Abstract
BACKGROUND AND PURPOSE
Collaterals may compensate for reduced blood flow in acute ischemic stroke, yet endurance and quality of collateral perfusion may vary. Collateral sustenance of penumbra may falter after initial recruitment, resulting in progressive ischemia and clinical deficits. Delayed collateral failure may extend the time window for revascularization, even after failed intravenous thrombolysis.
CASE DESCRIPTION
A 76-year-old woman returned to normal from National Institutes of Health Stroke Scale (NIHSS) score of 18 following intravenous thrombolysis, despite persistent occlusion of the left middle cerebral artery. Subsequent deterioration was successfully reversed with mechanical thrombectomy almost 14 hours after symptom onset.
CONCLUSIONS
Early clinical improvement or deterioration may reflect collateral perfusion, not necessarily recanalization or reocclusion. The definition of collateral failure must incorporate the expected role and endurance of collaterals. Further investigation of collateral pathophysiology may reveal predictive clinical or imaging features and disclose collateral therapeutic approaches to augment revascularization.
Keywords: Collaterals, MRI, angiography
Introduction
Dynamic clinical fluctuations may be observed following administration of intravenous tissue plasminogen activator (tPA) for acute ischemic stroke.1 Collateral perfusion may alter the extent of ischemic penumbra and dysfunctional parenchyma.2 Vascular correlates, including proximal arterial occlusion and compensatory collateral circulation, are not routinely monitored. The recent U.S. Food and Drug Administration (FDA) clearance of the Merci® Retriever System for endovascular thrombectomy in failed intravenous thrombolysis expands current treatment options, yet several aspects await clarification. The definition of failed intravenous thrombolysis remains unclear. Angiography may delineate collaterals and the vascular basis of early clinical fluctuations. We describe late mechanical thrombectomy with the Merci® device following failed intravenous tPA and explore the concept of collateral failure.
Case Description
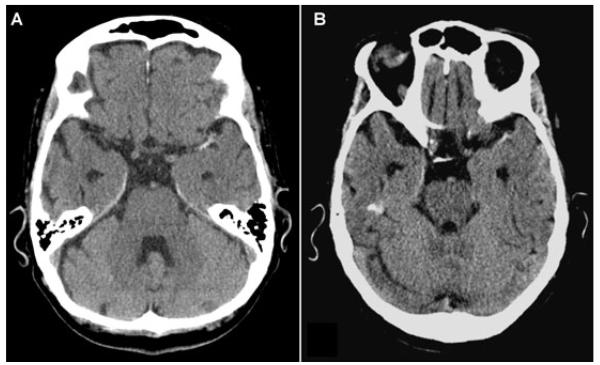
A 76-year-old woman with hypertension and peripheral vascular disease presented with acute right hemiparesis, right hemisensory loss, and aphasia. Noncontrast CT revealed left middle cerebral artery (MCA) hyperdensity (Fig 1 A). Intravenous tPA was started 2:07 from symptom onset. One hour after completion of tPA, NIHSS remained 18. Vigorous hydration and supine positioning for magnetic resonance imaging (MRI) led to complete resolution of clinical deficits (NIHSS 0 at 4:00). Scanner malfunction limited MRI acquisition, yet non-contrast CT showed persistent left MCA hyperdensity (Fig 1 B).
Fig 1.
Noncontrast CT pre- (A) and post- (B) intravenous thrombolysis reveals persistent left MCA hyperdensity.
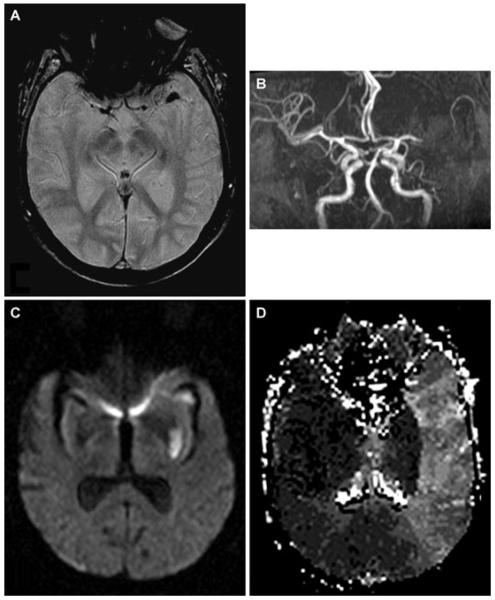
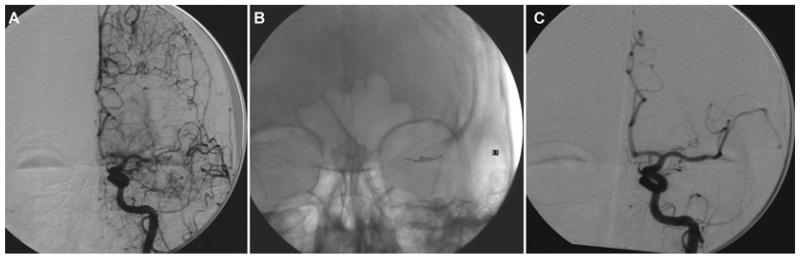
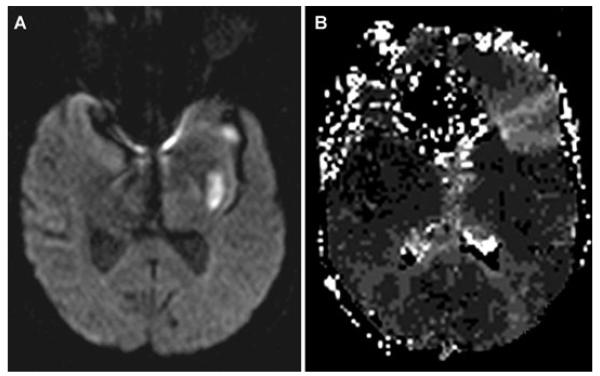
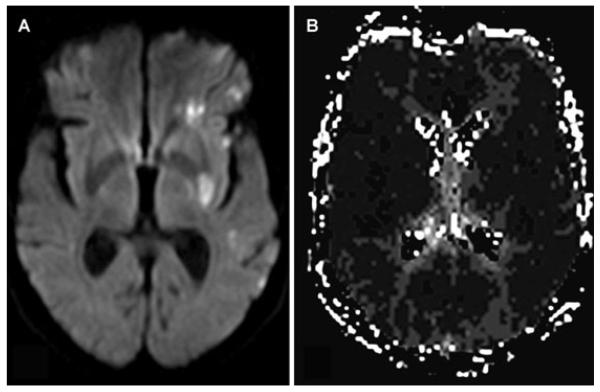
At 10:30, aphasia and right hemiparesis (NIHSS 8) reappeared despite no change in systemic hemodynamics or oxygenation. Emergent MRI/MRA revealed no hemorrhage, but persistent left MCA occlusion with extensive diffusion-perfusion mismatch (Fig 2). Immediate angiography demonstrated proximal left MCA occlusion with extensive retrograde leptomeningeal collateral perfusion (Fig 3 A). On second pass of the Merci® device (Fig 3 B), a 1.0 × 1.0 mm thrombus was withdrawn. After a third pass, a residual 3.0 × 1.0 mm thrombus was retrieved. Postthrombectomy angiography revealed M1 recanalization (Fig 3 C). Distal anterior division branch occlusion with collateral filling was also noted. Immediate resolution of hemiparesis with residual nonfluent aphasia corresponded to restricted inferior frontal hypoperfusion on postprocedural MRI at 3 hours (Fig 4 B).
Fig 2.
MRI/MRA after neurological deterioration demonstrates left MCA thrombus on gradient-echo images (A) and lack of antegrade flow on MRA (B). Subcortical ischemia in the lenticulostriate distribution on diffusion (C), with a larger extent of hypoperfusion on time-to-peak maps (D).
Fig 3.
Angiography demonstrates left MCA occlusion with retrograde leptomeningeal collateral filling (A). Clot retrieval with the Merci® device (B) results in M1 recanalization (C) at almost 14 hours.
Fig 4.
Postprocedural MRI 3 hours later reveals prior lenticulostriate infarction (A) with limited hypoperfusion in left inferior frontal region (B) corresponding to branch occlusion on angiography.
Residual isolated aphasia resolved over 2 days. Day 7 MRI revealed scattered diffusion lesions without hypoperfusion (Fig 5). Anticoagulation for previously undocumented atrial fibrillation was initiated prior to discharge home.
Fig 5.
Day 7 MRI demonstrates scattered diffusion lesions (A) without residual hypoperfusion (B).
Discussion
Collateral circulation mitigates ischemia induced by arterial occlusion, determining mismatch between established infarction and larger areas of hypoperfusion.2 Endurance of these alternative routes may define the therapeutic window for successful revascularization and influence the safety and efficacy of thrombolysis. Collateral circulation is an important variable in the clinical outcome of thrombolysis,3 but collateral persistence is erratic. Temporary sustenance of the ischemic territory is frequently noted on angiography and collateral failure is often invoked as the cause of subacute infarct evolution. Clinical predictors of collateral capacity or endurance remain unclear. Collateral failure is rarely defined by serial angiography and the pathophysiology of retrograde leptomeningeal blood flow remains obscure. Retrograde leptomeningeal collateral flow may temporarily maintain penumbra, yet such routes may fail to completely replace antegrade perfusion. The definition of collateral failure may therefore depend on the anticipated endurance or expected role of collaterals.
Dramatic resolution of initial neurological deficits and remarkable clinical response to late mechanical thrombectomy may be ascribed to exceptional collateral blood flow, whereas collateral failure may be responsible for deterioration following initial improvement. Exuberant collaterals may not have been expected given proximal embolic occlusion associated with atrial fibrillation, yet these collaterals permitted successful revascularization almost 14 hours after onset. Revascularization at 14 hours after onset is not ideal, as potential risk may increase during this delay. It should also be noted that use of the Merci® is generally limited to an 8-hour window. The decision to revascularize a patient, however, must always balance risk and benefit. In such a case with marked clinical improvement and the potential risks of endovascular complications, the balance may favor conservative treatment unless deterioration ensues. The basis for eventual failure of these extensive collaterals also remains unclear. Even following mechanical thrombectomy and distal branch occlusion, a similar pattern of initial collateral support and eventual collateral diminution was noted. Collateral sustenance and subsequent failure may parallel conventional roles of recanalization and reocclusion in explaining dynamic clinical fluctuations following intravenous tPA. Prior to recent FDA clearance of mechanical thrombectomy, such pathophysiologic events were irrelevant in clinical practice.
In the post-Merci® approval era, further revascularization may follow intravenous tPA failure. Clinical improvement may result from collateral perfusion, not successful thrombolysis. Transient recanalization and subsequent reocclusion seem unlikely with persistent CT hyperdensity of the MCA. The clinical response following intravenous tPA was used to determine further therapy, although collateral failure may not have been predicted. More reliable assessment of thrombolytic response in the future may utilize angiographic techniques. Vessel patency at a prespecified time point may be a practical outcome measure.4 Timing is likely a critical factor in determining intravenous tPA failure. The recommended dose and adequate time interval following thrombolytic infusion must be allowed to establish lack of thrombolytic effect.4 Imaging mismatch may also be used as a surrogate for collateral flow, possibly portending eventual collateral failure. Early infarct volume may not predict subsequent clinical events.
Consideration of collateral perfusion during early phases of acute stroke will require intensive imaging.2 Noninvasive studies may be corroborated with angiographic collaterals, yet standard, objective scales must be employed. Correlative studies may investigate clinical features of collateral dependence and collateral failure. Possible determinants of collateral failure, including systemic blood pressure or intravascular deoxygenation of retrograde blood flow,5 may also be elucidated.
Further evaluation of collaterals may also yield options to augment revascularization. Collateral variability and time-dependent discrepancies in mismatch may be defined. Although MRI studies are starting to explore vascular correlates of intravenous thrombolysis,6 collateral routes have been neglected. Collateral therapeutics, including hemodynamic interventions and oxygenation, may augment perfusion or extend the time window for revascularization.7 Most neuroprotective and thrombolytic trials failed to consider these potential confounding factors.8,9 Attention to physiological parameters in this case may have promoted early clinical improvement and facilitated late thrombectomy. Such potentially beneficial effects may be transient, however. Hemodynamic factors (eg induced hypertension) may also be limited with thrombolysis. Collateral therapeutics may maintain penumbral viability only until revascularization is achieved, although it is paradoxical to term this collateral failure.
Our case illustrates the integral and complex role of collaterals in acute ischemic stroke. Marked clinical fluctuations may reflect collateral perfusion, not necessarily recanalization or reocclusion. Mechanical thrombectomy for failed intravenous thrombolysis will require consideration of early thrombolytic outcome measures such as angiographic patency and perfusion. Detailed vascular imaging will clarify determinants of collateral perfusion, including the entity of collateral failure.
References
- 1.Grotta JC, Welch KM, Fagan SC, Lu M, Frankel MR, Brott T, Levine SR, Lyden PD. Clinical deterioration following improvement in the NINDS rt-PA Stroke Trial. Stroke. 2001;32:661–668. doi: 10.1161/01.str.32.3.661. [DOI] [PubMed] [Google Scholar]
- 2.Liebeskind DS. Collateral circulation. Stroke. 2003;34:2279–2284. doi: 10.1161/01.STR.0000086465.41263.06. [DOI] [PubMed] [Google Scholar]
- 3.Higashida RT, Furlan AJ, Roberts H, Tomsick T, Connors B, Barr J, Dillon W, Warach S, Broderick J, Tilley B, Sacks D. Trial design and reporting standards for intra-arterial cerebral thrombolysis for acute ischemic stroke. Stroke. 2003;34:e109–e137. doi: 10.1161/01.STR.0000082721.62796.09. [DOI] [PubMed] [Google Scholar]
- 4.Molina CA. Editorial comment–degree of arterial recanalization: an end point for efficacy in future intravenous thrombolytic trials. Stroke. 2004;35:114–115. doi: 10.1161/01.STR.0000110121.57772.18. [DOI] [PubMed] [Google Scholar]
- 5.Liebeskind DS, Ances BM, Weigele JB, Hurst RW. Intravascular deoxygenation of leptomeningeal collaterals detected with gradient-echo MRI. Stroke. 2004;35:266. [Google Scholar]
- 6.Derex L, Nighoghossian N, Hermier M, Adeleine P, Berthezene Y, Philippeau F, Honnorat J, Froment JC, Trouillas P. Influence of pretreatment MRI parameters on clinical outcome, recanalization and infarct size in 49 stroke patients treated by intravenous tissue plasminogen activator. J Neurol Sci. 2004;225:3–9. doi: 10.1016/j.jns.2004.05.020. [DOI] [PubMed] [Google Scholar]
- 7.Liebeskind DS. Collateral therapeutics for cerebral ischemia. Expert Rev Neurotherapeutics. 2004;4:255–265. doi: 10.1586/14737175.4.2.255. [DOI] [PubMed] [Google Scholar]
- 8.Liebeskind DS. Neuroprotection from the collateral perspective. IDrugs. 2005;8:222–228. [PubMed] [Google Scholar]
- 9.Weir CJ, Kaste M, Lees KR. Targeting neuroprotection clinical trials to ischemic stroke patients with potential to benefit from therapy. Stroke. 2004;35:2111–2116. doi: 10.1161/01.STR.0000136556.34438.b3. [DOI] [PubMed] [Google Scholar]