Abstract
This study describes how age and high fat diet affect the profile of NADPH oxidase (NOX). Specifically, NOX activity and subunit expression were evaluated in the frontal cerebral cortex of 7-, 16-, and 24-month old mice following a 4-month exposure to either Western diet (WD, 41% calories from fat) or very high fat lard diet (VHFD, 60% calories from fat). Data reveal a significant effect of age in on NOX activity, and show that NOX activity was only increased by VHFD, and only in 24-month old mice. NOX subunit expression was also increased by diet only in older mice. Quantification of protein carbonyls revealed significant age-related increases in protein oxidation, and indicate that only aged mice respond to high fat diet with enhanced protein oxidation. Histological analyses indicate prominent neuronal localization of both NOX subunits and protein carbonylation. Finally, data indicate that changes in reactive microgliosis, but not astrocytosis, mirror the pattern of diet-induced NOX activation and protein oxidation. Collectively, these data show that both age and dietary fat drive NOX activation, and further indicate that aged mice are preferentially sensitive to the effects of high fat diet. These data also suggest that high fat diets might exacerbate age-related oxidative stress in the brain via increased NOX.
Keywords: metabolic dysfunction, neurodegeneration, obesity, oxidative stress, Western diet
INTRODUCTION
Metabolic syndrome is a clinical condition characterized by systemic abnormalities that can include combinations of abdominal obesity, insulin resistance or glucose intolerance, dyslipidemia, hypertension, and increased expression of prothrombotic and proinflammatory markers (reviewed in [1]). In the United States and similar industrialized nations, obesity caused by long-term consumption of diets high in fat and calories appears to be the primary cause of metabolic syndrome, which is associated with a dramatically enhanced risk for a myriad of diseases, including type 2 diabetes, cardiovascular disease, gastrointestinal and respiratory difficulties, stroke, and many types of cancer (reviewed in [2]). Because so many of these clinical syndromes are also associated with aging, it can be hypothesized that obesity and high fat diet consumption modulate aging processes to promote and/or accelerate disease progression.
The brain may be one of the more crucial sites at the intersection of age and metabolic dysfunction, as an especially costly and debilitating deficit of aging is cognitive dysfunction and increased risk of dementia. Indeed, studies have reported deficits in learning, memory, and executive function in obese as compared to nonobese patients [3], [4], [5], and regression studies have demonstrated that both age and increased body weight are associated with decreased brain volume [6]. Other studies have confirmed alterations of brain morphology in overweight and obese young adults, and further show that clinical obesity is associated with reductions in focal gray matter volume and enlarged white matter, particularly in the frontal lobe [7]. Although the physiologic mechanisms whereby obesity adversely affects the brain are poorly understood, both experimental and epidemiological studies have shown that metabolic dysfunction is highly associated with increased oxidative stress (reviewed in [8]). This observation is noteworthy as oxidative stress is widely believed to be a key player in neurodegeneration [9], [10], and oxidative modifications specifically to proteins have been documented in both aging [11] and age-related dementia disorders such as Alzheimer’s disease [12]. Furthermore, several studies have shown that the administration of high fat or high calorie diets to rodents can specifically increase free radical generation [13] and protein oxidation [14] in the brain.
Collectively, the reports described above support the hypothesis that excess dietary fat can detrimentally affect the brain at least in part via increases in oxidative stress. While it remains unclear how obesity and/or high fat diets trigger brain oxidative stress, recent reports have implicated activation of NADPH oxidase (NOX) in the detrimental effects of diet-induced obesity. NOX is a superoxide-producing enzyme system consisting of membrane (gp91phox and p22phox) and cytosolic (p47phox, p67phox, and p40phox) components which assemble at the plasma membrane to form the active oxidase [15], [16]. Experimental studies in rats have shown that obesity-associated endothelial cell dysfunction is mediated by NOX-induced oxidative stress [17]. NOX has also been shown in human studies to mediate obesity-induced oxidative stress in blood mononuclear [18] and endothelial cells [19]. While the effects of diet-induced obesity on NOX in the CNS have not been widely studied, the expression of NOX subunits is increased in the brains of young rats exposed to high fat diets [13], and NOX has also been repeatedly implicated in the production of free radicals thought to contribute to CNS disease, particularly in aging [20], [21], [22]. This study was thus undertaken to determine whether high fat diets affect the age-related profile of NOX in the brain. Specifically, NOX enzymatic activity and subunit expression were measured in the cerebral cortex of 7-, 16-, and 24-month old mice that had been exposed for 4 months to 1 of 2 different high fat diets. The diets used were the so-called “Western” diet (WD, 41% calories from fat, and its control diet C-WD); and a high fat lard diet (VHFD, 60 percent calories from fat, and its control diet C-VHFD). In addition to evaluating NOX activity and subunit expression, the expression of markers of glial reactivity and oxidative stress were quantified to uncover potential mechanisms and consequences of NOX activation.
MATERIALS AND METHODS
Diets and Animals
All experimental animal procedures and protocols were compliant with NIH guidelines on the use of experimental animals, and were approved by the PBRC Institutional Animal Care and Use Committee. Male C57Bl/6 mice of increasing age (3-, 12-, 20-months old) were purchased from the contract colony maintained by the National Institute on Aging at Charles River Laboratories (Wilmington, MA) and placed for 16 weeks on Western diet (WD, D12079B), High Fat Lard diet (VHFD, D12492), or their corresponding low fat control diets (C-WD: 98052602; and C-VHFD: D12450B). The WD is composed of 41% fat (butterfat and corn oil) and 29% sucrose, while the VHFD is composed of 60% fat (pork lard), and the control diets were each composed of 10% fat. All diets were purchased from Research Diets (New Brunswick, NJ), and were provided in pelleted form. All mice were housed individually in standard caging with 12:12 light: dark cycle, and had ad libitum access to feed formulations and water throughout the study. Data were compiled from 2 separate cohorts of mice, with a total of 9–13 animals in each group.
Body weight of all mice was measured regularly throughout the duration of diet exposure. Near the end of the 16-week diet exposure period, body composition was measured using a Bruker minispec LF90 time domain NMR analyzer (Bruker Optics, Billerica MA). All mice were humanely euthanatized, and the cortical brain tissues were immediately collected and stored in −80°C.
Measures of NOX activity
Samples taken from the frontal cerebral cortex of mice of different ages/diets were homogenized in Tris-buffered saline (pH 7.4) lysis buffer containing protease inhibitor cocktail (Sigma-Aldrich, Inc., St. Louis, MO) at 4°C, and then subjected to differential centrifugation to isolate membranes. Membrane samples (10–25 µg total protein) were incubated with 5 µM lucigenin and 100 µM NADPH, and NOX activity was measured immediately by documenting the light produced by each sample at 37°C. Light emission was recorded from each sample in 10 second intervals for exactly 3 minutes. The specific role of NOX in the measured luminescence was determined by subtracting the background level of luminescence for each sample, which was generated by the inclusion of the flavoprotein inhibitor diphenyleneiodonium (DPI; 1 µM). NOX activity is presented as average luminescent counts per minute (CPM) per microgram protein.
Measures of protein expression by Western blot
Tissue samples were taken from the frontal cerebral cortex of diet-exposed mice and homogenized in a Tris-buffered saline (pH 7.4) lysis buffer containing 0.1% Triton X-100, 5 mM EDTA, and protease inhibitor cocktail (Sigma-Aldrich, Inc.). Samples were denatured in SDS, and equivalent amounts of protein were electrophoretically separated in polyacrylamide gels and blotted onto nitrocellulose. Blots were processed using the following primary antisera: anti-gp91phox (1:1000, BD Biosciences Inc., San Jose, California); anti -p47phox (1:1000, Millipore, Billerica, MA); anti-GFAP (1:5000, Abcam Inc., Cambridge, MA); anti-Iba-1 (1:500, Wako Chemicals USA Inc., Richmond, VA), and anti-tubulin (1:1000, Wako Chemicals USA Inc.). After incubation with primary antibodies, blots were washed and exposed to horseradish peroxidase-conjugated secondary antibodies, and visualized using a chemiluminescence system (Amersham Biosciences, Pittsburgh, PA). Blot images were scanned and densitometrically analyzed for quantification.
To ensure accurate quantification across multiple blots, samples from all groups in a given age (WD, HFD, and CD for both diets) were included in each individual blot. Data were calculated as a ratio of expression over tubulin expression, which was included as an internal loading control. Protein expression in WD or HFD mice was then calculated and presented as percent expression in CD mice of the same age.
Analyses of protein oxidation
Oxidative modifications to proteins were evaluated by quantifying protein carbonylation in brain tissue homogenates by spectrophotometric analyses using modifications of previously described procedures [23], [24]. Cortical tissue samples from all mice were homogenized in PBS (pH 7.0) containing protease inhibitor cocktail (Sigma Aldrich, Inc.). Homogenates (10 µg of total protein) were incubated with an excess of 2,4-dinitrophenylhydrazone (DNPH) for 20 minutes, followed by the addition of 12% sodium dodecylsulfate (SDS). Each experiment included samples that underwent the protein carbonyl detection procedure without the derivatization step (negative controls). Protein carbonyls were quantified by monitoring the absorbance at 370 nm in a quartz 96 well plate using a spectrophotometer, and calculated using extinction coefficient of 22.0 M−1 × cm−1 for aliphatic hydrazones. Data are reported as nmol protein carbonyls per mg of total protein.
Histological analyses
For histological analyses, an additional set of 20 month–old mice was given either VHFD or C-VHFD for 16 weeks, after which they were perfused with 4% paraformaldehyde and the brains were processed for paraffin embedding. Coronal brain sections (6 µm) at the level of the lateral ventricle were cut, collected, and processed for immunohistochemical analyses. To visualize the cellular distribution of NOX expression, sections were double-labeled for gp91phox and neuronal, microglial, or actrocytic cell markers using the following primary antisera: anti-gp91phox (1:100, Santa Cruz Biotechnology, Santa Cruz, CA); anti-NeuN (1:100, Abcam Inc., Cambridge, MA); anti-Iba-1 (1:100, Wako Chemicals, Richmond, VA); and anti-GFAP (1:500, Abcam Inc.). Sections were incubated with biotinylated or peroxidase-linked secondary antibodies, and then visualized using diaminobenzidine (DAB, for gp91 phox) or NOVAred (for NeuN, Iba1, GFAP) as chromagens following manufacturer’s instructions (Vector Laboratories, Burlingame, CA). To document non-specific staining, the primary antibodies were omitted from the staining protocol.
To visualize neuronal protein oxidation, sections were incubated with 2,4-dinitrophenyl hydrazine (DNPH) to derivitize protein carbonyls, and then probed with antibodies to dinitrophenylhydrazone (DNP) (Millipore, Billerica, MA), and visualized as described above.
Statistical analyses
All data are shown as mean ± standard error of measurement. Body weight, body composition, NOX activity, and protein carbonyl data were all analyzed with 2-way analyses of variance (ANOVA), followed by planned Bonferroni posttests to determine differences between high fat diet and control diet groups in individual age groups. Protein expression values generated by Western blot (ratios of expression over tubulin) were normalized to percent control diet for each age to reconcile data from multiple blots, and were analyzed by unpaired t-tests to determine if statistically significant differences exist between high fat and control diet groups of each age. Statistical significance for all analyses was accepted at p < 0.05, and *, **, and *** represent p < 0.05, p < 0.01, and p < 0.001, respectively.
RESULTS
Effects of HFD on body weight and composition
Male mice of increasing age were exposed to the Western diet (WD) composed of 41% fat (butterfat and corn oil) and 29% sucrose, or the very high fat lard diet (VHFD) composed of 60% fat (pork lard), or their respective control 10% low fat diets (C-WD, C-VHFD) for 4 months. During this period, body weights progressively diverged such that by the end of the diet exposure period, 24-monthold mice having consumed the VHFD diet weighed more than any other group, although both diets caused significant weight gain (Table 1). Statistically, ANOVA for age × diet in the WD and C-WD mouse groups revealed a significant main effect of age on body weight (F(2,68) = 3.652, p = 0.032). The effect of diet on body weight was also significant (F(1,68) = 80.69, p < 0.0001), but the interaction was not. ANOVA for age × diet in the VHFD and C-VHFD mice likewise revealed significant main effects of age (F(2,68) = 11.34, p < 0.0001) and diet (F(1,68) = 203.1, p < 0.0001), on body weight, but no interaction.
Table 1. Body weight and body fat composition in experimental animals of increasing age after 16 weeks of diet exposure.
Body weight and composition in male mice of increasing age (7 months, 16 months, and 24 months) was measured at the end of a 4-month exposure to either Western diet (WD) or very high fat lard diet (VHFD), or their respective control diets ( C-WD and C-VHFD) as described in Methods. Body weight data are expressed as mean body weight in grams ± SEM with 8–14 mice per group, while body composition is expressed as the percent of body mass comprised of fat, and is also presented as mean ± SEM.
| 7 month | 16 month | 24 month | ||
|---|---|---|---|---|
| Body Weight (grams) |
C-WD | 39.24 ± 1.18 | 42.12 ± 0.82 | 40.72 ± 1.10 |
| WD | 47.05 ± 1.19*** | 50.01 ± 1.19*** | 50.17 ± 1.25*** | |
| C-VHFD | 38.74 ± 0.85 | 42.20 ± 1.32 | 41.96 ± 0.72 | |
| VHFD | 51.09 ± 0.94*** | 55.27 ± 1.07*** | 59.29 ± 2.25*** | |
| Body Composition (% fat) |
C-WD | 32.38 ± 1.06 | 31.34 ± 0.70 | 24.67 ± 1.32 |
| WD | 40.90 ± 0.62*** | 40.07 ± 0.85*** | 39.12 ± 0.75*** | |
| C-VHFD | 31.26 ± 0.98 | 27.75 ± 1.25 | 30.99 ± 0.74 | |
| VHFD | 43.00 ± 0.98*** | 41.68 ± 1.13*** | 42.41 ± 0.47*** | |
indicates significant (p < 0.001) increases in body weight or fat percentage in mice of each age induced by either WD or VHFD.
In addition to body weight, total body fat was measured using NMR. Data show that unlike what was observed for body weight, aging was not associated with increased body fat (Table 1). However, both WD and VHFD induced significant and robust increases in body fat compared to aged-matched control diet mice (Table 1). Statistically, ANOVA for age × diet in WD/C-WD mice revealed significant main effects of age (F(2,49) = 14.75, p < 0.0001) and diet (F(1,49) = 172.1, p < 0.0001), on fat percentage, with a significant interaction (F(2,49) = 6.71, p < 0.0027). ANOVA for age × diet in the VHFD/C-VHFD mice revealed significant main effects of age (F(2,49) = 3.722, p = 0.0313) and diet (F(1,49) = 276.9, p < 0.0001) on body fat, but no interaction.
NOX activity in mice of increasing age exposed to WD or VHFD
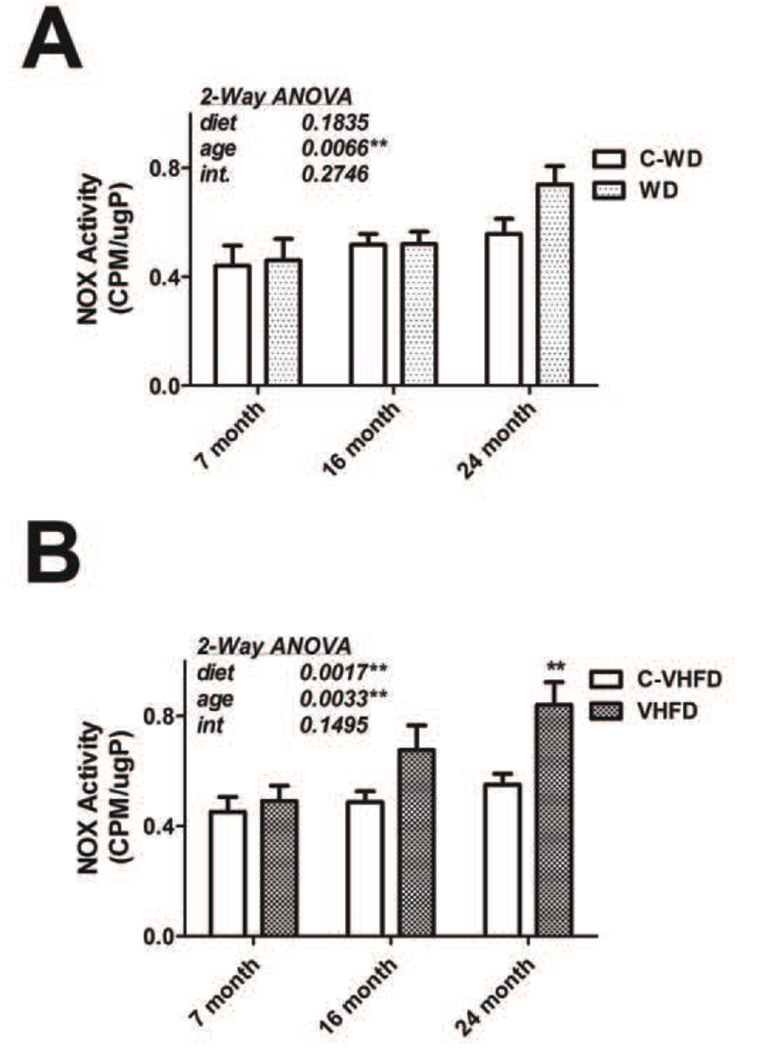
While reports have demonstrated that NOX expression is increased by dietary fat in animal models [13], the profile of NOX enzymatic activity in brain following high fat diet has not been evaluated, nor have the effects of age or different diet formulations. Initial experiments were thus designed to determine NOX activity in the frontal cerebral cortex of mice of increasing age (7-, 16-, and 24-months) at the end of a 16-week exposure to either WD or VHFD (or the C-WD and C-VHFD control diets) as described in Methods. NADPH-dependent superoxide production was evaluated in membrane preparations generated from the cortex using the lucigenin assay as described in Methods, and data were analyzed by 2-way ANOVA. Evaluation of data from mice exposed to WD and C-WD showed that there was a significant main effect of age (F(2,58) = 5.485, p = 0.0066) on NOX activity (Fig. 1A), but no significant effect of diet or significant interaction between diet and age on NOX activity. Planned comparisons of WD and C-WD groups did not reveal any significant increases in NOX activity induced by WD at any age. Conversely, evaluation of data from mice exposed to VHFD and C-VHFD demonstrated both a significant main effect of age (F(2,58) = 6.325, p = 0.0033) and diet (F(1,58) = 10.89, p = 0.0017) on NOX activity (Fig. 1B), but no significant interaction. Furthermore, planned comparisons of VHFD and C-VHFD mice revealed that consumption of VHFD by aged (24 month old) mice resulted in further significant increases in cortical NOX activity (Fig. 1B).
Figure 1. Effects of age and diet on NOX activity in mouse brain.
(A) NOX activity was measured in the cerebral cortex of mice of increasing age that had been exposed to WD or C-WD for 4 months, as described in Methods. Data were calculated as counts per minute (CPM) per microgram total protein, and are presented as mean and SEM. 2-way ANOVA indicated an overall significant main effect of age on NOX activity, but no effect of diet not any interaction (text insert). (B) Mice of increasing age were exposed to VHFD or C-VHFD for 4-months, and then NOX activity was measured in cortex as described in Methods. Data were calculated as counts per minute (CPM) per microgram total protein, and are presented as mean and SEM. 2-way ANOVA indicated an overall significant main effect of age and of diet on NOX activity, but no interaction (text insert). ** indicates significant (p < 0.01) increases in NOX activity in 24-month old mice that consumed VHFD as compared to 24-month old C-VHFD mice.
NOX subunit expression in mice of increasing age exposed to WD or VHFD
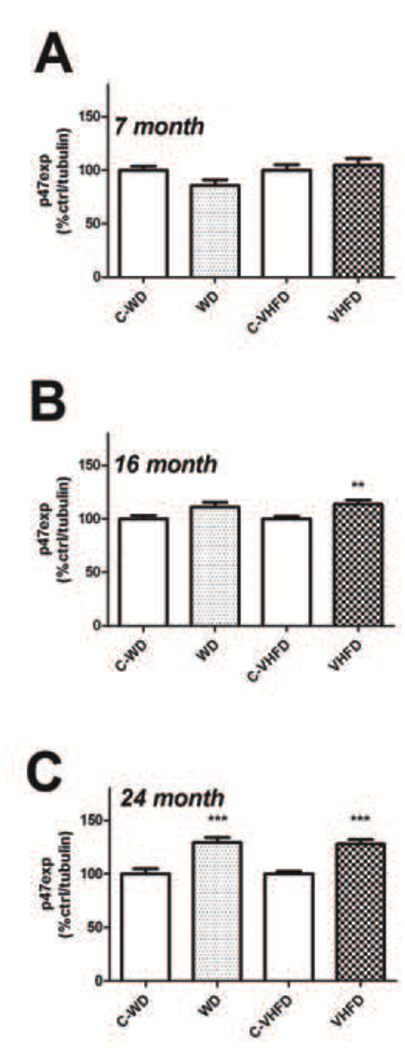
Experiments next evaluated if the observed increases in NOX activity were associated with increased expression of key NOX subunits. To this end, the expression profile of the regulatory subunit p47phox and the catalytic subunit gp91phox were measured in the cortex of mice after a 4-month exposure to WD, VHFD, or the C-WD and C-VHFD diets. Each age (7-, 16-, and 24-months) was measured separately by Western blot, and individual samples were normalized first to tubulin and then to control diet to reconcile multiple blots, as described in Methods. Thus, for each age, protein expression values from high fat diet-exposed mice were compared to values from control diet mice by t-test. Data show that neither diet increased expression of p47phox as compared to control diet in 7-month old (Fig. 2). However, 16-month old mice that had consumed VHFD, but not WD, had significantly higher p47phox expression (t(18) = 3.204, p = 0.0049) in the cortex as compared to control mice (Fig. 2B); and both diets increased p47phox expression relative to their respective control diets [(t(22) = 4.570, p = 0.0001) for WD, and (t(22) = 5.684, p < 0.0001), for VHFD] in 24-month old mice (Fig. 2C).
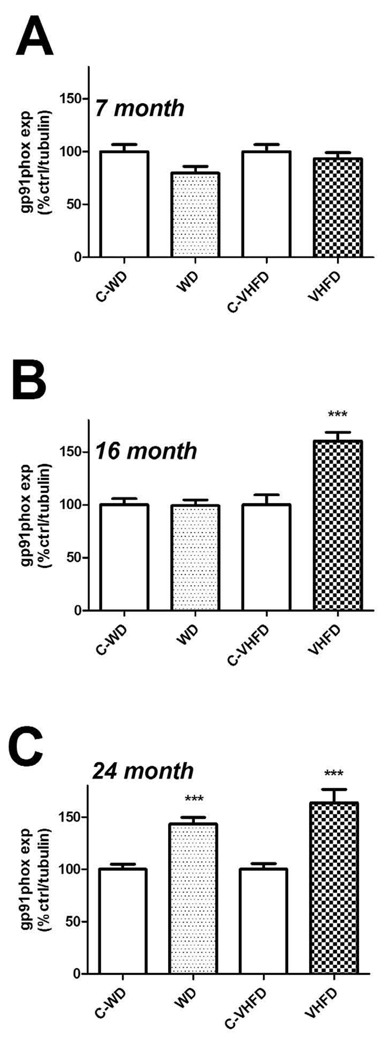
Figure 2. Effects of age and diet on gp91phox expression in mouse brain.
(A) Expression of the NOX catalytic subunit gp91phox was measured in cortex of 7-month old mice following a 4-month exposure to either WD or VHFD (or their respective control diets C-WD and C-VHFD), as described in Methods. Data were measured by Western blot, and individual samples were normalized first to tubulin and then to control diet, as described in Methods. (B) Cortical gp91phox expression in 16-month old mice following a 4-month exposure to either WD or VHFD (or their respective control diets C-WD and C-VHFD). Data were measured by Western blot, and individual samples were normalized first to tubulin and then to control diet, as described in Methods. *** indicates significant (p < 0.001) increases in gp91phox expression VHFD mice as compared age-matched C-VHFD mice. (C) Cortical gp91phox expression in 24-month old mice following a 4-month exposure to either WD or VHFD (or their respective control diets C-WD and C-VHFD). Data were measured by Western blot, and individual samples were normalized first to tubulin and then to control diet, as described in Methods. *** indicates significant (p < 0.001) increases in gp91phox expression in both VHFD mice and WD mice as compared age-matched control diet mice.
Evaluation of the effects of high fat diet on expression of the catalytic subunit gp91phox revealed a pattern similar to that observed for p47phox. Data show that neither diet increased gp91phox expression as compared to control diet in 7-month old mice (Fig. 3A). Conversely, 16- month old mice that had consumed VHFD, but not WD, had significantly higher gp91phox expression (t(18) = 4.855, p < 0.0001) as compared to control mice (Fig. 3B). Finally, both diets increased gp91phox expression relative to their respective control diets [(t(22) = 5.439, p < 0.0001) for WD, and (t(22) = 4.539, p = 0.0002), for VHFD] in 24-month old mice (Fig. 3C).
Figure 3. Effects of age and diet on p47phox expression in mouse brain.
(A) Expression of the NOX regulatory subunit p47phox was measured in cortex of 7-month old mice following a 4-month exposure to either WD or VHFD (or their respective control diets C-WD and C- VHFD), as described in Methods. Data were measured by Western blot, and individual samples were normalized first to tubulin and then to control diet, as described in Methods. (B) Cortical p47phox expression in 16-month old mice following a 4-month exposure to either WD or VHFD (or their respective control diets C-WD and C- VHFD). Data were measured by Western blot, and individual samples were normalized first to tubulin and then to control diet, as described in Methods. ** indicates significant (p < 0.01) increases in p47phox expression VHFD mice as compared age-matched C-VHFD mice. (C) Cortical p47phox expression in 24-month old mice following a 4-month exposure to either WD or VHFD (or their respective control diets C-WD and C- VHFD). Data were measured by Western blot, and individual samples were normalized first to tubulin and then to control diet, as described in Methods. ** indicates significant (p < 0.01) increases in p47phox expression in both VHFD mice and WD mice as compared age-matched control diet mice.
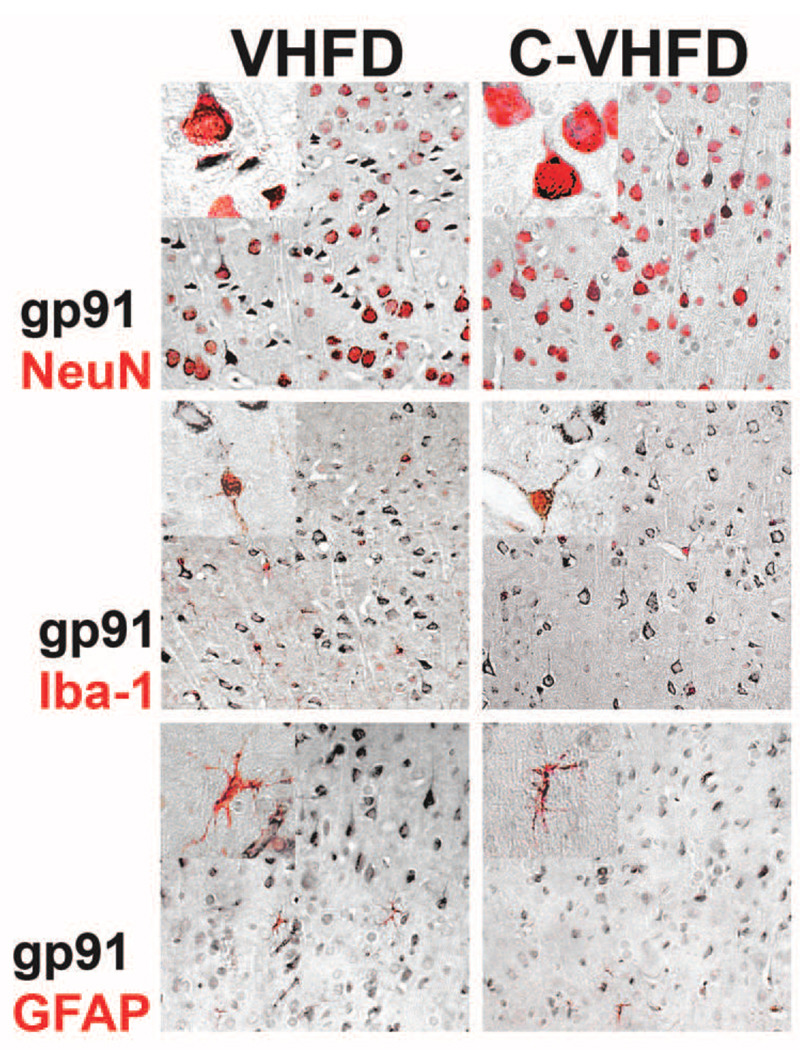
While the above data indicate that NOX activity and expression are increased in aged mice in response to high fat diet consumption, these data do not identify which cells are responsible for these phenomena. This issue is noteworthy as while the pathophysiologic consequences of increased NOX activity/expression in the brain are frequently thought to reflect toxic microglial over-activation, NOX subunits are expressed in neurons and astrocytes [25], [26]. Indeed, NOX has been shown to participate in neuronal synaptic physiology [27], [28], raising the possibility that altered NOX activity and/or expression in neurons could perturb their function. To resolve whether neuronal or glial NOX might participate in the increased NOX expression and activity detected in aged mice, the expression of gp91phox was evaluated immunohistochemically in an additional set of 20 month–old mice given either VHFD or C-VHFD for 16 weeks. To further identify the cell-type specific pattern of gp91phox expression, VHFD or C-VHFD sections were double-labeled for gp91 and cell markers specific for either neurons (NeuN), microglia (Iba-1), or astrocytes (GFAP). Sections were taken from the level of the lateral ventricle, and immunostaining was specifically evaluated in the dorsal frontoparietal cortex. No staining was observed when the primary antibody was omitted from the protocol (data not shown). Interestingly, evaluation of tissue sections double-labeled for gp91phox and NeuN showed very prominent neuronal localization of gp91phox expression (Fig. 4, top panel) demonstrating that neurons express relatively high levels of gp91phox, particularly in this area of the cortex devoted to motor function. Neuronal gp91phox expression was lower in somatosensory areas of the lateral frontoparietal cortex and was absent in the striatum (data not shown). A consistent pattern of gp91phox staining in superior frontoparietal cortex was also associated with microglia (Fig. 4, middle panel), and with astrocytes (Fig. 4, bottom panel) in both VHFD and C-VHFD sections, indicating that glia express NOX as well. However, the number of gp91phox-positive neurons was much greater than gp91phox-positive glia (Fig. 4), suggesting that NOX expression and activity specifically in cortical neurons may be increased in aged mice by high fat diet consumption.
Figure 4. Cell-type specific expression of cortical gp91phox in VHFD and C-VHFD brains.
Coronal brain sections from the level of the lateral ventricle were collected from VHFD and C-VHFD mice and analyzed for cell type-specific gp91phox expression specifically in cells of the dorsal frontoparietal cortex as described in Methods. (Top panel) Representative 20× low-magnification and 63× high-magnification (insert) images depict the pattern of gp91phox staining (black DAB stain) specifically in NeuN-positive neurons (NOVAred stain) in cortex of VHFD and C-VHFD brains. (Middle panel) Representative 20× low-magnification and 63× high-magnification (insert) images depict the pattern of gp91phox staining (black DAB stain) specifically in Iba-1 positive microglia (NOVAred stain) in cortex of VHFD and C-VHFD brains. (Bottom panel) Representative 20× low-magnification and 63× high-magnification (insert) images depict the pattern of gp91phox staining (black DAB stain) specifically in GFAP positive astrocytes (NOVAred stain) in cortex of VHFD and C-VHFD brains.
Oxidative stress in mice of increasing age exposed to WD or VHFD
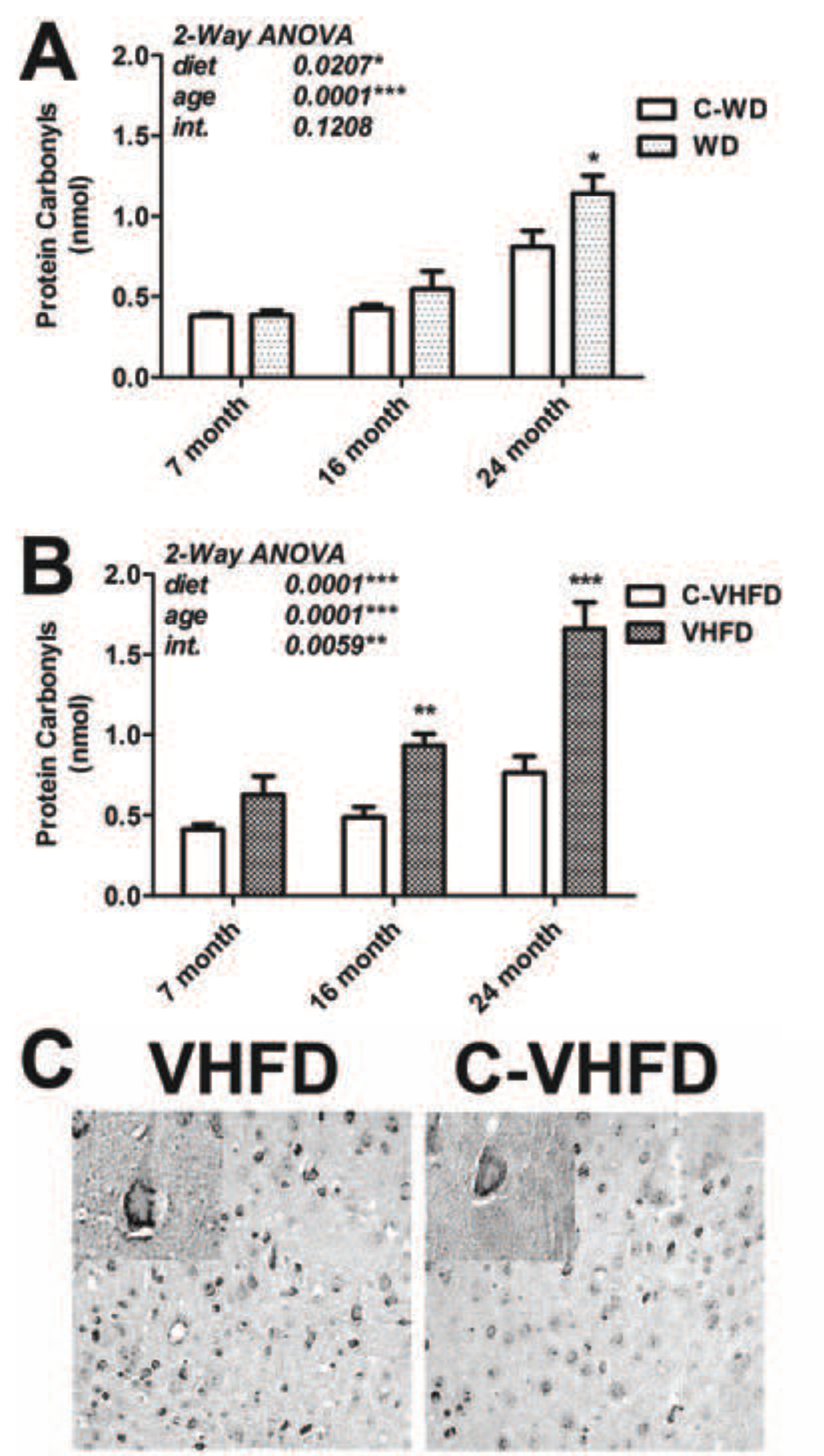
Previous reports have indicated that experimental obesity can increase free radical generation and/or oxidative stress in brains of rodents [13], [17], [14], but the specific effects of high fat diet on the aging brain have not been evaluated. To determine if the increases in NOX activity noted specifically in aged brains following high fat diet consumption were associated with enhanced oxidative stress, experiments were designed to evaluate the degree of protein carbonylation in cortical tissues from mice of increasing age exposed to WD and VHFD. Overall, data show that aged mice were the most vulnerable to diet-induced increases in protein oxidation, and that VHFD induced more robust increases in protein oxidation than WD (Fig. 5). Statistically, evaluation of data from mice exposed to WD/C-WD revealed significant main effects of age (F(2,42) = 32.75, p < 0.0001) and diet (F(1,42) = 5.78, p = 0.021) on protein oxidation (Fig. 5A), but no significant interaction between diet and age. Planned comparisons of WD and C-WD groups indicated that WD was only able to induce significant increases in protein oxidation in 24-month old mice (Fig. 5A). Conversely, evaluation of data from mice exposed to VHFD/C-VHFD demonstrated significant main effects of age (F(2,42) = 25.08, p < 0.0001), of diet (F(1,42) = 39.68, p < 0.0001), and a significant interaction between age and diet (F(2,42) = 5.82, p = 0.0059), on protein carbonylation (Fig. 5B). Furthermore, planned comparisons of VHFD and C-VHFD mice revealed that consumption of VHFD by both middle aged (16-month old) and aged (24-month old) mice resulted in significant increases in protein oxidation (Fig. 5B). Finally, evaluation of protein nitration and iNOS expression did not reveal any effects of either diet on iNOS expression or protein nitration in mice of any age (data not shown).
Figure 5. Effects of age and diet on protein carbonylation in mouse brain.
(A) Protein carbonyls were measured in cortex of mice of increasing age that had been exposed to WD or C-WD for 4 months, as described in Methods. Data were calculated as nmol carbonyl per milligram total protein, and are presented as mean and SEM. 2-way ANOVA indicated an overall significant main effect of age and diet on protein carbonyls, but no significant interaction of age and diet (text insert). * indicates significant (p < 0.05) increases in carbonylation in 24-month old mice that consumed WD as compared to 24-month old C-WD mice. (B) Mice of increasing age were exposed to VHFD or C-VHFD for 4-months, and then protein carbonyls were measured in cortex as described in Methods. Data were calculated as nmol per milligram total protein, and are presented as mean and SEM. 2-way ANOVA indicated an overall significant main effect of age and of diet, and a significant interaction of age and diet, on NOX activity (text insert). ** and *** indicate significant (p < 0.01 and p < 0.001, respectively) increases in levels of protein carbonyls in 16-month old and 24-month old mice that consumed VHFD as compared to aged-matched C-VHFD mice. (C) Coronal brain sections from the level of the lateral ventricle were collected from VHFD and C-VHFD mice and analyzed for protein carbonyls by immunocytochemistry as described in Methods. Representative 20× low-magnification and 63× high-magnification (insert) images depict the pattern of protein oxidation in cells of the dorsal frontoparietal cortex of VHFD and C-VHFD brains.
To determine if the histological pattern of protein oxidation mirrored that observed for NOX expression, protein carbonylation was evaluated immunohistochemically in brains from 20 month–old mice given either VHFD or C-VHFD for 16 weeks. Specifically, sections were again taken from the level of the lateral ventricle, and immunostaining for DNP was visualized as described in Methods in cells of the dorsal frontoparietal cortex. Examination of stained slides revealed prominent protein carbonylation in large, pyramidal-shaped cells of the cortex (Fig. 5C). No DNP staining was observed when the DNPH derivitization step was omitted from the protocol (not shown). While the DNPH derivitization step interfered with the pattern of NeuN immunostaining (not shown), staining of adjacent sections with NeuN indicated that the DNP-positive cells were indeed neurons. Thus, these investigations suggest that the same population of neurons that express relatively high levels of gp91phox also appear to show extensive evidence of protein oxidation.
Expression of glial markers in mice of increasing age exposed to WD or VHFD
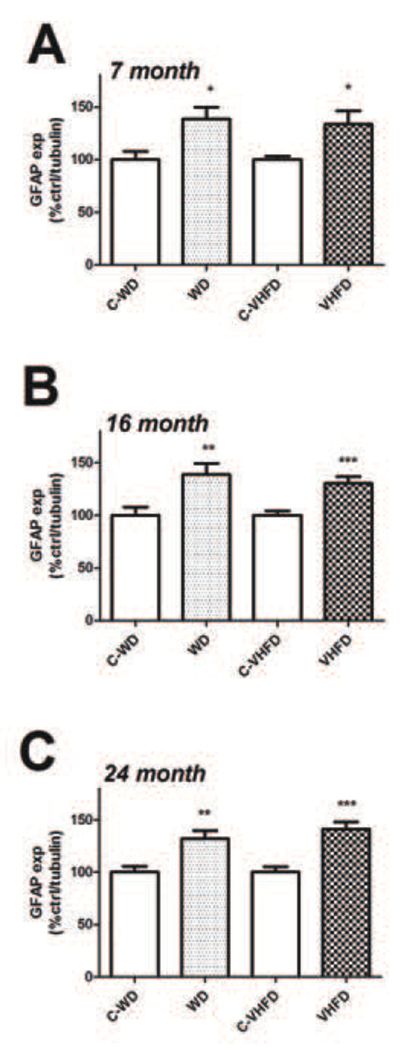
While the precise mechanisms whereby dietary fats alter brain physiology are unknown, several studies suggest that experimental obesity in rodents is associated with glial inflammation and hypertrophy [29], [30]. Furthermore, inflammatory reactions in glia are well known to accompany neuronal injury, particularly in the context of age [31], [32], [33], [34]. Thus, to clarify the relationship of reactive gliosis to diet-induced NOX activity and to further determine how high fat diets modulate glial reactivity in mice of increasing age, the expression of astrocyte and microglial markers were evaluated using Western blot as described in Methods. The intermediate filament protein glial fibrillary acidic protein (GFAP) was used to evaluate astrocyte hypertrophy [35]. Data show that both WD and VHFD increased GFAP expression relative to their respective control diets [(t(18) = 2.863, p = 0.0103) for WD, and (t(18) = 2.577, p = 0.019), for VHFD] in 7-month old mice (Fig. 6A). Likewise, both diets significantly increased GFAP expression in the cortex of 16-month old mice [(t(18) = 2.963, p = 0.0083) for WD, and (t(18) = 4.010, p = 0.0008), for VHFD] and 24-month old mice [(t(22) = 3.380, p = 0.0027) for WD, and (t(22) = 4.845, p < 0.0001), for VHFD] relative to control diet (Fig. 6B and C).
Figure 6. Effects of age and diet on GFAP expression in mouse brain.
(A) Expression of the astrocyte marker GFAP was measured in cortex of 7-month old mice following a 4-month exposure to either WD or VHFD (or their respective control diets C-WD and C- VHFD), as described in Methods. Data were measured by Western blot, and individual samples were normalized first to tubulin and then to control diet, as described in Methods. * indicates significant (p < 0.05) increases in GFAP expression in both VHFD mice and WD mice as compared age-matched control diet mice. (B) Cortical GFAP expression in 16-month old mice following a 4-month exposure to either WD or VHFD (or their respective control diets C-WD and C- VHFD). Data were measured by Western blot, and individual samples were normalized first to tubulin and then to control diet, as described in Methods. ** and *** indicate significant (p < 0.01 and p < 0.001, respectively) increases in GFAP expression in WD mice and VHFD mice as compared age-matched control diet mice. (C) Cortical GFAP expression in 24-month old mice following a 4-month exposure to either WD or VHFD (or their respective control diets C-WD and C- VHFD). Data were measured by Western blot, and individual samples were normalized first to tubulin and then to control diet, as described in Methods. *** indicates significant (p < 0.001) increases in GFAP expression in both VHFD mice and WD mice as compared age-matched control diet mice.
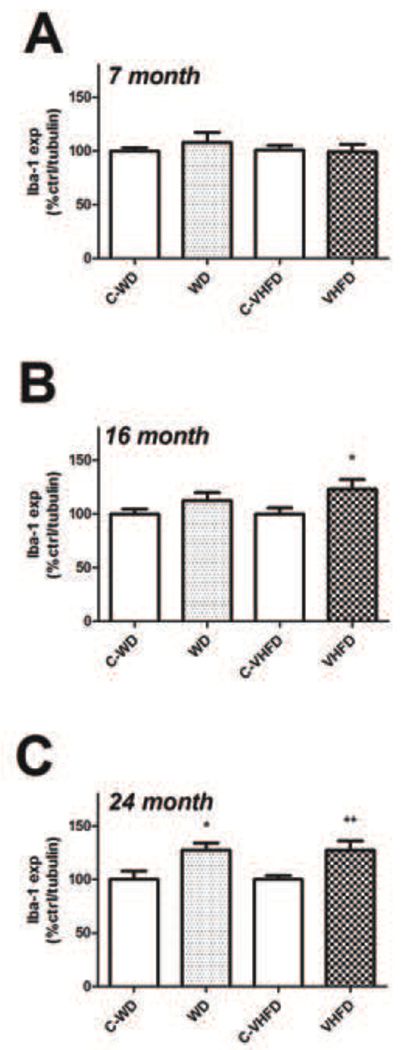
Microglial reactivity was evaluated by measuring expression of Iba-1, which is a 17-kDa calcium binding protein that is specifically expressed in macrophages/ microglia and is upregulated during the in vivo activation of these cells [36], [37], [38]. This particular marker is widely used to evaluate microglial reactivity as it can be used in denatured cell lysates, [31] to accurately quantify expression using Western blot. Data show that neither high fat diet increased expression of Iba-1 as compared to control diet in 7-month old mice (Fig. 7A). Conversely, 16-month old mice that had consumed VHFD, but not WD, had significantly higher Iba-1 expression (t(18) = 2.168, p = 0.0438) in the cortex as compared to control mice (Fig. 7B). Finally, both diets increased Iba-1 expression relative to their respective control diets [(t(18) = 2.684, p = 0.0152) for WD, and (t(22) = 2.884, p = 0.0086), for VHFD] in 24-month old mice (Fig. 7C).
Figure 7. Effects of age and diet on Iba-1 expression in mouse brain.
(A) Expression of the microglial marker Iba-1 was measured in cortex of 7-month old mice following a 4-month exposure to either WD or VHFD (or their respective control diets C-WD and C-VJFD), as described in Methods. Data were measured by Western blot, and individual samples were normalized first to tubulin and then to control diet, as described in Methods. (B) Cortical Iba-1 expression in 16-month old mice following a 4-month exposure to either WD or VHFD (or their respective control diets C-WD and C- VHFD). Data were measured by Western blot, and individual samples were normalized first to tubulin and then to control diet, as described in Methods. * indicates significant (p < 0.05) increases in Iba-1 expression in VHFD mice as compared age-matched C-VHFD mice. (C) Cortical Iba-1 expression in 24-month old mice following a 4-month exposure to either WD or VHFD (or their respective control diets C-WD and C- VHFD). Data were measured by Western blot, and individual samples were normalized first to tubulin and then to control diet, as described in Methods. * and ** indicate significant (p < 0.05 and p < 0.01, respectively) increases in Iba-1 expression in WD and VHFD mice as compared age-matched control diet mice.
DISCUSSION
This report describes data obtained from mice of increasing age following high fat diet administration. Specifically, samples were taken from the frontal cerebral cortex of 7-, 16-, and 24-month old mice following a 4-month exposure to either the Western diet (WD, 41% calories from fat) or very high fat lard diet (VHFD, 60% calories from fat) to determine if dietary fat could act synergistically with age to affect NOX activity and expression. Using a luminescent assay to detect NADPH-dependent free radical production, data show that only the VHFD significantly increased NOX activity over control levels, and only in aged mice. Evaluation of NOX subunit expression demonstrated that aged mice were more sensitive to the effects of diet on NOX expression, with only the 24-month old mice showing significant increases in NOX expression in response to WD or VHFD. Additionally, data show that consumption of VHFD and to a lesser extent WD, resulted in significant increases in protein oxidation in aged mice, demonstrating again the heightened sensitivity of aged mice to high levels of dietary fat. Immunohistochemical evaluation of cells in the frontal cortex revealed prominent neuronal localization of both gp91phox and protein oxidation. Finally, while both diets increased expression of the astrocyte marker GFAP in all age groups, the expression of microglial markers closely followed the pattern of NOX expression, indicating the effects of diet and age on NOX activity might lead to increased microglial activation and brain inflammation. While previous studies have provided evidence that NOX can be altered by diet-induced or genetic models of obesity [13], [17], and that NOX may be involved with age-related neurodegeneration [22], [39], these data link and extend these separate observations by demonstrating that increased NOX activity and expression can be specifically induced by high fat diet in aged, but not young, mice. Collectively, these findings suggest that NOX activity may be a player in the ability of high fat diets to detrimentally affect brain homeostasis in aged individuals, and further indicate that NOX activation may be associated with feed-forward cascades of oxidative stress and brain inflammation.
Our results suggest that age is a major factor in the vulnerability of the brain to the detrimental effects of high fat diet. While the etiology of brain aging is not well understood, the free radical theory of aging [40] describes a compelling mechanistic rationale for the age-related decline in function of many biological systems. This oxidative stress-based theory describes a redox imbalance, whereby the production of free radicals overtakes endogenous anti-oxidant capacity, leading to oxidative damage to critical cellular elements [41]. The free radical theory of aging may be particularly relevant for the brain, as the brain utilizes relatively high levels of oxygen but has relatively low levels of endogenous antioxidants. Indeed, oxidative stress has been repeatedly implicated in brain aging and in neurodegenerative disorders like AD [11], [42]. Thus, there is ample evidence that oxidative stress could participate in age-related declines in brain function, but the source of age-related oxidative stress is not fully understood. While evidence supports a role for a “mitochondrial cascade hypothesis”, in which age-related increases in mitochondrial ROS production and decreases in mitochondrial function lead to neuronal dysfunction and dementia [10], [43], data in this manuscript support the hypothesis that increased NOX could participate in age-associated oxidative stress, which is in keeping with data presented in previous publications [21], [44], [39]. Interestingly, recent expansion of the genome databases has led to identification of several novel homologues of gp91phox, which constitute the NOX family of oxidases [45], [46]. The human genome contains 5 NOX members: NOX1 through NOX5, with gp91phox as NOX2. Some of these novel oxidases have a fairly limited tissue expression [47]. For example, NOX1 is highly expressed in the colon and in vascular smooth muscle, whereas NOX3 is found almost exclusively in peripheral auditory tissues. However, in addition to NOX2, both NOX1 and NOX4 have been found in brain, and are expressed in neurons and glia [45], [47], [48], and thus might participate in the effects noted in this study. Indeed, the detrimental actions of NOX appear to be most strongly associated with age-related chronic disease processes, such as Parkinson’s disease, Alzheimer’s disease, and atherosclerosis (reviewed in [22]). Based on these observations, a concept termed “antagonistic pleiotropy” has been proposed to explain a potentially dual role of NOX in the brain, describing a scenario in which the physiologic production of reactive oxygen species (ROS) garners an advantage in early life, but the sustained or aberrant activation of NOX accumulates and culminates in harmful effects later in life [22].
While it is known that the brain can be affected by nutritional status (over nutrition or under nutrition), it is unclear whether the amplified effects of the VHFD diet compared to the WD diet reflect specific differences in diet composition, or rather are a consequence of the increased body weight and adiposity in these mice. The WD contains 41% butterfat and a relatively high sucrose content. The VHFD consists of 60% fat (from lard) and relatively low carbohydrate levels. Both diets are open source, and comparison of the specific fat composition of the two diets indicates that while the VHFD has a greater percentage of total fat (60% versus 41% for the WD), the WD actually has the higher percentage of saturated fat (62% versus 37%). Thus, the heightened vulnerability of aged mice to VHFD is likely not based on the particular composition of fat in the diet, but more likely reflects the increased quantity of dietary fat and/or the enhanced ability of this diet to induce large increases in body weight. Indeed, VHFD mice were heavier than WD mice at each age, and increased obesity per se could potentially mediate neurological perturbations noted in this study, as epidemiological and experimental data have established that the brain is sensitive to obesity and obesity-induced metabolic dysfunction (reviewed in [49]). For example, one of the earliest reports of adverse effects of experimental obesity on brain pathology reported that genetically obese (ob/ob) mice had decreased overall levels of myelin and marked alterations to the fatty acid composition of myelin as compared to wild type mice [50]. The use of a genetic model for these studies is significant in that it suggests that increased adiposity, rather than increased dietary lipids, is sufficient to alter the composition of brain myelin. This study is supported by more recent imaging studies in humans that also have revealed changes in white matter and myelin abnormalities in association with obesity [51], [52]. While the human studies have not been confirmed by histopathological investigations to identify the actual structural and biochemical changes to myelin in obese patients, an obvious consequence of altered myelination would be altered axonal transmission. Indeed, animal studies have confirmed that experimental obesity is associated with cognitive abnormalities [53], [54]. Studies designed to untangle the potentially divergent effects of type versus amount of dietary fat on brain aging are currently under development, and the use of aged mice that lack expression of specific NOX subunits will clarify the role that NOX plays the detrimental effects of high fat diet on age-related oxidative stress in the brain.
The physiologic mechanisms of NOX activation in brain are not well understood, and indeed, the molecular sources of free radicals in the aged brain are still subject to debate and investigation. The expression of the microglial marker Iba-1 mirrored the pattern of NOX expression and activity, suggesting that the observed increases in NOX activity might be a cause or consequence enhanced microglial reactivity. This potential scenario is supported by a body of literature implicating aberrant or excessive activation of microglia in the pathogenesis of brain aging (reviewed in [32], [55]). Activation of NOX is a characteristic feature of microglial activation both in vitro and in vivo, and experimental evidence suggests that ROS generated by activated microglia could directly contribute to brain injury by inducing lipid peroxidation, DNA fragmentation and protein oxidation in surrounding cells – a phenomena called “bystander lysis” [56]. However, immunohistochemical evaluation of NOX expression illustrated relatively high expression of pg91phox in cortical neurons. Further investigations indicated that the same population of neurons showed evidence of extensive protein oxidation. Thus, these data support a potentially important role for neuronal NOX in mediating the adverse effects of high fat diet. The widespread expression of NOX subunits in neurons has led to the recognition that deliberate ROS production by NOX plays an important role in many biological events, including neuronal signaling [27], [28]. Indeed, there is substantial evidence that NOX-based ROS can regulate synaptic plasticity and memory formation (reviewed in [57]). For example, cognitive dysfunction has been reported in human patients suffering from chronic granulamatosis [58], a clinical condition caused by mutation in the gp91phox gene. Furthermore, mice deficient in either gp91phox or p47phox have disrupted cognitive function and memory [59]. Interestingly, closer evaluation of experimental data reveal that a delicate balance of ROS is required for signaling, with either too little or too much ROS resulting in impairments in long-term potentiation (LTP) and memory formation [60]. In further relation to aging, data suggest an age-related shift in the role of ROS signaling in hippocampal LTP and memory formation [60], [61], [62]. For example, overexpression of the antioxidant enzyme superoxide dismutase has been shown to impair LTP in young mice, but preserves LTP in aged mice [62], [61], while chronic treatment of mice with small molecular weight antioxidant enzyme mimetics can reverse hippocampus-dependent learning deficits in aged mice [63]. Thus, these data support the hypothesis that aberrantly enhanced or sustained NOX activation in neurons could directly disrupt cellular and synaptic function, particularly in the context of aging. In summary, these data raise the possibility that NOX activation, particularly in neurons, could participate in cognitive deficits observed in association with age and diet-induced obesity in humans [3], [4], [5], [6].
ACKNOWLEDGEMENTS
The authors are grateful to Megan Purpera and Sun-Ok Fernandez-Kim for expert technical assistance and animal handling. This work was supported by grants from the NIH (NS46267, DA19398, and AG05119 to AJB-K; NS051570 and RR021945 to CDM), and funds from the Hibernia National Bank/Edward G. Schlieder Chair to JNK. This study also used PBRC Core facilities (Animal Phenotyping and Imaging) that are funded by the NIH (P20-RR021945 and P30-DK072476).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Olufadi R, Byrne CD. Clinical and laboratory diagnosis of the metabolic syndrome. J. Clin. Pathol. 2008;61:697–706. doi: 10.1136/jcp.2007.048363. [DOI] [PubMed] [Google Scholar]
- 2.Haslam DW, James WP. Obesity. Lancet Neurol. 2005;366:1197–1209. doi: 10.1016/S0140-6736(05)67483-1. [DOI] [PubMed] [Google Scholar]
- 3.Elias MF, Elias PK, Sullivan LM, Wolf PA, D'Agostino RB. Lower cognitive function in the presence of obesity and hypertension: the Framingham Heart Study. Int. J. Obes. Relat. Metab. Disord. 2003;27:260–268. doi: 10.1038/sj.ijo.802225. [DOI] [PubMed] [Google Scholar]
- 4.Elias MF, Elias PK, Sullivan LM, Wolf PA, D'Agostino RB. Obesity, diabetes and cognitive deficit: the Framingham Heart Study. Neurobiol. Aging. 2005;26:11–16. doi: 10.1016/j.neurobiolaging.2005.08.019. [DOI] [PubMed] [Google Scholar]
- 5.Waldstein SR, Katzel LI. Interactive relations of central versus total obesity and blood pressure to cognitive function. Int. J. Obes. (Lond.) 2006;30:201–207. doi: 10.1038/sj.ijo.0803114. [DOI] [PubMed] [Google Scholar]
- 6.Ward MA, Carlsson CM, Trivedi MA, Sager MA, Johnson SC. The effect of body mass index on global brain volume in middle-aged adults: a cross sectional study. BMC Neurol. 2005:23. doi: 10.1186/1471-2377-5-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pannacciulli N, Del Parigi A, Chen K, Le DS, Reiman EM, Tataranni PA. Brain abnormalities in human obesity: a voxel-based morphometric study. Neuroimage. 2006;31:1419–1425. doi: 10.1016/j.neuroimage.2006.01.047. [DOI] [PubMed] [Google Scholar]
- 8.Roberts CK, Sindhu KK. Oxidative stress and metabolic syndrome. Life Sci. 2009;84:705–712. doi: 10.1016/j.lfs.2009.02.026. [DOI] [PubMed] [Google Scholar]
- 9.Ansari MA, Roberts KN, Scheff SW. Oxidative stress and modification of synaptic proteins in hippocampus after traumatic brain injury. Free Radic. Biol. Med. 2008;45:443–452. doi: 10.1016/j.freeradbiomed.2008.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reddy PH. Mitochondrial medicine for aging and neurodegenerative diseases. Neuromolecular Med. 2008;10:291–315. doi: 10.1007/s12017-008-8044-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stadtman ER, Levine RL. Free radical-mediated oxidation of free amino acids and amino acid residues in proteins. Amino Acids. 2003;25:207–218. doi: 10.1007/s00726-003-0011-2. [DOI] [PubMed] [Google Scholar]
- 12.Smith MA, Perry G, Richey PL, Sayre LM, Anderson VE, Beal MF, Kowall N. Oxidative damage in Alzheimer's. Nature. 1996;382:120–121. doi: 10.1038/382120b0. [DOI] [PubMed] [Google Scholar]
- 13.Zhang X, Dong F, Ren J, Driscoll MJ, Culver B. High dietary fat induces NADPH oxidase-associated oxidative stress and inflammation in rat cerebral cortex. Exp. Neurol. 2005;191:318–325. doi: 10.1016/j.expneurol.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 14.Souza CG, Moreira JD, Siqueira IR, Pereira AG, Rieger DK, Souza DO, Souza TM, Portela LV, Perry ML. Highly palatable diet consumption increases protein oxidation in rat frontal cortex and anxiety-like behavior. Life Sci. 2007;81:198–203. doi: 10.1016/j.lfs.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 15.Babior BM. The respiratory burst oxidase and the molecular basis of chronic granulomatous disease. Am. J. Hematol. 1991;37:263–266. doi: 10.1002/ajh.2830370410. [DOI] [PubMed] [Google Scholar]
- 16.DeLeo FR, Quinn MT. Assembly of the phagocyte NADPH oxidase: molecular interaction of oxidase proteins. J. Leukoc. Biol. 1996;60:677–691. doi: 10.1002/jlb.60.6.677. [DOI] [PubMed] [Google Scholar]
- 17.Chinen I, Shimabukuro M, Yamakawa K, Higa N, Matsuzaki T, Noguchi K, Ueda S, Sakanashi M, Takasu N. Vascular lipotoxicity: endothelial dysfunction via fatty-acid-induced reactive oxygen species overproduction in obese Zucker diabetic fatty rats. Endocrinology. 2007;148:160–165. doi: 10.1210/en.2006-1132. [DOI] [PubMed] [Google Scholar]
- 18.Patel C, Ghanim H, Ravishankar S, Sia CL, Viswanathan P, Mohanty P, Dandona P. Prolonged reactive oxygen species generation and nuclear factor-kappaB activation after a high-fat, high-carbohydrate meal in the obese. J. Clin. Endocrinol. Metab. 2007;92:4476–4479. doi: 10.1210/jc.2007-0778. [DOI] [PubMed] [Google Scholar]
- 19.Silver AE, Beske SD, Christou DD, Donato AJ, Moreau KL, Eskurza I, Gates PE, Seals DR. Overweight and obese humans demonstrate increased vascular endothelial NAD(P)H oxidase-p47(phox) expression and evidence of endothelial oxidative stress. Circulation. 2007;115:627–637. doi: 10.1161/CIRCULATIONAHA.106.657486. [DOI] [PubMed] [Google Scholar]
- 20.Block ML. NADPH oxidase as a therapeutic target in Alzheimer's disease. BMC Neurosci. 2008;9 Suppl 2:S8. doi: 10.1186/1471-2202-9-S2-S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shimohama S, Tanino H, Kawakami N, Okamura N, Kodama H, Yamaguchi T, Hayakawa T, Nunomura A, Chiba S, Perry G, Smith MA, Fujimoto S. Activation of NADPH oxidase in Alzheimer's disease brains. Biochem. Biophys. Res. Commun. 2000;273:5–9. doi: 10.1006/bbrc.2000.2897. [DOI] [PubMed] [Google Scholar]
- 22.Lambeth JD. Nox enzymes, ROS, and chronic disease: an example of antagonistic pleiotropy. Free Radic. Biol. Med. 2007;43:332–347. doi: 10.1016/j.freeradbiomed.2007.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Forster MJ, Dubey A, Dawson KM, Strutts WA, Lal H, Sohal RS. Age-related losses of cognitive function and motor skills in mice are associated with oxidized protein damage in brain. Proc. Natl. Acad. Sci. U.S.A. 1996;93:4765–4769. doi: 10.1073/pnas.93.10.4765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Keller JN, Schmitt FA, Scheff SW, Ding Q, Chen Q, Butterfield DA, Markesbery WR. Evidence of increased oxidative damage in subjects with mild cognitive impairment. Neurology. 2005;64:1152–1156. doi: 10.1212/01.WNL.0000156156.13641.BA. [DOI] [PubMed] [Google Scholar]
- 25.Noh KM, Koh JY. Induction and activation by zinc of NADPH oxidase in cultured cortical neurons and astrocytes. J. Neurosci. 2000;20:1–5. doi: 10.1523/JNEUROSCI.20-23-j0001.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim MJ, Shin KS, Chung YB, Jung KW, Cha CI, Shin DH. Immunohistochemical study of p47Phox and gp91Phox distributions in rat brain. Brain Res. 2005;1040:178–186. doi: 10.1016/j.brainres.2005.01.066. [DOI] [PubMed] [Google Scholar]
- 27.Kishida KT, Pao M, Holland SM, Klann E. NADPH oxidase is required for NMDA receptor-dependent activation of ERK in hippocampal area CA1. J Neurochem. 2005;94:299–306. doi: 10.1111/j.1471-4159.2005.03189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tejada-Simon MV, Serrano F, Villasana LE, Kanterewicz BI, Wu GY, Quinn MT, Klann E. Synaptic localization of a functional NADPH oxidase in the mouse hippocampus. Mol. Cell Neurosci. 2005;29:97–106. doi: 10.1016/j.mcn.2005.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Choi JY, Jang EH, Park CS, Kang JH. Enhanced susceptibility to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine neurotoxicity in high-fat diet-induced obesity. Free Radic. Biol. Med. 2005;38:806–816. doi: 10.1016/j.freeradbiomed.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 30.Sriram K, Benkovic SA, Miller DB, O'Callaghan JP. Obesity exacerbates chemically induced neurodegeneration. Neuroscience. 2002;115:1335–1346. doi: 10.1016/s0306-4522(02)00306-8. [DOI] [PubMed] [Google Scholar]
- 31.Vega-Avelaira D, Moss A, Fitzgerald M. Age-related changes in the spinal cord microglial and astrocytic response profile to nerve injury. Brain Behav. Immun. 2007;21:617–623. doi: 10.1016/j.bbi.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 32.Rogers J, Mastroeni D, Leonard B, Joyce J, Grover A. Neuroinflammation in Alzheimer's disease and Parkinson's disease: are microglia pathogenic in either disorder? Int. Rev. Neurobiol. 2007;82:235–246. doi: 10.1016/S0074-7742(07)82012-5. [DOI] [PubMed] [Google Scholar]
- 33.Ling EA, Ng YK, Wu CH, Kaur C. Microglia: its development and role as a neuropathology sensor. Prog. Brain Res. 2001;132:61–79. doi: 10.1016/S0079-6123(01)32066-6. [DOI] [PubMed] [Google Scholar]
- 34.Cagnin A, Brooks DJ, Kennedy AM, Gunn RN, Myers R, Turkheimer FE, Jones T, Banati RB. In-vivo measurement of activated microglia in dementia. Lancet. 2001;358:461–467. doi: 10.1016/S0140-6736(01)05625-2. [DOI] [PubMed] [Google Scholar]
- 35.O'Callaghan JP, Sriram K. Glial fibrillary acidic protein and related glial proteins as biomarkers of neurotoxicity. Expert Opin. Drug Saf. 2005;4:433–442. doi: 10.1517/14740338.4.3.433. [DOI] [PubMed] [Google Scholar]
- 36.Hilton GD, Stoica BA, Byrnes KR, Faden AI. Roscovitine reduces neuronal loss, glial activation, and neurologic deficits after brain trauma. J. Cereb. Blood Flow Metab. 2008;28:1845–1859. doi: 10.1038/jcbfm.2008.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee CH, Hwang IK, Lee IS, Yoo KY, Choi JH, Lee BH, Won MH. Differential immunoreactivity of microglial and astrocytic marker protein in the hippocampus of the seizure resistant and sensitive gerbils. J. Vet. Med. Sci. 2008;70:1405–1409. doi: 10.1292/jvms.70.1405. [DOI] [PubMed] [Google Scholar]
- 38.Zecca L, Wilms H, Geick S, Claasen JH, Brandenburg LO, Holzknecht C, Panizza ML, Zucca FA, Deuschl G, Sievers J, Lucius R. Human neuromelanin induces neuroinflammation and neurodegeneration in the rat substantia nigra: implications for Parkinson's disease. Acta Neuropathol. 2008;116:47–55. doi: 10.1007/s00401-008-0361-7. [DOI] [PubMed] [Google Scholar]
- 39.Park L, Anrather J, Girouard H, Zhou P, Iadecola C. Nox2-derived reactive oxygen species mediate neurovascular dysregulation in the aging mouse brain. J. Cereb. Blood Flow Metab. 2007;27:1908–1918. doi: 10.1038/sj.jcbfm.9600491. [DOI] [PubMed] [Google Scholar]
- 40.Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- 41.Harman D. Free radical involvement in aging. Pathophysiology and therapeutic implications. Drugs Aging. 1993;3:60–80. doi: 10.2165/00002512-199303010-00006. [DOI] [PubMed] [Google Scholar]
- 42.Markesbery WR, Lovell MA. Four-hydroxynonenal, a product of lipid peroxidation, is increased in the brain in Alzheimer's disease. Neurobiol. Aging. 1998;19:33–36. doi: 10.1016/s0197-4580(98)00009-8. [DOI] [PubMed] [Google Scholar]
- 43.Swerdlow RH, Khan SMA. "mitochondrial cascade hypothesis" for sporadic Alzheimer's disease. Med Hypotheses. 2004;63:8–20. doi: 10.1016/j.mehy.2003.12.045. [DOI] [PubMed] [Google Scholar]
- 44.Niikura T, Yamada M, Chiba T, Aiso S, Matsuoka M, Nishimoto I. Characterization of V642I-AbetaPP-induced cytotoxicity in primary neurons. J. Neurosci. Res. 2004;77:54–62. doi: 10.1002/jnr.20139. [DOI] [PubMed] [Google Scholar]
- 45.Lambeth JD. NOX enzymes and the biology of reactive oxygen. Nat. Rev. Immunol. 2004;4:181–189. doi: 10.1038/nri1312. [DOI] [PubMed] [Google Scholar]
- 46.Geiszt M, Leto TL. The Nox family of NAD(P)H oxidases: host defense and beyond. J. Biol. Chem. 2004;279:51715–51718. doi: 10.1074/jbc.R400024200. [DOI] [PubMed] [Google Scholar]
- 47.Krause KH. Tissue distribution and putative physiological function of NOX family NADPH oxidases. Jpn. J. Infect. Dis. 2004;57:S28–S29. [PubMed] [Google Scholar]
- 48.Sorce S, Krause KH. NOX enzymes in the central nervous system: from signaling to disease. Antioxid. Redox Signal. 2009 doi: 10.1089/ars.2009.2578. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 49.Bruce-Keller AJ, Keller JN, Morrison CD. Obesity and vulnerability of the CNS. Biochim. Biophys. Acta. 2009;1792:395–400. doi: 10.1016/j.bbadis.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sena A, Sarliève LL, Rebel G. Brain myelin of genetically obese mice. J. Neurol. Sci. 1985;68:233–243. doi: 10.1016/0022-510x(85)90104-2. [DOI] [PubMed] [Google Scholar]
- 51.Jagust W. What can imaging reveal about obesity and the brain? Curr. Alzheimer Res. 2007;4:135–139. doi: 10.2174/156720507780362146. [DOI] [PubMed] [Google Scholar]
- 52.Gazdzinski S, Kornak J, Weiner MW, Meyerhoff DJ. Body mass index and magnetic resonance markers of brain integrity in adults. Ann. Neurol. 2008;63:652–657. doi: 10.1002/ana.21377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Winocur G, Greenwood CE. Studies of the effects of high fat diets on cognitive function in a rat model. Neurobiol. Aging. 2005;26:46–49. doi: 10.1016/j.neurobiolaging.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 54.White CL, Pistell PJ, Purpera MN, Gupta S, Fernandez-Kim SO, Hise TL, Keller JN, Ingram DK, Morrison CD, Bruce-Keller AJ. Effects of high fat diet on Morris maze performance, oxidative stress, and inflammation in rats: contributions of maternal diet. Neurobiol. Dis. 2009;35:3–13. doi: 10.1016/j.nbd.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Streit WJ, Miller KR, Lopes KO, Njie E. Microglial degeneration in the aging brain--bad news for neurons? Front. Biosci. 2008;13:3423–3438. doi: 10.2741/2937. [DOI] [PubMed] [Google Scholar]
- 56.McGeer PL, McGeer EG. The role of the immune system in neurodegenerative disorders. Mov. Disord. 1997;12:855–858. doi: 10.1002/mds.870120604. [DOI] [PubMed] [Google Scholar]
- 57.Kishida KT, Klann E. Sources and targets of reactive oxygen species in synaptic plasticity and memory. Antioxid. Redox Signal. 2007;9:233–244. doi: 10.1089/ars.2007.9.ft-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pao M, Wiggs EA, Anastacio MM, Hyun J, DeCarlo ES, Miller JT, Anderson VL, Malech HL, Gallin JI, Holland SM. Cognitive function in patients with chronic granulomatous disease: a preliminary report. Psychosomatics. 2004;45:230–234. doi: 10.1176/appi.psy.45.3.230. [DOI] [PubMed] [Google Scholar]
- 59.Kishida KT, Hoeffer CA, Hu D, Pao M, Holland SM, Klann E. Synaptic plasticity deficits and mild memory impairments in mouse models of chronic granulomatous disease. Mol. Cell Biol. 2006;26:5908–5920. doi: 10.1128/MCB.00269-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Knapp LT, Klann E. Role of reactive oxygen species in hippocampal long-term potentiation: contributory or inhibitory? J. Neurosci. Res. 2002;70:1–7. doi: 10.1002/jnr.10371. [DOI] [PubMed] [Google Scholar]
- 61.Kamsler A, Segal M. Hydrogen peroxide as a diffusible signal molecule in synaptic plasticity. Mol. Neurobiol. 2004;29:167–178. doi: 10.1385/MN:29:2:167. [DOI] [PubMed] [Google Scholar]
- 62.Hu D, Serrano F, Oury TD, Klann E. Aging-dependent alterations in synaptic plasticity and memory in mice that overexpress extracellular superoxide dismutase. J. Neurosci. 2006;26:3933–3941. doi: 10.1523/JNEUROSCI.5566-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liu R, Liu IY, Bi X, Thompson RF, Doctrow SR, Malfroy B, Baudry M. Reversal of age-related learning deficits and brain oxidative stress in mice with superoxide dismutase/catalase mimetics. Proc. Natl. Acad. Sci. U.S.A. 2003;100:8526–8531. doi: 10.1073/pnas.1332809100. [DOI] [PMC free article] [PubMed] [Google Scholar]