Abstract
Background
Self inflicted injury, including cutting or burning, is the most frequent reason for psychiatric visits to medical emergency departments. This behavior, particularly when there is no apparent suicidal intent, is poorly understood from both biological and clinical perspectives.
Objective
To examine the role of endogenous opioids and monoamine neurotransmitters in non-suicidal self-injury (NSSI).
Methods
We compared cerebrospinal fluid (CSF) levels of endogenous opioids, 5 hydroxyindolacetic acid (5-HIAA) and homovanillic acid (HVA) in individuals with a history of repetitive non-suicidal self-injury with a diagnostically-matched group of individuals who had never engaged in non-suicidal self-injury. History of suicidal behavior, demographic background and psychopathology was assessed. All patients were diagnosed with a Cluster B personality disorder (i.e. borderline, antisocial, narcissistic or histrionic) (N=29) and had a history of at least one suicide attempt. Fourteen participants had a history of repeated non suicidal self-injurious behavior (NSSI) in adulthood and 15 did not (no-NSSI).
Results
The NSSI group had significantly lower levels of CSF β-endorphin and met-enkephalin when compared with the non-NSSI group. CSF dynorphin, HVA and 5-HIAA levels did not differ. Severity of depression, hopelessness and overall psychopathology was greater in the NSSI group.
Conclusion
β-endorphin and met-enkephalin, opioids acting upon receptors involved in mediating stress-induced and physical pain analgesia respectively, are implicated in NSSI. Serotonergic and dopaminergic dysfunction do not appear to be related to NSSI. Based on our findings, we propose a model of non-suicidal self injury. Our results suggest that drugs acting on the opioid system warrant exploration as pharmacological treatments for NSSI.
INTRODUCTION
Non-suicidal self-injury (NSSI), defined as self injurious behavior without the intent to die, is a puzzling and poorly understood phenomenon. NSSI usually begins in adolescence, and most commonly takes the form of cutting, burning, or self-hitting (Nock and Favazza, 2009). The behavior often becomes habitual, persists into adulthood (Stanley et al., 1992; Nada-Raja et al., 2004) and is often treatment refractory (Perry et al., 2009). Individuals who exhibit NSSI are at increased risk for suicidal behavior (Stanley and Brodsky, 2005; Hawton and Harriss, 2006; Hawton and Harriss 2007) with many self-injurers having a history of multiple suicide attempts (Nock et al., 2006). Self-injurers often report a compulsive quality to their behavior, typically associated with a need to relieve tension, or alleviate emotional distress or pain (Klonsky, 2007). Some reports suggest disturbances in reward circuity or pain regulation in individuals who self-injure (Kemperman, 1997; Schmahl et al., 2004), while others have associated NSSI with emotion-focused and avoidance-focused coping styles (Williams and Hasking, 2009). NSSI occurs most often in individuals diagnosed with borderline personality disorder, a disorder characterized by self-destructive behavior, impulsivity and emotional lability (APA, 1994). However, several other disorders and syndromes have been associated with this behavior, including major depression (Langbehn and Pfohl, 1993), antisocial personality disorder (Isometsa et al., 1996), dissociative identity disorder (Saxe et al., 2002), posttraumatic stress disorder (Herman, 1992), eating disorders (Paul et al., 2002), autism (Nagamitsu et al., 1997), developmental disabilities (Nagamitsu, 1993) and Lesch-Nyhan syndrome (Anderson and Ernst, 1994).
While serotonergic and dopaminergic dysfunction have been associated with suicidal behavior (van Heeringen, 2003; Mann and Currier, 2007), several converging lines of evidence suggest the endogenous opioid system may play a more prominent role in self injury where there is no suicidal intent. There is a well-established relationship between pain perception and endogenous opioids (Urban and Gebhart, 1999; Fields, 2004; Przewlocki and Przewlocka 2001). β-endorphin and met-enkephalin, which are primarily mu- and delta- receptor agonists respectively, play a role in antinociception broadly, and are involved in a phenomenon known as stress-induced analgesia (Rubinstein et al., 1996; Yamada and Nabeshima 1995). Dynorphin, which binds primarily to the kappa opioid receptor, has a more complex role in the pain system, and has been found to be involved not only in analgesic states (Stevens and Yaksh, 1986), but also in stress-induced hyperalgesia (Vanderah et al., 2000) and the perpetuation of neuropathic pain (Lai et al., 2006). Research has demonstrated increased pain tolerance in individuals who self-injure (Claes et al. 2006) and in patients with borderline personality disorder (Ludascher et al., 2007). Also, regional activation of mu-opioid neurotransmission has been implicated in the suppression of the affective qualities of a pain stressor and in the negative internal affective states induced in a pain challenge paradigm. Zubieta and colleagues (2001; 2003) found dynamic changes in mu-opioid neurotransmission in response to an experimentally-induced negative affective state. Finally, β-endorphin plays an important role in the hypothalamic-pituitary adrenal stress response system via its role as a neuromodulator of corticotrophin-releasing hormone (Williams et al. 2003). There is also some evidence that the HPA axis is dysfunctional in individuals with BPD (Carrasco et al., 2007) and in individuals with a history of NSSI (Powers and McArdle, 2003).
Few studies have directly examined endogenous opioid levels in self injurious behavior. Coid et al. (1983) found plasma levels of met-enkephalin to be associated with the recency and severity of NSSI in habitual self-injurers with BPD, while reduced baseline (Weizman et al., 1988) but elevated post-NSSI (Sandman et al., 1997) plasma β-endorphin levels have been found in individuals with developmental disorders. Although these findings are suggestive of a relationship between the opioid system and NSSI, plasma neuropeptide levels may not accurately reflect central levels. Several studies have found no substantial correlation between plasma and CSF β-endorphin in humans (Nakao et al., 1980; Tsigos et al., 1993; Geracioti et al., 1997; De Riu et al., 1997). Research investigating central opioid functioning is therefore necessary if we are to understand their role in NSSI.
While the CSF concentration of endogenous opioids (β-endorphin, met-enkephalin and dynorphin) in individuals with a history of self-injury has not been examined, lower levels of endogenous opioids have been implicated in the psychiatric disorders in which a self destructive component exists. For example, Gillberg (1990) found reduced levels of CSF β-endorphin in individuals with autism. In addition, Brewerton et al. (1992) reported that CSF β-endorphin levels were lower in individuals with bulimia nervosa than in healthy controls, while Demitrack et al. (1993) found a decrease in CSF β-endorphin levels in underweight anorexic individuals. In contrast, both Brewerton et al. and Lesem et al. (1991) found no difference in CSF dynorphin concentrations between individuals with eating disorders and normal controls. Taken together, the evidence suggests that dysregulation of the endogenous opioid system, and the mu-opioid system in particular, could underlie NSSI.
There is limited research examining the relationship between monoamine function and NSSI. Although serotonergic dysfunction is hypothesized to underlie self-injurious behavior associated with Prader-Willi Syndrome (Hellings and Warnock, 1994; Kishore and Stamm, 2006) and dopamine is implicated in Lesch-Nyhan disease (Allen and Rice, 1996; Wong et al., 1996), both genetic disorders characterized by self injury, the findings in psychiatric samples with NSSI have been mixed.. In a study comparing adolescent girls with a history of NSSI and age-matched controls, Crowell et al. (2005) found lower peripheral serotonin levels in girls with a history of NSSI. However, as with the neuropeptides, peripheral measures are not strong indicators of central monoamine function (Sarrias et al. 1990). Using CSF measures, Gardner et al. (1990) found no difference between BPD individuals with NSSI and without NSSI in CSF levels of 5-HIAA and HVA.
In this study, we examine three endogenous opioids: β-endorphin; met-enkephalin; and dynorphin in depressed individuals with Cluster B personality disorders and a history of suicidal behavior. We hypothesized that CSF β-endorphin and met-enkephalin levels, but not dynorphin level, would be reduced in depressed individuals with a history of NSSI. Because dynorphin is appears to be primarily involved in the induction and maintenance of dysphoric states and neuropathic pain, we did not anticipate dynorphin levels to be associated with NSSI. In addition, we also examined the CSF levels of the monoamine metabolites 5-hydroxyindoleacetic acid (5-HIAA) and homovanillic acid (HVA). We hypothesized that serotonergic and dopaminergic dysfunction would not be altered in these individuals because we propose that these dysfunctions, while related to self injurious behavior with suicidal intent, would not be related to self injurious behavior in which the intent is primarily to regulate emotional state and reduce tension.
METHODS
Participants
Twenty-nine psychiatric patients at two large urban medical centers participated in the study after giving their written informed consent. The Institutional Review Boards approved the study which was part of a larger protocol investigating multiple clinical and biological aspects of NSSI and suicidal behavior. Inclusion criteria were: age 18-65 and DSM-IV (APA, 1994) Axis II diagnosis of a Cluster B personality disorder (i.e., borderline, antisocial, narcissistic, or histrionic). Exclusion criteria included: current substance abuse or dependence; psychotic disorders, a cognitive disorder which interfered with the patient’s ability to answer clinician-administered and self report rating scales; a history of head trauma resulting in coma; and mental retardation or other significant cognitive impairment. In order to control for the potential confounding effects of a history of suicidal behavior and to differentiate the neurobiology of non-suicidal self injury from suicidal behavior, all patients in the study had a history of one or more suicide attempts with an average of 3.0 attempts (S.D.= 2.4). The sample was divided into groups according to the presence or absence of a history of repeated non-suicidal NSSI, as indicated by a history of multiple episodes of self injury in adulthood. NSSI is defined as any purposeful self-harm, committed without the intent to die, resulting in bodily tissue damage, most frequently cutting, burning, and hitting. Individuals with only one episode of self injury were excluded. Subjects with a history of repeated non-suicidal self-injury comprised the NSSI group (n = 14), and those without a history of NSSI comprised the No NSSI group (n = 15). Patients were free medication for seven to fourteen days depending on the medication, prior to the lumbar puncture. The mean age of the sample was 35.5 ±9.1 (range 18-65). Most subjects were female (70%), Caucasians (79%), single (62%), without children (81%) and unemployed (83%). Sixty-two percent had attended college.
Diagnostic Assessment
Patients were given DSM-IV diagnoses following interviews with a trained clinician which included the Schedule for Interviewing Borderlines (Baron, 1980), and a clinical interview to obtain the information required to convert Research Diagnostic Criteria diagnoses to DSM-IV diagnoses. Raters were trained to an acceptable level of reliability by using videotaped assessments. Interrater reliability for the primary diagnosis was high (kappa=0.90). Depressive symptomatology was assessed in two ways. Clinician ratings were collected with the 21-item Hamilton Rating Scale for Depression (HRSD, Hamilton, 1960), administered in a semi-structured interview format. Participants also completed the Beck Depression Inventory, a self-report measure of depressive symptomatology (BDI; Beck et al.,, 1965). Global symptom severity was assessed with the Brief Psychiatric Rating Scale (BPRS, Overall et al., 1962), also administered in a semi-structured interview format, and the Clinical Global Impressions Scale (CGI). Hostility was assessed using the Buss-Durkee Hostility Inventory (BDHI, Buss and Durkee, 1957), a 75-item self-report questionnaire designed to measure several aspects of hostility and aggression
Lumbar Puncture
Cerebrospinal fluid (CSF) samples were collected via lumbar puncture, and concentrations of β-endorphin, met-enkephalin, dynorphin, the serotonin metabolite, 5-hydroxyindoleacetic acid (5-HIAA), and the dopamine metabolite, homovanillic acid (HVA), were determined for all participants. The lumbar puncture was performed between 8:00 a.m. and 10:00 a.m., following a night of supervised strict bed rest and a fast of at least 8 hours. The lumbar puncture was carried out under sterile conditions by a research psychiatrist using a fine-gauge (20 G) spinal needle, with the patient lying in a left lateral knee-chest position. CSF samples were consistently taken from the space between the third and fourth lumbar vertebrae. Eighteen ml of CSF were collected in three aliquots of 2 ml, 15 ml, and 1 ml. The 15-ml aliquot was centrifuged (750 g for 5 minutes) and divided into 1-ml aliquots. All aliquots were stored at −80°C until assayed.
Measurement of Endogenous Opioids, 5-HIAA and HVA in CSF Samples
Immunoreactive β-endorphin was quantified using a rabbit antiserum (K-7762) raised against unconjugated synthetic β-endorphin (Bramnert et al., 1982). The antiserum was used in a final dilution of 1:25,000. The specific recognition site was in the N-terminal region of β-endorphin. The cross-reactivity expressed as the ratio of the molar concentration of β-endorphin to met-enkephalin, causing 50% inhibition of tracer binding, was 9% (Bramnert et al., 1982). The detection limit was 10 pmol/L and the interassay coefficient of variation was <10%. Immunoreactive met-enkephalin was determined using a rabbit antiserum in a final dilution 1:20000. The antibody recognizes the C-terminus of met-enkephalin and does not cross-react with leucine-enkephalin, β-endorphin and dynorphin. The detection limit was 10 pmol/L and the interassay coefficient was below 10% (Samuelson et al., 1993). Immunoreactive dynorphin was determined using a rabbit antiserum in a final dilution of 1:12 500. The antiserum was directed against the C-terminal portion of dynorphin 1-13 and cross-reacted with dynorphin 1-9, 1-10, and 1-11 < 3.3%. The detection limit was 20 pmol/L and the interassay coefficient of variation as < 12% (Bramnert et al., 1982). 5-HIAA and HVA levels were determined in one of the 1-ml aliquots from the 15-ml sample by using high performance liquid chromatography with electrochemical detection. The interassay coefficient of variance for this procedure averages 4.9% for 5-HIAA and 5.0 for HVA (Meyendorff et al., 1986).
Data Analysis
The groups (NSSI/no NSSI) were compared on demographic, psychiatric, and biological measures using two-tailed Student’s t-tests for continuous variables, and Chi-square analysis for categorical variables. In addition, correlations were computed to examine relationships among biochemical and psychiatric measures. Age and height were initially included as covariates in the analyses because of their reported association with CSF metabolites (Roggenbach et al., 2002). In order to conserve degrees of freedom in the analyses, we removed covariates that did not account for a significant portion of the variance in metabolite levels.
RESULTS
The NSSI and no NSSI groups did not differ with respect to background characteristics. Age, sex, ethnicity, marital status, religious affiliation, education level, or employment rate did not differ between the groups (Table 1). All subjects were diagnosed with Cluster B personality disorder, all had BPD except one member of the NSSI group (histrionic) and two members of the no NSSI group (1 antisocial, 1 narcissistic). The rate of current major depression (about two thirds) did not differ between the groups. Approximately 21% of the NSSI group and 35% of the no NSSI had a family history of either suicide attempts or completed suicide in first-degree relatives. Mean number of psychiatric hospitalizations in each group was not significantly different (NSSI = 3.1 ± 3.8, no NSSI = 3.0 ± 3.0; t<1, df = 27; NS). There were no significant differences in having received prior psychiatric treatment (NSSI: 90%, no NSSI: 73%; Chi square = 2.47, df = 1, NS). Table 2 presents measures of psychopathology for the NSSI and no NSSI groups. Depression, hopelessness, and overall psychopathology as measured by the BPRS was greater in the NSSI group. The groups did not differ on the mean number of suicide attempts and lethality of most recent suicide attempt. Table 3 presents the results comparing CSF opioids, 5-HIAA and HVA levels in the two groups. The NSSI group had significantly lower levels of β-endorphin and met-enkephalin. There were no differences in CSF dynorphin, 5-HIAA and HVA levels between the groups.
Table 1.
Demographic Characteristics for NSSI and Non-NSSI Groups
| Variable | NSSI (N=14) |
Non- NSSI (N=15) |
Test | p |
|---|---|---|---|---|
| Gender | 86.7 | 69.6 | X2(1) = 2.32 | NS |
| (% Female) | ||||
| Age | 29.3±8.6 | 30.8±11.8 | t (27)<1 | NS |
| Ethnicity (%) | ||||
| White | 86.7 | 91.3 | X2 (3) < 1 | NS |
| Hispanic | 6.7 | 0 | ||
| Black | 3.3 | 0 | ||
| Asian | 3.3 | 8.7 | ||
| Marital Status | ||||
| Single | 73.3 | 65.2 | X2 (1) = 1.76 | NS |
| Married | 6.7 | 8.7 | ||
| Separated | 6.7 | 17.4 | ||
| Divorced | 13.3 | 8.7 | ||
| Children (%) | 21.7 | 17.4 | t (1) < 1 | NS |
| Education (%) | ||||
| Some HS | 3.3 | 13.0 | X2 (1) = 4.31 | NS |
| HS Graduate | 13.3 | 21.7 | ||
| Some College | 46.7 | 34.8 | ||
| College | 26.7 | 13.0 | ||
| Graduate | ||||
| Graduate | 10.0 | 17.4 | ||
| School | ||||
| Religion (%) | ||||
| Catholic | 3.3 | 40.9 | X2 (1) = 6.40 | NS |
| Protestant | 6.9 | 22.7 | ||
| Jewish | 24.1 | 4.6 | ||
| None | 20.7 | 13.6 | ||
| Other | 20.7 | 18.2 | ||
| Currently | 36.7 | 30.4 | X2 (1) < 1 | NS |
| Employed (%) | ||||
| Major | 66.7 | 60.9 | X2 (1) < 1 | NS |
| Depressive | ||||
| Disorder (%) | ||||
|
Family History
of Suicidal |
20.0 | 35.0 | X2 (1) = 1.46 | NS |
| Behavior (%) |
Table 2.
Clinical Ratings for NSSI and Non-NSSI Groups
| Measure | NSSI (N=14) |
Non- NSSI (N=15) |
t | p |
|---|---|---|---|---|
| Hamilton | 24.4±7.2 | 19.1±9.4 | 2.25 | <0.05 |
| Depression Scale | ||||
| Beck Hopelessness | 12.0±4.0 | 9.1±5.5 | 2.19 | <0.05 |
| Scale | ||||
| Brief Psychiatric | 40.1±8.4 | 35.2±8.8 | 1.90 | <0.05 |
| Rating Scale | ||||
| Buss-Durkee | 45.0±10.7 | 42.5±10.5 | <1 | NS |
| Hostility Inventory | ||||
| Clinical Global | 4.4±0.8 | 4.3±0.8 | <1 | NS |
| Impression Scale | ||||
| Mean # of Suicide | 3.1±2.4 | 2.9±2.4 | <1 | NS |
| Attempts | ||||
| Lethality of Most | 4.4±0.8 | 4.4±1.2 | <1 | NS |
| Recent Attempt | ||||
| (Range 1-8) |
Table 3.
CSF Metabolite Levels for NSSI and Non-NSSI Groups
| Metabolite | NSSI (N=14) |
Non-NSSI (N-15) |
t | p |
|---|---|---|---|---|
| β-endorphin | 91.4±14.1 | 105.9±19.2 | 2.18 | <0.05 |
| Met- enkephalin |
45.7±8.1 | 58.4±12.1 | 3.11 | <0.01 |
| Dynorphin | 20.1±7.9 | 21.7±7.9 | <1 | NS |
| 5-HIAA | 19.3±9.0 | 15.1±8.1 | 1.30 | NS |
| HVA | 36.9±17.9 | 26.8±12.0 | 1.77 | NS |
Note. All metabolite concentrations are in ng/ml.
DISCUSSION
This study is the first to report altered levels of CSF endogenous opioids in psychiatric patients with history of non-suicidal self injury. We found that patients in the NSSI group had lower CSF levels of β-endorphin and met-enkephalin than a matched group of patients without NSSI but no difference in dynorphin levels between the two groups. We also found that, while serotonergic and dopaminergic dysfunction has been reported in individuals with suicidal behavior (Mann and Currier, 2007), monoamine metabolite levels did not differ in relation to NSSI.
Importantly, we controlled for the possible confounding effects of suicidal behavior and psychiatric diagnosis by only including individuals with a suicide attempt history, a cluster B personality disorder and co-morbid depression. Gross-Isseroff et al. (1990) found that suicide was accompanied by a significant increase in mu-opioid receptor density in the young, but not the old, subjects compared to age-matched controls. We wanted to test for the independent relationship of non-suicidal self injury to opioids and, therefore, including only those individuals with prior suicidal behavior in both the experimental and control groups allowed us to make this comparison. Thus, our findings cannot be attributed to differences due to suicide attempt history or psychiatric diagnoses. Furthermore, we included only patients with several episodes of self injury ensuring that those who experimented with the behavior once or twice were exclude.
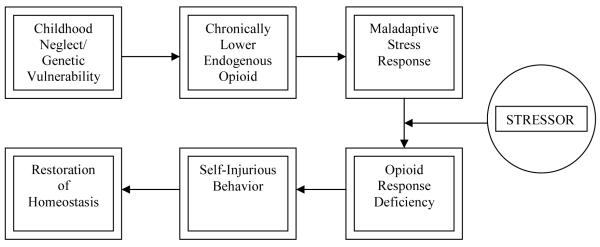
We propose a model in which opioid deficiency could result from chronic and severe childhood stress and trauma, such as abuse, neglect and loss or from a biological predisposition (Figure 1). Because endogenous opioids play a role in pain threshold and perception (Urban and Gebhart, 1999), they are likely candidates for involvement in self-inflicted injury, a behavior often associated with the need to feel pain or relieve emotional tension (Kemperman et al., 1997). Chronic stress can lead to a blunted endogenous opioid response to acute stress (Pike et al., 1997), and severe physical or psychological traumas may lead to a permanent deficiency state (Heffernan et al., 2000; van Goozen et al., 2000; Bremner, 2003), or perhaps habituation to higher levels of endogenous opioids. Several reports found persistent changes in opioid receptor binding after stress changes in animal models (Insel et al., 1990; Sanchez et al., 1992; Sanchez et al., 1996; Sanchez et al., 2000). Patients with abuse and neglect histories may require increased levels of endorphins to cope with stress as adults, and NSSI may be an effort at increasing the endogenous opioids to restore homeostasis. Childhood trauma and NSSI have a high co-occurrence (Zanarini et al., 2002; Gratz, 2003; Joyce et al., 2006).
Figure 1.
Homeostasis Model of NSSI
NSSI in borderline personality disorder is often followed by mood enhancement (Simeon et al., 1992). Specifically, a decrease in negative affect, an increase in positive affect, and an increase in dissociative symptoms has been reported (Kemperman et al., 1997) Simeon et al. (1992) suggest that self-injury may act as self-healing through restoration of positive affect, however brief. Studies in animals have demonstrated that the activation of mu-opioid receptor-mediated neurotransmission suppresses fear and stress responses to noxious threatening stimuli and mother-infant separation (Akil et al., 1984; Kalin et al., 1989; Good and Westbrook, 1995). The mu-opioid receptors also contribute to regulation of emotional memory (Quirate et al., 1998). It is worth noting that endogenous opioids may also mediate the mood-enhancing effect of exercise (Sher, 1998).
If the endogenous opioid system is central to repetitive self-injury, then treatment with a long-acting opioid antagonist could block the reward of enhanced endogenous opioids caused by such behaviors and subsequently lead to their extinction. Indeed, several reports (Sandman et al., 1983; Chabane et al., 2000; Garcia and Smith, 1999; Bystritsky and Strausser, 1996; McGee, 1997; Casner et al., 1996; Thompson et al., 1994; Lesem et al., 1991; Sandman, 2009) found that naloxone or naltrexone are useful in diminishing NSSI. Additionally, examining the efficacy of buprenorphine, a partial mu opioid agonist/antagonist, for NSSI may be a promising avenue of research.
Owing to limitations of our research design, we cannot rule out the possibility that individuals with NSSI may have had a differential response to the stress of a lumbar puncture than those without a history of NSSI. At the same time, using CSF for assessment of opioid function is a strength of this study. Further studies testing both this model and using other stress paradigms in self injurers and non self-injurers would help to evaluate whether the model is accurate, and whether our findings are due in part to the stress response of individuals with NSSI. Despite this limitation our findings suggest that patients with NSSI have abnormalities in the brain opioid system suggesting the possibility of disordered pain or reward circuitry. Studies of stressor and pain probes may further elucidate mechanisms of NSSI. Future studies should explore possible treatment approaches targeting the opioid system, such as buprenorphine, in NSSI.
Acknowledgements
None
Supported in part by NIMH grants #MH41847 and MH46745 to Dr. Stanley.
Funding Disclosure Funding for this study was provided by NIMH Grants #MH41847 and MH46745. The NIMH had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication
Footnotes
Financial Disclosures The authors of this manuscript have no financial or other relationships that are relevant to the subject matter of this research or the results described herein.
Conflict of Interest The authors of this manuscript have no financial or other relationships that are relevant to the subject matter of this research or the results described herein.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams ML, Morris DL, Brase DA, Dewey WL. Stereoselective effect of morphine on antinociception and endogenous opioid peptide levels in plasma but not cerebrospinal fluid of dogs. Life Sci. 1991;48(9):917–24. doi: 10.1016/0024-3205(91)90039-e. [DOI] [PubMed] [Google Scholar]
- Akil H, Watson SJ, Young E, Lewis ME, Khachaturian H, Walker JM. Endogenous opioids: biology and function. Annu Rev Neurosci. 1984;7:223–55. doi: 10.1146/annurev.ne.07.030184.001255. [DOI] [PubMed] [Google Scholar]
- Allen SM, Rice SN. Risperidone antagonism of self-mutilation in a Lesch-Nyhan patient. Progr Neuro-Psychopharmacol Biol Psychiatry. 1996;20:793–800. doi: 10.1016/0278-5846(96)00059-0. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 4th ed Author; Washington, DC: 1994. [Google Scholar]
- Anderson LT, Ernst M. Self-injury in Lesch-Nyhan disease. J Autism Dev Disord. 1994;24:67–81. doi: 10.1007/BF02172213. [DOI] [PubMed] [Google Scholar]
- Baker DG, West SA, Orth DN, Hill KK, Nicholson WE, Ekhator NN, Bruce AB, Wortman MD, Keck PE, Geracioti TD. Cerebrospinal fluid and plasma β -endorphin in combat veterans with post-traumatic stress disorder. Psychoneuroendocrinology. 1997;22:517–29. doi: 10.1016/s0306-4530(97)00053-x. [DOI] [PubMed] [Google Scholar]
- Baron M. The schedule for interviewing borderlines. New York State Psychiatric Institute; New York, NY: 1980. [Google Scholar]
- Basbaum AI, Fields HL. Endogenous pain control systems: brainstem spinal pathways and endorphin circuitry. Annu Rev Neurosci. 1984;7:309–38. doi: 10.1146/annurev.ne.07.030184.001521. [DOI] [PubMed] [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1965;12:63–70. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Bramnert M, Ekman R, Larson I, Thorell JI. Characterization and application of a radioimmunoassay for β-endorphin using an antiserum with negligible cross-reactivity against - lipotropin. Regul Pept. 1982;5:65–75. doi: 10.1016/0167-0115(82)90076-3. [DOI] [PubMed] [Google Scholar]
- Bremner JD. Long-term effects of childhood abuse on brain and neurobiology. Child Adolesc Psychiatr Clin N Am. 2003;12:271–92. doi: 10.1016/s1056-4993(02)00098-6. [DOI] [PubMed] [Google Scholar]
- Brewerton TD, Lydiard RB, Laraia MT, Shook JE, Ballenger JC. CSF β-endorphin and dynorphin in bulimia nervosa. Am J Psychiatry. 1992;149:1086–90. doi: 10.1176/ajp.149.8.1086. [DOI] [PubMed] [Google Scholar]
- Bystritsky A, Strausser BP. Treatment of obsessive-compulsive cutting behavior with naltrexone. J Clin Psychiatry. 1996;57:423–424. [PubMed] [Google Scholar]
- Buss AH, Durkee A. An inventory for assessing different kinds of hostility. J Consult Psychol. 1957;21:343–349. doi: 10.1037/h0046900. [DOI] [PubMed] [Google Scholar]
- Carrasco JL, Diaz-Marsa M, Pastrana JI, Molina R, Brotons L, Lopez-Ibor MI. Hypothalamic–pituitary–adrenal axis response in borderline personality disorder without post-traumatic features. Br J Psychiatry. 2007;190:357–358. doi: 10.1192/bjp.bp.106.022590. [DOI] [PubMed] [Google Scholar]
- Casner JA, Weinheimer B, Gualtieri CT. Naltrexone and self-injurious behavior: a retrospective population study. J Clin Psychopharmacol. 1996;16:389–394. doi: 10.1097/00004714-199610000-00008. [DOI] [PubMed] [Google Scholar]
- Chabane N, Leboyer M, Mouren-Simeoni MC. Opiate antagonists in children and adolescents. Eur Child Adolesc Psychiatry. 2000;9:I44–50. doi: 10.1007/s007870070018. [DOI] [PubMed] [Google Scholar]
- Claes L, Vandereycken W, Vertommen H. Pain experience related to self-injury in eating disorder patients. Eating behaviors. 2006;7:204–213. doi: 10.1016/j.eatbeh.2005.09.003. [DOI] [PubMed] [Google Scholar]
- Coid J, Allolio B, Rees LH. Raised plasma met-enkephalin in patients who habitually mutilate themselves. Lancet. 1983;2:545–6. doi: 10.1016/s0140-6736(83)90572-x. [DOI] [PubMed] [Google Scholar]
- Cross AJ, Hille C, Slater P. Subtraction autoradiography of opiate receptor subtypes in human brain. Brain Res. 1987;418:343–348. doi: 10.1016/0006-8993(87)90101-6. [DOI] [PubMed] [Google Scholar]
- Crowell SE, Beauchaine TP, McCauley E, Smith CJ, Stevens AL, Sylvers P. Psychological, autonomic, and serotonergic correlates of parasuicide among adolescent girls. Dev Psychopathol. 2005;17:1105–1127. doi: 10.1017/s0954579405050522. [DOI] [PubMed] [Google Scholar]
- Demitrack MA, Putnam FW, Rubinow DR, Pigott TA, Altemus M, Krahn DD, Gold PW. Relation of dissociative phenomena to levels of cerebrospinal fluid monoamine metabolites and β-endorphin in patients with eating disorders: a pilot study. Psychiatry Res. 1993;49:1–10. doi: 10.1016/0165-1781(93)90026-d. [DOI] [PubMed] [Google Scholar]
- De Riu PL, Petruzzi V, Caria MA, Mameli O, Casu AR, Nuvoli S, Spanu A, Madeddu G. β-endorphin and cortisol levels in plasma and CSF following acute experimental spinal traumas. Physiol Behav. 1997;62:1–5. doi: 10.1016/s0031-9384(97)00099-1. [DOI] [PubMed] [Google Scholar]
- Fields H. State-dependent opioid control of pain. Nat Rev Neurosci. 2004;5:565–575. doi: 10.1038/nrn1431. [DOI] [PubMed] [Google Scholar]
- Gabilondo AM, Meana JJ, Garcia-Sevilla JA. Increased density of mu-opioid receptors in the postmortem brain of suicide victims. Brain Res. 1995;682:245–50. doi: 10.1016/0006-8993(95)00333-l. [DOI] [PubMed] [Google Scholar]
- Garcia D, Smith RG. Using analog baselines to assess the effects of naltrexone on self-injurious behavior. Res Dev Disabil. 1999;20:1–21. doi: 10.1016/s0891-4222(98)00028-6. [DOI] [PubMed] [Google Scholar]
- Gardner DL, Lucas PB, Cowdry RW. CSF metabolites in borderline personality disorder compared with normal controls. Biol Psychiatry. 1990;28:247–254. doi: 10.1016/0006-3223(90)90580-u. [DOI] [PubMed] [Google Scholar]
- Geracioti TD, Nicholson WE, Orth DN, Ekhator NN, Loosen PT. Cholecytokinin in human cerebrospinal fluid: concentrations, dynamics, molecular forms and relationship to fasting and feeding in health, depression and alcoholism. Brain Res. 1993;629:260–268. doi: 10.1016/0006-8993(93)91329-q. [DOI] [PubMed] [Google Scholar]
- Gillberg C, Terenius L, Hagberg B, Witt-Engerström I, Eriksson I. CSF β-endorphins in childhood neuropsychiatric disorders. Brain and Development. 1990;12:88–92. doi: 10.1016/s0387-7604(12)80185-9. [DOI] [PubMed] [Google Scholar]
- Good AJ, Westbrook RF. Effects of a microinjection of morphine into the amygdala on the acquisition and expression of conditioned fear and hypoalgesia in rats. Behav Neurosci. 1995;109:631–41. doi: 10.1037//0735-7044.109.4.631. [DOI] [PubMed] [Google Scholar]
- Gratz KL. Risk Factors for and Functions of Deliberate Self-Harm: An Empirical and Conceptual Review. Clinical Psychology Science and Practice. 2003;10:192–225. [Google Scholar]
- Gross-Isseroff R, Dillon KA, Israeli M, Biegon A. Regionally selective increases in mu opioid receptor density in the brains of suicide victims. Brain Res. 1990;530:312–6. doi: 10.1016/0006-8993(90)91301-v. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawton K, Harriss L. Deliberate self-harm in people aged 60 years and over : Characteristics and outcome of a 20-year cohort. Int J Geriatr Psychiatry. 2006;21:572–581. doi: 10.1002/gps.1526. [DOI] [PubMed] [Google Scholar]
- Hawton K, Harriss L. Deliberate self-harm in young people : Characteristics and subsequent mortality in a 20 year cohort of patients presenting to hospital. J Clin Psychiatry. 2007;68:1574–1583. [PubMed] [Google Scholar]
- Heffernan K, Cloitre M, Tardiff K, Marzuk PM, Portera L, Leon AC. Childhood trauma as a correlate of lifetime opiate use in psychiatric patients. Addict Behav. 2000;25:797–803. doi: 10.1016/s0306-4603(00)00066-6. [DOI] [PubMed] [Google Scholar]
- Hellings JA, Warnock JK. Self-injurious behavior and serotonin in Prader-Willi syndrome. Psychopharmacol Bull. 1994;30:245–250. [PubMed] [Google Scholar]
- Helmstetter FJ, Fanselow MS. Effects of naltrexone on learning and performance of conditional fear-induced freezing and opioid analgesia. Physiol Behav. 1987;39:501–5. doi: 10.1016/0031-9384(87)90380-5. [DOI] [PubMed] [Google Scholar]
- Herman JL. Complex PTSD: A syndrome in survivors of prolonged and repeated trauma. J Trauma Stress. 1992;5:377–391. [Google Scholar]
- Insel TR, Kinsley CH, Mann PE, Bridges RS. Prenatal stress has long-term effects on brain opiate receptors. Brain Research. 1990;511(1):93–7. doi: 10.1016/0006-8993(90)90228-4. [DOI] [PubMed] [Google Scholar]
- Isometsa ET, Henriksson MM, Heikkinen ME, Aro HM, Marttunen MJ, Kuoppasalmi KI, Lonnqvist JK. Suicide among subjects with personality disorders. Am J Psychiatry. 1996;153:667–673. doi: 10.1176/ajp.153.5.667. [DOI] [PubMed] [Google Scholar]
- Joyce PR, McKenzie JM, Mulder RT, Luty SE, Sullivan PF, Miller AL, Kennedy MA. Genetic, developmental and personality correlates of self-mutilation in depressed patients. Aust N Z J Psychiatry. 2006;40:225–9. doi: 10.1080/j.1440-1614.2006.01778.x. [DOI] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE, Barksdale CM. Behavioral and physiologic effects of CRH administered to infant primates undergoing maternal separation. Neuropsychopharmacology. 1989;2:97–104. doi: 10.1016/0893-133x(89)90012-2. [DOI] [PubMed] [Google Scholar]
- Kemperman I. Pain assessment in self-injurious patients with borderline personality disorder using signal detection theory. Psychiatry Res. 1997;70:175–183. doi: 10.1016/s0165-1781(97)00034-6. [DOI] [PubMed] [Google Scholar]
- Kemperman I, Russ MJ, Shearin E. Self-injurious behavior and mood regulation in borderline patients. J Personal Disord. 1997;11:146–57. doi: 10.1521/pedi.1997.11.2.146. [DOI] [PubMed] [Google Scholar]
- Kishore S, Stamm S. The snoRNA HBII-52 regulates alternative splicing of the serotonin receptor 2C. Science. 2006;5758:230–232. doi: 10.1126/science.1118265. [DOI] [PubMed] [Google Scholar]
- Klonsky D. The functions of deliberate self-injury: A review of the evidence. Clin Psychol Review. 2007;27:226–239. doi: 10.1016/j.cpr.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Lai J, Luo MC, Chen Q, Ma S, Gardell LR, Ossipov MH, Porreca F. Dynorphin A activates bradykinin receptors to maintain neuropathic pain. Nat. Neurosci. 2006;9:1534–1540. doi: 10.1038/nn1804. [DOI] [PubMed] [Google Scholar]
- Land BB, Bruchas MR, Lemos JC, Xu M, Melief EJ, Chavkin C. The dysphoric component of stress is encoded by activation of the dynorphin kappa-opioid system. J Neurosci. 2008;28(2):407–14. doi: 10.1523/JNEUROSCI.4458-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langbehn DR, Pfohl B. Clinical correlates of self-mutilation among psychiatric inpatients. Ann Clin Psychiatry. 1993;5:45–51. doi: 10.3109/10401239309148923. [DOI] [PubMed] [Google Scholar]
- Lesem MD, Berrettini WH, Kaye WH, Jimerson DC. Measurement of CSF dynorphin A 1-8 immunoreactivity in anorexia nervosa and normal-weight bulimia. Biol Psychiatry. 1991;29:244–52. doi: 10.1016/0006-3223(91)91286-z. [DOI] [PubMed] [Google Scholar]
- Ludascher P, Bohus M, Lieb K, Philipsen A, Jochims A. Elevated pain thresholds correlate with dissociation and aversive arousal in patients with borderline personality disorder. Psychiatry Res. 2007;149:291–296. doi: 10.1016/j.psychres.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Maier SF, Davies S, Grau JW, Jackson RL, Morrison DH, Moye T, Madden J, Barchas JD. Opiate antagonists and long-term analgesic reaction induced by inescapable shock in rats. J Comp Physiol Psychol. 1980;94:1172–83. doi: 10.1037/h0077743. [DOI] [PubMed] [Google Scholar]
- Mann JJ, Currier D. A review of prospective studies of biological predictors of suicidal behavior in mood disorders. Arch Suicide Res. 2007;11:3–16. doi: 10.1080/13811110600993124. [DOI] [PubMed] [Google Scholar]
- McGee MD. Cessation of self-mutilation in a patient with borderline personality disorder treated with naltrexone. J Clin Psychiatry. 1997;58:32–33. doi: 10.4088/jcp.v58n0106e. [DOI] [PubMed] [Google Scholar]
- Meyendorff E, Jain A, Träskman-Bendz L, Stanley B, Stanley M. The effects of fenfluramine on suicidal behavior. Psychopharmacol Bull. 22:155–159. [PubMed] [Google Scholar]
- Nada-Raja S, Skegg K, Langley J, Morrison D, Sowerby P. Self-harming behaviors in a population-based sample of young adults. Suicide and Life-Threatening Behavior. 2004;34:177–186. doi: 10.1521/suli.34.2.177.32781. [DOI] [PubMed] [Google Scholar]
- Nagamitsu S. CSF β-endorphin levels in pediatric neurologic disorders. Kurume Med J. 1993;40:233–241. doi: 10.2739/kurumemedj.40.233. [DOI] [PubMed] [Google Scholar]
- Nagamitsu S, Matsuishi T, Kisa T, Komori H, Miyazaki M, Hashimoto T, Yamashita Y, Ohtaki E, Kato H. CSF β-endorphin levels in patients with infantile autism. J Autism Dev Disord. 1997;27:155–63. doi: 10.1023/a:1025839807431. [DOI] [PubMed] [Google Scholar]
- Nakao K, Oki S, Tanaka I, Horii K, Nakai Y, Furui T, Fukushima M, Kuwayama A, Kageyama N, Imura H. Immunoreactive β-endorphin and adrenocorticotropin in human cerebrospinal fluid. J Clin Invest. 1980;66:1383–90. doi: 10.1172/JCI109991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nock M, Favazza A. Nonsuicidal Self-Injury: Definition and Classification. In: Nock M, editor. Understanding Nonsuicidal Self–Injury. American Psychological Association; Washington, D.C.: 2007. pp. 9–36. [Google Scholar]
- Nock M, Joiner T, Gordon K, Lloyd-Richardson E, Prinstein M. Non-suicidal self-injury among adolescents: Diagnostic correlates and relation to suicide attempts. Psychiatry Res. 2006;144:65–72. doi: 10.1016/j.psychres.2006.05.010. [DOI] [PubMed] [Google Scholar]
- Overall JE, Gorham DR. The Brief Psychiatric Rating Scale. Psychol Rep. 1962;10:799–812. [Google Scholar]
- Paul T, Schroeter K, Dahme B, Nutzinger DO. Self-injurious behavior in women with eating disorders. Am J Psychiatry. 2002;159:408–411. doi: 10.1176/appi.ajp.159.3.408. [DOI] [PubMed] [Google Scholar]
- Perry CJ, Fowler JC, Bailey A, Clemence AJ, Plakun EM, Zheutlin B, Speanburg S. Improvement and recovery from suicidal and self-destructive phenomena in treatment-refractory disorders. J Ner Ment Dis. 2009;197:28–34. doi: 10.1097/NMD.0b013e3181927598. [DOI] [PubMed] [Google Scholar]
- Pfeiffer A, Brantl V, Herz A, Emrich HM. Psychotomimesis mediated by kappa opiate receptors. Science. 1986;233:774–776. doi: 10.1126/science.3016896. [DOI] [PubMed] [Google Scholar]
- Pike JL, Smith TL, Hauger RL, Nicassio PM, Patterson TL, McClintock J, Costlow C, Irwin MR. Chronic life stress alters sympathetic, neuroendocrine, and immune responsivity to an acute psychological stressor in humans. Psychosom Med. 59:447–457. doi: 10.1097/00006842-199707000-00015. [DOI] [PubMed] [Google Scholar]
- Powers SI, McArdle ET. Coping strategies moderate the relation of hypothalamus-pituitary-adrenal axis reactivity to self-injurious behavior. Ann N Y Acad Sci. 2003;1008:285–288. doi: 10.1196/annals.1301.033. [DOI] [PubMed] [Google Scholar]
- Przewlocki R, Przewlocka B. Opioids in chronic pain. Eur J Pharmacol. 2001;429:79–91. doi: 10.1016/s0014-2999(01)01308-5. [DOI] [PubMed] [Google Scholar]
- Quirarte GL, Galvez R, Roozendaal B, McGaugh JL. Norepinephrine release in the amygdala in response to footshock and opioid peptidergic drugs. Brain Research. 1998;808:134–40. doi: 10.1016/s0006-8993(98)00795-1. [DOI] [PubMed] [Google Scholar]
- Roth AS, Ostroff RB, Hoffman RE. Naltrexone as a treatment for repetitive self-injurious behaviour: an open-label trial. J Clin Psychiatry. 1996;57:233–237. [PubMed] [Google Scholar]
- Rubinstein M, Mogil JS, Japon M, Chan EC, Allen RG, Low MJ. Absence of opioid stress-induced analgesia in mice lacking β -endorphin by site-directed mutagenesis. Proc Natl Acad Sci. 1996;93:3395–4000. doi: 10.1073/pnas.93.9.3995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuelsson H, Ekman R, Hedner T. CSF neuropeptides in cancer pain: effects of spinal opioid therapy. Acta Anaesthesiol Scand. 1993;37:502–508. doi: 10.1111/j.1399-6576.1993.tb03755.x. [DOI] [PubMed] [Google Scholar]
- Sanchez MD, Milanes MV, Fuente T, Laorden ML. Prenatal stress alters the hypothalamic levels of methionine-enkephalin in pup rats. Neuropeptides. 1992;23:131–135. doi: 10.1016/0143-4179(92)90090-j. [DOI] [PubMed] [Google Scholar]
- Sanchez MD, Milanes MV, Pazos A, Diaz A, Laorden ML. Autoradiographic evidence of mu-opioid receptors down-regulation after prenatal stress in offspring rat brain. Brain Res Dev Brain Res. 1996;94:14–21. doi: 10.1016/0165-3806(96)00032-6. [DOI] [PubMed] [Google Scholar]
- Sanchez MD, Milanes MV, Pazos A, Diaz A, Laorden ML. Autoradiographic evidence of delta-opioid receptor downregulation after prenatal stress in offspring rat brain. Pharmacology. 2000;60:13–18. doi: 10.1159/000028341. [DOI] [PubMed] [Google Scholar]
- Sandman CA, Datta PC, Barron J, Hoehler FK, Williams C, Swanson JM. Naloxone attenuates self-abusive behavior in developmentally disabled clients. Appl Res Ment Retard. 1983;4:5–11. doi: 10.1016/s0270-3092(83)80014-9. [DOI] [PubMed] [Google Scholar]
- Sandman CA. Efficacy of Opioid Antagonists in Attenuating Self-Injurious Behavior. In: Dean R, Bilsky EJ, Negus SS, editors. Opiate Receptors and Antagonists From Bench to Clinic. Humana Press; New York: 2009. [Google Scholar]
- Sarrias MJ, Cabre P, Martinez E, Artigas F. Relationship between serotonergic measures in blood and cerebrospinal fluid simultaneously obtained in humans. Journal of Neurochemistry. 1990;54(3):783–786. doi: 10.1111/j.1471-4159.1990.tb02319.x. [DOI] [PubMed] [Google Scholar]
- Saxe GN, Chawla N, Van der Kolk B. Self-destructive behavior in patients with dissociative disorders. Suicide Life Threat Behav. 2002;32:313–320. doi: 10.1521/suli.32.3.313.22174. [DOI] [PubMed] [Google Scholar]
- Schmahl C, Greffrath W, Baumgärtner U, Schlereth T, Magerl W, Philipsen A, Lieb K, Bohus M, Treede R. Differential nociceptive deficits in patients with borderline personality disorder and self-injurious behavior: laser-evoked potentials, spatial discrimination of noxious stimuli, and pain ratings. Pain. 2004;110:470–479. doi: 10.1016/j.pain.2004.04.035. [DOI] [PubMed] [Google Scholar]
- Schmauss C. Spinal kappa-opioid receptor-mediated antinociception is stimulus-specific. Eur J Pharmacol. 1987;137:197–205. doi: 10.1016/0014-2999(87)90223-8. 1987. [DOI] [PubMed] [Google Scholar]
- Sher L. The endogenous euphoric reward system that reinforces physical training: a mechanism for mankind’s survival. Med Hypoth. 1998;51:449–450. doi: 10.1016/s0306-9877(98)90064-0. [DOI] [PubMed] [Google Scholar]
- Simeon D, Hollander E. Self injurious behaviors: Assessment and treatment. American Psychiatric Publishing, Inc.; Washington, DC: 2001. [Google Scholar]
- Shippenberg TS. The dynorphin/kappa opioid receptor system: a new target for the treatment of addiction and affective disorders? Neuropsychopharmacology. 2009;34(1):247. doi: 10.1038/npp.2008.165. Click here to read. [DOI] [PubMed] [Google Scholar]
- Sora I, Funada M, Uhl GR. The mu-opioid receptor is necessary for [D-Pen 2, D- Pen 5] enkephalin-induced analgesia. Eur J Pharmocol. 1997;324:R1–2. doi: 10.1016/s0014-2999(97)10016-4. [DOI] [PubMed] [Google Scholar]
- Stanley B, Brodsky B. Suicidal and self-injurious behavior in borderline personality disorder: The self-regulation action model. In: Gunderson J, Hoffman P, editors. Borderline Personality Disorder Perspectives: From Professional to Family Member, Sharing Knowledge, Building Partnerships. American Psychiatric Press; Washington, DC: 2005. [Google Scholar]
- Stanley B, Siever L. The interpersonal dimension of borderline personality disorder: Towards a neuropeptide model. Am J Psychiatry. doi: 10.1176/appi.ajp.2009.09050744. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley B, Winchel R, Molcho A, Simeon D, Stanley M. Suicide and the self-harm continuum: Phenomenological and biochemical evidence. Int Rev Psychiatry. 1992;4:149–155. [Google Scholar]
- Stevens CW, Yaksh TL. Dynorphin A and related peptides administered intrathecally in the rat: a search for putative kappa opiate receptor activity. J. Pharmacol. Exp. Ther. 1986;238:833–838. [PubMed] [Google Scholar]
- Thompson T, Hackenberg T, Cerutti D, Baker D, Axtell S. Opioid antagonist effects on self-injury in adults with mental retardation: response form and location as determinants of medication effects. Am J Ment Retard. 1994;99:85–102. [PubMed] [Google Scholar]
- Tsigos C, Crosby SR, Gibson S, Young RJ, White A. Proopiomelanocortin is the predominant adrenocorticotropin-related peptide in human cerebrospinal fluid. J Clin Endocrinol Metab. 1993;76:620–624. doi: 10.1210/jcem.76.3.8383142. [DOI] [PubMed] [Google Scholar]
- Urban M, Gebhart G. Central mechanisms in pain. Med Clin North Am. 1999;83:585–596. doi: 10.1016/s0025-7125(05)70125-5. [DOI] [PubMed] [Google Scholar]
- Vanderah TW, Gardell LR, Burgess SE, Ibrahim M, Dogrul A, Zhong CM, Zhang ET, Malan TP, Ossipov MH, Lai J, Porreca F. Dynorphin promotes abnormal pain and spinal opioid antinociceptive tolerance. J Neurosci. 2000;20:7074–7079. doi: 10.1523/JNEUROSCI.20-18-07074.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Goozen SH, van den Ban E, Matthys W, Cohen-Kettenis PT, Thijssen JH, van Engeland H. Increased adrenal androgen functioning in children with oppositional defiant disorder: a comparison with psychiatric and normal controls. J Am Acad Child Adolesc Psychiatry. 2000;39:1446–51. doi: 10.1097/00004583-200011000-00020. [DOI] [PubMed] [Google Scholar]
- van Heeringen K. The neurobiology of suicide and suicidality. Can J Psychiatry. 2003;48:292–300. doi: 10.1177/070674370304800504. [DOI] [PubMed] [Google Scholar]
- Weizman R, Gil-Ad I, Dick J, Tyano S, Szekely GA, Lavon Z. Low Plasma Immunoreactive [] β-Endorphin Levels in Autism. J Am Acad Child Adolesc Psychiatry. 1988;27:430–433. doi: 10.1097/00004583-198807000-00009. [DOI] [PubMed] [Google Scholar]
- Williams F, Hasking P. Emotion regulation, coping and alcohol use as moderators in the relationship between non-suicidal self injury and psychological distress. Prev Sci. 2009 Aug; doi: 10.1007/s11121-009-0147-8. 2009.epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Williams KL, Ko MC, Rice KC, Woods JH. Effect of opioid receptor antagonists on hypothalamic– pituitary– adrenal activity in rhesus monkeys. Psychoneuroendocrinology. 2003;28:513–528. doi: 10.1016/s0306-4530(02)00037-9. [DOI] [PubMed] [Google Scholar]
- Wong DF, Harris JC, Naidu S, Yokoi F, Marenco S, Dannals RF, Ravert HT, Yasters M, Evans A, Rousset O, Bryan RN, Gjedde A, Kuhar MJ, Breese GR. Dopamine transporters are markedly reduced in Lesch-Nyhan disease in vivo. Proc Natl Acad Sci. 1996;93:5539–5543. doi: 10.1073/pnas.93.11.5539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K, Nabeshima T. Stress-induced behavioral responses and multiple opioid systems in the brain. Behav Brain Res. 1995;67:133–145. doi: 10.1016/0166-4328(94)00150-e. [DOI] [PubMed] [Google Scholar]
- Zanarini MC, Yong L, Frankenburg FR, Hennen J, Reich DB, Marino MF, Vujanovic AA. Severity of reported childhood sexual abuse and its relationship to severity of borderline psychopathology and psychosocial impairment among borderline inpatients. J Nerv Ment Dis. 2002;190:381–7. doi: 10.1097/00005053-200206000-00006. [DOI] [PubMed] [Google Scholar]
- Zubieta JK, Ketter TA, Bueller JA, Xu Y, Kilbourn MR, Young EA, Koeppe RA. Regulation of human affective responses by anterior cingulate and limbic mu-opioid neurotransmission. Arch Gen Psychiatry. 2003;60:1145–53. doi: 10.1001/archpsyc.60.11.1145. [DOI] [PubMed] [Google Scholar]
- Zubieta JK, Smith YR, Bueller JA, Xu Y, Kilbourn MR, Jewett DM, Meyer CR, Koeppe RA, Stohler CS. Regional mu opioid receptor regulation of sensory and affective dimensions of pain. Science. 2001;293:311–315. doi: 10.1126/science.1060952. [DOI] [PubMed] [Google Scholar]