SUMMARY
Background
Chronic obstructive pulmonary disease (COPD) patients have lower levels of physical activity compared to age-matched controls, and they limit physical activities requiring normal exertion. Our purpose was to compare the effectiveness of a traditional exercise therapy (TET) program with a behavioral lifestyle activity program (LAP) in promoting physical activity.
Methods
Moderate physical activity (kcal/week) was assessed in 176 COPD patients using the Community Health Activities Model for Seniors questionnaire. Patients were randomized to either a three month TET program that meet thrice weekly or a LAP. The LAP was designed to teach behavioral skills that encouraged the daily accumulation of self-selected physical activities of at least moderate intensity. Interventionist contact was similar (36 hours) between the two groups. Patients were assessed at baseline and 3, 6 and 12 months.
Results
Compared to baseline values, self-reported moderate physical activity increased three months post-randomization with no significant difference (p = 0.99) found between the TET (2,501 ± 197 kcal/week) and the LAP (2,498 ± 211 kcal/week). At 6 and 12 months post-randomization, there were no significant differences (p = 0.37 and 0.69, respectively) in self-reported levels of moderate physical activity between the TET (2,210 ± 187 and 2,213 ± 218 kcal/week, respectively) and the LAP (2,456 ± 198 and 2,342 ± 232 kcal/week, respectively).
Conclusion
Although there was no difference between treatment groups, the TET and the LAP were both effective at in increasing moderate levels of physical activity at 3 months and maintaining moderate physical activity levels 12 months post-randomization.
Keywords: Chronic obstructive pulmonary disease, Exercise, Physical activity, Behavioral intervention
INTRODUCTION
Chronic obstructive pulmonary disease (COPD) patients report limitations in physical activities requiring normal exertion (30) and lower physical activity levels when compared to age matched healthy adults. (22; 25; 34) Consequently, COPD patients experience decreases in physical function and quality of life resulting in further inactivity and a cycle of further deconditioning. Short-term exercise therapy can interrupt this cycle and improve physical function and quality of life. (20; 21; 32) Furthermore, results contrasting short-term (3 months) and long-term (18 months) exercise therapy has demonstrated the superiority of long-term therapy on improved physical function and quality of life. (5; 11) Recent data from Garcia-Aymerich et al. have shown that regular physical activity is associated with a reduction in COPD hospitalizations and respiratory mortality. (12) Collectively, these data support the benefit and need of promoting long-term physical activity in COPD patients.
Unfortunately long-term adherence with exercise programs for individuals with a chronic disease mirrors the unfavorable reports found in asymptomatic populations. (23) Given that the current exercise therapy model for COPD patients is to provide 8–12 weeks of center-based exercise therapy with no additional intervention (13), the goal of the present study was to contrast this approach with a lifestyle activity program (LAP) on long-term (12-month) maintenance of physical activity levels in COPD patients. The LAP, based on our past successful experience with a similar program developed for individuals at risk for or those with cardiovascular disease (26), was designed to phase out supervised, center-based activity over an initial 3-month period using behavior change procedures that encouraged patients to increase their daily physical activity levels such that they met established physical activity guidelines (1; 41). Our hypothesis was that the LAP would result in higher levels of moderate physical activity at the end of 12 months as compared to a traditional three-month exercise therapy (TET) program.
METHODS
Design
This was a single center, single blind, randomized controlled clinical trial. Patient were recruited over a 4 year period in waves of approximately 12 to 15 patients per wave via community based advertising in the local media and physician referral. No financial remuneration was offered to patients for their participation, and all patients were informed they had the option to drop out of the study at any time with no adverse effects on their treatment. Patients within each wave were randomized to either the LAP or the TET following completion of baseline visits. Evaluation of participants occurred at baseline and at 3, 6 and 12 months post-randomization. Staff members blinded to the participant's treatment assignment completed all data collection. All analyses allocated participants to their originally randomized groups and used all available follow-up data.
Participants
Participants were eligible if they had an expiratory airflow limitation such that the FEV1/FVC was ≤ 70% and the FEV1 was ≥ 20% of predicted. Patients had to report difficulty in performing at least one of the following activities due to dyspnea: walking a city block, grocery shopping, doing household chores, lifting objects chest height or higher, walking up stairs and getting out of a chair. Patients had to be free of severe cardiovascular or peripheral vascular disease, not undergoing active treatment for cancer, free from uncontrolled hypertension or diabetes, have not participated in a pulmonary rehabilitation or exercise program for the previous three months, and be willing to accept random assignment into either intervention arm. Determination of medical diagnoses which would preclude participation in the study was done through a medical history, physical exam and a graded exercise test. All participants remained under the care of their personal physician and signed an informed consent approved by the University's Institutional Review Board.
Behavioral Run-In
Because the LAP required participants to engage in self monitoring of physical activity for 12 months, all participants completed a one week behavioral run-in prior to randomization. Participants were asked to record information regarding their levels of physical activity during this period and were queried as to their perceived difficulty with this process. They were also questioned about their willingness to continue this process for an additional 12 months. Those that were unable to perform self monitoring during this one week period or unwilling to continue this process for an additional 12 months were excluded from the study.
Randomization
Randomization was performed using a web-based randomization application. All baseline measurements were obtained prior to randomization. Only the statisticians were unblinded to the randomization scheme, which was stratified by gender and study period (1st, 2nd, and final third of participants randomized). Block sizes varied randomly between 4 and 6.
Power
This study was designed to have 90% power to detect a 400 kcal/week average difference during follow-up with 85 evaluable participants per group if the correlation between baseline and follow-up measurements was 0.60 as observed by Stewart et al. (38), and 85% power if the correlation was 0.50.
Interventions
Both the LAP and the TET groups received identical center-based exercise therapy and bimonthly education classes at the clinic site during the first three months of the study. Both interventions had 36 contact hours between interventionists and participants. The TET group met for one-hour exercise sessions thrice weekly for 12 weeks. Each session consisted of a brief warm-up, 30 – 35 minutes of walking at a rating of perceived dyspnea of 3 – 5 (moderate to somewhat hard) on the Borg categorical scale (6), 10 – 15 minutes of strength training using elastic resistance bands and a brief cool-down. Following completion of the 36 center-based sessions, participants were encouraged to continue exercising; however, an option to continue at the clinic site was not provided. Participants were provided information on community sites that conducted similar programs similar to the one completed.
The LAP also consisted of 36 hours of participant contact; however, the structure, timing, and goals of these sessions differed from the TET. A basic principle underlying these contacts and their sequencing was one of gradually weaning participants from the dependency on staff and the center-based program toward independent promotion and self-regulation of physical activity at home. This process was one of a phased increase in the ratio of personal responsibility in conjunction with a phased decrease in staff, group and clinic dependency. In the LAP intervention, the principles of group dynamics were used to systematically develop group formation and identity, create the group's common motivational base (i.e., independent physical activity), and establish exercise and adherence expectancies in members that were of consequence to the group. In other words, the group was used to promote an independent lifestyle of physical activity by (a) making this the group goal, (b) teaching and practicing this approach within the group, (c) gaining commitment to independent exercise for each member from within the group, and then, (d) avoiding group dependence by weaning participants from the group so that these individuals sustain independent, long-term, daily activity at home and/or in the community.
For the LAP, there were four different types of contact that the patients had with the staff to help establish the above goals. These were center-based exercise/group sessions. During center-based exercise/group sessions, participants met at the rehabilitation facility for one hour of center-based exercise training. These exercise sessions were the same as those used with the TET program. Following the exercise sessions, participants engaged in a 15-minute period of instruction and participated in group discussion regarding the self-regulatory aspect of learning long-term maintenance of physical activity. The second type of contact the LAP patients had with staff were center-based exercise training sessions. During center-based exercise training sessions, participants met at the rehabilitation facility for one hour exercise training. The third type of contact patients had with staff were 30 minute individual counseling sessions to review and evaluate the patient's ability to sustain independent long-term physical activity. The fourth type of contact patients had with staff were 15 minute individual phone contacts to review and evaluate the patient's progress in sustaining physical activity.
When center-based training sessions were being reduced, patients were encouraged to increase home-based training so that total weekly exercise sessions occurred three times per week. This home-based training was prescribed at an intensity comparable to center-based training. However, patients were told that their exercise sessions could be divided into smaller units of no less than 10 minutes. Thus, participants might choose to perform their home exercise using four 10-min bouts, two 20-min bouts or a single 40-min bout. Patients in the LAP group were given instruction in and assistance in planning for goals that included integrating alternative physical activities into their daily lifestyles. This involved activities such as yard work, choosing to use stairs rather than elevators, taking the option of walking instead of sitting to wait for someone, and/or becoming more involved in recreational sports activities. The objective was to make daily living as active as possible emphasizing that benefits can also be obtained from physical activity that is moderate in intensity and, in some instances, relatively brief.
The timing of all patient contact for the LAP was structured so the patients would not have contact with the staff for one month prior to a follow-up testing visit. A detailed description of the TET and LAP contacts is shown in Table 1.
Table 1.
LAP and TET contact schedule
| Month | LAP Contacts | Contact Hours | Total Contact Hours | TET Contacts | Contact Hours | Total Contact Hours |
|---|---|---|---|---|---|---|
| 1 | 6 Center-Based Exercise/Group Session | 7.50 | 7.50 | 12 Center-Based Exercise Sessions | 12 | 12 |
|
| ||||||
| 2 | 1 Individual Counseling Session | 0.50 | 12 Center-Based Exercise Sessions | 12 | 24 | |
| 4 Center-Based Exercise/Group Session | 5.00 | 13.0 | ||||
|
| ||||||
| 3 | 1 Individual Phone Contact | 0.25 | 12 Center-Based Exercise Sessions | 12 | 36 | |
| 3 Center-Based Exercise/Group Session | 3.75 | 17.0 | ||||
|
| ||||||
| 4 | 1 Individual Counseling Session | 0.50 | ||||
| 3 Center-Based Exercise/Group Session | 3.75 | 21.25 | ||||
|
| ||||||
| 5 | 1 Individual Phone Contact | 0.25 | ||||
| 3 Center-Based Exercise/Group Session | 3.75 | 25.25 | ||||
|
| ||||||
| 6 | 1 Individual Counseling Session | 0.50 | ||||
| 2 Center-Based Exercise/Group Session | 2.5 | 28.25 | ||||
|
| ||||||
| 7 | 2 Center-Based Exercise/Group Session | 2.5 | 30.75 | |||
|
| ||||||
| 8 | 2 Center-Based Exercise/Group Session | 2.5 | 33.25 | |||
|
| ||||||
| 9 | 1 Individual Phone Contact | 0.25 | ||||
| 1 Center-Based Exercise Session | 1.00 | 34.5 | ||||
|
| ||||||
| 10 | 1 Individual Phone Contact | 0.25 | ||||
| 1 Center-Based Exercise Session | 1.00 | 35.75 | ||||
|
| ||||||
| 11 | 1 Individual Phone Contact | 0.25 | 36.00 | |||
|
| ||||||
| 12 | 0 Contacts | 0 | 36.00 | |||
| Key: | ||
|---|---|---|
| Contact Type | Hours | Minutes |
| Center-Based Exercise/Group Session | 1.25 | 75 |
| Center-Based Exercise Session | 1.00 | 60 |
| Individual Counseling Session | 0.50 | 30 |
| Individual Phone Contact | 0.25 | 15 |
Primary Outcome
Assessment of the primary outcome and all secondary outcomes were performed by assessors blinded to the patients' treatment randomization. The primary outcome was weekly energy expenditure from moderate physical activity measured as kcals/week as estimated using the Community Health Activities Model Program for Seniors (CHAMPS) physical activity questionnaire. (38) The CHAMPS was specifically designed to assess physical activity of older adults and to evaluate interventions designed to increase physical activity in older adults. It has been shown to be appropriate for use across diverse samples of older adults and to be sensitive to change in a number of studies with older adults. (18; 31; 38; 40). It is interviewer administered and comprised of 41 items of which 27 pertain to physical activity and are used for scoring. From the instrument, typical weekly energy expenditure (kcal/week) and frequency of moderate and total physical activity can be estimated. Six-month intraclass correlation coefficients for energy expenditure in all and moderate activities were 0.66 and 0.67, respectively (39). While the CHAMPS defines moderate physical activity as a metabolic equivalent (MET) of 3 or more, we defined moderate physical activity as any activity with a MET value greater than 1.84 METs. Our rationale for doing this was based on the fact that moderate physical activity is defined in Physical Activity and Health: A Report of the Surgeon General as any activity requiring an intensity greater than or equal to 45% of the maximal MET capacity. (41) The average measured maximal MET capacity of the participants in this current investigation measured during their baseline visit was 4.1 METs. Thus 45% of 4.1 METs was 1.84 METs.
Secondary Outcomes
Physical Function
Physical function was assessed using a series of tests that have all been used previously with COPD patients. (2; 10; 15; 28) These included the six minute walk distance, stair climb time and the Short Physical Performance Battery (SPPB) which consisted of a four meter walk speed, chair rise time and balance time. The six minute walk was performed in a dedicated gymnasium that measured 8 meters by 14 meters according to the guidelines of the American Thoracic Society. (2) For the timed stair climb, participants were asked to ascend two flights of stairs as quickly as possible while holding onto the handrail. Each step had a seven inch rise and a 12 inch run. The total vertical ascent was 12.6 feet. The participants did not receive encouragement or feedback on their performance.
Walking speed in the SPPB was assessed by asking the participants to walk at their usual pace over four meters. Participants were allowed to use walking aids (cane, walker, or other walking aid) if necessary, but not the assistance of another person. Time in seconds needed to complete the entire distance was recorded. Two trials were administered and the faster of the two was used to compute walking speed. The chair rise test was performed using a straight-backed chair placed with its back against a wall. Participants were first asked to stand from a sitting position without using their arms. If they were able to perform the task, they were then asked to stand up and sit five times as quickly as possible. The time to complete the task was recorded. For the test of standing balance, participants were asked to maintain balance in three positions characterized by a progressive narrowing of the base support: feet together (side by side position), the heel of one foot beside the big toe of the other foot (semi tandem position), and the heel of one foot in front of and touching the toes of the other foot (tandem position). For each of the three positions, participants were timed to a maximum of 10 seconds. Scores are summed for the measure of balance for a range of 0 to 30 seconds. Each of the three performance measures was assigned a score ranging from 0 to 4, with 4 indicating the highest level of performance and 0 the inability to complete the test according to the recommendations of Guralnick. (15) A summary performance score ranging from 0 (worst performers) to 12 (best performers) was calculated by adding walking speed, chair stands and standing balance scores. This scale has proven valid for predicting institutionalization, hospital admission, mortality and disability. (14; 15)
Self-reported Disability
Self-reported disability was determined using a 23-item questionnaire that assessed the physical disability of the participant. For each item, participants were queried as to how much difficulty they experienced while performing physical activities during the past month. The questionnaire is scored on a 6-point scale with a score of one indicating “usually did with no difficulty” and a score of six indicating “usually did not do for other reasons.” (27; 29)
Health-related Quality of Life
Health-related quality of life was measured using both generic (8; 37) and a disease specific instruments. (16) Psychological functioning was assessed using the short form of the CES-D (8), whereas subscales from the RAND 36-item Health Survey (37) were used to assess general health, pain and social functioning. The Chronic Respiratory Disease Questionnaire was used as a disease-specific measure of health related quality of life. (16) This measure assessed quality of life in the domains of dyspnea, mastery, fatigue and emotion.
Exercise Capacity
Exercise capacity was expressed as peak oxygen consumption (VO2 peak) and total time during a graded exercise test performed on a treadmill. (4) Peak oxygen consumption was determined during a graded exercise test performed in the morning prior to the use of any bronchodilators. Each patient performed a modified Naughton protocol on a treadmill (Quinton Q-4000) in which the grade and/or belt speed was increased by a specified amount at two-minute stages. Oxygen consumption was measured using a Medical Graphics Corporation CPX-D metabolic cart. All values were collected during a 60-second period and reported as minute values. The highest oxygen consumption value measured for a complete 60-second period represented the VO2peak. Calibration of the system occurred prior to every test according to the manufacturer's specifications. All calibration gases were certified standard gases verified via Haldane analysis.
Pulmonary Function
Pulmonary function tests and lung volume determinations were performed according to ATS guidelines using a Medical Graphics Corporation 1085D plethysmograph. (3)
Compliance
Compliance was defined as the number of sessions completed divided by the total number of sessions prescribed and was expressed as a percentage. Compliance was calculated based on all randomized patients. If a participant planned to miss a session, an exercise leader prepared an exercise prescription for that participant to perform while away and verified compliance once the participant returned.
Safety Monitoring
Serious adverse events were defined based on the Code of Federal Regulations, 21CFR 312.32 and included any of the following outcomes: death, a life-threatening adverse event, inpatient hospitalization or prolongation of existing hospitalization, or a persistent or significant disability/incapacity.
Statistical Analysis
Baseline characteristics were compared between groups using chi-square tests for categorical variables and the two sample t-test for continuous variables. An examination of the compliance data revealed non-normal distributions. As a result median data are reported for this measure.
The effect of the intervention on the primary outcome averaged across both follow-up measurements was analyzed using mixed effects analysis of covariance models for repeated outcomes. The model testing this hypothesis included factors used to stratify randomization ((gender, study period (1st, 2nd, and final third of participants randomized)), the baseline measure of energy expenditure, the intervention effect, and a factor measuring the time effect (3 months, 6 months, and 12 months) and an intervention by time interaction term. An unstructured covariance matrix was used to account for covariance between repeated measures and estimation was done using maximum likelihood. Follow-up means were estimated as least-squares means. The primary hypothesis was tested as a contrast representing the difference in the average effect between intervention groups across all time points, using a two-sided 0.05 level of Type I error.
Secondary analyses of the primary and secondary outcomes were conducted for differential effects of the intervention at different follow-up points (i.e. an interaction between the intervention effect and the time effect).
RESULTS
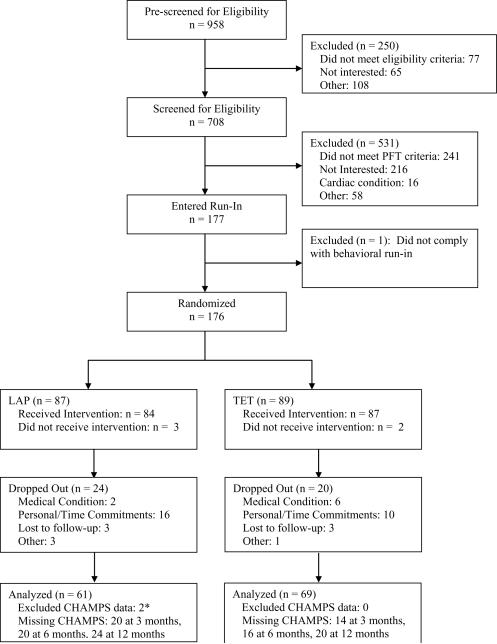
Recruitment occurred over a three year period. Participant flow is illustrated in Figure 1. Following the screening visits, 176 individuals were randomized to either the LAP (n = 87) or TET (n = 89). There were no differences between the groups in the baseline characteristics shown in Table 2. When comparing baseline characteristics between those that dropped out versus those that completed the study, the drop outs were significantly younger and there were a relatively lower percentage with comorbidity illness. There were no other differences between these two groups. During the course of the study, 24 patients dropped out of the LAP program, and 20 dropped out of the TET program. Dropout rates were not significantly different between the two groups.
Figure 1.
* CHAMPS data from two participants was excluded from analysis due to improper administration of the baseline questionnaire to the participants. Given baseline values were used as a covariate, data from these participants were also excluded from the follow-up analysis. An analysis that did not control for baseline CHAMPS levels, but included all follow-up data for these participants produced similar results and did not change the overall conclusions.
Table 2.
Participant Characteristics.
| Characteristic | TET | LAP | P Value |
|---|---|---|---|
| Men/Women, n/n | 48/41 | 47/40 | 0.99 |
| Age, yr (mean ± SD) | 66 ± 10 | 66 ±10 | 0.96 |
| Mass, kg (mean ± SD) | 84.4 ± 23.9 | 82.2 ± 20.3 | 0.50 |
| Pulmonary Function (mean ± SD) | |||
| FEV1 (1) | 1.48 ± 0.63 | 1.46 ± 0.69 | 0.79 |
| FEV1 (% of predicted) | 53.0 ± 18.5 | 50.5 ± 20.2 | 0.41 |
| FEV1/FVC (%) | 52.3 ± 12.2 | 51.1 ± 13.6 | 0.55 |
| RV/TLC (%) | 56.6 ± 13.0 | 58.7 ± 11.6 | 0.40 |
| Disease Severity (n with) | |||
| Mild | 5 | 7 | 0.56 |
| Moderate | 44 | 34 | 0.26 |
| Severe | 30 | 33 | 0.71 |
| Very Severe | 10 | 13 | 0.53 |
| Smoking Status | |||
| Current, n | 26 | 29 | 0.51 |
| Previous, n | 59 | 53 | |
| Pack Years (mean ± SD) | 24 ± 12 | 27 ± 17 | 0.23 |
| Co-Morbid Illnesses (n with) | |||
| Arthritis | 49 | 35 | 0.06 |
| Hypertension | 47 | 39 | 0.30 |
| Circulatory Problems | 17 | 16 | 0.90 |
| Heart Disease | 39 | 40 | 0.77 |
| Diabetes | 8 | 14 | 0.15 |
| Cancer | 20 | 20 | 0.93 |
| Co-Morbid (n with) | |||
| 0 | 10 | 18 | 0.29 |
| 1 | 18 | 16 | |
| >2 | 30 | 27 | |
The p values for comparing the two treatment arms were obtained using the Chi-square test for categorical variables and the two sample t-test for continuous variables. FEV1 is the forced expiratory volume in one second. FVC is the forced vital capacity. RV is the residual volume. TLC is the total lung capacity.
Compliance with the Interventions
Compliance data were not normally distributed, therefore median data are reported. Compliance with the TET was 80.6 percent and with the LAP was 74.3 percent. Results from the Wilcoxon test showed these values not to be significantly different (p = 0.21).
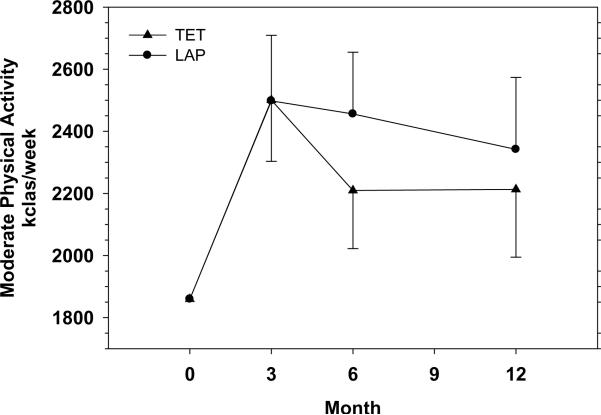
Moderate Physical Activity
Figure 2 illustrates moderate physical activity levels. Over 12 months, physical activity levels were not significantly different between the LAP and TET groups (p = 0.57, average effect across all follow-up visits). The adjusted difference in physical activity levels (kcals/week) between the two groups was 3 at 3 months (p = .99), 246 at 6 months (p = .37) and 129 at 12 months (p = .69). Within the LAP group, there were significant increases in moderate physical activity when comparing 3 (p = 0.004), 6 (p = 0.005) and 12 month (p = 0.048) follow up data to baseline values. Within the TET group, there were significant increases in moderate physical activity when comparing 3 (p = 0.002) and 6 month follow up (p = 0.039) data to baseline values. For the TET, 12 month follow up physical activity levels were not significantly different from baseline values (p = 0.089).
Figure 2.
Moderate physical activity levels in kcals per week by intervention arm at baseline and follow-up. Data are reported as mean ± standard error of the mean. Means were estimated from mixed effects analysis of covariance for repeated measures adjusted for gender and baseline values.
Physical Function
Table 3 provides the results for measures of physical function. Over 12 months of follow-up, none of the measures of physical function were significantly different between the LAP and the TET groups. There were no significant differences in measures of physical function between the groups at any of the follow-up time points. Within group analysis showed significant improvements in selected measures of physical function for both groups. More specifically, the TET had significant improvements in six-minute walk distance, stair climb time, SPPB score, four-meter walk distance and chair rise times at 3 months as compared to baseline. The TET group maintained improvements in six-minute walk distance, SPPB, four-meter walk distance and chair rise time at 12 months. The LAP had significant improvements in six-minute walk distance, SPPB score, four-meter walk distance and chair rise times at 3 months as compared to baseline. The LAP group maintained improvements in SPPB score, four-meter walk distance and chair rise time at 12 months.
Table 3.
Physical function outcomes by intervention arm at baseline and follow-up.
| Variable | P value TET vs LAP, average effect across all follow-up visits | ||||
|---|---|---|---|---|---|
| Baseline | 3 Mo | 6 Mo | 12 Mo | ||
| Six-min Walk (m) | |||||
| TET | 410.7 | 428.7 ± 8.3 (0.03) | 439.8 ± 9.9 (<0.01) | 430.5 ± 10.0 (0.05) | 0.41 |
| LAP | 410.7 | 434.8 ± 8.8 (<0.01) | 426.7 ± 10.3 (0.12) | 408.1 ± 10.5 (0.81) | |
| P value, TET vs LAP | 0.61 | 0.36 | 0.13 | ||
| Stair Climb (sec) | |||||
| TET | 15.1 | 14.0 ± 0.5 (0.02) | 14.1 ± 0.6 (0.07) | 14.3 ± 0.6 (0.17) | 0.54 |
| LAP | 15.1 | 14.7 ± 0.5 (0.37) | 14.6 ± 0.6 (0.39) | 14.5 ± 0.6 (0.30) | |
| P value, TET vs LAP | 0.36 | 0.52 | 0.83 | ||
| SPPB | |||||
| TET | 10.6 | 11.0 ± 0.2 (0.01) | 11.0 ± 0.2 (0.04) | 11.2 ± 0.2 (<0.01) | 0.61 |
| LAP | 10.6 | 11.0 ± 0.2 (0.03) | 11.4 ± 0.2 <0.01) | 11.1 ± 0.2 (<0.01) | |
| P value, TET vs LAP | 0.89 | 0.11 | 0.81 | ||
| Four Meter Walk (sec) | |||||
| TET | 3.9 | 3.5 ± 0.1 (<0.01) | 3.6 ± 0.1 (<0.01) | 3.5 ± 0.1 (<0.01) | 0.80 |
| LAP | 3.9 | 3.6 ± 0.1 (<0.01) | 3.5 ± 0.1 (<0.01) | 3.5 ± 0.1 (<0.01) | |
| P value, TET vs LAP | 0.68 | 0.54 | 0.73 | ||
| Chair Rise (sec) | |||||
| TET | 12.8 | 10.2 ± 0.4 (<0.01) | 9.8 ± 0.4 (<0.01) | 10.3 ± 0.4 (<0.01) | 0.74 |
| LAP | 12.8 | 10.5 ± 0.4 (<0.01) | 10.1 ± 0.4 (<0.01) | 10.0 ± 0.4 (<0.01) | |
| P value, TET vs LAP | 0.49 | 0.53 | 0.64 | ||
| Balance (sec) | |||||
| TET | 29.1 | 28.6 ± 0.3 (0.09) | 28.8 ± 0.4 (0.38) | 29.1 ± 0.3 (0.98) | 0.10 |
| LAP | 29.1 | 29.3 ± 0.4 (0.71) | 29.6 ± 0.4 (0.20) | 29.6 ± 0.3 (0.12) | |
| P value, TET vs LAP | 0.16 | 0.13 | 0.27 | ||
All values are estimated least squares means (± standard error of the mean) from repeated measures analysis of covariance adjusted for gender and baseline value. Baseline value is overall mean used to obtain least squares means at follow-up visits. P values for within group analysis comparing follow up visits to baseline values are reported adjacent to individual mean values.
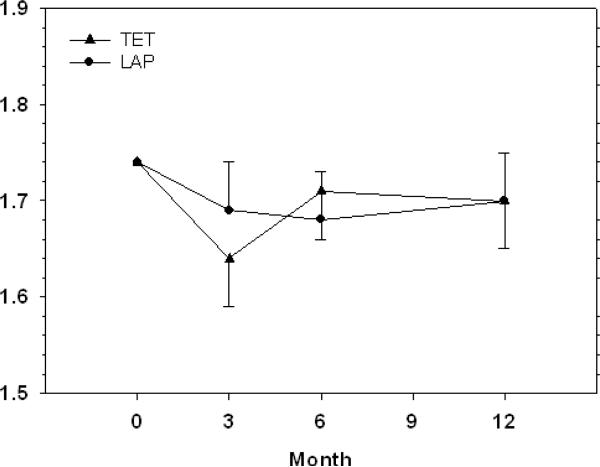
Self-reported Disability
Figure 3 illustrates estimated mean levels of self-reported disability. Over 12 months of follow-up, self-reported disability levels were not significantly different between the groups (p = 0.91 for average effect across all follow-up visits). There were no significant differences in self-reported disability levels between the two intervention groups at 3 (p = 0.46), 6 (p = 0.74) or 12 months (p = 0.97) of follow-up. Within the LAP group, there were no significant differences in self-reported disability when comparing 3 (p = 0.23), 6 (p = 0.23) and 12 month (p = 0.43) follow up data to baseline values. Within the TET group, there were significant increases in self-reported disability when comparing 3 month follow up (p = 0.027) data to baseline values. For the TET, 6 and 12 month follow up self-reported disability were not significantly different from baseline values (p = 0.44 and 0.43, respectively).
Figure 3.
Self-reported disability by intervention arm at baseline and follow-up. Data are reported as mean ± standard error of the mean. Means were estimated from mixed effects analysis of covariance for repeated measures adjusted for gender and baseline values.
Quality of Life
Table 4 contains quality of life results. Over 12 months of follow-up, quality of life measures were not significantly different between the groups. There were no significant differences in quality of life measures between the groups at any of the follow-up time points. The TET had significant improvements in the SF-36 physical scale and the CRQ at 3 months as compared to baseline. The TET group maintained improvements in the CRQ at 12 months. The LAP had significant improvements in the CRQ at 3 months as compared to baseline and maintained these improvements at 12 months.
Table 4.
Quality of life measures by intervention arm at baseline and follow-up.
| Variable | P value TET vs LAP, average effect across all follow-up visits | ||||
|---|---|---|---|---|---|
| Baseline | 3 Mo | 6 Mo | 12 Mo | ||
| CESD | |||||
| TET | 11.6 | 10.8 ± 0.9 (0.36) | 11.5 ± 0.9 (0.89) | 13.0 ± 1.0 (0.18) | 0.82 |
| LAP | 11.6 | 12.0 ± 1.0 (0.73) | 12.3 ± 0.9 (0.51) | 11.8 ± 1.1 (0.87) | |
| P value, TET vs LAP | 0.38 | 0.57 | 0.43 | ||
| SF-36 - Physical Scale | |||||
| TET | 34.9 | 36.7 ± 0.9 (0.03) | 36.5 ± 0.9 (0.08) | 36.2 ± 1.1 (0.23) | 0.45 |
| LAP | 34.9 | 36.0 ± 0.9 (0.22) | 35.7 ± 1.0 (0.40) | 35.2 ± 1.2 (0.77) | |
| P value, TET vs LAP | 0.55 | 0.54 | 0.55 | ||
| SF-36 - Mental Scale | |||||
| TET | 51.6 | 51.9 ± 1.0 (0.80) | 52.3 ± 1.0 (0.49) | 51.1 ± 1.1 (0.65) | 0.50 |
| LAP | 51.6 | 51.3 ± 1.1 (0.79) | 51.1 ± 1.0 (0.64) | 50.3 ± 1.2 (0.29) | |
| P value, TET vs LAP | 0.71 | 0.42 | 0.64 | ||
| CRQ | |||||
| TET | 4.3 | 4.8 ± 0.1 (<0.01) | 4.7 ± 0.1 (<0.01) | 4.6 ± 0.1 (0.02) | 0.32 |
| LAP | 4.3 | 4.6 ± 0.1 (<0.01) | 4.5 ± 0.1 (0.04) | 4.6 ± 0.1 (0.03) | |
| P value, TET vs LAP | 0.11 | 0.27 | 0.98 | ||
All values are estimated least squares means (± standard error of the mean) from repeated measures analysis of covariance adjusted for gender and baseline value. Baseline value is overall mean used to obtain least squares means at follow-up visits. CESD is the Center for Epidemiological Studies Depression scale. SF-36 is the Medical Outcomes Study 36-item Short Form. The CRQ is the Chronic Respiratory Disease Questionnaire. P values for within group analysis comparing follow up visits to baseline values are reported adjacent to individual mean values.
Exercise Capacity
Table 5 contains exercise capacity results. Over 12 months of follow-up, neither VO2 peak nor total treadmill time was significantly different between the groups. There were no significant differences in either measure between the two groups at any of the follow-up time points.
Table 5.
Exercise capacity measures by intervention arm at baseline and follow-up
| Variable | P value TET vs LAP, average effect across all follow-up visits | |||
|---|---|---|---|---|
| Baseline | 3 Mo | 12 Mo | ||
| VO2 peak(ml/kg/min) | ||||
| TET | 14.4 | 14.9 ± 0.6 (0.42) | 16.5 ± 0.8 (0.01) | 0.23 |
| LAP | 14.4 | 15.2 ± 0.6 (0.14) | 14.3 ± 0.8 (0.87) | |
| P value, TET vs LAP | 0.65 | 0.06 | ||
| Total Treadmill Time (s) | ||||
| TET | 335 | 416 ± 14 (0.09) | 427 ± 20 (0.08) | 0.73 |
| LAP | 335 | 411 ± 14 (0.21) | 419 ± 21 (0.22) | |
| P value, TET vs LAP | 0.79 | 0.75 | ||
All values are estimated least squares means (± standard error of the mean) from repeated measures analysis of covariance adjusted for gender and baseline value. VO2 peak is peak oxygen consumption. Baseline value is overall mean used to obtain least squares means at follow-up visits. P values for within group analysis comparing follow up visits to baseline values are reported adjacent to individual mean values.
Serious Adverse Events
Serious adverse events for each intervention arm are presented in Table 6. Twenty-three of the participants in the TET group and 24 of the participants in the LAP group reported at least one serious adverse event. There was no significant differences between the two interventions in the number participants reporting serious adverse events (p = 0.92).
Table 6.
Serious Adverse Events by Intervention Arm.
| TET | LAP | P Value | |
|---|---|---|---|
| Serious Adverse Event | |||
| Cardiac | 7 | 5 | 0.58 |
| Dental | 1 | 0 | 1.00 |
| Gastrointestinal | 2 | 1 | 1.00 |
| Genitourinary/Reproductive | 1 | 4 | 0.21 |
| Lymphatic | 1 | 0 | 1.00 |
| Metabolic | 1 | 0 | 1.00 |
| Musculoskeletal | 2 | 1 | 1.00 |
| Neurologic | 2 | 1 | 1.00 |
| Pulmonary | 8 | 13 | 0.22 |
Values are the number of events. The p values for comparing the two treatment arms were obtained using the Chi-square test for categorical variables.
DISCUSSION
The results are somewhat surprising as they show no difference in physical activity levels and secondary outcomes at one year between patients in the TET and LAP. Because interventions that have used similar cognitive-behavioral strategies have been shown to be effective in the promotion and maintenance of physical activity in healthy older adults and those with cardiovascular disease (7; 26; 42), we hypothesized that the LAP participants would exhibit higher physical activity levels versus those in the TET at the end of the trial. Our results did show the LAP group to have higher activity levels at 12 months as compared to their baseline values and the TET group showed a trend (p = 0.09) for higher activity levels at 12 months as compared to baseline. We also thought that the LAP participants would maintain the benefits in secondary outcomes, whereas the TET participants would experience a loss. Our results showed both groups maintaining a number of improvements in physical function and quality of life at 12 months when compared to baseline.
While most studies show a return of health benefits to baseline values in follow-up testing following termination of a structured rehabilitation program similar to our TET, some studies have shown that participants maintain these benefits. Casaburi et al. examined endurance time in COPD patients randomized to either an 8 week pulmonary rehabilitation program while receiving tiotropium or an 8 week pulmonary rehabilitation program while receiving a placebo. (9) Their results showed that all patients, regardless of treatment, maintained improvements in exercise capacity 3 months after the completion of the pulmonary rehabilitation program. In a follow-up paper, they found that 3 months following the completion of the program patients in the placebo group were at activity levels similar to those reported during the program. (17) Thus, it appears that over the short term COPD patients do maintain increases in physical activity levels following an exercise rehabilitation program and this is accompanied with the maintenance of exercise therapy derived benefits.
Our results and those of Casaburi et al. show that participants in exercise rehabilitation programs can maintain physical activity levels and associated benefits 3 to 9 months following the completion of such a program. There is evidence that these benefits are not maintained much beyond 12 to 24 months. (5; 33) Ries at al. evaluated the efficacy of a 12 month telephone-based maintenance program in COPD patients that had completed an eight week rehabilitation program. (33) During the 12 month intervention, they found exercise tolerance and overall health status was better maintained in the experimental group. By 24 months, these group differences had disappeared, and patients returned to levels close to baseline values. The authors concluded that a maintenance program consisting of weekly calls and monthly supervised sessions produced only modest improvements in the maintenance of benefits following rehabilitation and that these improvements are not durable in the absence of an ongoing intervention. In retrospect, it would have informative to extend out study out to 24-months to examine group differences at this more distant time point.
In a previous trial, we reported on patients who completed a 3 or 18 month center-based exercise intervention. (5) Those in the 3 month intervention were encouraged to continue exercising; however, they were not provided with the opportunity to continue at our center. At the end of 18 months, those in the 3 month intervention reported lower levels of physical activity, physical function and a reduced quality of life as compared to those in the 18 month program. More importantly, those in the 18 month intervention maintained the early benefits archived from participating. Recently, Steele and colleagues evaluated the efficacy of a 12 week exercise adherence intervention initiated after patients completed an 8 week rehabilitation program. (36) As compared to a control group that only completed the 8 week rehabilitation program, those patients in the exercise adherence intervention had smaller declines in exercise adherence and exercise capacity at the completion of the 12 week exercise adherence intervention. However, at one year of follow-up, there were no differences between the groups in daily activity levels, and there were no long-term benefits with respect to exercise capacity. Based on the results of these previously published studies and this investigation, it appears that COPD patients who participate in short-term exercise therapy programs can maintain the benefits for up to one year. However, following that, it appears that the benefits begin to diminish as time progresses. Collectively, this research points to the need for intervention studies that examine adherence 2 to 4 years following the onset of treatment and to formally test behavioral interventions that directly address the question of how to best promote long term adherence.
A limitation with the current study is the lack of a control group that received no exercise intervention. Given previous studies documenting the effectiveness of exercise at improving physical function in COPD patients, we did not feel it would be ethical to withhold a proven treatment. Since both the TET and LAP had similar levels of physical activity and physical function at the completion of the trial, it could be argued that neither intervention was effective at increasing physical activity or improvement secondary outcomes. However, despite the fact that there were no between group differences both LAT and TET both groups did improve their physical activity habits from baseline to follow-up assessments and this was complemented by improvement in selected measures of physical function and quality of life. Results from previous studies have shown that physical function will decline in COPD patients not involved in exercise. Stav et al. followed patients assigned to either a three year pulmonary rehabilitation program versus a no exercise control group. At the end of one, two, and three years, physical function had declined in the control group, whereas physical function was improved and maintained throughout the study in the exercise group.(35)
An additional limitation to our study is the fact that traditional measures used in evaluating the efficacy of pulmonary rehabilitation (i.e, six minute walk and the CRQ) did not show clinically meaningful improvements at 3 or 12 months. Our interventions were designed so that both groups had similar exercise prescriptions while performing center based exercise. We set these levels so that those in the LAP would be able to mimic these same levels when exercising unsupervised at home. While it may be argued that these intensity levels were too low to elicit clinically meaningful improvements in traditional measures used in pulmonary rehabilitation, we did see clinically meaningful changes in the SPPB in both groups at 12 months. (19; 24) Given the SPPB is a valid measure of lower extremity function that has been used with COPD patients, we would argue that both interventions, albeit while not significantly different from each other, were effective in improving long-term physical function.
In conclusion, this study shows that a LAP approach to exercise therapy with COPD patients is safe and effective at maintaining increased physical activity levels at one year. Additionally, both the LAP and TET result in the maintenance of selected health benefits at one year. It is important to note that all participants in this study had to pass a behavior run-in prior to being deemed eligible for participation. This is relevant to the external validity of the study results. Additional intervention research is needed to determine whether the long-term effects of LAP and TET on exercise adherence and health benefits in patients with COPD extend beyond 12 months.
Acknowledgments
Supported grants HL 53755, AG 21332 and M01 RR07122 from the National Institutes of Health (Bethesda, MD).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This clinical trial is registered with ClinicalTrials.gov. Its identifier is NCT00328484.
Reference List
- 1.American College of Sports Medicine Position Stand Exercise and physical activity for older adults. Med Sci Sports Exerc. 1998;30:992–1008. [PubMed] [Google Scholar]
- 2.American Thoracic Society ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166:111–117. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 3.American Thoracic Society Standardization of spirometry - 1987 update. Am Rev Respir Dis. 1987;136:1285–1298. doi: 10.1164/ajrccm/136.5.1285. [DOI] [PubMed] [Google Scholar]
- 4.Berry MJ, Adair NE, Rejeski WJ. Use of peak oxygen consumption in predicting physical function and quality of life in COPD patients. Chest. 2006;129:1516–1522. doi: 10.1378/chest.129.6.1516. [DOI] [PubMed] [Google Scholar]
- 5.Berry MJ, Rejeski WJ, Adair NE, Ettinger WH, Jr., Zaccaro DJ, Sevick MA. A randomized, controlled trial comparing long-term and short-term exercise in patients with chronic obstructive pulmonary disease. J Cardiopulm Rehabil. 2003;23:60–68. doi: 10.1097/00008483-200301000-00011. [DOI] [PubMed] [Google Scholar]
- 6.Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14:377–381. [PubMed] [Google Scholar]
- 7.Brawley LR, Rejeski WJ, Lutes L. A group-mediated cognitive-behavioral intervention for increasing adherence to physical activity in older adults. Journal of Applied Biobehavioral Research. 2000;5:47–64. [Google Scholar]
- 8.Burnam MA, Wells KB, Leake B, Landsverk J. Development of a brief screening instrument for detecting depressive disorders. Med Care. 1988;26:775–789. doi: 10.1097/00005650-198808000-00004. [DOI] [PubMed] [Google Scholar]
- 9.Casaburi R, Kukafka D, Cooper CB, Witek TJ, Jr., Kesten S. Improvement in exercise tolerance with the combination of tiotropium and pulmonary rehabilitation in patients with COPD. Chest. 2005;127:809–817. doi: 10.1378/chest.127.3.809. [DOI] [PubMed] [Google Scholar]
- 10.Eisner MD, Blanc PD, Yelin EH, Sidney S, Katz PP, Ackerson L, Lathon P, Tolstykh I, Omachi T, Byl N, Iribarren C. COPD as a systemic disease: impact on physical functional limitations. Am J Med. 2008;121:789–796. doi: 10.1016/j.amjmed.2008.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Foy CG, Rejeski WJ, Berry MJ. Gender moderates the effects of long-term exercise therapy upon health-related quality of life among COPD patients. Chest. 2001;119:70–76. doi: 10.1378/chest.119.1.70. [DOI] [PubMed] [Google Scholar]
- 12.Garcia-Aymerich J, Lange P, Benet M, Schnohr P, Anto JM. Regular physical activity reduces hospital admission and mortality in chronic obstructive pulmonary disease: a population based cohort study. Thorax. 2006;61:772–778. doi: 10.1136/thx.2006.060145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garvey C. 2007 Reimbursement for pulmonary rehabilitation. American Thoracic Society; 2007. Online. [Google Scholar]
- 14.Guralnik JM, Ferrucci L, Pieper CF, Leveille SG, Markides KS, Ostir GV, Studenski S, Berkman LF, Wallace RB. Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol A Biol Sci Med Sci. 2000;55:M221–M231. doi: 10.1093/gerona/55.4.m221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, Scherr PA, Wallace RB. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–M94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 16.Guyatt GH, Berman LB, Townsend M, Pugsley SO, Chambers LW. A measure of quality of life for clinical trials in chronic lung disease. Thorax. 1987;42:773–778. doi: 10.1136/thx.42.10.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kesten S, Casaburi R, Kukafka D, Cooper CB. Improvement in self-reported exercise participation with the combination of tiotropium and rehabilitative exercise training in COPD patients. Int J Chron Obstruct Pulmon Dis. 2008;3:127–136. doi: 10.2147/copd.s2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.King AC, Pruitt LA, Phillips W, Oka R, Rodenburg A, Haskell WL. Comparative effects of two physical activity programs on measured and perceived physical functioning and other health-related quality of life outcomes in older adults. J Gerontol A Biol Sci Med Sci. 2000;55:M74–M83. doi: 10.1093/gerona/55.2.m74. [DOI] [PubMed] [Google Scholar]
- 19.Kwon S, Perera S, Pahor M, Katula JA, King AC, Groessl EJ, Studenski SA. What is a meaningful change in physical performance? Findings from a clinical trial in older adults (the LIFE-P study) J Nutr Health Aging. 2009;13:538–544. doi: 10.1007/s12603-009-0104-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lacasse Y, Goldstein R, Lasserson TJ, Martin S. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2006:CD003793. doi: 10.1002/14651858.CD003793.pub2. [DOI] [PubMed] [Google Scholar]
- 21.Lacasse Y, Guyatt GH, Goldstein RS. The components of a respiratory rehabilitation program: a systematic overview. Chest. 1997;111:1077–1088. doi: 10.1378/chest.111.4.1077. [DOI] [PubMed] [Google Scholar]
- 22.McGlone S, Venn A, Walters EH, Wood-Baker R. Physical activity, spirometry and quality-of-life in chronic obstructive pulmonary disease. COPD. 2006;3:83–88. doi: 10.1080/15412550600651263. [DOI] [PubMed] [Google Scholar]
- 23.Oldridge NB, Streiner DL. The health belief model: predicting compliance and dropout in cardiac rehabilitation. Med Sci Sports Exerc. 1990;22:678–683. doi: 10.1249/00005768-199010000-00020. [DOI] [PubMed] [Google Scholar]
- 24.Perera S, Mody SH, Woodman RC, Studenski SA. Meaningful change and responsiveness in common physical performance measures in older adults. J Am Geriatr Soc. 2006;54:743–749. doi: 10.1111/j.1532-5415.2006.00701.x. [DOI] [PubMed] [Google Scholar]
- 25.Pitta F, Troosters T, Spruit MA, Probst VS, DeCramer M, Gosselink R. Characteristics of physical activities in daily life in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2005;171:972–977. doi: 10.1164/rccm.200407-855OC. [DOI] [PubMed] [Google Scholar]
- 26.Rejeski WJ, Brawley LR, Ambrosius WT, Brubaker PH, Focht BC, Foy CG, Fox LD. Older adults with chronic disease: benefits of group-mediated counseling in the promotion of physically active lifestyles. Health Psychol. 2003;22:414–423. doi: 10.1037/0278-6133.22.4.414. [DOI] [PubMed] [Google Scholar]
- 27.Rejeski WJ, Ettinger WHJ, Schumaker S, James P, Burns R, Elam JT. Assessing performance-related disability in patients with knee osteoarthritis. Osteoarthritis Cartilage. 1995;3:157–167. doi: 10.1016/s1063-4584(05)80050-0. [DOI] [PubMed] [Google Scholar]
- 28.Rejeski WJ, Foley KO, Woodard CM, Zaccaro DJ, Berry MJ. Evaluating and understanding performance testing in COPD patients. J Cardiopulm Rehabil. 2000;20:79–88. doi: 10.1097/00008483-200003000-00001. [DOI] [PubMed] [Google Scholar]
- 29.Rejeski WJ, Ip EH, Marsh AP, Miller ME, Farmer DF. Measuring disability in older adults: the International Classification System of Functioning, Disability and Health (ICF) framework. Geriatr Gerontol Int. 2008;8:48–54. doi: 10.1111/j.1447-0594.2008.00446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rennard S, DeCramer M, Calverley PM, Pride NB, Soriano JB, Vermeire PA, Vestbo J. Impact of COPD in North America and Europe in 2000: subjects' perspective of Confronting COPD International Survey. Eur Respir J. 2002;20:799–805. doi: 10.1183/09031936.02.03242002. [DOI] [PubMed] [Google Scholar]
- 31.Resnick B, King A, Riebe D, Ory M. Measuring physical activity in older adults: use of the Community Health Activities Model Program for Seniors Physical Activity Questionnaire and the Yale Physical Activity Survey in three behavior change consortium studies. West J Nurs Res. 2008;30:673–689. doi: 10.1177/0193945907311320. [DOI] [PubMed] [Google Scholar]
- 32.Ries AL, Bauldoff GS, Carlin BW, Casaburi R, Emery CF, Mahler DA, Make B, Rochester CL, ZuWallack R, Herrerias C. Pulmonary Rehabilitation: Joint ACCP/AACVPR Evidence-Based Clinical Practice Guidelines. Chest. 2007;131:4S–42S. doi: 10.1378/chest.06-2418. [DOI] [PubMed] [Google Scholar]
- 33.Ries AL, Kaplan RM, Myers R, Prewitt LM. Maintenance after pulmonary rehabilitation in chronic lung disease: a randomized trial. Am J Respir Crit Care Med. 2003;167:880–888. doi: 10.1164/rccm.200204-318OC. [DOI] [PubMed] [Google Scholar]
- 34.Serres I, Gautier V, Varray A, Prefaut C. Impaired skeletal muscle endurance related to physical inactivity and altered lung function in COPD patients. Chest. 1998;113:900–905. doi: 10.1378/chest.113.4.900. [DOI] [PubMed] [Google Scholar]
- 35.Stav D, Raz M, Shpirer I. Three years of pulmonary rehabilitation: inhibit the decline in airflow obstruction, improves exercise endurance time, and bodymass index, in chronic obstructive pulmonary disease. BMC Pulm Med. 2009;9:26. doi: 10.1186/1471-2466-9-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Steele BG, Belza B, Cain KC, Coppersmith J, Lakshminarayan S, Howard J, Haselkorn JK. A randomized clinical trial of an activity and exercise adherence intervention in chronic pulmonary disease. Arch Phys Med Rehabil. 2008;89:404–412. doi: 10.1016/j.apmr.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 37.Stewart AL, Hays RD, Ware JEJ. The MOS short-form general health survey. Reliability and validity in a patient population. Med Care. 1988;26:724–735. doi: 10.1097/00005650-198807000-00007. [DOI] [PubMed] [Google Scholar]
- 38.Stewart AL, Mills KM, King AC, Haskell WL, Gillis D, Ritter PL. CHAMPS physical activity questionnaire for older adults: outcomes for interventions. Med Sci Sports Exerc. 2001;33:1126–1141. doi: 10.1097/00005768-200107000-00010. [DOI] [PubMed] [Google Scholar]
- 39.Stewart AL, Mills KM, King AC, Haskell WL, Gillis D, Ritter PL. CHAMPS physical activity questionnaire for older adults: outcomes for interventions. Medicine and Science in Sports and Exercise. 2001;33:1126–1141. doi: 10.1097/00005768-200107000-00010. [DOI] [PubMed] [Google Scholar]
- 40.Stewart AL, Mills KM, Sepsis PG, King AC, McLellan BY, Roitz K, Ritter PL. Evaluation of CHAMPS, a physical activity promotion program for older adults. Ann Behav Med. 1997;19:353–361. doi: 10.1007/BF02895154. [DOI] [PubMed] [Google Scholar]
- 41.U.S.Department of Health and Human Services U.S, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion; Atlanta, GA: Physical Activity and Health: A Report of the Surgeon General. 1996;33
- 42.Wilcox S, Dowda M, Leviton LC, Bartlett-Prescott J, Bazzarre T, Campbell-Voytal K, Carpenter RA, Castro CM, Dowdy D, Dunn AL, Griffin SF, Guerra M, King AC, Ory MG, Rheaume C, Tobnick J, Wegley S. Active for life: final results from the translation of two physical activity programs. Am J Prev Med. 2008;35:340–351. doi: 10.1016/j.amepre.2008.07.001. [DOI] [PubMed] [Google Scholar]