Abstract
Purpose
Current rabbit stroke models often depend on symptoms as endpoints for embolization and produce wide variation in location, size, and severity of strokes. To further refine our angiographic embolic stroke model we correlated localized infarctions to neurological deficits. Our goal is a rabbit model for long term studies of therapies after stroke.
Materials and Methods
New Zealand White rabbits (4–5 kg) (n=71) had selective internal carotid artery (ICA) angiography and a single clot was injected. At 24 hours neurological assessment scores (NAS) were measured on a 0=normal to 10=dead scale. Brains were removed and stained to identify stroke areas. All animals with single strokes, N=31, were analyzed by specific brain structure involvement and NAS values were correlated.
Results
Stroke incidence differed by location with cortex, subcortical, and basal ganglia regions highest. Distributions of middle cerebral artery (MCA) at 52% and anterior cerebral artery (ACA) at 29% were most commonly involved with largest stroke volumes in the ACA distribution. Brain stem and cerebellum strokes had disproportionately severe neurological deficits, scoring 2.25±1.0 vs. cortex (0.5±0.2), subcortical (1.3±0.4) and basal ganglia (0.5±0.3) all in the frontal or parietal regions on NAS (P≤0.02).
Conclusions
MCA and ACA distributions included 81% of strokes. These sites were relatively silent (potentially allowing longer term survival studies) while others in the posterior circulation produced disproportionately severe symptoms. Symptoms were not reliable indicators of stroke occurrence and other endpoints such as imaging may be required. These are important steps towards refinement of the rabbit stroke model.
Keywords: Embolic Stroke, Brain, Rabbit, Animal Models, Neurological Deficit
INTRODUCTION
Stroke is the third-leading cause of death in the United States. Mortality rates depend greatly on the type of stroke and anatomic location with brain stem strokes being the most devastating (1). The percentage of stroke survivors returning to work can vary from 20 to 70% (2–6). This large variation in functional neurological recovery depends on the anatomic location of the stroke in addition to the volume of infarction and presence of hemorrhage.
Human cerebral vascular patterns usually supply discrete regions of brain. They include numerous anatomical variations but are generally consistent so that specific occlusions give rise to distinct symptom patterns. These parameters of stroke location and outcome have been extensively examined clinically in humans (7–10).
Animal models of ischemic stroke have usually failed to successfully transition into human clinical practice. This is especially the case with mouse and rat models which have been the most commonly used models but have never made a successful transition into human application (11). Larger animals apparently are required, but the selection is limited by other factors including unfavorable anatomy. The rabbit model is the one exception to this and has been successful in tPA therapy development (12–15) leading to tPA as the standard of care in human stroke. However, most rabbit models show wide variability in the strokes thus limiting precision. They use relatively short survival of a few hours to two days while deaths and severe symptoms limit longer term studies (16–19).
Adult New Zealand rabbits are large enough to provide adequate arterial detail to mimic human anatomy. A modified technique (20–22) of angiographic selection of the internal carotid artery (ICA) through femoral artery access with subsequent single clot embolization allowed us to produce similar strokes in a series of rabbits with a low death rate. At 24 hours we examined functional deficits, incidence, and anatomical stroke location. This allows the study of stroke location and its relation to neurological function deficits.
This is an important step towards refinement and further validation of the animal model and can lead to its future use in long term comparison of new therapies.
MATERIALS AND METHODS
Animals
New Zealand White rabbits (4–5 kg) (n=71) had selective angiography of the ICA. Of the 71 animals, only n=31 produced single strokes and thus were utilized for this study described as follows. Rabbits were gavaged with aspirin (10 mg/kg) the day of the procedure then anesthetized with ketamine (35 mg/kg body weight) and xylazine (5mg/Kg). Anesthesia was maintained with mask ventilation using isoflurane at 1–1.5% maintenance. All animals received care in compliance with the American Association for Laboratory Animal Science. Approvals for these studies were obtained from the Institutional Animal Care and Use Committee.
Embolization Procedures
Clot was produced using autologous blood drawn 3–6 hours before embolization which was allowed to clot in 1 mm internal diameter tubing. An angiographic 3F angled tip catheter (SlipCath, Cook, Bloomington IN) was advanced to the common carotid artery from a femoral artery approach. Magnification angiography visualized the major branches including the ICA (Fig 1). The ICA was then selected for further magnification angiography and embolization (10,20,21). A single red autologous clot (0.6×0.6×1.0 mm after shrinkage) was injected with steady flush of 2 cc of saline over 10–15 seconds in all animals. This type of clot is pliable, very smooth, and routinely passes intact through the 3F catheter. All embolizations were performed by the same individual to minimize variability in technique. Angiography was then repeated at 1 and 30 minutes post embolization. The catheter was removed and the femoral artery ligated. Rabbits were recovered and neurological assessment was scored on the following day.
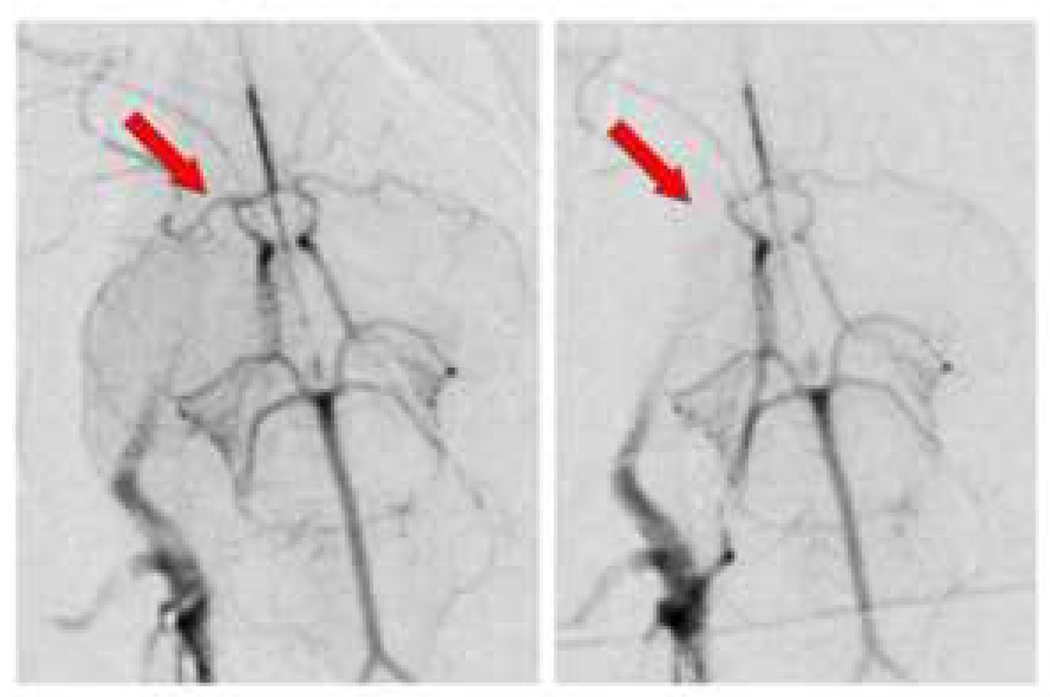
Figure 1. Rabbit angiographic stroke model.
ICA sub-selective angiogram (left panel) and follow up angiogram after embolus delivery (right panel); note MCA and occlusion of MCA (arrows).
Therapeutics
Four test groups included a control and three therapeutic groups. Animals from all groups were included in the localization study. Treatments did not influence stroke location. Therapeutic results will be reported separately.
Functional Evaluations
At 24 hours all animals were functionally evaluated using the rabbit Wryneck Evaluation (Neurological Assessment Score [NAS]). Scores are determined by evaluation of neurological signs. These signs include tests for torsion of the neck, righting reflexes, paw flex tests, circling tests and postural reflex. The range is from normal at 0 to 9 with death at 10. The Wryneck Evaluation has been successfully used previously in the rabbit (23–26).
Identification of Stroke Area
Twenty-four hours after embolization, animals were euthanized and brains were carefully removed to preserve morphology. After one hour in ice cold saline they were placed in a Harvard Apparatus Coronal matrix device for sectioning into precise 4 mm sections from the anterior olfactory bulb to the caudal face of the cerebellum. The sections were incubated in 1% triphenyltetrazolium chloride (TTC) (Sigma, St. Louis, MO) at 37°C, for approximately 1 hour. The caudal face of each section was digitally photographed and measured using the NIH Image J Software, Version 0.99i (Fig 2). Structures with infarction were identified, measured and recorded. Infarct volume was computed as a percent of the total volume of the brain. The tissue sections were then placed in formalin for 2–3 days at 4°C and histological examination performed.
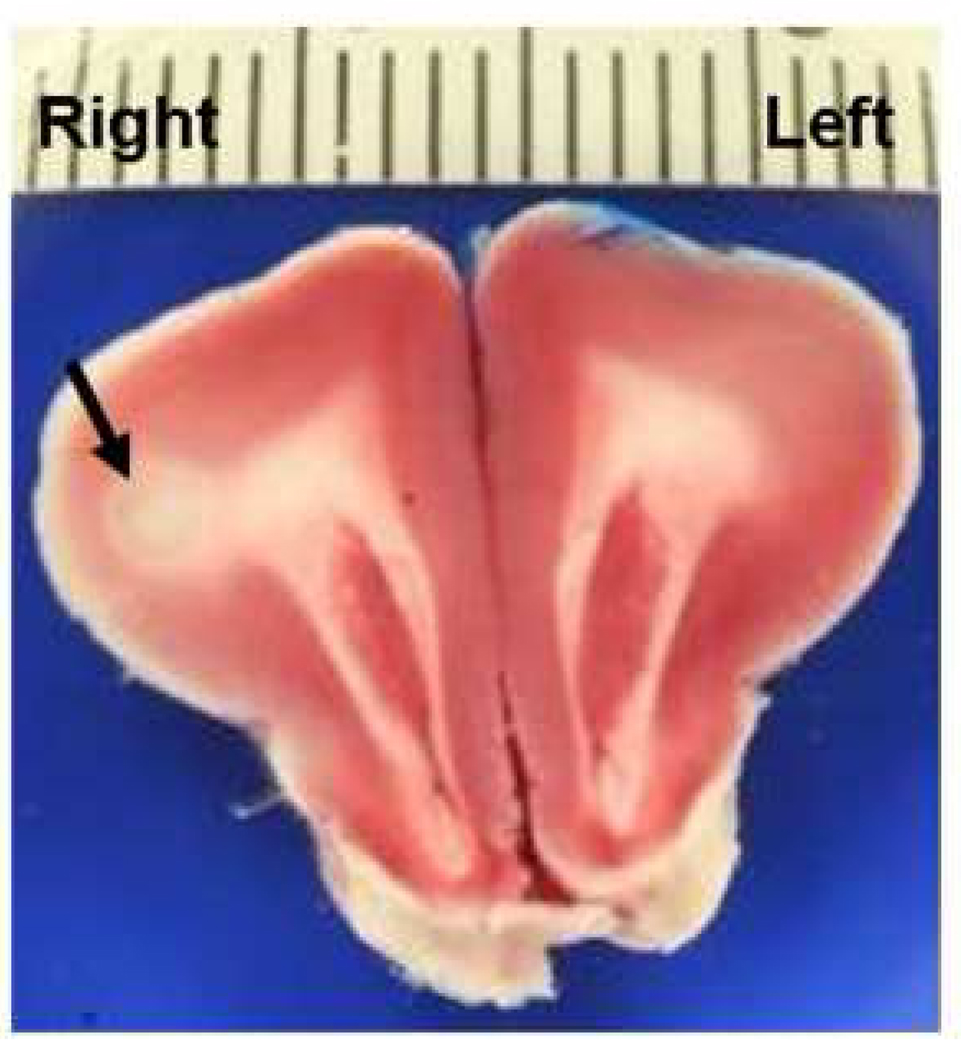
Figure 2. Representative Illustration of Stroke Area on TTC.
The light staining stroke area in the right frontal cortex and subcortical white matter (arrow) was associated with an NAS score of 5. Bar scale represents mm increments.
Singular Strokes
Those cases that exhibited a definite single infarct on TTC stained sections were chosen for correlative functional analysis. This was to minimize the neurological effects of positive infarcted structures.
Anatomical Location Identifiers
Anatomic locations of stroke areas were identified using an atlas of the rabbit brain (27). The list of anatomic structures with infarction is given by region in Table 1. Table 2 associates structures with appropriate arterial supplies.
Table 1.
Anatomical Structures with Stroke
| I. Cortex | II. Subcortical | III. Basal ganglia |
|---|---|---|
| Frontal cortex | Corpus callosum | Caudate nucleus |
| Parietal cortex | Corona radiate* | Tail of caudate nucleus* |
| Temporal cortex* | Internal capsule | Putamen* |
| Occipital cortex* | Hippocampus dorsal | Globus pallidus* |
| Olfactory tubercle | Hippocampus ventral | Claustrum |
| Cingulate cortex* | Amygdala | |
| IV. Diencephalon | V. Brain stem + cerebellum | |
| Thalamus | Red nucleus* | |
| Hypothalamus | Superior colliculus | |
| Inferior colliculus* | ||
| Cerebellum | ||
| Pons* |
Anatomical structures that had identifiable areas of stroke are listed.
The asterisks indicate sites seen only in animals with multiple strokes.
Table 2.
Structures in Arterial Distribution
| I. Anterior Cerebral Art | II. Middle Cerebral Art | III. Posterior Cerebral Art |
|---|---|---|
| Frontal cortex | Tail of caudate* | Occipital cortex* |
| Olfactory tubercle | Caudate nucleus | Thalamus |
| Hypothalamus (anterior)* | Internal capsule | Hypothalamus (posterior) |
| Corpus callosum | Putamen* | Crus cerebri* |
| Cingular cortex* | Claustrum | Red nucleus* |
| Parietal Cortex | Superior colliculus | |
| Hippocampus dorsal | Inferior colliculus* | |
| Hippocampus ventral | ||
| Temporal cortex* | ||
| Globus pallidus* | IV. Basilar Art | |
| Amygdala | Cerebellum | |
| Pons* |
All structures with infarcts are associated with the appropriate major blood supply.
The asterisks indicate sites seen only in animals with multiple strokes.
Brain Histology
Brain sections were processed, paraffin embedded, and sectioned at 6 µm for histochemical and immunohistochemical staining. To corroborate non-TTC stained areas as infarct, hematoxylin and eosin (H&E) stained sections were examined by a veterinary pathologist in a blinded fashion.
Statistical Analysis
Statistical analysis was performed using analysis of variance (ANOVA) and the Chi square test compared stroke occurrence by region and its trends.
RESULTS
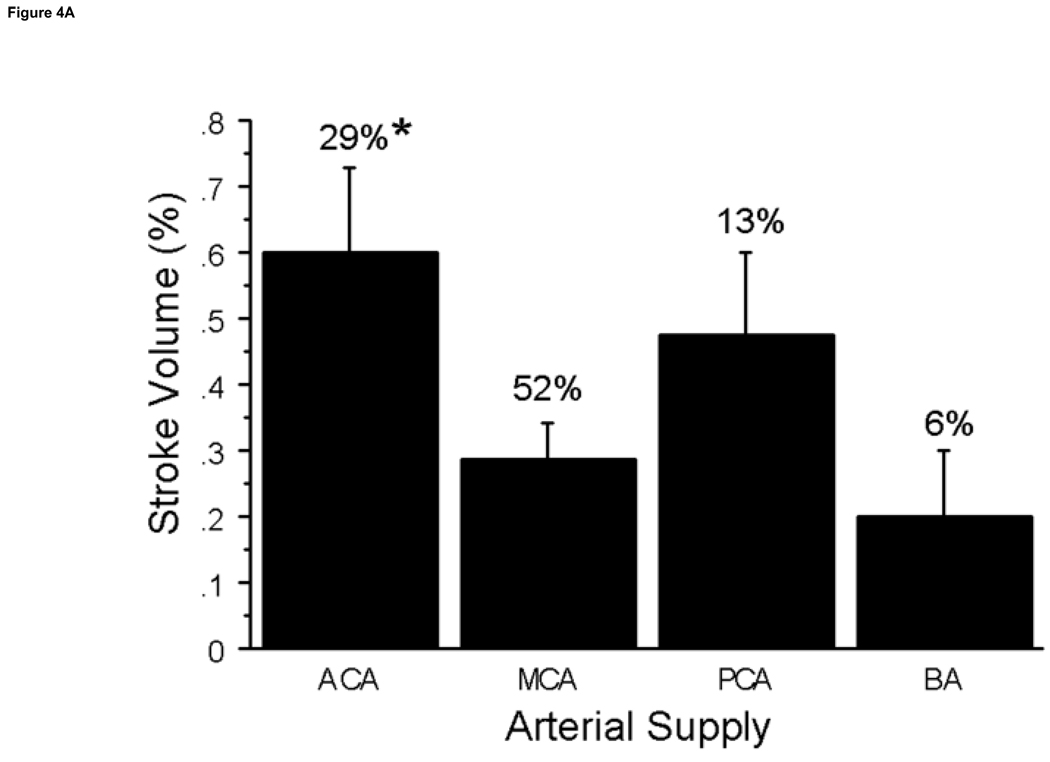
All post-embolization rabbits had angiographic evidence of clot embolus delivery to the internal carotid artery (Fig 1). All but one of 71 survived 24 hours. Among the 31 animals with a single stroke, the observable stroke incidence was significantly different (P=0.03) between the cortex, subcortical, and basal ganglia regions, 81%, compared to the diencephalon and brain stem + cerebellum areas, 19%. The greatest percentage of strokes occurred in the cortex (39%) followed by the subcortical area (29%), basal ganglia (13%), brain stem (13%) and diencephalon (6%). The structures involved for each region are listed in Table 1. Strokes were unilateral and on the targeted side in 87% of animals. Contralateral strokes included the olfactory tubercle which was served bilaterally by the targeted anterior cerebral artery (ACA), one cerebellum stroke served by the basilar artery, and one in the thalamus and one in the hippocampus which were not explained by arterial details. The middle cerebral artery (MCA) and ACA (Table 2) were associated with the highest number of stroke observations at 52% and 29% (Fig 3a). By Chi square analysis at P≤0.0001 these distributions were significantly different from other arteries. Mean stroke volumes were 0.60%±0.1% in the ACA, 0.29±0.06% in the MCA, 0.48%±0.1% in the posterior cerebral artery, and 0.20%±0.1% in the basilar artery distributions (Fig 3b).
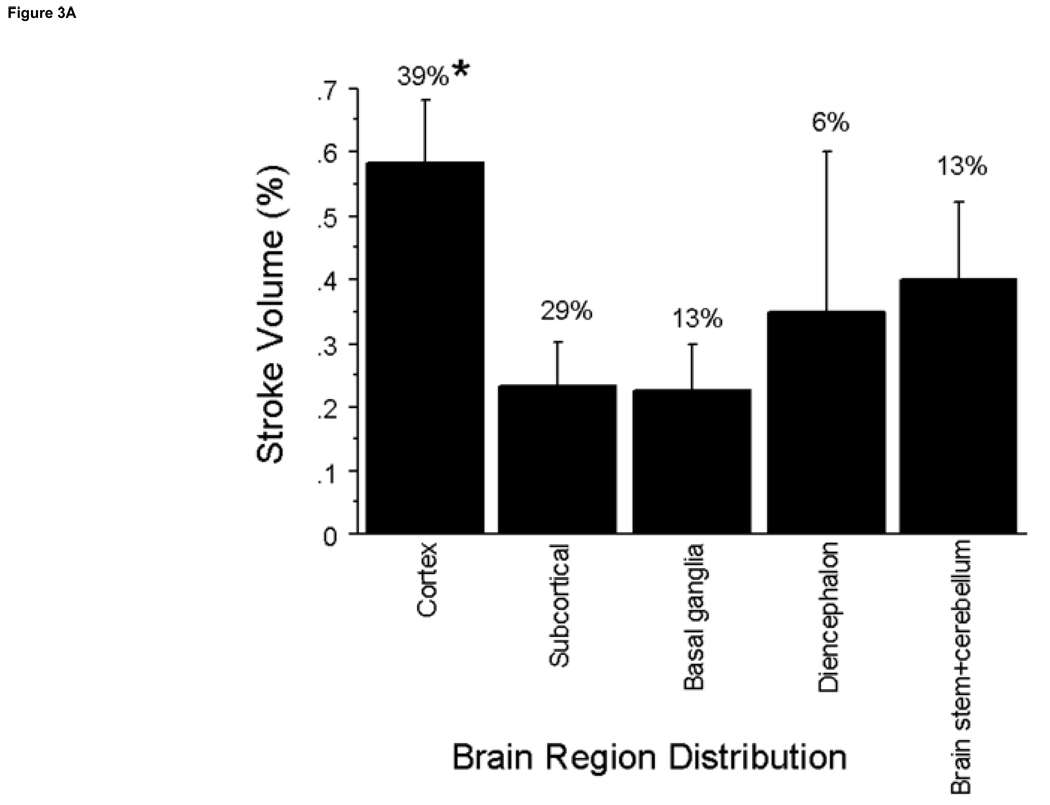
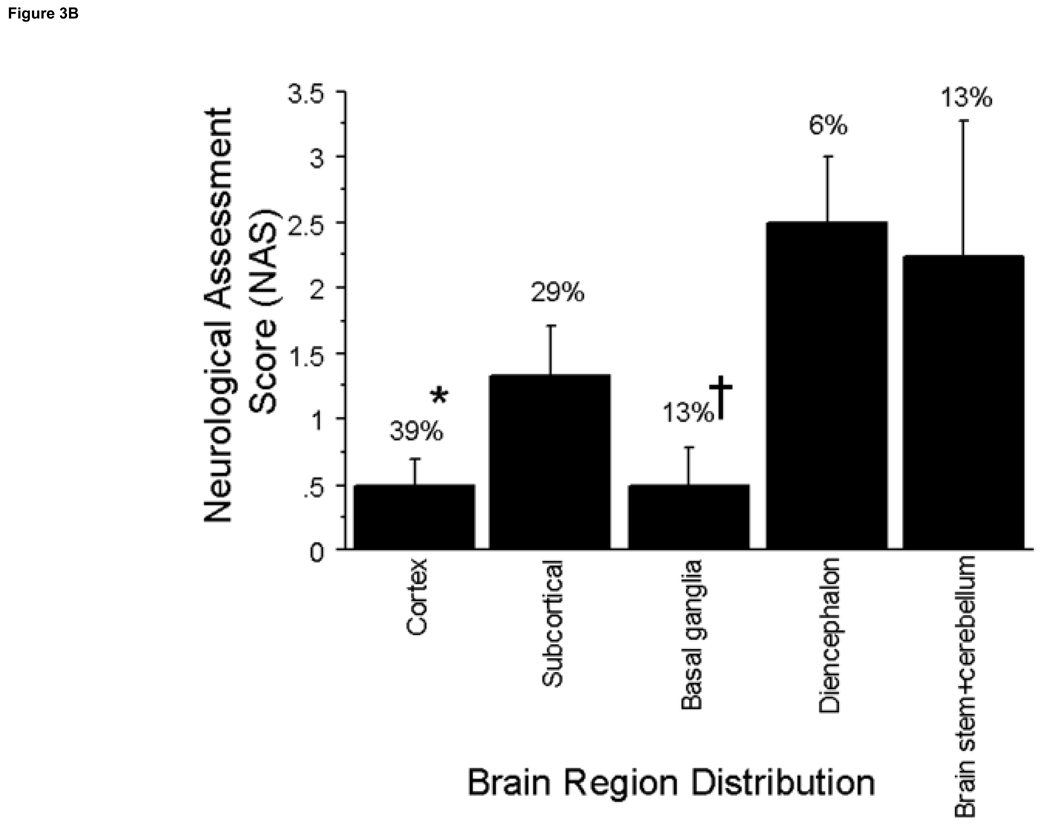
Figure 3. Regional Distribution of Stroke Volume and NAS.
A. The incidence of stroke in brain regions was determined. ANOVA analysis indicates significant differences between stroke volumes in the cortex compared to the subcortical, and basal ganglia regions at (P≤0.03) with the greatest volume and percent distribution occurring in the cortex. B. The brain regions with the highest incidence of neurological deficits were the cortex and subcortical regions. The brain regions with the highest neurological deficits were the diencephalon and the brainstem + cerebellum which were significantly higher in neurological deficits vs. the cortex at *P≤0.02 and vs. the basal ganglia at †P≤0.04.
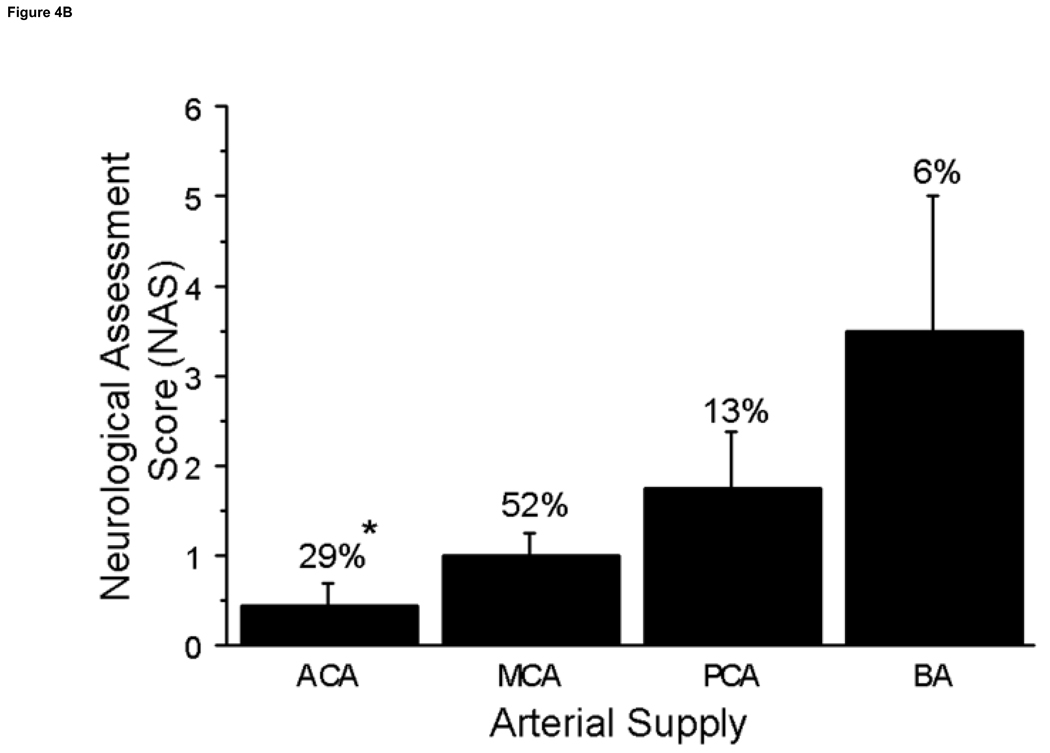
Neurological deficits (NAS values) were significantly different according to brain region involved (p=0.02) (Fig 4a). Although the cerebral cortex had the highest percentage of stroke incidence at 39%, it was among the lowest in NAS (0.5± 0.2). The brain regions that produced the highest NAS values were the diencephalon and brain stem + cerebellum (2.5±0.5 and 2.25±1.0, respectively). Strokes in the brain stem + cerebellum produced increased neurological deficits compared to the cerebral cortex, 0.5±0.2, subcortical, 1.3±0.4, and basal ganglia regions, 0.5 ±0.3 (P≤0.02); strokes in the diencephalon produced increased neurological deficits compared to the cortex and basal ganglia regions (P≤0.04). Some brain structures with high incidence of stroke, including the corpus callosum and olfactory tubercle, produced no neurological deficits (0 NAS values). Neurological deficits (NAS values) were significantly increased in the posterior cerebral artery (PCA) and basilar artery (BA) distributions compared to the ACA (p<0.04) and the BA compared to the MCA distribution (P=0.003) (Fig 4b).
Figure 4. Arterial Supply of Stroke Volume and NAS.
A. The incidence of stroke in the perfusion territories of the major cerebral arteries was determined with the greatest percentage occurrence in the MCA at 52%. Whereas the ACA had a lower incidence of stroke, it also had the greater stroke volume compared to the MCA territory at *P=0.01. B. The NAS are presented by arterial territory and percent of stroke incidence. The arterial territories with the greatest incidence were the ACA and MCA however NAS values were very low. The PCA and BA had the higher NAS values vs. the ACA at *P≤0.04. Abbreviations: Anterior Cerebral Artery (ACA), Middle Cerebral Artery (MCA), Posterior Cerebral Artery (PCA) and Basilar Artery (BA).
DISCUSSION
Findings in animal models of ischemic stroke usually fail in transition to clinical application into human use (11). Mice and rats have been very disappointing, but results from a rabbit model for evaluating acute stroke and stroke therapies have successfully transitioned into human therapeutic use (12,15). Current standard care with tPA is based on this work (12). Long term rabbit outcomes have not been studied in depth, although recovery after several months is an important goal in human studies. While some rabbit cerebral embolic models do not recover the animals in order to evaluate neurological function and instead rely on histological findings for neurological outcome (16–19), others do survive animals for up to 48 hours but rely on a simple dichotomous rating system of normal vs neurological abnormality or one including death (12,28). Published methods of evaluating neurological function in rabbits are limited. However the Wryneck test has been used to evaluate neurological function in several publications and may provide for more objective detection and a wider range scale for neurological deficit measurement rather than a dichotomous determination (23–26).
This study utilized a single clot embolus procedure to determine the incidence of infarct in specific anatomical structures. We compared functional differences after recovery from anesthetic and with passage of time similar to that used to define a transient ischemic attack in humans. Normal physiologic thrombolysis and collateral vessel recruitment to supply ischemic sites are thought to contribute to improvement in transient ischemic attacks. Without animal recovery and neurological assessment following embolization, evaluation of potential therapies can’t include these factors and is thus limited.
Though they did not functionally evaluate the animals following embolization, Kirchhof et al (22), noted that very small red thrombi selectively placed in the distal portions of the ICA could impact the basal ganglia and kill rabbits in <6 hours. They discarded rabbits with MCA duplication. While we did not discard them in our study, we found that the rate of occurrence was 29% in our rabbits, a potentially high failure rate. Clot composition may have determined the impact of hemorrhage in these infarcts with red clot being most detrimental to the animal. Our emboli were uniform single red clots with a smooth cylindrical shape, a carefully measured length, and of pliable consistency that could deform during transit through the catheter and emerge intact.
Another recent report used very delicate flow directed microcatheters to subselect the MCA where thrombin was used to induce clotting at that location and well beyond the catheter tip (29). The catheter used was too small to allow injection of solid clots of the size we used. This raised questions of appropriate nature of the induced clot, its extensive distal branch thrombosis which may impede physiological collateral formation, and the high cost of each experiment (30).
The most successful standard techniques have used either a shower of small emboli or a series of single large clot fragments injected through a catheter into a surgically isolated segment of the distal common carotid artery and flushed into the ICA and the Circle of Willis. The endpoint for embolization in these studies (31–33) is the appearance of any neurological deficit in the conscious animal. These investigators report no system of measuring clinical stroke severity, simply the presence or absence of any of several findings. Infarcts were often found to be bilateral (31–33).
The technique herein delivers formed clot similar in size to the MCA to the ICA for flow directed travel to the brain. This is similar to the human condition and similar to some of the animal models. The angiographic findings routinely show MCA and ACA occlusions from the clot. The angiographic technique does not occlude the common carotid artery so inflow is preserved in this model. In the surgical model compromised inflow and thrombus formation in the surgically isolated common carotid segment could further cloud the issue. The variation in anatomical structures involved with observed infarct indicates that this is a complex process including factors such as congenital vascular variations, flow patterns on injection, and the presence of collateral blood flow and autolysis of clot. In our study it is of interest that more than one focus of ischemic stroke was found in most animals, usually in the same arterial distribution suggesting collaterals competing to supply the ischemic area or fragmentation of the clot and distal embolization causing reduced areas of actual infarct. A few animals with remote embolization to the posterior circulation or even to the other side of the brain probably represent the effect of total MCA and ACA occlusion with a fragment of clot embolizing elsewhere due to redirected blood flow. Over vigorous flushing to deliver clot or follow up angiography could also have caused this delivery to non-target sites. The common duplication of the MCA could also have contributed to this problem since the vessels might be too small to accommodate all of the clot volume, thereby sending it elsewhere. These processes could have great clinical impact when non-silent portions of the brain, the brainstem or other fragile areas of the posterior circulation, were the sites of eventual ischemia.
This model shows 81% of histological strokes occur in the MCA and ACA distribution, with 87% on the anticipated ipsilateral side confirming the angiographic appearance, and proving the targeting of the modified technique (Fig 3). This may be more uniform in localization than small fragment embolization, constitutes a proximal occlusion instead of tiny distal branch occlusions, and is very different from the shower of bilateral infarcts produced in that model (34). Posterior cerebral artery and basilar artery strokes were very uncommon in this study, but were common with showers of small clots, or even large clot, techniques. They were associated with the higher NAS values (Fig 4).
In an attempt to duplicate earlier publications, we performed cut downs on external carotid arteries and injected contrast into the common carotid artery through a short catheter. These few preliminary animals were not included in this report. The prescribed volumes of up to 6 cc caused massive reflux into the aortic arch even when the proximal carotid had been ligated, due to flow through the Circle of Willis and vertebral arteries. Without common carotid ligation, it was more pronounced. These surgical common carotid injection techniques could often send emboli to the posterior circulation and thereby cause more symptoms than limited angiographic injections which produced proportionally more anterior circulation filling. This explains the bilateral strokes often seen in those studies (34).
In humans, the location of the embolus impacts recovery and functional outcomes for months and years following stroke occurrence (7–10,35). Several studies have noted compensation of non-stroked areas following infarcts in some cortical regions; whereas, motor function is often severely impacted without as much compensation (9). A long term animal model would be of value in studying functional recovery with therapies.
Limitations
The cortex in this study had the highest stroke incidence at 39% compared to other brain regions but was not associated with increased neurological deficit. This, in part, may reflect the absence of measurement of higher cortical functions as assessed by the NAS test. The subcortical and basal ganglia brain regions also had high incidence of stroke occurrence and were also not associated with increased NAS scores. However, the diencephalon and brain stem + cerebellum were low in incidence but very high in NAS values, a pattern reflecting the difficulty in using neurological defects alone as an end point for embolization. Other measurements of stroke such as infarct volumes measured on MRI or post mortem sections may prove more sensitive and appropriate in analysis of stroke therapies.
The inclusion of animals with therapies may have obscured some signs, however the distribution of lesions was similar in all groups. Clot lysis, either by therapy or autolysis, is certainly one of the important factors in stroke outcomes, therefore we are continuing this study with insoluble emboli to exclude that possibility. Another limitation is that very small strokes may be missed between surfaces of the 4mm thick sections. We are performing MRI studies to determine if this is a significant risk.
Summary
In summary, the presence of neurologic deficits relates best to diencephalon, and brain stem + cerebellum strokes but not as well to cortex, subcortical or basal ganglia lesions. The low level of symptoms with MCA and ACA lesions using this model makes long term survival studies a realistic goal. This also more closely mimics the clinical situation and is an important step towards refinement of the animal model for future transitional research.
Acknowledgements
None
Funding: This work was supported by a grant from the National Institutes of Health, NIH R01HL82481.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented at the 34th Annual Society of Interventional Radiology Meeting, March 2009, San Diego, CA
REFERENCES
- 1.Rosamond WD, Folsom AR, Chambless LE, et al. Stroke incidence and survival among middle-aged adults: 9-year follow-up of the Atherosclerosis Risk in Communities (ARIC) cohort. Stroke. 1999;30:736–743. doi: 10.1161/01.str.30.4.736. [DOI] [PubMed] [Google Scholar]
- 2.Duncan PW, Zorowitz R, Bates B, et al. Management of adult stroke rehabilitation care a clinical practice guideline. Stroke. 2005;36:e100–e143. doi: 10.1161/01.STR.0000180861.54180.FF. [DOI] [PubMed] [Google Scholar]
- 3.Executive Committee for the Asymptomatic Carotid Atherosclerosis Study. Endarterectomy for asymptomatic carotid artery stenosis. JAMA. 1995;273:1421–1428. [PubMed] [Google Scholar]
- 4.Biller J, Feinberg WM, Castaldo JE, et al. Guidelines for carotid endarterectomy: a statement for healthcare professionals from a Special Writing Group of the Stroke Council, American Heart Association. Circulation. 1998;97:501–509. doi: 10.1161/01.cir.97.5.501. [DOI] [PubMed] [Google Scholar]
- 5.North American Symptomatic Carotid Endarterectomy Trial Collaborators. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N Engl J Med. 1991;325:445–453. doi: 10.1056/NEJM199108153250701. [DOI] [PubMed] [Google Scholar]
- 6.Randomised trial of endarterectomy for recently symptomatic carotid stenosis: final results of the MRC European Carotid Surgery Trial (ECST) Lancet. 1998;351:1379–1387. [PubMed] [Google Scholar]
- 7.Chen CL, Tang FT, Chen HC, Chung CY, Wong MK. Brain lesion size and location: effects on motor recovery and functional outcome in stroke patients. Arch Phys Med Rehabil. 2000;81:447–452. doi: 10.1053/mr.2000.3837. [DOI] [PubMed] [Google Scholar]
- 8.Feydy A, Carlier R, Roby-Brami A, et al. Longitudinal study of motor recovery after stroke: recruitment and focusing of brain activation. Stroke. 2002;33:1610–1617. doi: 10.1161/01.str.0000017100.68294.52. [DOI] [PubMed] [Google Scholar]
- 9.Shelton FN, Reding MJ. Effect of lesion location on upper limb motor recovery after stroke. Stroke. 2001;32:107–112. doi: 10.1161/01.str.32.1.107. [DOI] [PubMed] [Google Scholar]
- 10.Fries W, Danek A, Scheidtmann K, Hamburger C. Motor recovery following capsular stroke. Role of descending pathways from multiple motor areas. Brain. 1993;116:369–382. doi: 10.1093/brain/116.2.369. [DOI] [PubMed] [Google Scholar]
- 11.Donnan GA. The 2007 Feinberg Lecture: A New Road Map for Neuroprotection. Stroke. 2008;39:242–248. doi: 10.1161/STROKEAHA.107.493296. [DOI] [PubMed] [Google Scholar]
- 12.Zivin JA, Fisher M, DeGirolami U, Hemenway CC, Stashak JA. Tissue Plasminogen Activator Reduces Neurological Damage After Cerebral Embolism. Science. 1985;230:1289–1292. doi: 10.1126/science.3934754. [DOI] [PubMed] [Google Scholar]
- 13.Bednar MM, Gross CE, Russell SR, Short D, Giclas PC. Activation of complement by tissue plasminogen activator, but not acute cerebral ischemia, in a rabbit model of thromboembolic stroke. J Neurosurg. 1997;86:139–142. doi: 10.3171/jns.1997.86.1.0139. [DOI] [PubMed] [Google Scholar]
- 14.Hamilton MG, Lee JS, Cummings PJ, Zabramski JM. A comparison of intra-arterial and intravenous tissue-type plasminogen activator on autologous arterial emboli in the cerebral circulation of rabbits. Stroke. 1994;25:651–655. doi: 10.1161/01.str.25.3.651. [DOI] [PubMed] [Google Scholar]
- 15.Hoyte L, Kaur J, Buchan AM. Lost in translation: taking neuroprotection from animal models to clinical trials. Exp Neuro. 2004;188:200–204. doi: 10.1016/j.expneurol.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 16.Reasoner DK, Ryu KH, Hindman BJ, Cutkomp J, Smith T. Marked hemodilution increases neurologic injury after focal cerebral ischemia in rabbits. Anesth Analg. 1996;82:61–67. doi: 10.1097/00000539-199601000-00011. [DOI] [PubMed] [Google Scholar]
- 17.Maynard KI, Kawamata T, Ogilvy CS, Perez F, Arango P, Ames A., 3rd Avoiding stroke during cerebral arterial occlusion by temporarily blocking neuronal functions in the rabbit. J Stroke Cerebrovasc Dis. 1998;7:287–295. doi: 10.1016/s1052-3057(98)80045-1. [DOI] [PubMed] [Google Scholar]
- 18.Murphy BD, Chen X, Lee TY. Serial changes in CT cerebral blood volume and flow after 4 hours of middle cerebral occlusion in an animal model of embolic cerebral ischemia. AJNR Am J Neuroradiol. 2007;28:743–749. [PMC free article] [PubMed] [Google Scholar]
- 19.Jahan R, Stewart D, Vinters HV, et al. Middle cerebral artery occlusion in the rabbit using selective angiography: application for assessment of thrombolysis. Stroke. 2008;39:1613–1615. doi: 10.1161/STROKEAHA.107.507376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Culp BC, Brown A, Erdem E, Lowery J, Culp WC. Selective intracranial magnification angiography of the rabbit: Basic techniques and anatomy. JVIR. 2007;18:187–192. doi: 10.1016/j.jvir.2006.12.720. [DOI] [PubMed] [Google Scholar]
- 21.Caldwell CB, Flores R, Lowery J, Culp WC. Angiographic variations in the circle of willis in the New Zealand white rabbit. JVIR. 2008;19:S29. doi: 10.1016/j.jvir.2011.01.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kirchhof K, Welzel T, Zoubaa S, et al. New method of embolus preparation for standardized embolic stroke in rabbits. Stroke. 2002 Sep;33:2329–2333. doi: 10.1161/01.str.0000027436.82700.73. [DOI] [PubMed] [Google Scholar]
- 23.Zhao BQ, Suzuki Y, Kondo K, Kawano K, Ikeda Y, Umemura K. Cerebral hemorrhage due to heparin limits its neuroprotective effects: studies in a rabbit model of photothrombotic middle cerebral artery occlusion. Brain Res. 2001;902:30–39. doi: 10.1016/s0006-8993(01)02285-5. [DOI] [PubMed] [Google Scholar]
- 24.Zhao BQ, Suzuki Y, Kondo K, Kawano K, Ikeda Y, Umemura K. A novel MCA occlusion model of photothrombotic ischemia with cyclic flow reductions: development of cerebral hemorrhage induced by heparin. Brain Res Protoc. 2002;9:85–92. doi: 10.1016/s1385-299x(01)00124-6. [DOI] [PubMed] [Google Scholar]
- 25.Zhao BQ, Suzuki Y, Kondo K, Ikeda Y, Umemura K. Combination of a free radical scavenger and heparin reduces cerebral hemorrhage after heparin treatment in a rabbit middle cerebral artery occlusion model. Stroke. 2001;32:2157–2163. doi: 10.1161/hs0901.095640. [DOI] [PubMed] [Google Scholar]
- 26.Chen ZZ, Jiang XD, Zhang LL, et al. Beneficial effect of autologous transplantation of bone marrow stromal cells and endothelial progenitor cells on cerebral ischemia in rabbits. Neurosci Lett. 2008;445:36–41. doi: 10.1016/j.neulet.2008.08.039. [DOI] [PubMed] [Google Scholar]
- 27.Shek JW, Wen GY, Wisniewski HM. Atlas of the rabbit brain and spinal cord. S Karger AG: Zurich; 1986. [Google Scholar]
- 28.Lapchak PA. The phenylpropanoid micronutrient chlorogenic acid improves clinical rating scores in rabbits following multiple infarct ischemic strokes: synergism with tissue plasminogen activator. Exp Neurol. 2007;205:407–413. doi: 10.1016/j.expneurol.2007.02.017. [DOI] [PubMed] [Google Scholar]
- 29.Brekenfeld C, Schroth G, El-Koussy M, et al. Mechanical thromboectomy for acute ischemic stroke: comparison of the Catch thromboectomy device and the Merci Retriever in vivo. Stroke. 2008;39:1213–1219. doi: 10.1161/STROKEAHA.107.495614. [DOI] [PubMed] [Google Scholar]
- 30.Yousaf M, Atherton ME, Culp WC. Incomplete modeling of the thromboembolectomy technique. Stroke. 2008;39:e127. doi: 10.1161/STROKEAHA.107.524256. [DOI] [PubMed] [Google Scholar]
- 31.Lapchak PA, Araujo DM, Pakola S, Song D, Wei J, Zivin JA. Microplasmin: a novel thrombolytic that improves behavioral outcome after embolic strokes in rabbits. Stroke. 2002;33:2279–2284. doi: 10.1161/01.str.0000028267.09604.7b. [DOI] [PubMed] [Google Scholar]
- 32.Lapchak PA, Araujo DM, Zivin JA. Comparison of tenecteplase with alteplase on clinical rating scores following small clot embolic strokes in rabbits. Exp Neuro. 2004;185:154–159. doi: 10.1016/j.expneurol.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 33.Lapchak PA, Zivin JA. Ebselen, a seleno-organic antioxidant, is neuroprotective after embolic strokes in rabbits: synergism with low-dose tissue plasminogen activator. Stroke. 2003;34:2013–2018. doi: 10.1161/01.STR.0000081223.74129.04. [DOI] [PubMed] [Google Scholar]
- 34.Lapchak PA, Araujo DM, Song D, Wei J, Zivin JA. Neuroprotective effects of the spin trap agent disodium-[(tert-butylimino)methyl]benzene-1,3-disulfonate N-oxide (generic NXY-059) in a rabbit small clot embolic stroke model: combination studies with the thrombolytic tissue plasminogen activator. Stroke. 2002;33:1411–1415. doi: 10.1161/01.str.0000015346.00054.8b. [DOI] [PubMed] [Google Scholar]
- 35.Hendrikse J, Petersen ET, Chèze A, Chng SM, Venketasubramanian N, Golay X. Relation between cerebral perfusion territories and location of cerebral infarcts. Stroke. 2009;40:1617–1622. doi: 10.1161/STROKEAHA.108.539866. [DOI] [PubMed] [Google Scholar]