intravenously (Wood et al., 2004). This reinforcement cannot be explained merely by peripheral actions of androgens on muscle fatigue and joint pain, because even intra-cerebral injections of testosterone can induce conditioned place preference (Packard et al., 1997) or persistent self-administration (Wood et al., 2004).
The mechanisms of these hedonic effects are reviewed in detail elsewhere (Wood, 2008). Briefly, chronic (but not acute) AAS administration appears to increase mesolimbic dopamine synthesis, thus likely causing reinforcement via the same mechanism as natural rewards (e.g., food, sex) and classical addictive drugs. Additionally AAS interact with brain opioid systems, possibly inducing positive and reinforcing mood states through chronic inhibition of the dynorphin/kappa opioid system in the nucleus accumbens. The link between AAS and opioidergic effects is further supported by observations that AAS and opioid dependence frequently co-occur in humans (Kanayama et al., 2009a), possibly because AAS trigger opioid-mediated or modulated neuropathways involved in positive reinforcement, thus sensitizing the brain to the positive and motivational effects of taking opioids.
6.2. Treatment implications
The evidence in section 6.1 suggests that pharmacological and psychosocial treatments effective for substance dependence in general and opioid dependence in particular might also benefit AAS dependence. Looking first at pharmacotherapies, there are three FDA-approved agents for treating opioid dependence: methadone, buprenorphine, and naltrexone. Methadone and buprenorphine are both experienced as positively reinforcing, and thus would seem unsuitable for dependent AAS users unless they have failed other treatments and are comorbidly dependent on opioids. Naltrexone, however, is not positively reinforcing, and it blocks the experience of opioid-mediated positive reinforcement in humans and also blocks testosterone self-administration in hamsters (Peters and Wood, 2005). Moreover, long-acting intramuscular naltrexone has shown efficacy in treating human alcohol dependence, possibly via blocking the effects of endogenous opioids, whose release is enhanced by alcohol (Mannelli et al., 2007; O'Malley et al., 2007). By extension, naltrexone might benefit AAS dependence, since AAS are also non-opioid substances that likely enhance endogenous opioid activity. Should naltrexone be tried, however, it must be remembered that it will precipitate withdrawal in individuals currently taking opioids, and might conceivably precipitate opioid-like withdrawal symptoms in currently dependent AAS users—as suggested by one human case report (Tennant et al., 1988) and by animal observations (Peters and Wood, 2005). In such cases, withdrawal should be treated symptomatically with clonidine, non-narcotic analgesics, and agents for nausea or diarrhea as needed.
Although little has been written on psychosocial treatments for AAS dependence (Brower, 1989; Corcoran and Longo, 1992; Kilmer et al., 2005; Pope and Brower, 2008), it seems plausible that psychosocial treatments for drug dependence, many of which show effectiveness across different drugs (Carroll and Onken, 2005; Stitzer and Vandrey, 2008; Woody, 2003), might also be useful for AAS dependence—especially in the common situation where AAS dependence is comorbid with other substance-use disorders (Kanayama et al., 2009c; Skarberg et al., 2009). As with all substance abusers, AAS-dependent individuals may initially benefit from motivational therapies to address denial and minimization, thus encouraging commitment to treatment (Carroll et al., 2006; Miller and Rollnick, 2002). Additional psychological therapies, empirically validated for the case of opioid dependence, include contingency management, behavioral couples therapy or behavioral family counseling, and supportive-expressive therapy (American Psychiatric Association, 2007). Couples therapy may be particularly indicated, since women may suffer abuse from AAS-using male partners (Choi and Pope, 1994). Importantly, some of these treatments have also been specifically validated for use in conjunction with naltrexone (Carroll and Rounsaville, 2007).
Psychosocial treatment models must acknowledge that AAS users frequently show antisocial traits (Handelsman and Gupta, 1997; Kanayama et al., 2003b; Kindlundh et al., 2001) and more generally features of “cluster B” personality disorders (Perry et al., 2003; Porcerelli and Sandler, 1995; Yates et al., 1990). These traits likely precede AAS use, rather than being purely a consequence of it (Kanayama et al., 2009c), and they may be associated with cognitive deficits in impulsivity, risk-taking, and decision-making (Bickel and Marsch, 2001; de Wit, 2008; Dom et al., 2005; Reynolds, 2006; Verdejo-Garcia and Bechara, 2009; Verdejo-Garcia et al., 2008). These deficits, sometimes characterized as “myopia for the future” (Verdejo-Garcia and Bechara, 2009), may collectively mark an endophenotype (Gottesman and Gould, 2003) associated with an elevated risk of substance dependence (Verdejo-Garcia et al., 2008). Individuals with these attributes may perhaps be particularly vulnerable to the hedonic effects of substances in general, including AAS (Kanayama et al., 2009a). This vulnerability may be further exacerbated by AAS exposure, since androgens may increase sensitivity to reward and decrease sensitivity to punishment, as demonstrated in both animals (Bing et al., 1998; Boissy and Bouissou, 1994) and humans (Hermans et al., 2007; van Honk et al., 2004). However, such traits do not necessarily contraindicate psychotherapy or guarantee adverse treatment outcomes (Woody et al., 1985).
7. Goals of treatment
Summarizing the evidence of the above sections, and extrapolating from the literature on treating other forms of substance dependence, we would tentatively propose six goals for treatment of AAS dependence: 1) build motivation to initiate and maintain abstinence from AAS and all other addictive substances as well as nonmedical use of prescription medications; 2) assist initiation of abstinence by alleviating distressing withdrawal symptoms, which may require pharmacological intervention; and 3) address substance-induced and co-occurring medical and psychiatric disorders, including muscle dysmorphia and persistent suppression of the HPG axis. In addition, patients should be helped to 4) develop a social support system that favors recovery; 5) improve coping skills and self-efficacy for managing stress that may increase risk for relapse; and 6) balance exercise-related behaviors with alternative rewarding activities.
8. Conclusions
Unlike conventional drugs of abuse, AAS may induce dependence via at least three separate pathways—the anabolic, androgenic, and hedonic mechanisms. Human and animal studies in the last decade have greatly expanded our understanding of these three mechanisms, thus suggesting a theoretical model for approaching the treatment of AAS dependence. This model indicates that clinicians should be particularly sensitive to, and prepared to treat 1) underlying body image disorders such as muscle dysmorphia; 2) AAS-induced hypogonadism and possible consequent major depression; and 3) “hedonic” dependence on AAS, with possible comorbid dependence on classical drugs of abuse.
Several limitations of our analysis should be acknowledged. First, our recently proposed diagnostic criteria for AAS dependence (Kanayama et al., 2009b) have yet to be tested for reliability and validity. Thus, there may be many AAS users who do not meet these criteria, but nevertheless suffer from adverse effects of AAS, and hence be candidates for treatment. Second, few published studies have evaluated treatments for AAS dependence, and our discussion might thus seem premature. However, as suggested in section 2, the number of AAS users seeking treatment may be about to increase substantially, as the leading edge of the AAS-using population grows old enough to enter the age of risk for cardiac and psychoneuroendocrine complications. Therefore, it does not seem premature to start evaluating the treatment approaches suggested above. Third, it should be recognized that for each of our three proposed mechanisms of AAS dependence, the causal pathways are likely bidirectional, in that body image disorders, vulnerability to hypogonadal symptoms, and risk-taking/decision-making deficits may each predispose to AAS use, and then in turn be further exacerbated by AAS. However, the direction of causality is not critical to the immediate question of therapeutic intervention, since these attributes deserve treatment regardless of their place in the chain of causality. Future longitudinal studies will be needed to elucidate the nature of these hypothesized causal pathways and the efficacy of our suggested treatment modalities.
Acknowledgments
Author disclosures: Role of funding source: Funding for this study was provided by NIDA Grant DA016744 to Drs. Pope, Kanayama, and Hudson, and by NIDA Grant DA 12843 to Dr. Wood; NIDA had no further role in the preparation or writing of the report; or in the decision to submit the paper for publication Contributors: All authors have materially participated to the research and manuscript preparation. Drs. Kanayama and Pope performed the initial literature search and drafted the first five sections of the manuscript. Drs. Wood and Brower wrote the first draft of section 6 of the manuscript. All five authors contributed to successive revisions of the entire manuscript and have approved the final manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: Dr. Pope has provided expert testimony in legal cases involving anabolic-androgenic steroids on five occasions in the last three years. Dr. Pope declares no other conflicts of interest. All other authors declare that they have no conflicts of interest.
References
- Alexander GM, Packard MG, Hines M. Testosterone has rewarding affective properties in male rats: implications for the biological basis of sexual motivation. Behav. Neurosci. 1994;108:424–428. doi: 10.1037//0735-7044.108.2.424. [DOI] [PubMed] [Google Scholar]
- Allen A, Hadley SJ, Kaplan A, Simeon D, Friedberg J, Priday L, Baker BR, Greenberg JL, Hollander E. An open-label trial of venlafaxine in body dysmorphic disorder. CNS Spectr. 2008;13:138–144. doi: 10.1017/s1092852900016291. [DOI] [PubMed] [Google Scholar]
- Allnutt S, Chaimowitz G. Anabolic steroid withdrawal depression: a case report. Can. J. Psychiatry. 1994;39:317–318. doi: 10.1177/070674379403900525. [DOI] [PubMed] [Google Scholar]
- Almeida OP, Waterreus A, Spry N, Flicker L, Martins RN. One year follow-up study of the association between chemical castration, sex hormones, beta-amyloid, memory and depression in men. Psychoneuroendocrinology. 2004;29:1071–1081. doi: 10.1016/j.psyneuen.2003.11.002. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. Third Edition, Revised American Psychiatric Association; Washington, DC: 1987. [Google Scholar]
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders. fourth edition (DSM-IV) American Psychiatric Association; Washington, DC: 1994. [Google Scholar]
- American Psychiatric Association Practice guideline for the treatment of patients with substance use disorders, second edition. Am. J. Psychiatry. 2007;164(Supplemnt 4):75–123. [PubMed] [Google Scholar]
- Arnedo MT, Salvador A, Martinez-Sanchis S, Gonzalez-Bono E. Rewarding properties of testosterone in intact male mice: a pilot study. Pharmacol. Biochem. Behav. 2000;65:327–332. doi: 10.1016/s0091-3057(99)00189-6. [DOI] [PubMed] [Google Scholar]
- Arnedo MT, Salvador A, Martinez-Sanchis S, Pellicer O. Similar rewarding effects of testosterone in mice rated as short and long attack latency individuals. Addiction Biol. 2002;7:373–379. doi: 10.1080/1355621021000005955. [DOI] [PubMed] [Google Scholar]
- Baker JS, Graham MR, Davies B. Steroid and prescription medicine abuse in the health and fitness community: A regional study. Eur. J. Intern. Med. 2006;17:479–484. doi: 10.1016/j.ejim.2006.04.010. [DOI] [PubMed] [Google Scholar]
- Ballard CL, Wood RI. Intracerebroventricular self-administration of commonly abused anabolicandrogenic steroids in male hamsters (Mesocricetus auratus): nandrolone, drostanolone, oxymetholone, and stanozolol. Behav. Neurosci. 2005;119:752–758. doi: 10.1037/0735-7044.119.3.752. [DOI] [PubMed] [Google Scholar]
- Bickel WK, Marsch LA. Toward a behavioral economic understanding of drug dependence: delay discounting processes. Addiction. 2001;96:73–86. doi: 10.1046/j.1360-0443.2001.961736.x. [DOI] [PubMed] [Google Scholar]
- Bing O, Heilig M, Kakoulidis P, Sundblad C, Wiklund L, Eriksson E. High doses of testosterone increase anticonflict behaviour in rat. Eur Neuropsychopharmacol. 1998;8:321–323. doi: 10.1016/s0924-977x(97)00095-3. [DOI] [PubMed] [Google Scholar]
- Blouin AG, Goldfield GS. Body image and steroid use in male bodybuilders. Int. J. Eat. Disord. 1995;18:159–165. doi: 10.1002/1098-108x(199509)18:2<159::aid-eat2260180208>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Boissy A, Bouissou MF. Effects of androgen treatment on behavioral and physiological responses of heifers to fear-eliciting situations. Horm. Behav. 1994;28:66–83. doi: 10.1006/hbeh.1994.1006. [DOI] [PubMed] [Google Scholar]
- Boyadjiev NP, Georgieva KN, Massaldjieva RI, Gueorguiev SI. Reversible hypogonadism and azoospermia as a result of anabolic-androgenic steroid use in a bodybuilder with personality disorder. A case report. J. Sports Med. Phys. Fitness. 2000;40:271–274. [PubMed] [Google Scholar]
- Brower K. Rehabilitation for anabolic-androgenic steroid dependence. Clin. Sports Med. 1989;1:171–181. [PubMed] [Google Scholar]
- Brower KJ. Anabolic steroid abuse and dependence. Curr. Psychiatry Rep. 2002;4:377–387. doi: 10.1007/s11920-002-0086-6. [DOI] [PubMed] [Google Scholar]
- Brower KJ. Anabolic steroid abuse and dependence in clinical practice. Physician and Sportsmedicine. 2009;37:1–11. doi: 10.3810/psm.2009.12.1751. [DOI] [PubMed] [Google Scholar]
- Brower KJ, Blow FC, Eliopulos GA, Beresford TP. Anabolic androgenic steroids and suicide. Am. J. Psychiatry. 1989;146:1075. doi: 10.1176/ajp.146.8.1075a. [DOI] [PubMed] [Google Scholar]
- Brower KJ, Blow FC, Hill EM. Risk factors for anabolic-androgenic steroid use in men. J. Psychiatr. Res. 1994;28:369–380. doi: 10.1016/0022-3956(94)90019-1. [DOI] [PubMed] [Google Scholar]
- Cafri G, Thompson JK, Ricciardelli L, McCabe M, Smolak L, Yesalis C. Pursuit of the muscular ideal: Physical and psychological consequences and putative risk factors. Clin. Psychol. Rev. 2005;25:215–239. doi: 10.1016/j.cpr.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Carroll KM, Ball SA, Nich C, Martino S, Frankforter TL, Farentinos C, Kunkel LE, Mikulich-Gilbertson SK, Morgenstern J, Obert JL, Polcin D, Snead N, Woody GE. Motivational interviewing to improve treatment engagement and outcome in individuals seeking treatment for substance abuse: a multisite effectiveness study. Drug Alcohol Depend. 2006;81:301–312. doi: 10.1016/j.drugalcdep.2005.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll KM, Onken LS. Behavioral therapies for drug abuse. Am. J. Psychiatry. 2005;162:1452–1460. doi: 10.1176/appi.ajp.162.8.1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll KM, Rounsaville BJ. A perfect platform: combining contingency management with medications for drug abuse. Am. J. Drug Alcohol Abuse. 2007;33:343–365. doi: 10.1080/00952990701301319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi PY, Pope HG., Jr. Violence toward women and illicit androgenic-anabolic steroid use. Ann. Clin. Psychiatry. 1994;6:21–25. doi: 10.3109/10401239409148835. [DOI] [PubMed] [Google Scholar]
- Cohen J, Collins R, Darkes J, Gwartney D. A league of their own: demographics, motivations and patterns of use of 1,955 male adult non-medical anabolic steroid users in the United States. J. Int. Soc. Sports Nutr. 2007;4:12. doi: 10.1186/1550-2783-4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole JC, Smith R, Halford JC, Wagstaff GF. A preliminary investigation into the relationship between anabolic-androgenic steroid use and the symptoms of reverse anorexia in both current and ex-users. Psychopharmacology (Berl) 2003;166:424–429. doi: 10.1007/s00213-002-1352-3. [DOI] [PubMed] [Google Scholar]
- Collins R. Legal Muscle: Anabolics in America. Legal Muscle Publishing; East Meadow, NY: 2002. [Google Scholar]
- Corcoran JP, Longo ED. Psychological treatment of anabolic-androgenic steroid-dependent individuals. J. Subst. Abuse Treat. 1992;9:229–235. doi: 10.1016/0740-5472(92)90065-v. [DOI] [PubMed] [Google Scholar]
- D'Andrea A, Caso P, Salerno G, Scarafile R, De Corato G, Mita C, Di Salvo G, Severino S, Cuomo S, Liccardo B, Esposito N, Calabro R. Left ventricular early myocardial dysfunction after chronic misuse of anabolic androgenic steroids: a Doppler myocardial and strain imaging analysis. Br. J. Sports Med. 2007;41:149–155. doi: 10.1136/bjsm.2006.030171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Beun R, Jansen E, Slangen JL, Van de Poll NE. Testosterone as appetitive and discriminative stimulus in rats: sex- and dose-dependent effects. Physiol. Behav. 1992;52:629–634. doi: 10.1016/0031-9384(92)90389-j. [DOI] [PubMed] [Google Scholar]
- de Wit H. Impulsivity as a determinant and consequence of drug use: a review of underlying processes. Addict. Biol. 2009;14:22–31. doi: 10.1111/j.1369-1600.2008.00129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodge T, Litt D, Seitchik A, Bennett S. Drive for Muscularity and Beliefs about Legal Performance Enhancing Substances as Predictors of Current Use and Willingness to Use. J. Health Psychol. 2008;13:1173–1179. doi: 10.1177/1359105308095970. [DOI] [PubMed] [Google Scholar]
- Dom G, Sabbe B, Hulstijn W, van den Brink W. Substance use disorders and the orbitofrontal cortex: systematic review of behavioural decision-making and neuroimaging studies. Br. J. Psychiatry. 2005;187:209–220. doi: 10.1192/bjp.187.3.209. [DOI] [PubMed] [Google Scholar]
- Frye CA. Some rewarding effects of androgens may be mediated by actions of its 5alpha-reduced metabolite 3alpha-androstanediol. Pharmacol. Biochem. Behav. 2007;86:354–367. doi: 10.1016/j.pbb.2006.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galduroz JC, Noto AR, Nappo SA, Carlini EA. Household survey on drug abuse in Brazil: study involving the 107 major cities of the country--2001. Addict. Behav. 2005;30:545–556. doi: 10.1016/j.addbeh.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Gazvani MR, Buckett W, Luckas MJ, Aird IA, Hipkin LJ, Lewis-Jones DI. Conservative management of azoospermia following steroid abuse. Hum. Reprod. 1997;12:1706–1708. doi: 10.1093/humrep/12.8.1706. [DOI] [PubMed] [Google Scholar]
- Gottesman II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. Am. J. Psychiatry. 2003;160:636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- Gray PB, Yang CF, Pope HG., Jr. Fathers have lower salivary testosterone levels than unmarried men and married non-fathers in Beijing, China. Proc. Biol. Sci. 2006;273:333–339. doi: 10.1098/rspb.2005.3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guay AT, Jacobson J, Perez JB, Hodge MB, Velasquez E. Clomiphene increases free testosterone levels in men with both secondary hypogonadism and erectile dysfunction: who does and does not benefit? Int. J. Impot. Res. 2003;15:156–165. doi: 10.1038/sj.ijir.3900981. [DOI] [PubMed] [Google Scholar]
- Hall RC, Hall RC. Abuse of supraphysiologic doses of anabolic steroids. South. Med. J. 2005;98:550–555. doi: 10.1097/01.SMJ.0000157531.04472.B2. [DOI] [PubMed] [Google Scholar]
- Hall RC, Hall RC, Chapman MJ. Psychiatric complications of anabolic steroid abuse. Psychosomatics. 2005;46:285–290. doi: 10.1176/appi.psy.46.4.285. [DOI] [PubMed] [Google Scholar]
- Handelsman DJ, Gupta L. Prevalence and risk factors for anabolic-androgenic steroid abuse in Australian high school students. Int. J. Androl. 1997;20:159–164. doi: 10.1046/j.1365-2605.1997.d01-285.x. [DOI] [PubMed] [Google Scholar]
- Hartgens F, Kuipers H. Effects of androgenic-anabolic steroids in athletes. Sports Med. 2004;34:513–554. doi: 10.2165/00007256-200434080-00003. [DOI] [PubMed] [Google Scholar]
- Herlitz LC, Markowitz GS, Farris AB, Schwimmer JA, Stokes MB, Kunis C, Colvin RB, D'Agati VD. Development of focal segmental glomerulosclerosis after anabolic steroid abuse. J. Am. Soc. Nephrol. 2009 doi: 10.1681/ASN.2009040450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermans EJ, Putman P, Baas JM, Gecks NM, Kenemans JL, van Honk J. Exogenous testosterone attenuates the integrated central stress response in healthy young women. Psychoneuroendocrinology. 2007;32:1052–1061. doi: 10.1016/j.psyneuen.2007.08.006. [DOI] [PubMed] [Google Scholar]
- Hildebrandt T, Langenbucher JW, Carr SJ, Sanjuan P. Modeling population heterogeneity in appearance- and performance-enhancing drug (APED) use: applications of mixture modeling in 400 regular APED users. J. Abnorm. Psychol. 2007;116:717–733. doi: 10.1037/0021-843X.116.4.717. [DOI] [PubMed] [Google Scholar]
- Hildebrandt T, Schlundt D, Langenbucher J, Chung T. Presence of muscle dysmorphia symptomology among male weightlifters. Compr. Psychiatry. 2006;47:127–135. doi: 10.1016/j.comppsych.2005.06.001. [DOI] [PubMed] [Google Scholar]
- Hochberg Z, Pacak K, Chrousos GP. Endocrine withdrawal syndromes. Endocr. Rev. 2003;24:523–538. doi: 10.1210/er.2001-0014. [DOI] [PubMed] [Google Scholar]
- Hollander E, Allen A, Kwon J, Aronowitz B, Schmeidler J, Wong C, Simeon D. Clomipramine vs desipramine crossover trial in body dysmorphic disorder: selective efficacy of a serotonin reuptake inhibitor in imagined ugliness. Arch. Gen. Psychiatry. 1999;56:1033–1039. doi: 10.1001/archpsyc.56.11.1033. [DOI] [PubMed] [Google Scholar]
- Holt RI, Sonksen PH. Growth hormone, IGF-I and insulin and their abuse in sport. Br. J. Pharmacol. 2008;154:542–556. doi: 10.1038/bjp.2008.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ipser JC, Sander C, Stein DJ. Pharmacotherapy and psychotherapy for body dysmorphic disorder. Cochrane Database Syst. Rev. 2009:CD005332. doi: 10.1002/14651858.CD005332.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarow JP, Lipshultz LI. Anabolic steroid-induced hypogonadotropic hypogonadism. Am. J. Sports Med. 1990;18:429–431. doi: 10.1177/036354659001800417. [DOI] [PubMed] [Google Scholar]
- Johansson P, Lindqvist A, Nyberg F, Fahlke C. Anabolic androgenic steroids affects alcohol intake, defensive behaviors and brain opioid peptides in the rat. Pharmacol. Biochem. Behav. 2000;67:271–279. doi: 10.1016/s0091-3057(00)00365-8. [DOI] [PubMed] [Google Scholar]
- Johnson LR, Wood RI. Oral testosterone self-administration in male hamsters. Neuroendocrinology. 2001;73:285–292. doi: 10.1159/000054645. [DOI] [PubMed] [Google Scholar]
- Johnston LD, O'Malley PM, Bachman JG, Schulenberg JE. Monitoring the Future national survey results on drug use, 1975-2008. Volume II: College students and adults ages 19-50 (NIH Publication No. 09-7402) National Institute on Drug Abuse; Bethesda, MD: 2009. [Google Scholar]
- Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am. J. Psychiatry. 2005;162:1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- Kanayama G, Amiaz R, Seidman S, Pope HG., Jr. Testosterone supplementation for depressed men: current research and suggested treatment guidelines. Exp. Clin. Psychopharmacol. 2007a;15:529–538. doi: 10.1037/1064-1297.15.6.529. [DOI] [PubMed] [Google Scholar]
- Kanayama G, Barry S, Hudson JI, Pope HG., Jr. Body image and attitudes toward male roles in anabolic-androgenic steroid users. Am. J. Psychiatry. 2006;163:697–703. doi: 10.1176/ajp.2006.163.4.697. [DOI] [PubMed] [Google Scholar]
- Kanayama G, Boynes M, Hudson JI, Field AE, Pope HG., Jr. Anabolic steroid abuse among teenage girls: An illusory problem? Drug Alcohol Depend. 2007b;88:156–162. doi: 10.1016/j.drugalcdep.2006.10.013. PMC 1978191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanayama G, Brower KJ, Wood RI, Hudson JI, Pope HG. Anabolic-androgenic steroid dependence: an emerging disorder. Addiction. 2009a;104:1966–1978. doi: 10.1111/j.1360-0443.2009.02734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanayama G, Brower KJ, Wood RI, Hudson JI, Pope HG., Jr. Issues for DSM-V: clarifying the diagnostic criteria for anabolic-androgenic steroid dependence. Am. J. Psychiatry. 2009b;166:642–645. doi: 10.1176/appi.ajp.2009.08111699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanayama G, Cohane GH, Weiss RD, Pope HG. Past anabolic-androgenic steroid use among men admitted for substance abuse treatment: an underrecognized problem? J. Clin. Psychiatry. 2003a;64:156–160. doi: 10.4088/jcp.v64n0208. [DOI] [PubMed] [Google Scholar]
- Kanayama G, Hudson JI, Pope HG. Illicit anabolic-androgenic steroid use. Hormones and Behavior. doi: 10.1016/j.yhbeh.2009.09.006. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanayama G, Hudson JI, Pope HG., Jr. Long-term psychiatric and medical consequences of anabolic-androgenic steroid abuse: a looming public health concern? Drug Alcohol Depend. 2008;98:1–12. doi: 10.1016/j.drugalcdep.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanayama G, Hudson JI, Pope HG., Jr. Features of men with anabolic-androgenic steroid dependence: A comparison with nondependent AAS users and with AAS nonusers. Drug Alcohol Depend. 2009c;102:130–137. doi: 10.1016/j.drugalcdep.2009.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanayama G, Pope HG, Cohane G, Hudson JI. Risk factors for anabolic-androgenic steroid use among weightlifters: a case-control study. Drug Alcohol Depend. 2003b;71:77–86. doi: 10.1016/s0376-8716(03)00069-3. [DOI] [PubMed] [Google Scholar]
- Kashkin KB, Kleber HD. Hooked on hormones? An anabolic steroid addiction hypothesis. J.A.M.A. 1989;262:3166–3170. doi: 10.1001/jama.262.22.3166. [DOI] [PubMed] [Google Scholar]
- Kasikcioglu E, Oflaz H, Umman B, Bugra Z. Androgenic anabolic steroids also impair right ventricular function. Int. J. Cardiol. 2009;134:123–125. doi: 10.1016/j.ijcard.2007.12.027. [DOI] [PubMed] [Google Scholar]
- Kilmer J, Cronce J, Palmer R. Relapse prevention for abuse of club drugs, hallucinogens, inhalants, and steroids. In: Marlatt G, Donovan D, editors. Relapse prevention: maintenance strategies in the treatment of addictive behaviors. second edition Guilford Press; New York: 2005. [Google Scholar]
- Kindlundh AM, Hagekull B, Isacson DG, Nyberg F. Adolescent use of anabolic-androgenic steroids and relations to self-reports of social, personality and health aspects. Eur. J. Public Health. 2001;11:322–328. doi: 10.1093/eurpub/11.3.322. [DOI] [PubMed] [Google Scholar]
- Knuth UA, Maniera H, Nieschlag E. Anabolic steroids and semen parameters in bodybuilders. Fertil. Steril. 1989;52:1041–1047. doi: 10.1016/s0015-0282(16)53172-0. [DOI] [PubMed] [Google Scholar]
- Kokkevi A, Fotiou A, Chileva A, Nociar A, Miller P. Daily exercise and anabolic steroids use in adolescents: a cross-national European study. Subst. Use Misuse. 2008;43:2053–2065. doi: 10.1080/10826080802279342. [DOI] [PubMed] [Google Scholar]
- Kouri EM, Pope HG, Jr., Katz DL, Oliva P. Fat-free mass index in users and nonusers of anabolic-androgenic steroids. Clin. J. Sport Med. 1995;5:223–228. doi: 10.1097/00042752-199510000-00003. [DOI] [PubMed] [Google Scholar]
- Krieg A, Scharhag J, Albers T, Kindermann W, Urhausen A. Cardiac tissue Doppler in steroid users. Int. J. Sports Med. 2007;28:638–643. doi: 10.1055/s-2007-964848. [DOI] [PubMed] [Google Scholar]
- Kurling S, Kankaanpaa A, Seppala T. Sub-chronic nandrolone treatment modifies neurochemical and behavioral effects of amphetamine and 3,4-methylenedioxymethamphetamine (MDMA) in rats. Behav Brain Res. 2008;189:191–201. doi: 10.1016/j.bbr.2007.12.021. [DOI] [PubMed] [Google Scholar]
- Li J, Al-Azzawi F. Mechanism of androgen receptor action. Maturitas. 2009;63:142–148. doi: 10.1016/j.maturitas.2009.03.008. [DOI] [PubMed] [Google Scholar]
- Lindqvist AS, Johansson-Steensland P, Nyberg F, Fahlke C. Anabolic androgenic steroid affects competitive behaviour, behavioural response to ethanol and brain serotonin levels. Behav. Brain Res. 2002;133:21–29. doi: 10.1016/s0166-4328(01)00408-9. [DOI] [PubMed] [Google Scholar]
- Litt D, Dodge T. A longitudinal investigation of the Drive for Muscularity Scale: predicting use of performance enhancing substances and weightlifting among males. Body Image. 2008;5:346–351. doi: 10.1016/j.bodyim.2008.04.002. [DOI] [PubMed] [Google Scholar]
- Liu PY, Death AK, Handelsman DJ. Androgens and cardiovascular disease. Endocr. Rev. 2003;24:313–340. doi: 10.1210/er.2003-0005. [DOI] [PubMed] [Google Scholar]
- Llewellyn W. Molecular Nutrition. 9th Edition Jupiter; Florida: 2009. Anabolics. [Google Scholar]
- Malone DA, Jr., Dimeff RJ. The use of fluoxetine in depression associated with anabolic steroid withdrawal: a case series. J. Clin. Psychiatry. 1992;53:130–132. [PubMed] [Google Scholar]
- Malone DA, Jr., Dimeff RJ, Lombardo JA, Sample RH. Psychiatric effects and psychoactive substance use in anabolic-androgenic steroid users. Clin. J. Sport Med. 1995;5:25–31. doi: 10.1097/00042752-199501000-00005. [DOI] [PubMed] [Google Scholar]
- Mannelli P, Peindl K, Masand PS, Patkar AA. Long-acting injectable naltrexone for the treatment of alcohol dependence. Expert. Rev. Neurother. 2007;7:1265–1277. doi: 10.1586/14737175.7.10.1265. [DOI] [PubMed] [Google Scholar]
- Maravelias C, Dona A, Stefanidou M, Spiliopoulou C. Adverse effects of anabolic steroids in athletes. A constant threat. Toxicol. Lett. 2005;158:167–175. doi: 10.1016/j.toxlet.2005.06.005. [DOI] [PubMed] [Google Scholar]
- McBride AJ, Williamson K, Petersen T. Three cases of nalbuphine hydrochloride dependence associated with anabolic steroid use. Br. J. Sports Med. 1996;30:69–70. doi: 10.1136/bjsm.30.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay D. Two-year follow-up of behavioral treatment and maintenance for body dysmorphic disorder. Behav. Modif. 1999;23:620–629. doi: 10.1177/0145445599234006. [DOI] [PubMed] [Google Scholar]
- McKay D, Todaro J, Neziroglu F, Campisi T, Moritz EK, Yaryura-Tobias JA. Body dysmorphic disorder: a preliminary evaluation of treatment and maintenance using exposure with response prevention. Behav. Res. Ther. 1997;35:67–70. doi: 10.1016/s0005-7967(96)00082-4. [DOI] [PubMed] [Google Scholar]
- Medras M, Tworowska U. Treatment strategies of withdrawal from long-term use of anabolicandrogenic steroids. Pol. Merkur Lekarski. 2001;11:535–538. [PubMed] [Google Scholar]
- Menon DK. Successful treatment of anabolic steroid-induced azoospermia with human chorionic gonadotropin and human menopausal gonadotropin. Fertil. Steril. 2003;79(Suppl 3):1659–1661. doi: 10.1016/s0015-0282(03)00365-0. [DOI] [PubMed] [Google Scholar]
- Miller WR, Rollnick S. Motivational Interviewing: Preparing People for Change. second edition Guilford Press; New York: 2002. [Google Scholar]
- Nilsson S, Baigi A, Marklund B, Fridlund B. The prevalence of the use of androgenic anabolic steroids by adolescents in a county of Sweden. Eur. J. Public Health. 2001;11:195–197. doi: 10.1093/eurpub/11.2.195. [DOI] [PubMed] [Google Scholar]
- O'Malley SS, Garbutt JC, Gastfriend DR, Dong Q, Kranzler HR. Efficacy of extended-release naltrexone in alcohol-dependent patients who are abstinent before treatment. J. Clin. Psychopharmacol. 2007;27:507–512. doi: 10.1097/jcp.0b013e31814ce50d. [DOI] [PubMed] [Google Scholar]
- Olivardia R, Pope HG, Jr., Hudson JI. Muscle dysmorphia in male weightlifters: a case-control study. Am. J. Psychiatry. 2000;157:1291–1296. doi: 10.1176/appi.ajp.157.8.1291. [DOI] [PubMed] [Google Scholar]
- Packard MG, Cornell AH, Alexander GM. Rewarding affective properties of intra-nucleus accumbens injections of testosterone. Behav. Neurosci. 1997;111:219–224. doi: 10.1037//0735-7044.111.1.219. [DOI] [PubMed] [Google Scholar]
- Papazisis G, Kouvelas D, Mastrogianni A, Karastergiou A. Anabolic androgenic steroid abuse and mood disorder: a case report. Int. J. Neuropsychopharmacol. 2007;10:291–293. doi: 10.1017/S1461145706007243. [DOI] [PubMed] [Google Scholar]
- Perry PJ, Kutscher EC, Lund BC, Yates WR, Holman TL, Demers L. Measures of aggression and mood changes in male weightlifters with and without androgenic anabolic steroid use. J. Forensic Sci. 2003;48:646–651. [PubMed] [Google Scholar]
- Peters KD, Wood RI. Androgen dependence in hamsters: overdose, tolerance, and potential opioidergic mechanisms. Neuroscience. 2005;130:971–981. doi: 10.1016/j.neuroscience.2004.09.063. [DOI] [PubMed] [Google Scholar]
- Petersson A, Garle M, Holmgren P, Druid H, Krantz P, Thiblin I. Toxicological findings and manner of death in autopsied users of anabolic androgenic steroids. Drug Alcohol Depend. 2006;81:241–249. doi: 10.1016/j.drugalcdep.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Phillips KA. An open-label study of escitalopram in body dysmorphic disorder. Int Clin Psychopharmacol. 2006;21:177–179. doi: 10.1097/01.yic.0000194378.65460.ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips KA, Albertini RS, Rasmussen SA. A randomized placebo-controlled trial of fluoxetine in body dysmorphic disorder. Arch. Gen. Psychiatry. 2002;59:381–388. doi: 10.1001/archpsyc.59.4.381. [DOI] [PubMed] [Google Scholar]
- Phillips KA, Najjar F. An open-label study of citalopram in body dysmorphic disorder. J. Clin. Psychiatry. 2003;64:715–720. doi: 10.4088/jcp.v64n0615. [DOI] [PubMed] [Google Scholar]
- Phillips KA, Rasmussen SA. Change in psychosocial functioning and quality of life of patients with body dysmorphic disorder treated with fluoxetine: a placebo-controlled study. Psychosomatics. 2004;45:438–444. doi: 10.1176/appi.psy.45.5.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope H, Phillips K, Olivardia R. The Adonis complex: The secret crisis of male body obsession. Simon & Schuster; New York: 2000a. [Google Scholar]
- Pope HG, Brower KJ. Treatment of anabolic-androgenic steroid-related disorders. In: Galanter M, Kleber H, editors. The American Psychiatric Publishing Textbook of Substance Abuse Treatment. Fourth Edition American Psychiatric Publishing; Washington, DC: 2008. pp. 237–246. [Google Scholar]
- Pope HG, Brower KJ. Anabolic-Androgenic Steroid-Related Disorders. In: Sadock B, Sadock V, editors. Comprehensive Textbook of Psychiatry. Ninth Edition Lippincott Williams & Wilkins; Philadelphia, PA: 2009. pp. 1419–1431. [Google Scholar]
- Pope HG, Jr., Gruber AJ, Choi P, Olivardia R, Phillips KA. Muscle dysmorphia. An underrecognized form of body dysmorphic disorder. Psychosomatics. 1997;38:548–557. doi: 10.1016/S0033-3182(97)71400-2. [DOI] [PubMed] [Google Scholar]
- Pope HG, Jr., Gruber AJ, Mangweth B, Bureau B, deCol C, Jouvent R, Hudson JI. Body image perception among men in three countries. Am. J. Psychiatry. 2000b;157:1297–1301. doi: 10.1176/appi.ajp.157.8.1297. [DOI] [PubMed] [Google Scholar]
- Pope HG, Jr., Katz DL. Affective and psychotic symptoms associated with anabolic steroid use. Am. J. Psychiatry. 1988;145:487–490. doi: 10.1176/ajp.145.4.487. [DOI] [PubMed] [Google Scholar]
- Pope HG, Jr., Katz DL, Hudson JI. Anorexia nervosa and “reverse anorexia” among 108 male bodybuilders. Compr. Psychiatry. 1993;34:406–409. doi: 10.1016/0010-440x(93)90066-d. [DOI] [PubMed] [Google Scholar]
- Pope HG, Jr., Kouri EM, Hudson JI. Effects of supraphysiologic doses of testosterone on mood and aggression in normal men: a randomized controlled trial. Arch Gen Psychiatry. 2000c;57:133–140. doi: 10.1001/archpsyc.57.2.133. discussion 155-136. [DOI] [PubMed] [Google Scholar]
- Pope HG, Jr., Olivardia R, Borowiecki JJ, 3rd, Cohane GH. The growing commercial value of the male body: a longitudinal survey of advertising in women's magazines. Psychother. Psychosom. 2001;70:189–192. doi: 10.1159/000056252. [DOI] [PubMed] [Google Scholar]
- Pope HG, Jr., Olivardia R, Gruber A, Borowiecki J. Evolving ideals of male body image as seen through action toys. Int. J. Eat. Disord. 1999;26:65–72. doi: 10.1002/(sici)1098-108x(199907)26:1<65::aid-eat8>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Pope HG, Kanayama G, Ionescu-Pioggia M, Hudson JI. Anabolic steroid users' attitudes towards physicians. Addiction. 2004;99:1189–1194. doi: 10.1111/j.1360-0443.2004.00781.x. [DOI] [PubMed] [Google Scholar]
- Pope HG, Katz DL. Psychiatric effects of exogenous anabolic-androgenic steroids. In: Wolkowitz OM, Rothschild AJ, editors. Psychoneuroendocrinology: The scientific basis of clinical practice. American Psychiatric Press; Washington, DC: 2003. pp. 331–358. [Google Scholar]
- Porcerelli JH, Sandler BA. Narcissism and empathy in steroid users. Am. J. Psychiatry. 1995;152:1672–1674. doi: 10.1176/ajp.152.11.1672. [DOI] [PubMed] [Google Scholar]
- Repcekova D, Mikulaj L. Plasma testosterone response to HCG in normal men without and after administration of anabolic drug. Endokrinologie. 1977;69:115–118. [PubMed] [Google Scholar]
- Reyes-Fuentes A, Veldhuis JD. Neuroendocrine physiology of the normal male gonadal axis. Endocrinol. Metab. Clin. North Am. 1993;22:93–124. [PubMed] [Google Scholar]
- Reynolds B. A review of delay-discounting research with humans: relations to drug use and gambling. Behav. Pharmacol. 2006;17:651–667. doi: 10.1097/FBP.0b013e3280115f99. [DOI] [PubMed] [Google Scholar]
- Rosen JC, Reiter J, Orosan P. Cognitive-behavioral body image therapy for body dysmorphic disorder. J. Consult. Clin. Psychol. 1995;63:263–269. doi: 10.1037//0022-006x.63.2.263. [DOI] [PubMed] [Google Scholar]
- Santen RJ. Feedback control of luteinizing hormone and follicle-stimulating hormone secretion by testosterone and estradiol in men: physiological and clinical implications. Clin. Biochem. 1981;14:243–251. doi: 10.1016/s0009-9120(81)90964-4. [DOI] [PubMed] [Google Scholar]
- Schmidt PJ, Berlin KL, Danaceau MA, Neeren A, Haq NA, Roca CA, Rubinow DR. The effects of pharmacologically induced hypogonadism on mood in healthy men. Arch. Gen. Psychiatry. 2004;61:997–1004. doi: 10.1001/archpsyc.61.10.997. [DOI] [PubMed] [Google Scholar]
- Skarberg K, Nyberg F, Engstrom I. Multisubstance use as a feature of addiction to anabolicandrogenic steroids. Eur. Addict. Res. 2009;15:99–106. doi: 10.1159/000199045. [DOI] [PubMed] [Google Scholar]
- Stitzer ML, Vandrey R. Contingency management: utility in the treatment of drug abuse disorders. Clin. Pharmacol. Ther. 2008;83:644–647. doi: 10.1038/sj.clpt.6100508. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration National Survey on Drug Use and Health (formerly called the National Household Survey) United States Department of Health and Human Services. 1994 SAMHSA. Available online at: http://www.oas.samhsa.gov/nhsda.htm; data for 1994-B survey available online at: http://www.icpsr.umich.edu/cgi-bin/SDA/SAMHDA/hsda?samhda+nhsda94b.
- Swerdloff RS, Wang C, Hikim APS. In: Hypothalamic-pituitary-gonadal axis in men. Pfaff D, Arnold A, Etgen A, Fahrbach S, Rubin R, editors. Hormones, Brain and Behavior; Elsevier, San Diego: 2002. pp. 1–36. [Google Scholar]
- Tan RS, Carrejo MH, Chen D. An unusual case of vascular hypogonadism treated with clomiphene citrate and testosterone replacement. Andrologia. 2009;41:63–65. doi: 10.1111/j.1439-0272.2008.00891.x. [DOI] [PubMed] [Google Scholar]
- Tan RS, Scally MC. Anabolic steroid-induced hypogonadism--towards a unified hypothesis of anabolic steroid action. Med. Hypotheses. 2009;72:723–728. doi: 10.1016/j.mehy.2008.12.042. [DOI] [PubMed] [Google Scholar]
- Tan RS, Vasudevan D. Use of clomiphene citrate to reverse premature andropause secondary to steroid abuse. Fertil. Steril. 2003;79:203–205. doi: 10.1016/s0015-0282(02)04550-8. [DOI] [PubMed] [Google Scholar]
- Tennant F, Black DL, Voy RO. Anabolic steroid dependence with opioid-type features. N. Engl. J. Med. 1988;319:578. doi: 10.1056/NEJM198809013190910. [DOI] [PubMed] [Google Scholar]
- Thiblin I, Lindquist O, Rajs J. Cause and manner of death among users of anabolic androgenic steroids. J. Forensic Sci. 2000;45:16–23. [PubMed] [Google Scholar]
- Thiblin I, Runeson B, Rajs J. Anabolic androgenic steroids and suicide. Ann. Clin. Psychiatry. 1999;11:223–231. doi: 10.1023/a:1022313529794. [DOI] [PubMed] [Google Scholar]
- Trenton AJ, Currier GW. Behavioural manifestations of anabolic steroid use. CNS Drugs. 2005;19:571–595. doi: 10.2165/00023210-200519070-00002. [DOI] [PubMed] [Google Scholar]
- Turek PJ, Williams RH, Gilbaugh JH, 3rd, Lipshultz LI. The reversibility of anabolic steroid-induced azoospermia. J. Urol. 1995;153:1628–1630. [PubMed] [Google Scholar]
- Urhausen A, Torsten A, Wilfried K. Reversibility of the effects on blood cells, lipids, liver function and hormones in former anabolic-androgenic steroid abusers. J. Steroid Biochem. Mol. Biol. 2003;84:369–375. doi: 10.1016/s0960-0760(03)00105-5. [DOI] [PubMed] [Google Scholar]
- van Breda E, Keizer HA, Kuipers H, Wolffenbuttel BH. Androgenic anabolic steroid use and severe hypothalamic-pituitary dysfunction: a case study. Int. J. Sports Med. 2003;24:195–196. doi: 10.1055/s-2003-39089. [DOI] [PubMed] [Google Scholar]
- van Honk J, Schutter DJ, Hermans EJ, Putman P, Tuiten A, Koppeschaar H. Testosterone shifts the balance between sensitivity for punishment and reward in healthy young women. Psychoneuroendocrinology. 2004;29:937–943. doi: 10.1016/j.psyneuen.2003.08.007. [DOI] [PubMed] [Google Scholar]
- Veale D, Gournay K, Dryden W, Boocock A, Shah F, Willson R, Walburn J. Body dysmorphic disorder: a cognitive behavioural model and pilot randomised controlled trial. Behav. Res. Ther. 1996;34:717–729. doi: 10.1016/0005-7967(96)00025-3. [DOI] [PubMed] [Google Scholar]
- Verdejo-Garcia A, Bechara A. A somatic marker theory of addiction. Neuropharmacology. 2009;56(Suppl 1):48–62. doi: 10.1016/j.neuropharm.2008.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdejo-Garcia A, Lawrence AJ, Clark L. Impulsivity as a vulnerability marker for substance-use disorders: review of findings from high-risk research, problem gamblers and genetic association studies. Neurosci. Biobehav. Rev. 2008;32:777–810. doi: 10.1016/j.neubiorev.2007.11.003. [DOI] [PubMed] [Google Scholar]
- Wade N. Anabolic steroids: doctors denounce them, but athletes aren't listening. Science. 1972;176:1399–1403. doi: 10.1126/science.176.4042.1399. [DOI] [PubMed] [Google Scholar]
- Weiner RB, Kanayama G, Hudson JI, Hutter AM, Picard MH, Pope HG, Baggish AL. Abstract 3048: Chronic anabolic-androgenic steroid use is associated with left ventricular systolic and diastolic dysfunction. Circulation. 2009;120:S741. doi: 10.1161/CIRCHEARTFAILURE.109.931063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wines JD, Jr., Gruber AJ, Pope HG, Jr., Lukas SE. Nalbuphine hydrochloride dependence in anabolic steroid users. Am. J. Addict. 1999;8:161–164. doi: 10.1080/105504999305965. [DOI] [PubMed] [Google Scholar]
- Winters SJ, Janick JJ, Loriaux DL, Sherins RJ. Studies on the role of sex steroids in the feedback control of gonadotropin concentrations in men. II. Use of the estrogen antagonist, clomiphene citrate. J. Clin. Endocrinol. Metab. 1979;48:222–227. doi: 10.1210/jcem-48-2-222. [DOI] [PubMed] [Google Scholar]
- Winters SJ, Troen P. Evidence for a role of endogenous estrogen in the hypothalamic control of gonadotropin secretion in men. J. Clin. Endocrinol. Metab. 1985;61:842–845. doi: 10.1210/jcem-61-5-842. [DOI] [PubMed] [Google Scholar]
- Wood RI. Anabolic-androgenic steroid dependence? Insights from animals and humans. Front Neuroendocrinol. 2008;29:490–506. doi: 10.1016/j.yfrne.2007.12.002. PMC 2585375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood RI, Johnson LR, Chu L, Schad C, Self DW. Testosterone reinforcement: intravenous and intracerebroventricular self-administration in male rats and hamsters. Psychopharmacology (Berl) 2004;171:298–305. doi: 10.1007/s00213-003-1587-7. [DOI] [PubMed] [Google Scholar]
- Woody GE. Research findings on psychotherapy of addictive disorders. Am. J. Addict. 2003;12(Suppl 2):S19–26. [PubMed] [Google Scholar]
- Woody GE, McLellan AT, Luborsky L, O'Brien CP. Sociopathy and psychotherapy outcome. Arch. Gen. Psychiatry. 1985;42:1081–1086. doi: 10.1001/archpsyc.1985.01790340059009. [DOI] [PubMed] [Google Scholar]
- Yang CF, Gray P, Pope HG., Jr. Male body image in Taiwan versus the West: Yanggang Zhiqi meets the Adonis complex. Am. J. Psychiatry. 2005;162:263–269. doi: 10.1176/appi.ajp.162.2.263. [DOI] [PubMed] [Google Scholar]
- Yates WR, Perry PJ, Andersen KH. Illicit anabolic steroid use: a controlled personality study. Acta. Psychiatr. Scand. 1990;81:548–550. doi: 10.1111/j.1600-0447.1990.tb05496.x. [DOI] [PubMed] [Google Scholar]