Summary
BACKGROUND
Corticotropin-releasing hormone (CRH) in maternal blood originates primarily from gestational tissues and elevated levels in midpregnancy have been linked to adverse pregnancy outcomes. Investigators have hypothesized that high levels of maternal stress might lead to elevated CRH levels in pregnancy. Yet a few studies have measured maternal CRH levels among subgroups of women who experience disproportionate socioeconomic disadvantage, such as African-American and Hispanic women, and found that these groups have lower CRH levels in pregnancy. Our goal was to identify maternal characteristics related to CRH levels in midpregnancy and examine which if any of these factors help to explain race differences in CRH levels.
METHODS
The Pregnancy Outcomes and Community Health (POUCH) Study prospectively enrolled women at 15–27 weeks’ gestation from 52 clinics in five Michigan communities (1998–2004). Data from the POUCH Study were used to examine maternal demographics, anthropometrics, health behaviors, and psychosocial factors (independent variables) in relation to midpregnancy blood CRH levels modeled as log CRH pg/ml (dependent variable). Analyses were conducted within a subcohort from the POUCH Study (671 non-Hispanic Whites, 545 African Americans) and repeated in the subcohort subset with uncomplicated pregnancies (n=746). Blood levels of CRH and independent variables were ascertained at the time of enrollment. All regression models included week of enrollment as a covariate. In addition, final multivariable regression models alternately incorporated different psychosocial measures along with maternal demographics and weight. Psychosocial variables included measures of current depressive symptoms, perceived stress, coping style, hostility, mastery, anomie, and a chronic stressor (history of abuse as a child and adult).
RESULTS
In subcohort models, the adjusted mean CRH level was significantly lower in African Americans vs. non-Hispanic whites; the difference was −0.48 pg/ml (P<0.01). This difference was reduced by 21% (−0.38 pg/ml, P<0.01) after inclusion of other relevant covariates. Adjusted mean CRH levels were also lower among women with < 12 years vs. ≥ 12 years of education (minimal difference =−0.19 pg/ml, P<0.05), and among women with high levels of depressive symptoms who did not use antidepressants vs. women with lower levels of depressive symptoms and no antidepressant use (minimal difference =−0.13 pg/ml, P<0.01). CRH levels were inversely associated with maternal weight (−0.03 pg/ml per 10 pound increase, P<.05) but unrelated to smoking and all other psychosocial measures. Results were similar in the subset of women with uncomplicated pregnancies, except that lower CRH levels were also linked to higher perceived stress.
CONCLUSION
African-American women have lower blood CRH levels at midpregnancy and the race difference in CRH levels is reduced modestly after adjustment for other maternal characteristics. CRH levels were not elevated among women with high levels of perceived stress or more chronic stressors. The inverse association between CRH levels and maternal weight is likely due to a hemodilution effect. Relations among maternal CRH levels and maternal race, educational level, and depressive symptoms are difficult to explain and invite further investigation. Our results highlight a group of covariates that merit consideration in studies that address CRH in the context of pregnancy and/or post-partum complications.
Keywords: corticotropin releasing hormone, pregnancy, race, weight, depression
Introduction
Corticotropin-releasing hormone (CRH), produced by the hypothalamus, is a component of the HPA axis (hypothalamic-pituitary-adrenal axis). Hypothalamic CRH stimulates the secretion of adrenocorticotropin hormone (ACTH) which in turn increases cortisol production and release from the adrenal glands. ACTH and cortisol levels exert a negative feedback on hypothalamic CRH. The HPA axis is a major neuroendocrine system involved in the stress response and in various body processes including digestion, the immune system, mood, and energy usage (Watson and Mackin 2006).
The production of CRH by the placenta, fetal membranes and decidua is a distinct characteristic of primates (Petraglia, Sawchenko et al. 1987; Petraglia, Tabanelli et al. 1992; Smith 2007). Maternal blood CRH levels during pregnancy primarily originate from gestational tissues (Grino, Chrousos et al. 1987) and levels increase exponentially with advancing pregnancy, peaking at the time of delivery (Emanuel, Robinson et al. 1994).
Investigators have hypothesized that CRH may play a critical role in the onset of parturition (Ellis, Livesey et al. 2002; McLean, Bisits et al. 1995; Smith 2007; Warren, Patrick et al. 1992). For reasons that are not entirely clear, higher maternal blood CRH levels in the second and third trimesters have been associated with an increased risk of adverse pregnancy outcomes such as preterm delivery (PTD), low birth weight, and preeclampsia (Emanuel, Torday et al. 2000; Grammatopoulos and Hillhouse 1999; Hobel, Arora et al. 1999; Holzman, Jetton et al. 2001; Karteris, Vatish et al. 2005; Leung, Chung et al. 2000; Makrigiannakis, Zoumakis et al. 2003; McLean, Bisits et al. 1999; Purwosunu, Sekizawa et al. 2007; Sibai, Meis et al. 2005; Wadhwa, Garite et al. 2004; Warren, Patrick et al. 1992).
Evidence suggests that CRH in gestational tissues has multiple autocrine, paracrine, and endocrine effects. Prior to and in the early stages of pregnancy, CRH can influence decidualization (Ferrari, Petraglia et al. 1995; Karteris, Papadopoulou et al. 2004), implantation (Makrigiannakis, Zoumakis et al. 2001), and trophoblast growth (Choy, Leung et al. 2004). Later effects include vasodilatation, (Clifton, Read et al. 1994) and modulation of myometrial contractility (Grammatopoulos and Hillhouse 1999; Quartero and Fry 1989). In addition, placental CRH may cross over to the fetus, stimulate the fetal pituitary adrenal axis, and thereby increase the production of both fetal dehydroepiandrosterone (DHEA-S) and cortisol (Florio, Severi et al. 2002; Smith 2007). The fetal DHEA-S serves as a precursor to estrogen synthesis in the placenta, and fetal cortisol may stimulate placental CRH synthesis in a feed-forward loop (Majzoub, McGregor et al. 1999). Given the importance of CRH during pregnancy, relatively little is known about maternal characteristics and pregnancy circumstances associated with levels of this biomarker in complicated and uncomplicated pregnancies.
Studies have probed links between CRH levels in pregnancy and maternal psychosocial factors such as stress, anxiety, and depression, but results to date have been mixed (Hobel, Dunkel-Schetter et al. 1999; Mancuso, Schetter et al. 2004; Rich-Edwards, Mohllajee et al. 2008; Susman, Schmeelk et al. 1999; Yim, Glynn et al. 2009). This line of inquiry is motivated, in part, by two potential scenarios that could lead to associations between CRH levels and psychosocial factors. First, women with depression or excess stress might have higher cortisol levels, which according to some in-vitro and in-vivo studies could result in stimulation of placental CRH production (Jones, Brooks et al. 1989; Robinson, Emanuel et al. 1988; Smith 2007). This maternal feed-forward mechanism, like the fetal feed-forward mechanism for placental CRH, is opposite to cortisol’s negative feedback effects on hypothalamic CRH, but parallels the ability of cortisol to increase CRH in the amygdala of the brain (Kalin, Takahashi et al. 1994; Makino, Gold et al. 1994). Second, placenta-derived CRH might affect maternal pituitary-adrenal axis activity, perhaps influencing her appraisal of stress or symptoms of depression. One study suggested that mothers appraised events as less stressful as pregnancy progresses (Glynn, Schetter et al. 2004) but this may or may not be related to CRH levels. The scenarios identified above are difficult to study, especially given the complexity of a dynamic psychosocial milieu and the physiologic ‘regulation’ systems in place. Some of these CRH regulation systems include: 1) a CRH binding protein (CRH-BP) produced in the maternal liver and in gestational tissues that affects the bioavailability of placenta-derived CRH, (Thomson 1998); 2) glucocorticoid receptors in gestational tissues which affect cortisol stimulation of CRH (Lee, Wang et al. 2005); 3) influences of other molecules such as estrogen and progesterone on CRH production (Karalis, Goodwin et al. 1996; Ni, Hou et al. 2004); 4) a cortocosteroid binding globulin (CBG) that alters cortisol bioavailabilty (Benassayag, Souski et al. 2001); 5) 11βHSD-2, an enzyme highly expressed in the placenta that reduces the bioavailability of cortisol by converting it into cortisone (Yang 1997); and 6) the up and down regulation of receptors along the mother’s HPA axis, (Brunton, Russell et al. 2008; Kammerer, Adams et al. 2002; Nierop, Bratsikas et al. 2006; Schulte, Weisner et al. 1990).
In light of the above hypothesized scenarios, and considering the link between elevated maternal CRH levels and adverse pregnancies outcomes, one might expect that women who experience multiple societal stressors and have poorer pregnancies outcomes, such as African-American women and women with low educational levels, would have higher maternal CRH levels. In the limited number of studies reporting on race and CRH levels, however, African-American (Glynn, Schetter et al. 2007; Holzman, Jetton et al. 2001) and Hispanic women had significantly lower blood levels of CRH in pregnancy (Ruiz, Fullerton et al. 2002; Siler-Khodr, Forthman et al. 2003). Within each racial/ethnic group, high CRH levels remained associated with adverse pregnancy outcomes (Holzman, Jetton et al. 2001). One study noted lower CRH levels among women with lower educational levels (Guendelman, Kosa et al. 2008). Other studies have considered maternal behaviors often linked to stress, such as smoking, and reported no association with maternal CRH levels in unadjusted (Guendelman, Kosa et al. 2008) and adjusted analyses. (Gillman, Rich-Edwards et al. 2006). Taken together these studies on maternal factors and CRH levels in pregnancy leave many unanswered questions.
The aim of this study was to investigate maternal demographics, anthropometrics, health behaviors, and psychosocial factors in relation to CRH levels in mid-pregnancy. We were particularly interested in determining if CRH levels differed by race, as in our previous study, and if race differences could be eliminated after adjusting for other maternal characteristics. Factors associated with maternal CRH levels were evaluated in a prospectively recruited sample of pregnant women and, for comparison, in a subset of this sample with relatively uncomplicated pregnancies (i.e., term deliveries, infant birth weight appropriate for gestational age, and no maternal hypertensive disorders before/during pregnancy).
Methods
Study design and population
The Pregnancy Outcomes and Community Health (POUCH) study recruited pregnant women from August 1998 to June 2004 in 52 clinics from five Michigan communities. The inclusion criteria were maternal age ≥15 years, English-speaking, singleton pregnancy without known congenital anomaly, screening for maternal serum alpha-fetoprotein (MSAFP) between 15–22 weeks’ gestation and no history of pre-pregnancy diabetes. Women were enrolled in the 15th–27th week of pregnancy. Those with unexplained high MSAFP (≥2 multiples of the median (MOM) were oversampled (i.e. 7% of the cohort) because MSAFP has been repeatedly linked to preterm delivery and therefore of particular interest to the POUCH study. Of the 3,038 women who had consented and enrolled, 3,019 participants (99.4%) completed the study.
Gestational age was determined by the first day of the last menstrual period (LMP) or early ultrasound (US) data. US data were used only when a gestational age based on LMP differed from that estimated by US by more than two weeks. PTD was defined as deliveries before completion of 37 weeks’ gestation.
Data from POUCH study participants were compared with data recorded on birth certificates from the five study communities in 2000. POUCH Study women were similar to community mothers with respect to factors such as age, parity, education levels, the proportions with Medicaid insurance, and prevalence of preterm delivery, previous stillbirth and previous low birth weight infants. The only exception was that the percentage of African-American women over 30 years of age in the POUCH study was lower than that found on birth certificates.
A sub-cohort was sampled and studied in greater detail to examine biomarkers and placental pathology. The sub-cohort included all the women who delivered preterm, all the women with unexplained high MSAFP, and a random sample of women with term deliveries and normal MSAFP, with over-sampling of African Americans in this latter group. Among the 1,371 sub-cohort women, 57 were excluded from this analysis due to either a missing or low volume blood sample and 57 Hispanics, 23 Asians and 18 women of ‘other race/ethnic’ background were excluded due to the small numbers and the resulting imprecision in estimates of their CRH levels (Figure 1). Thus the final sample included 1,216 women. In a second set of analyses we considered a subset (n=746) which excluded sub-cohort women with pregnancy complications linked to CRH levels, i.e. preterm delivery, preeclampsia, chronic hypertension, gestational hypertension, or infant small for gestational age (SGA). SGA was defined as <10th percentile birthweight for gestational week based on sex-specific birth weight distributions in singletons (Alexander, Himes et al. 1996). This subset analysis furthered our goal to identify maternal factors other than pregnancy complications that influence CRH levels
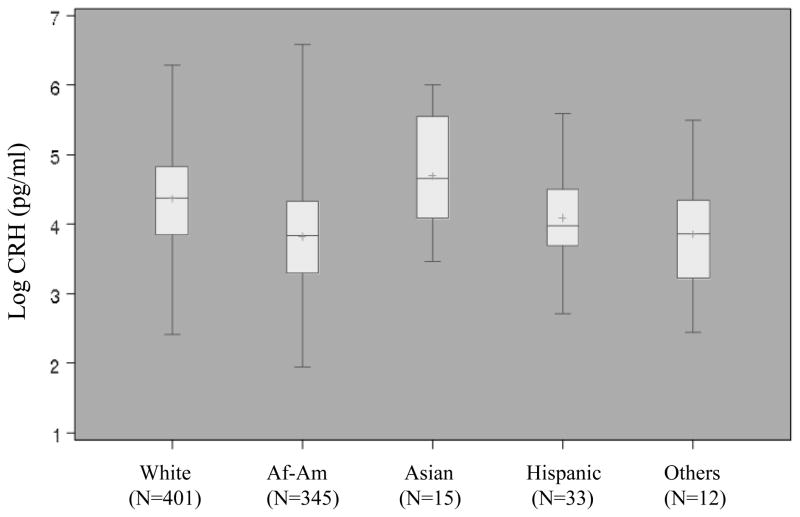
Figure 1.
Distribution of log CRH by racial/ethnic group among term, uncomplicated pregnancies. Bars in box represent median and inter-quartile range, + indicates mean
Maternal demographics and health behaviors
At enrollment, study nurses collected information about demographics, current pregnancy, reproductive history, health behaviors (e.g. smoking), social and psychological factors. Maternal race was determined by self-report. Women who reported being of more than one race group were asked to select one race group for purposes of categorization. Enrollment in the public insurance plan, Medicaid, was used here as one of several indicators of lower socioeconomic status.
Maternal anthropometrics
Maternal weight and height were measured at the time of enrollment and used to calculate a body mass index (BMI) kg/m2. We examined both weight and BMI in relation to CRH levels because weight is used to adjust for maternal size in routine prenatal screening (quad or triple tests) and BMI is typically used as a proxy for adiposity.
Maternal psychosocial measures
Our analyses included multiple psychosocial measures, two that evaluate recent maternal affect (i.e. depressive symptoms, perceived stress) and five that represent maternal attitudes, circumstances, coping styles, and stressful events over the lifecourse (i.e. anomie, hostility, mastery, effortful coping, and history of physical/sexual abuse). Depressive symptoms were assessed through a well-established screening tool, the Center for Epidemiological Studies-Depression Scale (CES-D) (Radloff 1977). Antidepressant and anti-anxiety medication taken during pregnancy were ascertained by abstracting prenatal and labor and delivery medical records. Medications included, hereafter referred to collectively as antidepressants, represent selective serotonin reuptake inhibitors, HT receptor antagonists, norepinephrine and dopamine reuptake inhibitors, selective serotonin 1A receptor antagonists, and selective serotonin and norepinephrine reuptake inhibitors. A four-level variable was created by stratifying women according to CES-D score, <16 vs. ≥ 16 (typical cut point for a positive depression screen) and antidepressant use during pregnancy, yes vs. no. The rationale for this four-level variable was as follows. Screening tools measure mood over a brief period whereas antidepressant use is indicative of a prolonged period of symptoms. Medication use typically signals more severe symptoms, but is not a prefect proxy for severity because not all women are diagnosed and treated, and some discontinue use in pregnancy despite severity. Medications may be used to treat problems other than depression such as anxiety. Maternal hormonal milieu may be related to mood and medication use and these may each have unique effects on maternal CRH levels. Finally, in other POUCH study analyses, the subgroup of women who had elevated CES-D levels and used psychotropic medication were at increased risk of PTD (Gavin, Holzman et al. in press).
Standard, validated instruments were used for five additional psychosocial measures. These included: 1) Cohen’s Perceived Stress (Cohen, Kamarck et al. 1983); 2) the Mastery Scale, (Pearlin and Schooler 1978); 3) the abbreviated nine item Cooke-Medley Hostility Scale (Barefoot, Larsen et al. 1995); 4) an eight item “effortful coping” scale, John Henryism, (James, Hartnett et al. 1983); and 5) a five item version of the Srole Anomie Scale, a measure of alienation and distrust in the ‘establishment’ (Rennie and Srole 1956). Continuous scores for each measure were grouped into quintiles with the highest quintile reflecting greater perceived stress, mastery, hostility, effortful coping, and anomie (Tiedje, Holzman et al. 2008). Women were also asked about their history of abuse, physical or sexual, during childhood and adulthood. Responses were grouped into a three-level variable: 1) no history of abuse (referent category); 2) abuse during one period, childhood or adulthood; and 3) abuse during both childhood and adulthood. In previous POUCH Study analyses, women with abuse in both periods had the highest levels of depressive symptoms during pregnancy (Holzman, Eyster et al. 2006).
Blood collection and laboratory assay
Plasma samples were collected in EDTA tubes at enrollment and then chilled briefly before being processed in a cooled centrifuge (−4° C), aliquoted, and stored at −80° C until assayed. Methanol (3.5 ml) was used to extract CRH from serum (0.5 ml). The precipitate was separated by centrifugation and the methanol-extracted CRH was dried. The residue was suspended in assay diluent (250 ul) before the assay. CRH was measured using a specific and sensitive radioimmunoassay similar to that described by Siler-Khodr et al for GnRH (Siler-Khodr, Khodr et al. 1984), except CRH in these samples was first extracted free of the CRH binding protein as described. Aliquots of resuspended extract were assayed in duplicate. Antiserum to CRH (TS-6) (100 uL) and CRH samples or a standard CRH (100 uL) were pre-incubated for 2 days at 4° C, and then 125I-CRH (10 pg/mL) was added. Tyr-CRH was radioiodinated by the method of Hunter and Greenwood (Hunter and Greenwood 1962). After addition of label, incubation was continued for 3 days at 4° C. Separation of bound and free CRH was done with anti-rabbit gamma globulin conjugated to magnetic beads. Assay sensitivity was 5 pg/tube or approximately 30 pg/mL when corrected for extraction loss. Intra-assay and inter-assay coefficients of variation were 3% and 10% respectively (Holzman, Jetton et al. 2001).
Analytic strategy
We examined each maternal characteristic in relation to CRH levels, and subsequently developed multivariable models. Sampling weights were applied to account for over-sampling of women with unexplained high MSAFP into the cohort and the sampling scheme used to construct the sub-cohort. CRH levels were transformed into natural log values to correct for positive skewing. Analyses were preformed on the sub-cohort and again on a subset of uncomplicated pregnancies (described above). Gestational week at blood sampling was included as a covariate in all analyses.
Regression analyses with sub-cohort sampling weights modeled log CRH levels as the dependent variable and each maternal characteristic (demographic, anthropometric, and psychosocial) as the independent variable (SAS Surveyreg procedure, SAS 9.01; SAS Institute, Cary, NC). Maternal characteristics related to log CRH levels (i.e., a criterion of P≤0.20) were later considered for final models. After observing a trend across maternal weight and BMI categories, we opted to use a continuous measure of weight in the final models; a more complete rationale for this choice is presented in the results and discussion sections. Criteria for eliminating covariates in the final model included: 1) P value > 0.10 in the likelihood ratio test; and 2) removal of the variable did not affect the adjusted mean of retained variables by more than 15%.
Three demographic variables were strongly correlated (i.e., maternal age, Medicaid Insurance status and maternal education), and when included in the same model were not statistically significant. Stepwise and backwards elimination procedures were used to determine which of these variable(s) would get carried forward into the final models. Results showed that maternal education was the only demographic variable of the three to be retained, and the reduced models produced the best fit (assessed based on AIC and BIC values). The psychosocial measures that qualified for final models were also correlated. Because they captured conceptually distinct elements of maternal circumstances and psychosocial functioning we incorporated each into a separate final model. Within final models we evaluated all potential covariate interactions.
Results
Table 1 provides distributions of selected maternal characteristics among the subcohort and the subset of uncomplicated pregnancies. Among the subcohort, log CRH levels were normally distributed with a range of 1.95–6.86 CRH pg/ml and a mean of 4.12 pg/ml (SD=0.76 pg/ml). Values were similar in the term subset. In both samples the mean log CRH (adjusted for gestational week at blood sampling) was significantly lower in association with the following characteristics: African-American race, < 12 years of education, enrollment in Medicaid, age ≤ 29 years, high weight, high BMI, smoking during pregnancy, CES-D score ≥ 16/no antidepressant, and higher levels of perceived stress, anomie, and hostility (Tables 1 & 2).
Table 1.
Maternal characteristics and log CRH levels in POUCH study women
| SUB-COHORT (N= 1216) |
TERM ONLYa (N=746) |
|||||
|---|---|---|---|---|---|---|
| N (Weighted %) | Log CRH (pg/ml)b Adjusted Mean (Standard Error) | Log CRH (pg/ml)b Mean difference (95% C.I.) | N (Weighted %) | Log CRH (pg/ml)b Adjusted Mean (Standard Error) | Log CRH (pg/ml)b Mean difference (95% CI) | |
| Race | ||||||
| Whites | 671 (75.5) | 4.33 (0.03) | 401 (76.4) | 4.33 (0.03) | ||
| African Americans | 545 (24.5) | 3.85 (0.03) | −0.48 (−0.56, −0.39)c | 345 (23.6) | 3.83 (0.04) | −0.49 (−0.60, −0.39)c |
| Maternal Education (years) | ||||||
| <12 | 275 (17.7) | 3.93 (0.05) | −0.34 (−0.45, −0.22)c | 164 (16.5) | 3.94 (0.07) | −0.32 (−0.46, −0.17)c |
| >=12 | 941 (82.3) | 4.27 (0.03) | 582 (83.5) | 4.26 (0.03) | ||
| Medicaidd | ||||||
| Yes | 687 (46.8) | 4.05 (0.03) | −0.30 (−0.39, −0.21)c | 411 (45.0) | 4.05 (0.04) | −0.30 (−0.41, −0.19)c |
| No | 527 (53.2) | 4.35 (0.03) | 335 (55.0) | 4.34 (0.04) | ||
| Maternal Age (years) | ||||||
| <20 | 213 (13.7) | 4.03 (0.06) | −0.15 (−0.29, −0.02)c | 120 (11.9) | 3.99 (.08) | −0.20 (−0.37, −0.03)c |
| 20–29 (referent) | 687 (56.9) | 4.19 (0.03) | 433 (59.0) | 4.19 (.04) | ||
| >=30 | 316 (29.4) | 4.33 (0.04) | 0.14 (0.03, 0.24)c | 193 (29.2) | 4.33 (.05) | 0.13 (0.01, 0.26)c |
| Maternal Weight | ||||||
| Q1 (referent) | 333 (25.5) | 4.29 (0.05) | 193 (25.0) | 4.32 (0.06) | ||
| Q2 | 289 (25.9) | 4.33 (0.05) | 0.04 (−0.10, 0.17) | 184 (26.9) | 4.32 (0.05) | 0.01 (−0.16, 0.17) |
| Q3 | 289 (25.4) | 4.21 (0.04) | −0.08 (−0.22, 0.05) | 191 (26.0) | 4.18 (0.05) | −0.13 (−0.29, 0.02) |
| Q4 | 305 (23.2) | 3.98 (0.05) | −0.32 (−0.45, −0.18)c | 178 (22.0) | 3.98 (0.05) | −0.34 (−0.50, −0.18)c |
| BMI Quartiles | ||||||
| Q1 (referent) | 334 (26.3) | 4.31 (0.05) | 175 (24.3) | 4.31 (0.07) | ||
| Q2 | 276 (25.5) | 4.36 (0.04) | −0.05 (−0.08, 0.18) | 185 (25.9) | 4.33 (0.05) | −0.02 (−0.19, 0.15) |
| Q3 | 303 (25.8) | 4.16 (0.04) | −0.14 (−0.28, −0.01)c | 194 (27.0) | 4.19 (0.05) | −0.13 (−0.29, 0.03) |
| Q4 | 303 (22.3) | 3.98 (0.04) | −0.32 (−0.46, −0.19)c | 192 (22.8) | 3.98 (0.05) | −0.33 (−0.49, −0.17)c |
| Smoking during Pregnancy | ||||||
| Did not smoke during pregnancy | 862 (72.0) | 4.25 (0.03) | 610 (83.8) | 4.24 (0.03) | ||
| Smoked during pregnancy | 354 (28.0) | 4.10 (0.04) | −0.15 (−0.26, −0.05)c | 136 (16.2) | 4.05 (0.06) | −0.19 (−0.32, −0.05)c |
| Parity/PTD History | ||||||
| No Previous Live Birth (referent) | 512 (41.3) | 4.24 (0.04) | 291 (39.0) | 4.22 (0.04) | ||
| Previous Live Birth no PTD | 638 (54.6) | 4.18 (0.03) | −0.05 (−0.15, 0.04) | 431 (58.0) | 4.20 (0.04) | −0.02 (−0.13, 0.09) |
| Previous Live Birth PTD | 66 (4.06) | 4.24 (0.14) | 0.00 (−0.29, 0.29) | 24 (3.02) | 4.14 (0.25) | −0.09 (−0.58, 0.41) |
Abbreviations: CRH = corticotropin-releasing hormone, CI= Confidence Interval, BMI = body mass index, PTD = Preterm Delivery
Excludes women with preeclampsia, gestational hypertension, chronic hypertension, and infants small for gestational age
Weighted for sampling scheme and adjusted for gestational week at blood sampling
p<0.05compared to referent group
Data for two women missing
Table 2.
Psychosocial measures and log CRH levels in POUCH study women
| SUB-COHORT (N=1216)a |
TERM ONLYb (N=746)a |
|||||
|---|---|---|---|---|---|---|
| N (Weighted %) | Log CRH (pg/ml)c Adjusted Mean | Log CRH (pg/ml)c Mean difference (95% CI) | N (Weighted %) | Log CRH (pg/ml)c Adjusted Mean | Log CRH (pg/ml)c Mean difference (95% CI) | |
| Depressive Symptoms and Antidepressant Meds Use | ||||||
| CES-D<16 and Meds: No (referent) | 721 (62.2) | 4.29 (0.03) | 451 (63.9) | 4.30 (0.03) | ||
| CES-D<16 and Meds: Yes | 35 (3.49) | 4.19 (0.12) | −0.10 (−0.35, 0.15) | 19 (2.95) | 4.21 (0.19) | −0.09 (−0.47, 0.29) |
| CES-D>=16 and Meds: No | 385 (27.9) | 4.04 (0.04) | −0.25 (−0.34, −0.15)d | 236 (27.2) | 4.02 (0.05) | −0.28 (−0.39, −0.16)d |
| CES-D>=16 and Meds: Yes | 69 (6.36) | 4.18 (0.13) | −0.10 (−0.37, 0.16) | 36 (5.93) | 4.09 (0.16) | −0.21 (−0.53, 0.11) |
| Perceived Stress Quartiles | ||||||
| Q1 (referent) | 296 (27.4) | 4.29 (0.05) | 178 (27.1) | 4.33 (0.06) | ||
| Q2 | 312 (26.7) | 4.29 (0.04) | 0.00 (−0.13, 0.12) | 192 (26.0) | 4.29 (0.05) | −0.04 (−0.19, 0.11) |
| Q3 | 292 (22.0) | 4.19 (0.05) | −0.10 (−0.24, 0.04) | 176 (21.6) | 4.18 (0.06) | −0.15 (−0.32, 0.01) |
| Q4 | 309 (23.9) | 4.04 (0.05) | −0.25 (−0.38, −0.11)d | 195 (25.4) | 4.02 (0.06) | −0.31 (−0.47, −0.15)d |
| Anomie Quartiles | ||||||
| Q1 (referent) | 328 (31.9) | 4.34 (0.04) | 216 (34.2) | 4.33 (0.05) | ||
| Q2 | 290 (26.1) | 4.24 (0.05) | −0.10 (−0.22, 0.03) | 176 (25.7) | 4.27 (0.05) | −0.06 (−0.20, 0.08) |
| Q3 | 271 (21.1) | 4.13 (0.05) | −0.21 (−0.34, −0.08)d | 156 (19.8) | 4.10 (0.06) | −0.23 (−0.39, −0.07)d |
| Q4 | 312 (20.9) | 4.05 (0.05) | −0.29 (−0.43, −0.16)d | 189 (20.3) | 4.04 (0.06) | −0.28 (−0.44, −0.13)d |
| Hostility Quartiles | ||||||
| Q1 (referent) | 213 (21.3) | 4.36 (0.05) | 132 (21.0) | 4.36 (0.06) | ||
| Q2 | 390 (35.9) | 4.25 (0.04) | −0.11 (−0.23, 0.01) | 250 (37.6) | 4.25 (0.04) | −0.10 (−0.25, 0.04) |
| Q3 | 203 (15.8) | 4.20 (0.07) | −0.16 (−0.32, 0.01) | 118 (15.3) | 4.17 (0.08) | −0.18 (−0.38, 0.01) |
| Q4 | 406 (27.0) | 4.03 (0.04) | −0.34 (−0.47, −0.21)d | 243 (26.1) | 4.03 (0.05) | −0.33 (−0.48, −0.17)d |
| Mastery Quartiles | ||||||
| Q1 (referent) | 246 (19.4) | 4.17 (0.06) | 142 (19.0) | 4.19 (0.07) | ||
| Q2 | 256 (21.2) | 4.22 (0.05) | 0.05 (−0.10, 0.19) | 260 (35.0) | 4.21 (0.04) | 0.03 (−0.14, 0.19) |
| Q3 | 403 (33.6) | 4.18 (0.04) | 0.01 (−0.12, 0.15) | 157 (21.0) | 4.14 (0.06) | −0.05 (−0.23, 0.13) |
| Q4 | 308 (25.8) | 4.26 (0.05) | 0.09 (−0.05, 0.24) | 185 (25.0) | 4.28 (0.06) | 0.09 (−0.09, 0.27) |
| Effortful Coping Quartiles | ||||||
| Q1 (referent) | 219 (20.6) | 4.26 (0.05) | 223 (33.7) | 4.23 (0.05) | ||
| Q2 | 266 (22.8) | 4.18 (0.05) | −0.08 (−0.21, 0.06) | 174 (25.0) | 4.23 (0.05) | 0.00 (−0.15, 0.14) |
| Q3 | 347 (30.2) | 4.24 (0.05) | −0.02 (−0.16, 0.11) | 194 (24.3) | 4.17 (0.06) | −0.06 (−0.21, 0.09) |
| Q4 | 381 (26.4) | 4.15 (0.05) | −0.12 (−0.25, 0.02) | 153 (17.0) | 4.18 (0.07) | −0.05 (−0.21, 0.11) |
| Abuse | ||||||
| None (referent) | 710 (58.4) | 4.23 (0.03) | 440 (58.7) | 4.23 (0.04) | ||
| Either Childhood or Adulthood | 410 (34.4) | 4.16 (0.04) | −0.07 (−0.17, 0.02) | 256 (35.4) | 4.17 (0.04) | −0.06 (−0.18, 0.05) |
| Both Childhood and Adulthood | 91 (7.18) | 4.22 (0.11) | −0.02 (−0.25, 0.22) | 47 (5.94) | 4.19 (0.15) | −0.04 (−0.34, 0.25) |
Abbreviations: CRH = corticotropin-releasing hormone, CI= Confidence Interval, Meds = medications
Data missing for some psychosocial variables for 2 to 15 women
Excludes women with preeclampsia, gestational hypertension, chronic hypertension, and infants small for gestational age
Weighted for sampling scheme and adjusted for gestational week at blood sampling
p<0.05 compared to referent group
In the final multivariable models for the sub-cohort two demographic characteristics, race and education, remained significantly associated with CRH levels (Table 3). There was a maximum of 21% decrease in the mean race difference (from −0.48 to −0.38 pg/ml) after adjustment for education level, weight, and anomie (Table 3, final model #3). The maternal education difference (< 12 vs. ≥ 12 years) declined from −0.34 to −0.19 pg/ml (44 % decrease) after adjustment for race, weight, and hostility levels (Table 3, final model # 4). Maternal weight at the time of blood sampling continued to be inversely associated with adjusted mean log CRH levels and its effect was the same across all final models. Smoking during pregnancy did not meet criteria for inclusion and only one psychosocial measure, CES-D score ≥ 16/no antidepressant, remained associated with lower CRH levels. Final model results in the subset of term deliveries were similar to those in the sub-cohort. There were two exceptions, however; women with a CES-D score ≥ 16 had a lower CRH level that was similar in magnitude irrespective of antidepressant use (Table 4, Model #1) though only those without antidepressants reached statistical significance, most likely due to their larger sample size. In addition high levels of perceived stress were linked to lower CRH levels. (Table 4, Model #2)
Table 3.
Maternal characteristics and psychosocial measures and log CRH levels in POUCH Sub-cohort women (N=1216) a
| Mean difference (95% CI) in log CRH (pg/ml)b | ||||
|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | Model 4 | |
| Race | ||||
| African Americans vs. Whites | −0.39 (−0.48, −0.30)c | −0.40 (−0.49, −0.31)c | −0.38 (−0.48, −0.28)c | −0.39 (−0.49, −0.29)c |
| Maternal Education (years) | ||||
| <12 vs. >=12 | −0.20 (−0.32, −0.07)c | −0.20 (−0.32, −0.07)c | −0.20 (−0.33, −0.08)c | −0.19 (−0.31, −0.07)c |
| Maternal Weight (per 10 pound increase) | −0.03 (−0.04, −0.02)c | −0.03 (−0.04, −0.02)c | −0.03 (−0.04, −0.02)c | −0.03 (−0.04, −0.02)c |
| Depressive Symptoms and Antidepressant Meds Use | ||||
| CES-D<16 and Meds: No (referent) | ||||
| CES-D<16 and Meds: Yes | −0.09 (−0.33, 0.15) | |||
| CES-D>=16 and Meds: No | −0.13 (−0.22, −0.03)c | |||
| CES-D>=16 and Meds: Yes | −0.06 (−0.31, 0.20) | |||
| Perceived Stress Quartiles | ||||
| Q1 (referent) | ||||
| Q2 | 0.05 (−0.07, 0.17) | |||
| Q3 | 0.01 (−0.12, 0.15) | |||
| Q4 | −0.12 (−0.25, 0.02) | |||
| Anomie Quartiles | ||||
| Q1 (referent) | ||||
| Q2 | −0.04 (−0.16, 0.07) | |||
| Q3 | −0.09 (−0.22, 0.04) | |||
| Q4 | −0.09 (−0.22, 0.05) | |||
| Hostility Quartiles | ||||
| Q1 (referent) | ||||
| Q2 | −0.06 (−0.18, 0.06) | |||
| Q3 | −0.08 (−0.24, 0.08) | |||
| Q4 | −0.10 (−0.24, 0.03) | |||
Abbreviations: CRH = corticotropin-releasing hormone, CI= Confidence Interval, Meds = medications
Data missing for some psycosocial variables for 2 to 15 women
Weighted for sampling scheme and adjusted for gestational week at blood sampling and all other variables listed in the table
p<0.05compared to referent group
Table 4.
Maternal characteristics and psychosocial measures and log CRH levels in POUCH Term only women a (N=746) b
| Mean difference (95% CI) in log CRH (pg/ml)c | ||||
|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | Model 4 | |
| Race | ||||
| African Americans vs. Whites | −0.41 (−0.52, −0.30)d | −0.41 (−0.52, −0.31)d | −0.40 (−−0.51, −0.28)d | −0.41 (−0.52, −0.29)d |
| Maternal Education (years) | ||||
| <12 vs. >=12 | −0.16 (−0.30, −0.01)d | −−0.15 (−0.29, −0.003)d | −0.16 (−0.31, −0.01)d | −0.16 (−0.30, −0.01)d |
| Maternal (Weight per 10 pound increase) | −0.03 (−0.04, −0.02)d | −0.03 (−0.04, −0.02)d | −0.03 (−0.04, −0.02)d | −0.03 (−0.04, −0.02)d |
| Depressive Symptoms and Antidepressant Meds Use | ||||
| CES-D<16 and Meds: No (referent) | ||||
| CES-D<16 and Meds: Yes | −0.08 (−0.44, 0.28) | |||
| CES-D>=16 and Meds: No | −0.15 (−0.26, −0.04)d | |||
| CES-D>=16 and Meds: Yes | −0.15 (−0.48, 0.17) | |||
| Perceived Stress Quartiles | ||||
| Q1 (referent) | ||||
| Q2 | 0.00 (−0.14, 0.14) | |||
| Q3 | −0.04 (−0.20, 0.12) | |||
| Q4 | −0.19 (−0.35, −0.03)d | |||
| Anomie Quartiles | ||||
| Q1 (referent) | ||||
| Q2 | −0.02 (−0.15, 0.12) | |||
| Q3 | −0.10 (−0.26, 0.06) | |||
| Q4 | −0.08 (−0.23, 0.08) | |||
| Hostility Quartiles | ||||
| Q1 (referent) | ||||
| Q2 | −0.06 (−0.20, 0.08) | |||
| Q3 | −0.11 (−0.30, 0.09) | |||
| Q4 | −0.09 (−0.25, 0.07) | |||
Abbreviations: CRH = corticotropin-releasing hormone, CI= Confidence Interval, Meds = medications
Excludes women with preeclampsia, gestational hypertension, chronic hypertension, and infants small for gestational age
Data missing for some psychosocial variables for 2 to 15 women
Weighted for sampling scheme and adjusted for gestational week at blood sampling and all other variables listed in the table
p<0.05 compared to referent group
All covariate interactions had P values greater than 0.20. Although maternal weight and BMI are highly correlated, we examined the effect of putting both in the same model. In this instance only maternal weight remained significantly associated with CRH levels. We also conducted a subgroup analysis with women who had the week of pregnancy determined mainly according to ultrasound examinations to test whether race/ethnic differences in CRH levels might be explained by misclassification of gestational age at blood sampling. Results showed that the CRH-Race association persisted and was not attenuated (data not shown).
Discussion
We found that three maternal characteristics, being African American, having a low educational level, and having a higher weight, were each independently associated with lower mid-pregnancy CRH levels, both in the entire sub-cohort and in the subset of uncomplicated pregnancies. In light of our previous study, (Holzman, Jetton et al. 2001) and the study by Glynn et al (Glynn, Schetter et al. 2007) showing lower maternal CRH levels in African Americans, we were particularly interested in seeing if we could account for race differences in CRH levels, but the differences we observed were only modestly attenuated after adjusting for education, weight, and psychosocial factors. CRH levels were unrelated to most psychosocial measures, with the exception that CRH appeared to be lower in women with higher levels of depressive symptoms.
Few in vivo studies have addressed functional differences in placental CRH production and release, making it difficult to explain observed race differences in maternal blood CRH levels. Some studies have examined race differences in HPA axis function and found that among non-pregnant women exposed to physical exercise or administered ovine CRH, African Americans had higher levels of ACTH than that in Whites (Yanovski, Yanovski et al. 2000; Yanovski, Yanovski et al. 1996). However, race differences in ACTH levels did not result in race differences in cortisol levels, suggesting variation in regulation points along the HPA axis, perhaps in response to chronic stressors. At least one study has noted race differences in a CRH related gene (Shimmin, Natarajan et al. 2007). The relevancy of these studies to racial/ethnic differences in pregnancy-related CRH levels remains uncertain.
The link between lower mid-pregnancy CRH levels and lower maternal educational levels in our study has been noted by at least one other research team (Guendelman, Kosa et al. 2008). This finding, and perhaps the race differences discussed above, suggests that social disadvantage may somehow be aligned with decreases in CRH levels. In the POUCH study, our protocol included an examination of the subcohorts’ delivered placentas. We looked to see if placental size might partially explain racial and educational level differences in maternal CRH levels. We found no relation between placental size and CRH levels and therefore no support for this hypothesis (data not shown).
Our finding of an inverse association between maternal weight and CRH levels is most likely due to a hemodilution effect. The concept of hemodilution due to weight is more commonly considered in calculations of drug doses based on weight. The greater the weight the larger the dose needed to achieve a certain blood level. In the instance of pregnancy, the fetus or placenta delivers certain substances that are exogenous to the mother (dosing the mother). A larger maternal vascular space would result in lower concentrations of these substances if all else were constant. The hemodilution effect is observed with pregnancy biomarkers such as MSAFP (Drugan, Dvorin et al. 1989) which is fetal in origin and crosses over to maternal blood. To account for the hemodilution effect, prenatal screening tests are routinely adjusted for maternal weight at the time of screening. Similarly CRH from gestational tissues enters the maternal circulation and the concentration of CRH in maternal blood depends on the volume of the diluent (maternal vascular space) which is related to maternal weight. In the example of CRH, however, there are multiple considerations that might complicate the straight-forward hemodilution effect. First, maternal weight is correlated with adiposity, and adiposity may increase the risk of placental vascular problems (Galtier-Dereure, Boegner et al. 2000; Roman, Robillard et al. 2007) which have been linked to higher maternal blood CRH levels (Hobel, Arora et al. 1999; Karteris, Vatish et al. 2005). Second, these same placental vascular problems are associated with decreases in the normal pregnancy-related expansion of the maternal vascular space (Salas, Marshall et al. 2006). Finally, adiposity has been linked to alterations in HPA axis regulation in non-pregnant populations (Anagnostis, Athyros et al. 2009; Chalew, Nagel et al. 1995; Chan, Inouye et al. 2005; Marin, Darin et al. 1992; Rosmond, Chagnon et al. 2000; Vicennati, Ceroni et al. 2006) and this link may persist in pregnancy. In our analysis of pregnancies ending at term without complications we excluded women with clinical signs of vascular complications and found that the maternal weight effect on CRH levels was similar to that in the entire sub-cohort. However our term subset may have included women with high BMI and associated subclinical placental vascular pathology. Our findings emphasize the need to consider maternal weight not only as a confounding element, but also as a highly correlated measure of adiposity which could be in the causal pathway. We therefore recommend comparisons of different statistical models that include and exclude measures of maternal weight and BMI when evaluating factors related to CRH levels. With respect to smoking, our finding of no association with maternal CRH levels agreed with two previous studies (Gillman, Rich-Edwards et al. 2006; Guendelman, Kosa et al. 2008).
In studies that have included maternal depressive symptoms and blood CRH levels during pregnancy, one found a positive association (Rich-Edwards, Mohllajee et al. 2008), another an inverse association, (Susman, Schmeelk et al. 1999) and the third reported no significant association (Yim, Glynn et al. 2009). Our results are most consistent with that of Susman et al (1999) showing lower CRH levels among women with more depressive symptoms. The varied results across studies may in part be due to differences in populations studied, exclusion criteria, tools used to measure depressive symptoms, gestational age at blood sampling, and covariates used in modeling. Susman’s study included 59 low-income, predominantly white adolescents. The Rich-Edwards’ et al. sample had a large percentage of older, well-educated, White women with relatively low prevalences of obesity and preterm delivery. Yim et al’s study included primarily non-Hispanic white and Hispanic women. Most were married (79%), college graduates (52%) who delivered at term (97%). Complicated pregnancies were either controlled for or excluded from the analysis in Rich-Edwards’ study, but not mentioned by Susman et al. (1999).
The links between stress-related biomarkers and psychosocial functioning are complex even in non-pregnant populations. Although elevated levels of hypothalamic CRH in cerebrospinal fluid have been associated with melancholic depression (Kasckow, Baker et al. 2001; Nemeroff, Widerlov et al. 1984), people experiencing depression may or may not present with unusually high (Carroll, Curtis et al. 1976) or unusually low (Oldehinkel, van den Berg et al. 2001) systemic cortisol levels, indications that HPA axis dysregulation is variable across individuals. Pregnancy adds other complexities including new sources of CRH (gestational tissues) and often new psychosocial stressors for the mother. In addition, pregnancy-related complications could influence the level of gestational CRH production and escape into maternal blood. Two studies with repeated measures of cortisol and CRH in maternal blood reported that levels of both biomarkers were higher among pregnancies that ended preterm vs. at term (Erickson, Thorsen et al. 2001; Sandman, Glynn et al. 2006). In one study the CRH group-difference preceded the cortisol group-difference (Erickson, Thorsen et al. 2001) and in the other study the order was reversed (Sandman, Glynn et al. 2006). It will not be easy to unravel these interrelations and their association with maternal affect/stress because of the many intervening factors related to pregnancy and the episodic and heterogeneous nature of conditions such as stress and depression.
Two studies have reported that women with high levels of prenatal anxiety or stress, which often overlap with depressive symptoms, show increases in CRH levels during pregnancy (Hobel, Dunkel-Schetter et al. 1999; Mancuso, Schetter et al. 2004). But women can have prenatal anxiety due to their awareness of complications, and some of these complications are related to elevated levels of CRH. Disentangling the causal order of these factors presents a challenge. We included multiple psychosocial measures that cover recent and more ‘chronic’ indicators of maternal unease or stress. Most of the psychosocial measures showed an inverse relation to CRH levels that disappeared after adjusting for race, educational level and maternal weight.
A major strength of our study was the composition of the cohort, which was sampled from diverse settings representing a wide range of socioeconomic backgrounds. In addition maternal weight was measured at enrollment, the same time as blood collection for CRH, and used to adjust for possible hemodilution effects. Psychosocial measures were obtained prospectively before maternal knowledge of pregnancy outcomes, thereby avoiding recall bias. By considering both the subcohort and a subset without pregnancy complications we reduced the likelihood that pathology and maternal pregnancy-related concerns would operate as confounders. The wide range of available variables allowed for a deeper probing of potential explanations for previously observed race differences in CRH levels during pregnancy (Glynn, Schetter et al. 2007; Holzman, Jetton et al. 2001).
One limitation is that maternal CRH levels and depressive symptoms were measured only once, thereby precluding the chance to examine time-order and changes in the association across the span of pregnancy. Our assessment of depression was limited to a screening tool, the CES-D, which lacks specificity and represents a very brief period of symptoms (the past week). To address this brief period limitation we also considered antidepressant use during pregnancy, a potential indicator of severity and chronicity of maternal depression/anxiety disorders. Although we had medical record information on specific antidepressant medications used, we did not have detailed data on timing of use or maternal compliance.
CRH remains a key peptide in investigations of pregnancy outcomes and maternal mental health. It is important to identify maternal factors associated with this biomarker, in part because it will lead to more careful modeling of CRH in relation to outcomes of interest. In addition these CRH-related covariates offer clues to the complexity of interpreting CRH levels and motivate future studies. The observed lower CRH levels in relation to social disadvantage (race, education), when in fact it is high CRH that marks elevated risk for adverse pregnancy outcome, remains an enigma that deserves further exploration.
Acknowledgments
We would like to thank Dr. Bertha Bullen for her assistance with study logistics. We also acknowledge the POUCH Study team of nurses who conducted interviews and collected samples.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alexander GR, Himes JH, et al. A United States national reference for fetal growth. Obstet Gynecol. 1996;87(2):163–168. doi: 10.1016/0029-7844(95)00386-X. [DOI] [PubMed] [Google Scholar]
- Anagnostis P, V, Athyros G, et al. Clinical review: The pathogenetic role of cortisol in the metabolic syndrome: a hypothesis. J Clin Endocrinol Metab. 2009;94(8):2692–2701. doi: 10.1210/jc.2009-0370. [DOI] [PubMed] [Google Scholar]
- Barefoot JC, Larsen S, et al. Hostility, incidence of acute myocardial infarction, and mortality in a sample of older Danish men and women. Am J Epidemiol. 1995;142(5):477–484. doi: 10.1093/oxfordjournals.aje.a117663. [DOI] [PubMed] [Google Scholar]
- Benassayag C, Souski I, et al. Corticosteroid-binding globulin status at the fetomaternal interface during human term pregnancy. Biol Reprod. 2001;64(3):812–821. doi: 10.1095/biolreprod64.3.812. [DOI] [PubMed] [Google Scholar]
- Brunton PJ, Russell JA, et al. Adaptive responses of the maternal hypothalamic-pituitary-adrenal axis during pregnancy and lactation. J Neuroendocrinol. 2008;20(6):764–776. doi: 10.1111/j.1365-2826.2008.01735.x. [DOI] [PubMed] [Google Scholar]
- Carroll BJ, Curtis GC, et al. Cerebrospinal fluid and plasma free cortisol concentrations in depression. Psychol Med. 1976;6(2):235–244. doi: 10.1017/s0033291700013775. [DOI] [PubMed] [Google Scholar]
- Chalew S, Nagel H, et al. The hypothalamic-pituitary-adrenal axis in obesity. Obes Res. 1995;3(4):371–382. doi: 10.1002/j.1550-8528.1995.tb00163.x. [DOI] [PubMed] [Google Scholar]
- Chan O, Inouye K, et al. Insulin alone increases hypothalamo-pituitary-adrenal activity, and diabetes lowers peak stress responses. Endocrinology. 2005;146(3):1382–1390. doi: 10.1210/en.2004-0607. [DOI] [PubMed] [Google Scholar]
- Choy MY, Leung TN, et al. Corticotropin-releasing hormone peptide and human first-trimester placental growth. Early Hum Dev. 2004;79(1):77–80. doi: 10.1016/j.earlhumdev.2004.04.010. [DOI] [PubMed] [Google Scholar]
- Clifton VL, Read MA, et al. Corticotropin-releasing hormone-induced vasodilatation in the human fetal placental circulation. J Clin Endocrinol Metab. 1994;79(2):666–669. doi: 10.1210/jcem.79.2.8045990. [DOI] [PubMed] [Google Scholar]
- Cohen S, Kamarck T, et al. A global measure of perceived stress. J Health Soc Behav. 1983;24(4):385–396. [PubMed] [Google Scholar]
- Drugan A, Dvorin E, et al. The inadequacy of the current correction for maternal weight in maternal serum alpha-fetoprotein interpretation. Obstet Gynecol. 1989;74(5):698–701. [PubMed] [Google Scholar]
- Ellis MJ, Livesey JH, et al. Plasma corticotropin-releasing hormone and unconjugated estriol in human pregnancy: gestational patterns and ability to predict preterm delivery. Am J Obstet Gynecol. 2002;186(1):94–99. doi: 10.1067/mob.2002.119188. [DOI] [PubMed] [Google Scholar]
- Emanuel RL, Robinson BG, et al. Corticotrophin releasing hormone levels in human plasma and amniotic fluid during gestation. Clin Endocrinol (Oxf) 1994;40(2):257–262. doi: 10.1111/j.1365-2265.1994.tb02477.x. [DOI] [PubMed] [Google Scholar]
- Emanuel RL, Torday JS, et al. Direct effects of corticotropin-releasing hormone and thyrotropin-releasing hormone on fetal lung explants. Peptides. 2000;21(12):1819–1829. doi: 10.1016/s0196-9781(00)00343-0. [DOI] [PubMed] [Google Scholar]
- Erickson K, Thorsen P, et al. Preterm birth: associated neuroendocrine, medical, and behavioral risk factors. J Clin Endocrinol Metab. 2001;86(6):2544–2552. doi: 10.1210/jcem.86.6.7607. [DOI] [PubMed] [Google Scholar]
- Ferrari A, Petraglia F, et al. Corticotropin releasing factor decidualizes human endometrial stromal cells in vitro. Interaction with progestin. J Steroid Biochem Mol Biol. 1995;54(5–6):251–255. doi: 10.1016/0960-0760(95)00142-m. [DOI] [PubMed] [Google Scholar]
- Florio P, Severi FM, et al. Placental stress factors and maternal-fetal adaptive response: the corticotropin-releasing factor family. Endocrine. 2002;19(1):91–102. doi: 10.1385/endo:19:1:91. [DOI] [PubMed] [Google Scholar]
- Galtier-Dereure F, Boegner C, et al. Obesity and pregnancy: complications and cost. Am J Clin Nutr. 2000;71(5 Suppl):1242S–1248S. doi: 10.1093/ajcn/71.5.1242s. [DOI] [PubMed] [Google Scholar]
- Gavin A, Holzman C, et al. Maternal depressive symptoms, depression and psychiatric medication use in relation to risk of preterm delivery. Womens’ Health Issues. doi: 10.1016/j.whi.2009.05.004. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillman MW, Rich-Edwards JW, et al. Maternal corticotropin-releasing hormone levels during pregnancy and offspring adiposity. Obesity (Silver Spring) 2006;14(9):1647–1653. doi: 10.1038/oby.2006.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glynn LM, Schetter CD, et al. Ethnic differences in adrenocorticotropic hormone, cortisol and corticotropin-releasing hormone during pregnancy. Peptides. 2007;28(6):1155–1161. doi: 10.1016/j.peptides.2007.04.005. [DOI] [PubMed] [Google Scholar]
- Glynn LM, Schetter CD, et al. Pregnancy affects appraisal of negative life events. J Psychosom Res. 2004;56(1):47–52. doi: 10.1016/S0022-3999(03)00133-8. [DOI] [PubMed] [Google Scholar]
- Grammatopoulos DK, Hillhouse EW. Role of corticotropin-releasing hormone in onset of labour. Lancet. 1999;354(9189):1546–1549. doi: 10.1016/S0140-6736(99)03418-2. [DOI] [PubMed] [Google Scholar]
- Grino M, Chrousos GP, et al. The corticotropin releasing hormone gene is expressed in human placenta. Biochem Biophys Res Commun. 1987;148(3):1208–1214. doi: 10.1016/s0006-291x(87)80261-9. [DOI] [PubMed] [Google Scholar]
- Guendelman S, Kosa JL, et al. Exploring the relationship of second-trimester corticotropin releasing hormone, chronic stress and preterm delivery. J Matern Fetal Neonatal Med. 2008;21(11):788–795. doi: 10.1080/14767050802379031. [DOI] [PubMed] [Google Scholar]
- Hobel CJ, Arora CP, et al. Corticotrophin-releasing hormone and CRH-binding protein. Differences between patients at risk for preterm birth and hypertension. Ann N Y Acad Sci. 1999;897:54–65. doi: 10.1111/j.1749-6632.1999.tb07878.x. [DOI] [PubMed] [Google Scholar]
- Hobel CJ, Dunkel-Schetter C, et al. Maternal plasma corticotropin-releasing hormone associated with stress at 20 weeks’ gestation in pregnancies ending in preterm delivery. Am J Obstet Gynecol. 1999;180(1 Pt 3):S257–263. doi: 10.1016/s0002-9378(99)70712-x. [DOI] [PubMed] [Google Scholar]
- Holzman C, Eyster J, et al. A life course perspective on depressive symptoms in mid-pregnancy. Matern Child Health J. 2006;10(2):127–138. doi: 10.1007/s10995-005-0044-0. [DOI] [PubMed] [Google Scholar]
- Holzman C, Jetton J, et al. Second trimester corticotropin-releasing hormone levels in relation to preterm delivery and ethnicity. Obstet Gynecol. 2001;97(5 Pt 1):657–663. doi: 10.1016/s0029-7844(00)01209-6. [DOI] [PubMed] [Google Scholar]
- Hunter WM, Greenwood FC. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- James SA, Hartnett SA, et al. John Henryism and blood pressure differences among black men. J Behav Med. 1983;6(3):259–278. doi: 10.1007/BF01315113. [DOI] [PubMed] [Google Scholar]
- Jones SA, Brooks AN, et al. Steroids modulate corticotropin-releasing hormone production in human fetal membranes and placenta. J Clin Endocrinol Metab. 1989;68(4):825–830. doi: 10.1210/jcem-68-4-825. [DOI] [PubMed] [Google Scholar]
- Kalin NH, Takahashi LK, et al. Restraint stress increases corticotropin-releasing hormone mRNA content in the amygdala and paraventricular nucleus. Brain Res. 1994;656(1):182–186. doi: 10.1016/0006-8993(94)91382-x. [DOI] [PubMed] [Google Scholar]
- Kammerer M, Adams D, et al. Pregnant women become insensitive to cold stress. BMC Pregnancy Childbirth. 2002;2(1):8. doi: 10.1186/1471-2393-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karalis K, Goodwin G, et al. Cortisol blockade of progesterone: a possible molecular mechanism involved in the initiation of human labor. Nat Med. 1996;2(5):556–560. doi: 10.1038/nm0596-556. [DOI] [PubMed] [Google Scholar]
- Karteris E, Papadopoulou N, et al. Expression and signalling characteristics of the corticotrophin-releasing hormone receptors during the implantation phase in the human endometrium. J Mol Endocrinol. 2004;32(1):21–32. doi: 10.1677/jme.0.0320021. [DOI] [PubMed] [Google Scholar]
- Karteris E, Vatish M, et al. Preeclampsia is associated with impaired regulation of the placental nitric oxide-cyclic guanosine monophosphate pathway by corticotropin-releasing hormone (CRH) and CRH-related peptides. J Clin Endocrinol Metab. 2005;90(6):3680–3687. doi: 10.1210/jc.2004-2210. [DOI] [PubMed] [Google Scholar]
- Kasckow JW, Baker D, et al. Corticotropin-releasing hormone in depression and post-traumatic stress disorder. Peptides. 2001;22(5):845–851. doi: 10.1016/s0196-9781(01)00399-0. [DOI] [PubMed] [Google Scholar]
- Lee MJ, Wang Z, et al. Expression and regulation of glucocorticoid receptor in human placental villous fibroblasts. Endocrinology. 2005;146(11):4619–4626. doi: 10.1210/en.2005-0235. [DOI] [PubMed] [Google Scholar]
- Leung TN, Chung TK, et al. Analysis of mid-trimester corticotrophin-releasing hormone and alpha-fetoprotein concentrations for predicting pre-eclampsia. Hum Reprod. 2000;15(8):1813–1818. doi: 10.1093/humrep/15.8.1813. [DOI] [PubMed] [Google Scholar]
- Majzoub JA, McGregor JA, et al. A central theory of preterm and term labor: putative role for corticotropin-releasing hormone. Am J Obstet Gynecol. 1999;180(1 Pt 3):S232–241. doi: 10.1016/s0002-9378(99)70707-6. [DOI] [PubMed] [Google Scholar]
- Makino S, Gold PW, et al. Corticosterone effects on corticotropin-releasing hormone mRNA in the central nucleus of the amygdala and the parvocellular region of the paraventricular nucleus of the hypothalamus. Brain Res. 1994;640(1–2):105–112. doi: 10.1016/0006-8993(94)91862-7. [DOI] [PubMed] [Google Scholar]
- Makrigiannakis A, Zoumakis E, et al. Corticotropin-releasing hormone promotes blastocyst implantation and early maternal tolerance. Nat Immunol. 2001;2(11):1018–1024. doi: 10.1038/ni719. [DOI] [PubMed] [Google Scholar]
- Makrigiannakis A, Zoumakis E, et al. Corticotropin-releasing hormone (CRH) and immunotolerance of the fetus. Biochem Pharmacol. 2003;65(6):917–921. doi: 10.1016/s0006-2952(02)01547-2. [DOI] [PubMed] [Google Scholar]
- Mancuso RA, Schetter CD, et al. Maternal prenatal anxiety and corticotropin-releasing hormone associated with timing of delivery. Psychosom Med. 2004;66(5):762–769. doi: 10.1097/01.psy.0000138284.70670.d5. [DOI] [PubMed] [Google Scholar]
- Marin P, Darin N, et al. Cortisol secretion in relation to body fat distribution in obese premenopausal women. Metabolism. 1992;41(8):882–886. doi: 10.1016/0026-0495(92)90171-6. [DOI] [PubMed] [Google Scholar]
- McLean M, Bisits A, et al. Predicting risk of preterm delivery by second-trimester measurement of maternal plasma corticotropin-releasing hormone and alpha-fetoprotein concentrations. Am J Obstet Gynecol. 1999;181(1):207–215. doi: 10.1016/s0002-9378(99)70461-8. [DOI] [PubMed] [Google Scholar]
- McLean M, Bisits A, et al. A placental clock controlling the length of human pregnancy. Nat Med. 1995;1(5):460–463. doi: 10.1038/nm0595-460. [DOI] [PubMed] [Google Scholar]
- Nemeroff CB, Widerlov E, et al. Elevated concentrations of CSF corticotropin-releasing factor-like immunoreactivity in depressed patients. Science. 1984;226(4680):1342–1344. doi: 10.1126/science.6334362. [DOI] [PubMed] [Google Scholar]
- Ni X, Hou Y, et al. Progesterone receptors A and B differentially modulate corticotropin-releasing hormone gene expression through a cAMP regulatory element. Cell Mol Life Sci. 2004;61(9):1114–1122. doi: 10.1007/s00018-004-4030-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nierop A, Bratsikas A, et al. Prolonged salivary cortisol recovery in second-trimester pregnant women and attenuated salivary alpha-amylase responses to psychosocial stress in human pregnancy. J Clin Endocrinol Metab. 2006;91(4):1329–1335. doi: 10.1210/jc.2005-1816. [DOI] [PubMed] [Google Scholar]
- Oldehinkel AJ, van den Berg MD, et al. Urinary free cortisol excretion in elderly persons with minor and major depression. Psychiatry Res. 2001;104(1):39–47. doi: 10.1016/s0165-1781(01)00300-6. [DOI] [PubMed] [Google Scholar]
- Pearlin LI, Schooler C. The structure of coping. J Health Soc Behav. 1978;19(1):2–21. [PubMed] [Google Scholar]
- Petraglia F, Sawchenko PE, et al. Evidence for local stimulation of ACTH secretion by corticotropin-releasing factor in human placenta. Nature. 1987;328(6132):717–719. doi: 10.1038/328717a0. [DOI] [PubMed] [Google Scholar]
- Petraglia F, Tabanelli S, et al. Human decidua and in vitro decidualized endometrial stromal cells at term contain immunoreactive corticotropin-releasing factor (CRF) and CRF messenger ribonucleic acid. J Clin Endocrinol Metab. 1992;74(6):1427–1431. doi: 10.1210/jcem.74.6.1375601. [DOI] [PubMed] [Google Scholar]
- Purwosunu Y, Sekizawa A, et al. Cell-free mRNA concentrations of CRH, PLAC1, and selectin-P are increased in the plasma of pregnant women with preeclampsia. Prenat Diagn. 2007;27(8):772–777. doi: 10.1002/pd.1780. [DOI] [PubMed] [Google Scholar]
- Quartero HW, Fry CH. Placental corticotrophin releasing factor may modulate human parturition. Placenta. 1989;10(5):439–443. doi: 10.1016/0143-4004(89)90054-4. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CES-D Scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1(3):385–401. [Google Scholar]
- Rennie TA, Srole L. Social class prevalence and distribution of psychosomatic conditions in an urban population. Psychosom Med. 1956;18(6):449–456. doi: 10.1097/00006842-195611000-00001. [DOI] [PubMed] [Google Scholar]
- Rich-Edwards JW, Mohllajee AP, et al. Elevated midpregnancy corticotropin-releasing hormone is associated with prenatal, but not postpartum, maternal depression. J Clin Endocrinol Metab. 2008;93(5):1946–1951. doi: 10.1210/jc.2007-2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson BG, Emanuel RL, et al. Glucocorticoid stimulates expression of corticotropin-releasing hormone gene in human placenta. Proc Natl Acad Sci U S A. 1988;85(14):5244–5248. doi: 10.1073/pnas.85.14.5244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman H, Robillard PY, et al. Obstetrical and neonatal outcomes in obese women. West Indian Med J. 2007;56(5):421–426. [PubMed] [Google Scholar]
- Rosmond R, Chagnon YC, et al. A glucocorticoid receptor gene marker is associated with abdominal obesity, leptin, and dysregulation of the hypothalamic-pituitary-adrenal axis. Obes Res. 2000;8(3):211–218. doi: 10.1038/oby.2000.24. [DOI] [PubMed] [Google Scholar]
- Ruiz RJ, Fullerton J, et al. Predicting risk of preterm birth: the roles of stress, clinical risk factors, and corticotropin-releasing hormone. Biol Res Nurs. 2002;4(1):54–64. doi: 10.1177/1099800402004001007. [DOI] [PubMed] [Google Scholar]
- Salas SP, Marshall G, et al. Time course of maternal plasma volume and hormonal changes in women with preeclampsia or fetal growth restriction. Hypertension. 2006;47(2):203–208. doi: 10.1161/01.HYP.0000200042.64517.19. [DOI] [PubMed] [Google Scholar]
- Sandman CA, Glynn L, et al. Elevated maternal cortisol early in pregnancy predicts third trimester levels of placental corticotropin releasing hormone (CRH): priming the placental clock. Peptides. 2006;27(6):1457–1463. doi: 10.1016/j.peptides.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Schulte HM, Weisner D, et al. The corticotrophin releasing hormone test in late pregnancy: lack of adrenocorticotrophin and cortisol response. Clin Endocrinol (Oxf) 1990;33(1):99–106. doi: 10.1111/j.1365-2265.1990.tb00470.x. [DOI] [PubMed] [Google Scholar]
- Shimmin LC, Natarajan S, et al. Corticotropin releasing hormone (CRH) gene variation: comprehensive resequencing for variant and molecular haplotype discovery in monosomic hybrid cell lines. DNA Seq. 2007;18(6):434–444. doi: 10.1080/10425170701388719. [DOI] [PubMed] [Google Scholar]
- Sibai B, Meis PJ, et al. Plasma CRH measurement at 16 to 20 weeks’ gestation does not predict preterm delivery in women at high-risk for preterm delivery. Am J Obstet Gynecol. 2005;193(3 Pt 2):1181–1186. doi: 10.1016/j.ajog.2005.06.036. [DOI] [PubMed] [Google Scholar]
- Siler-Khodr TM, Forthman G, et al. Maternal serum corticotropin-releasing hormone at midgestation in Hispanic and white women. Obstet Gynecol. 2003;101(3):557–564. doi: 10.1016/s0029-7844(02)03072-7. [DOI] [PubMed] [Google Scholar]
- Siler-Khodr TM, Khodr GS, et al. Immunoreactive gonadotropin-releasing hormone level in maternal circulation throughout pregnancy. Am J Obstet Gynecol. 1984;150(4):376–379. doi: 10.1016/s0002-9378(84)80142-8. [DOI] [PubMed] [Google Scholar]
- Smith R. Parturition. N Engl J Med. 2007;356(3):271–283. doi: 10.1056/NEJMra061360. [DOI] [PubMed] [Google Scholar]
- Susman EJ, Schmeelk KH, et al. Corticotropin-releasing hormone and cortisol: longitudinal associations with depression and antisocial behavior in pregnant adolescents. J Am Acad Child Adolesc Psychiatry. 1999;38(4):460–467. doi: 10.1097/00004583-199904000-00020. [DOI] [PubMed] [Google Scholar]
- Thomson M. Does the CRH binding protein shield the anterior pituitary from placental CRH? Endocrine. 1998;9(3):221–226. doi: 10.1385/ENDO:9:3:221. [DOI] [PubMed] [Google Scholar]
- Tiedje L, Holzman CB, et al. Hostility and anomie: links to preterm delivery subtypes and ambulatory blood pressure at mid-pregnancy. Soc Sci Med. 2008;66(6):1310–1321. doi: 10.1016/j.socscimed.2007.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicennati V, Ceroni L, et al. Sex difference in the relationship between the hypothalamic-pituitary-adrenal axis and sex hormones in obesity. Obesity (Silver Spring) 2006;14(2):235–243. doi: 10.1038/oby.2006.30. [DOI] [PubMed] [Google Scholar]
- Wadhwa PD, Garite TJ, et al. Placental corticotropin-releasing hormone (CRH), spontaneous preterm birth, and fetal growth restriction: a prospective investigation. Am J Obstet Gynecol. 2004;191(4):1063–1069. doi: 10.1016/j.ajog.2004.06.070. [DOI] [PubMed] [Google Scholar]
- Warren WB, Patrick SL, et al. Elevated maternal plasma corticotropin-releasing hormone levels in pregnancies complicated by preterm labor. Am J Obstet Gynecol. 1992;166(4):1198–1204. doi: 10.1016/s0002-9378(11)90606-1. discussion 1204–1197. [DOI] [PubMed] [Google Scholar]
- Watson S, Mackin P. HPA axis function in mood disorders. Psychiatry. 2006;5(5):166–170. [Google Scholar]
- Yang K. Placental 11 beta-hydroxysteroid dehydrogenase: barrier to maternal glucocorticoids. Rev Reprod. 1997;2(3):129–132. doi: 10.1530/ror.0.0020129. [DOI] [PubMed] [Google Scholar]
- Yanovski JA, Yanovski SZ, et al. Hypothalamic-pituitary-adrenal axis activity during exercise in African American and Caucasian women. J Clin Endocrinol Metab. 2000;85(8):2660–2663. doi: 10.1210/jcem.85.8.6708. [DOI] [PubMed] [Google Scholar]
- Yanovski JA, Yanovski SZ, et al. Differences in the hypothalamic-pituitary-adrenal axis of black girls and white girls. J Pediatr. 1996;129(1):130–135. doi: 10.1016/s0022-3476(96)70199-3. [DOI] [PubMed] [Google Scholar]
- Yim IS, Glynn LM, et al. Risk of postpartum depressive symptoms with elevated corticotropin-releasing hormone in human pregnancy. Arch Gen Psychiatry. 2009;66(2):162–169. doi: 10.1001/archgenpsychiatry.2008.533. [DOI] [PMC free article] [PubMed] [Google Scholar]