SUMMARY
Homelessness represents a context of extreme poverty and risk for child development. This study compared the relative influence of two classes of risk in the context of homelessness. Levels of socioeconomic resource-related risk and negative lifetime events were examined with respect to morning cortisol levels and cortisol response to a set of cognitive tasks. Participants were 66 children between the ages of 4 and 7 years staying in an emergency shelter for families. Adversities largely reflecting family level negative life events predicted higher levels of morning cortisol and differences in initial level and change over the course of the session of cognitive tasks. In contrast, a socioeconomic cumulative risk score was not associated with morning or session-related differences in cortisol.
Keywords: Homelessness, Early Childhood, Cortisol, Adversity, Risk, Family, Stress
INTRODUCTION
Overview
Understanding pathways of developmental risk for disadvantaged children is crucial for effective prevention and efforts to promote healthy development in these children. Past research on socioeconomic status (SES) disparities in health and behavior implicates the possible role of altered physiological stress response systems, including the hypothalamic-pituitary-adrenal (HPA) axis, in these risk pathways linking low SES and associated adversities to problems in multiple domains across the lifespan (e.g., Shonkoff, Boyce, & McEwen, 2009). Yet, little is known about the relative impact of various kinds of risk on developing stress response systems. The purpose of this study was to examine associations between cortisol levels, reflecting a component of neuroendocrine regulation, and the experience of stressful life events and other risk factors in the context of extreme childhood poverty in the United States. The study focused on children living in a family homeless shelter, a socioeconomically disadvantaged group with high risk for adversity exposure as well as developmental problems (Rog & Buckner, 2007).
Lower socioeconomic status (SES) has been robustly linked to many negative physical and mental health outcomes in children and adults, but the mechanisms that produce these associations have not been satisfactorily explicated (Adler et al., 1994; Anderson & Armstead, 1995; McLoyd, 1998; Lupien et al., 2001; Chen et al., 2002). Dynamic models that emphasize the interplay of biology and environment over time have seen renewed interest, especially those that include the role of psychosocial stress in development (e.g., McEwen, 2000; Boyce & Ellis, 2005; Ellis et al., 2006; Evans & Kim, 2007). Individuals from low SES backgrounds experience stressful situations more frequently and report greater perceived stress than individuals of higher SES (Duncan & Brooks-Gunn, 1997; McLoyd, 1998; Evans & English, 2002). Chronic activation of physiological stress response systems may help explain how poverty can “get under the skin” and produce disparities (McEwen, 1998; Lupien et al., 2001; Evans & Schamberg, 2009). Yet it remains unclear which aspects of the environmental conditions or experiences of poverty, such as exposure to poor housing conditions or increased likelihood of family disruptions, may be most influential in the development of differences in stress physiology. More refined delineation of these influences is important to advance understanding of the processes that account for the physical and mental health risks observed among disadvantaged children.
Homelessness as a context of extreme poverty and risk
Children living in homeless families represent one of the most disadvantaged groups in the United States. By 2005, about 34% (241,765) of all homeless shelter residents in the United States (from February 1 to April 30, 2005) were persons in families with children. Children accompanied by an adult represented about 21% (149,248) of all shelter residents, and 11% of the total were children under the age of 6 (U.S. Department of Housing and Urban Development, 2007).
While considerable diversity exists, homelessness is generally accompanied by exposure to multiple developmental risk factors (e.g., low family income, parental unemployment and low educational attainment, exposure to unsafe and violent contexts), which have been linked to negative developmental outcomes in children (Bassuk et al., 1996; Rog & Buckner, 2007; Wilder Research, 2007). Homeless children also frequently experience substandard housing and are exposed to worse conditions in their physical environments (U. S. Department of Housing and Urban Development, 2007). They are at increased risk for negative and highly stressful life events, such as child maltreatment, parental conflict, domestic violence, parental substance use and mental illness, and other risks associated with family instability (Masten et al., 1993; Bassuk et al., 1997; Rog & Buckner, 2007; Wilder Research, 2007; Gewirtz et al., 2008). This combination of risk factors in the environment and increased rates of stressful negative situations put homeless children at risk for a wide range of poor outcomes (Rafferty & Shinn, 1991; Masten et al., 1993; Buckner et al., 1999; Obradovic et al., 2009).
The hypothalamic-pituitary-adrenal axis
Experiencing significant stress and adversity, especially early in life, has been linked to lasting alterations in hypothalamic-pituitary-adrenal (HPA) axis functioning (Gunnar & Vasquez, 2001, 2006). The HPA axis plays a central role in many homeostatic processes, including reactions to a variety of somatic and psychosocial stressors. Under basal conditions, cortisol levels typically follow a circadian rhythm with levels highest in the morning and declining throughout the day. When activated in response to a stressor, the HPA axis participates in a cascade of neuroendocrine responses, beginning with the secretion of corticotrophin releasing hormone (CRH) from the hypothalamus. CRH travels to the anterior pituitary to release adrenocorticotropic hormone (ACTH). ACTH acts on the adrenal gland where cortisol is produced and released. Stress also activates other aspects of the sympathetic nervous system to produce arousal (the “fight-or-flight” response). Cortisol acts to interfere with many of the biological effects produced during the fight-flight period of stress responding while also acting to inhibit its own production through negative feedback regulation at the levels of the hippocampus, hypothalamus and pituitary. Thus, a typical HPA stress response involves a period of increased cortisol in circulation followed by a return to baseline levels (Sapolsky, 1992; Herman & Cullinan, 1997). This brief elevation following the onset of a stressor not only serves to inhibit hypothalamic production of CRH, but also assists in restoring other systems that are affected by the stress response. Partial failures in the inhibition of this cascade can result in chronic overproduction of cortisol, which has been linked to a variety of poor physical and mental health outcomes (McEwen, 1998; Sapolsky et al., 2000; Boyce & Ellis, 2005; Herbert et al., 2006).
Effects of poverty and chronic stress on HPA axis functioning
Chronic stress in childhood has been shown to predict differences in HPA axis functioning and diurnal cortisol profiles, with some children displaying elevated levels and others attenuated ones (Flinn & England, 1997,2003; De Bellis et al., 1999; De Bellis, 2001; Gunnar & Vasquez, 2001, 2006; Evans & Kim, 2007; Miller et al., 2007; Heim et al, 2008). Animal models examining maternal separation experiences in many species suggest that high levels of chronic stress early in development contribute to differences in HPA axis functioning throughout life (see Francis & Meaney, 1999; Dettling et al., 2002; Pryce et al., 2005).
An increasing number of studies document associations between socioeconomic status and cortisol levels in young children. Preschoolers whose families experienced challenging economic circumstances when the children were infants and continued to do so during the preschool period exhibited higher levels of cortisol than children whose families never experienced difficult economic circumstances or did so only during one of these developmental periods (Essex et al., 2002). Lupien also noted elevated cortisol levels for lower SES school-aged children, but not for teenagers (Lupien et al., 2001). However, Evans and Kim (2007) noted that overnight cortisol production was positively correlated with the number of years children had lived in poverty in a study of 13-year-olds. Notably, research on socioeconomic status and cortisol levels in children has moved beyond correlational analyses. Recently, Fernald and Gunnar (2009) showed that preschool-aged children in rural Mexico whose families were assigned to participation in a conditional cash transfer program had lower baseline cortisol levels compared to children reared in families of comparable pre-program poverty who were not given the opportunity to take part in the cash transfer program. Thus, there is good evidence that poverty, or factors associated with it, may produce chronic increases in cortisol production in children.
The importance of identifying more specific risk factors
Human development reflects the co-action and interaction of many processes across multiple system levels (from genes to physiological function to social relations to broader factors such as culture and public policy) and across time to produce the characteristics of an individual in a probabilistic fashion (Gottlieb, 1991; Gottlieb & Halpern, 2002; Yates et al., 2003). This principle likely applies to the development of stress response systems including the HPA axis. Indeed, differences in genotype, temperament, caregiving environment, exposure to stressful life events, community and school level variables, certain broader social factors, and developmental history all have been found to influence an individual’s HPA axis functioning as reflected in cortisol measures (Gunnar et al., 1992; Gunnar & Donzella, 2002; Chen & Paterson, 2006; Gunnar & Vazquez, 2006). Furthermore, researchers are only beginning to appreciate the complexities of these influences, leading studies away from broad and heterogeneous groups or indicators, such as ‘maltreated children’ or ‘poverty,’ and towards greater specificity in measurement of psychosocial and developmental risk (Brooks-Gunn & Duncan, 1997; Cicchetti & Rogosch, 2001a; Chen et al., 2002). This underscores the importance of considering theoretically grounded processes that take into account more proximal risk factors. Such an approach will serve to better elucidate basic explanatory models of the impact of childhood adversity while recommending potential targets for more effective interventions. This study attempts to move beyond the broad and heterogeneous risk factor of ‘poverty’ to more specific sources of risk among homeless children with respect to differences in cortisol levels.
Other efforts have attempted to identify which specific aspects of poverty may account for elevations in diurnal cortisol levels. Evans and Kim (2007; see also Evans & English, 2002) found that children who experience longer durations of poverty show higher levels of overnight urinary free cortisol. However, a cumulative risk score reflecting the amount of psychosocial and physical risk factors in the child’s life failed to mediate the poverty-cortisol relationship. Flinn and England (1997; Flinn, 1999) failed to find an effect of income on health among children living in a rural Caribbean village, but reported that stress related to unstable family environments was linked to higher cortisol levels during the day. They concluded that important intermediates between low SES and poor health could be found in psychosocial stressors that threaten or disrupt the family and consequently impact neuroendocrine and immune functioning. While it appears that low SES is associated with higher levels of diurnal cortisol in children, it remains unclear which aspects of poverty are most influential in this link. Neither income nor stress indices that highlight physical living conditions explain the association. As noted by Flinn and England (1997), the most promising factors involve family functioning.
While low SES appears to be associated with higher diurnal cortisol levels, much less is known about the relationship between socioeconomic status and differences in cortisol response to a challenging or stressful task. Evans and Kim (2007) found a link between poverty exposure and the stress response via blood pressure reactivity, although they did not consider cortisol. This poverty-stress response link was mediated by the degree of cumulative psychosocial and physical risk in a child’s life. Nonetheless, it remains unclear whether this association is the result of differences in HPA axis functioning, or if it operates through other pathways.
To our knowledge, there is no definitive study of socioeconomic effects on children’s cortisol response to any challenge. However, findings have linked differences in cortisol response with certain family-related adversities that occur more frequently in the lives of low SES children (Whitbeck & Hoyt, 1999; Pardeck, 2005a, 2005b; Rog & Buckner, 2007). Saltzman and colleagues (2005) found that children who had lived in families with marital violence showed elevated cortisol levels compared to controls both before and after a potentially distressing interview about domestic violence. Yet, there was no group difference in the cortisol response during the interview. Documented child maltreatment is also more prevalent among low SES families, and multiple forms of abuse and neglect have been linked with alterations in HPA axis functioning in the form of diurnal patterns (e.g., De Bellis et al., 1999; Cicchetti & Rogosch, 2001a) as well as in response to either psychosocial stress or physiological challenge (see DeBellis, 2001; Cicchetti, 2003; Gunnar & Vazquez, 2006). Yet, the direction of these differences (elevated versus attenuated responding; high versus low baseline levels) appear to vary as a function of the timing, type, and severity of the child’s negative experiences and their psychological and behavioral sequelae (Hart, Gunnar, & Cicchetti, 1995; Cicchetti & Rogosch, 2001a, 2001b; Gunnar & Vazquez, 2001; Cicchetti, 2003; Gunnar & Vazquez, 2006).
Attenuated versus elevated cortisol levels
While the literature on SES differences in cortisol suggests that morning levels are typically higher for children experiencing lower socioeconomic status, in some cases exposure to chronic stress has been associated with lower levels of cortisol. Termed ‘hypocortisolism’, such patterns have been found in some samples of high-risk children and are believed to be a product of down-regulation of the HPA axis following chronic elevations (Heim et al., 2000; Fries et al., 2005). However, it is not clear if this effect applies to all types of childhood adversities. Hypocortisolism has been documented in infants and young children who experience extreme forms of deprivation and neglect (Gunnar, 2000; Gunnar & Vasquez, 2001; Fisher et al., 2005; Dozier et al., 2006; Fisher et al., 2006; Bruce et al., 2009), young children of mothers with high levels of depression symptoms living in extreme urban poverty in the third world (Fernald et al., 2008), and school-aged children who have experienced repeated and recent traumatic events (Bevans et al., 2008). In contrast, many other studies of children reared in poverty or exposed to maltreatment have reported elevated cortisol levels, such as those noted above. Thus, while we recognize that the adversities examined in this report might produce a down-regulation of the HPA axis, based on the preponderance of the evidence, we expected positive associations between measures of adverse conditions and measures of cortisol.
Study goals and hypotheses
The goal of the present study was to investigate the relative contributions of two categories of risk on cortisol levels among homeless 4 to 7 year old children: cumulative socioeconomic risk linked to the child’s experience of economic resources; and cumulative negative lifetime events that largely index problems of family function. Based on broader research on children experiencing poverty, we hypothesized that lifetime event scores largely related to family level negative experiences and disruptions (e.g., Flinn & England, 1997) would be associated with higher morning cortisol levels, beyond any differences related to cumulative socioeconomic risk (e.g., Evans & Kim, 2007). Also, we expected a positive relationship between lifetime event scores and cortisol response to the session tasks, given past evidence suggesting that family difficulties that are more common in low income contexts (e.g., child maltreatment, exposure to domestic violence) are linked with differences in the stress response to psychosocial challenge.
METHODS
Participants
Parents and children were recruited from a large, urban, emergency shelter for families in the mid-western United States in 2006, prior to the rapid increase in foreclosures that emerged in force during 2007. Demographic characteristics of families in this shelter were comparable to national figures (U.S. Department of Housing and Urban Development, 2007). Inclusion criteria were families with a child between 4 and 7.3 years old (M=5.95 years, SD = .68) with no known developmental delay and who spoke proficient English (child and parent). The age range was selected because the current data are part of a larger project interested in early school success. Only one child participated per family. When there were multiple eligible children in a family, the child entering kindergarten was preferred, otherwise the parent chose which child participated.
All families with a child in the age range were invited to participate and approximately 86% of eligible families agreed. Five families were excluded from participation, all due to a lack of English proficiency. This resulted in a sample of 66 children (25 females). All caregivers provided informed consent. An effort was made to include only families who had stayed in the shelter for at least three nights to allow for some time to adjust to the context; yet two (3%) families participated prior to their third night in shelter. Nearly all participants had ethnic minority backgrounds [n = 64; 97%: 53 (80.3%) African American; 10 (15.2%) multiracial; and 1 (1.5%) Native American]. Two (3.0%) children were Caucasian.
Measures
Children and their primary caregivers completed independent assessment sessions that each lasted approximately 1.5 hours. All assessments occurred in specially designated rooms in the emergency shelter. Sessions occurred either in the morning (9 am to 12 pm), or afternoon (1 to 4:30 pm). Children were administered a number of developmentally appropriate cognitive tasks and provided saliva samples, while parents reported on child behavior, family characteristics, cumulative socioeconomic risk, and negative lifetime event histories. Parents received a $20 gift card as an honorarium for their time and children received toys valued around $10.
Cognitive tasks
Children were administered a series of cognitive tasks. These tasks served as the challenge to elicit a cortisol response, as found in past work with low SES preschoolers (Blair et al., 2005; Willoughby et al., 2007). First, children completed three subscales of the Wechsler Preschool and Primary Scale of Intelligence – Third Edition (Wechsler, 2002): Block Design, Matrix Reasoning, and Vocabulary. Then a set of widely used tasks were employed to assess executive function skills, including Simon Says (Kochanska et al., 1997), Peg Tapping (Diamond & Taylor, 1996), the Computerized Pointing Stroop task (Berger et al., 2000), and the Dimensional Change Card Sort (Frye et al., 1995). These tasks primarily require the use of inhibitory control of a prepotent response.
Salivary Cortisol
Children’s salivary cortisol was collected in two contexts: 1) during the assessment session as a measure of HPA reactivity to the tasks, and 2) in the morning prior to breakfast to assess diurnal cortisol levels following wake up. During the assessment session, three samples were taken at roughly 30 minute intervals following a 15 minute consent process and an initial 5 minute rapport building period. The three cortisol values were analyzed using linear mixed modeling (described below) to test for associations with initial sample values (intercept) and with differences in change over the session (slope).
Morning cortisol was collected three times on three separate days after the session. Investigators met each child and parent either in the shelter’s cafeteria during breakfast, or in the lobby as children were preparing to leave for school. Every effort was made to obtain the sample at least 15 minutes following wake up and before the child had eaten. On average, these samples were taken 46 minutes (SD = 24 minutes) after the parent reported that the child woke up. Values were averaged for each child for a single indicator of morning cortisol level.
Saliva was obtained by having the child dip a 1.5” cotton dental roll in about 0.025 g of cherry flavored Kool-Aid™ mix before placing the cotton roll into his/her mouth. The use of this small amount of Kool-Aid™ mix does not meaningfully affect the cortisol assay (Talge et al., 2005), while increasing the amount of salivation. The child held the cotton roll in his/her mouth until it became saturated. A needleless syringe was used to express the saliva into a 1.5 ml Eppendorf Safe-Lock microtube, which was then frozen at −20° C until assaying.
Samples were assayed in duplicate for cortisol concentration with a time-resolved fluorescence immunoassay (DELFIA). Intra-assay coefficient of variation was between 4.0% and 6.7%, and the inter-assay coefficients of variation were between 7.1% and 9.0% for this assay. Duplicates were highly correlated in our sample, r = .995, p < .001.
Cumulative Socioeconomic Risk
Primary caregiver interviews included information related to demographic and other poverty related risks in the physical environment. From these interviews, each child was assigned a cumulative socioeconomic risk score by summing six dichotomized (present vs. absent) risk factors (Sameroff et al., 1987; Sameroff et al., 2003; Evans, 2004). These particular risk factors were selected to index the family’s level of risk with respect to physical resources and human capital, with an attempt to avoid risk factors related to disruptions and disturbances in family functioning (see Table 1). This allowed for a comparison of cumulative socioeconomic risk and of negative lifetime events weighted toward family disturbances (see below).
Table 1.
Cumulative socioeconomic risk indicators and endorsement rates. Total sample N = 66.
|
Risk factors (thresholds for dichotomization) |
n |
% |
|---|---|---|
| Low parental education (< high school degree) | 20 | 30% |
| Parental unemployment | 56 | 85% |
| No family income last month ($0) | 17 | 26% |
| Unsafe neighborhood at last residence | 18 | 27% |
| Could not afford rent at last residence | 33 | 50% |
| Substandard or unsafe housing at last residence | 13 | 20% |
Negative Lifetime Events
Lifetime event scores were sums of the responses on the Lifetime Events Questionnaire (Masten et al., 1993) completed by the primary caregiver. This questionnaire elicits the number of stressful events that the child experienced during his or her lifetime, including events and situations that threaten the child directly as well as those experienced by the child that threaten a parent, a family member, or the integrity of the family (see Table 2).
Table 2.
Negative lifetime events and endorsement rates. Total sample N = 66.
|
Has this child… |
n |
% |
|---|---|---|
| Lived in a home with fights or severe relationship problems between parents or adults taking care of him/her |
26 | 39% |
| Experienced the divorce or permanent separation of his/her parents | 25 | 38% |
| Been separated from his/her parents for more than 2 weeks | 24 | 36% |
| Had a parent who was in prison | 23 | 35% |
| Seen violence happening to other people | 23 | 35% |
| Seen a parent injured by another person | 14 | 21% |
| Been hospitalized | 14 | 21% |
| Lived with a parent who had a serious mental illness | 9 | 14% |
| Lived with a parent who had a serious alcohol or drug problem | 7 | 11% |
| Lived in a foster home | 4 | 6% |
| Lived with a parent who had a serious physical illness | 4 | 6% |
| Other (unlisted) major life event | 4 | 6% |
| Experienced death of a parent | 2 | 3% |
| Experienced death of a brother or sister | 2 | 3% |
| Been the victim of physical violence | 2 | 3% |
| Experienced any other severe threat to his/her life or safety | 0 | 0% |
Potential Confounds
Parents completed a brief daily checklist every day a cortisol sample was collected. This checklist asked about daily factors that may have influenced a child’s cortisol levels. Six of these factors were endorsed, indicating that the child had recently: used an inhaler, taken a psychostimulant, taken other medication, was ill, had eaten in the past hour, or had some particularly stressful event occur that morning. Three children were actively taking medications that involved the direct administration of corticosteroids, or had mechanisms of action on physiological systems directly related to HPA axis functioning. Therefore, cortisol values from these three children were dropped from the dataset and estimated through statistical techniques (described below). Other endorsed factors were not related to cortisol (coefficients not reported), nor was length of stay in shelter (median: 11.5 days). Therefore, these factors were not included in analyses.
Missing Data
Despite great effort, the realities of collecting data in an emergency homeless shelter and working with families actively in crisis resulted in some missing data. No family lacked demographic, cumulative socioeconomic risk, or lifetime event data. However, as noted above, all session cortisol data for 3 children (4.5%) were excluded because of medication issues. Six (9.1%) children lacked only a third session sample, and one child lacked the 2nd and 3rd samples. Most commonly, data were missing because the family interrupted the session to attend meals which were offered at specific times in shelter. Fifty-six (84.8%) children had values for all three session samples, with 181 of a possible 198 (91.4%) session cortisol values obtained.
Collecting morning cortisol in shelter proved especially challenging. Accommodations were made to collect samples from children who did not attend breakfast or did not regularly ride the bus to school. Fifty-four (81.8%) children provided at least 1 morning cortisol sample, 44 (66.7%) at least 2 samples, and 36 (54.5%) provided all three morning samples.
Statistical Analyses
The relative contributions of cumulative socioeconomic risk and negative lifetime event scores to differences in morning cortisol were tested with a multiple regression analysis. Morning cortisol level was simultaneously regressed on cumulative socioeconomic risk and negative lifetime events while controlling for age, gender, and time since waking up. Additional models were run to inform the impact of lifetime events without socioeconomic risk considered, and of socioeconomic risk without lifetime events considered. Missing values for morning cortisol were imputed using PROC MI of SAS version 9.1. Data appeared to be “missing at random” (MAR), meaning that missing values were conditional on other observed variables (see Schafer & Graham, 2002), according to analyses of missingness (not presented). The imputation estimates missing values through an iterative process: Maximum likelihood estimates are made using an expectation-maximization (EM) algorithm. The EM estimates then serve as a starting point for a Markov chain Monte Carlo (MCMC) method to create twenty imputed datasets (Schafer & Graham, 2002). Regression analyses were run individually on each of twenty imputed datasets and test estimates and other coefficients were combined as recommended by Rubin (1987). This allows for a more robust estimation of missing data (Schafer & Graham, 2002). As an additional check, analyses with morning cortisol were completed using imputed and non-imputed datasets. Results were not meaningfully different. Estimates based on the imputed datasets are reported for statistics involving morning cortisol values.
The relationships between negative lifetime events, cumulative socioeconomic risk, and cortisol response to cognitive tasks over the session were tested using a linear mixed model (LMM; Fitzmaurice et al., 2004), controlling for age, sex, and time of day the session occurred. Again, additional models were run to inform the impact of lifetime events without socioeconomic risk considered, and of socioeconomic risk without lifetime events. LMM allows for relationships to be tested with respect to the initial cortisol level at the start of the session (intercept), and the pattern of change in cortisol over the course of the session (slope). Observed means suggested non-linear trajectories, but a quadratic polynomial could not be fit because the total degrees of freedom were limited by the availability of only 3 samples. Instead, we used a log transformation of the time metric [time = log (sample number)] which can accommodate near-linear and simple non-linear trajectories (Royston et al., 1999). Distributions of session cortisol levels violated normality assumptions and were corrected with a log-transformation.
RESULTS
Morning cortisol
First, hypotheses were tested with regard to the relationship between cumulative socioeconomic risk, lifetime events, and cortisol (see Table 3 for means). The distributions of cumulative socioeconomic risk and lifetime event scores did not violate normality assumptions (cumulative socioeconomic risk: std. skew = 0.62; std. kurtosis = −0.91; lifetime events: std. skew = 1.45; std. kurtosis = −1.41). Morning cortisol values violated normality assumptions, so a corrective logarithmic (log10) transformation was applied. Simple correlations between variables in these analyses are provided in Table 4. The results of the regression models are provided in Table 5. Only participants who had at least one valid morning cortisol value, after excluding children who provided no samples or whose samples were implausible due to medication use, were included in the imputation and subsequent analysis of morning cortisol levels to improve the accuracy of the prediction (n = 55). As expected, higher levels of negative lifetime events predicted higher morning cortisol levels (β = .40; p < .01) in the full model, controlling for age, gender, and time since the child woke up. However, cumulative socioeconomic risk scores were not associated with morning cortisol levels (β = .07; ns).1 This held in the full model that included socioeconomic risk and lifetime events simultaneously, as well as each separately.
Table 3.
Means for cumulative socioeconomic risk, negative lifetime events, and cortisol levels.
| Socioeconomic Risk (SD): | 2.38 (1.16) |
| Range (min., max.): | 5 (0, 5) |
| Median: | 2 |
| Lifetime Events (SD): | 2.77 (2.19) |
| Range (min., max.): | 8 (0, 8) |
| Median: | 2 |
| Morning Cortisol, µg/dl (SD) | 0.62 (0.23) |
| Session Cortisol Sample #1, µg/dl (SD) | 0.17 (0.12) |
| Session Cortisol Sample #2, µg/dl (SD) | 0.19 (0.20) |
| Session Cortisol Sample #3, µg/dl (SD) | 0.17 (0.17) |
Table 4.
Correlations between age, cumulative socioeconomic risk, negative lifetime events, and cortisol variables.
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | ||
|---|---|---|---|---|---|---|---|---|---|
| 1. | Age | --- | |||||||
| 2. | Socioeconomic Risk | −0.10 | --- | ||||||
| 3. | Lifetime Events | 0.10 | −0.15 | --- | |||||
| 4. | Time of session (Session cortisol only) |
0.11 | 0.03 | 0.06 | --- | ||||
| 5. | Time since wake up (Morning cortisol only) |
−0.08 | 0.16 | 0.00 | --- | --- | |||
| 6. | Morning Cortisol | −0.17 | −0.02 | 0.41** | --- | −0.26 | --- | ||
| 7. | Session Cortisol #1 | 0.00 | 0.12 | 0.22 | −0.44*** | --- | 0.07 | --- | |
| 8. | Session Cortisol #2 | −0.07 | −0.00 | 0.11 | −0.29* | --- | 0.20 | 0.49*** | --- |
| 9. | Session Cortisol #3 | −0.05 | −0.02 | 0.04 | −0.28* | --- | 0.15 | 0.37** | 0.87*** |
p < .05;
p < .01;
p <.001
Table 5.
Regression coefficients predicting morning cortisol level.
| Full Model | Without Socioeconomic Risk |
Without Lifetime Events |
|
|---|---|---|---|
| β | β | β | |
| Age | −0.17 | −0.16 | −0.19 |
| Gender | −0.02 | −0.02 | −0.11 |
| Time since wake-up | −0.29† | −0 28† | −0.30† |
| Socioeconomic Riska | 0.07 | -- | 0.03 |
| Lifetime Eventsb | 0.40** | 0.39** | -- |
| Total R2 | 0.28c | 0.28d | 0.13 |
p < 0.1;
p < 0.01;
Resource-related cumulative risk score;
Child and family related lifetime events;
Mean p-value across imputed datasets = 0.01, SD = 0.01;
Mean p = 0.004, SD = 0.01.
Cortisol in response to session
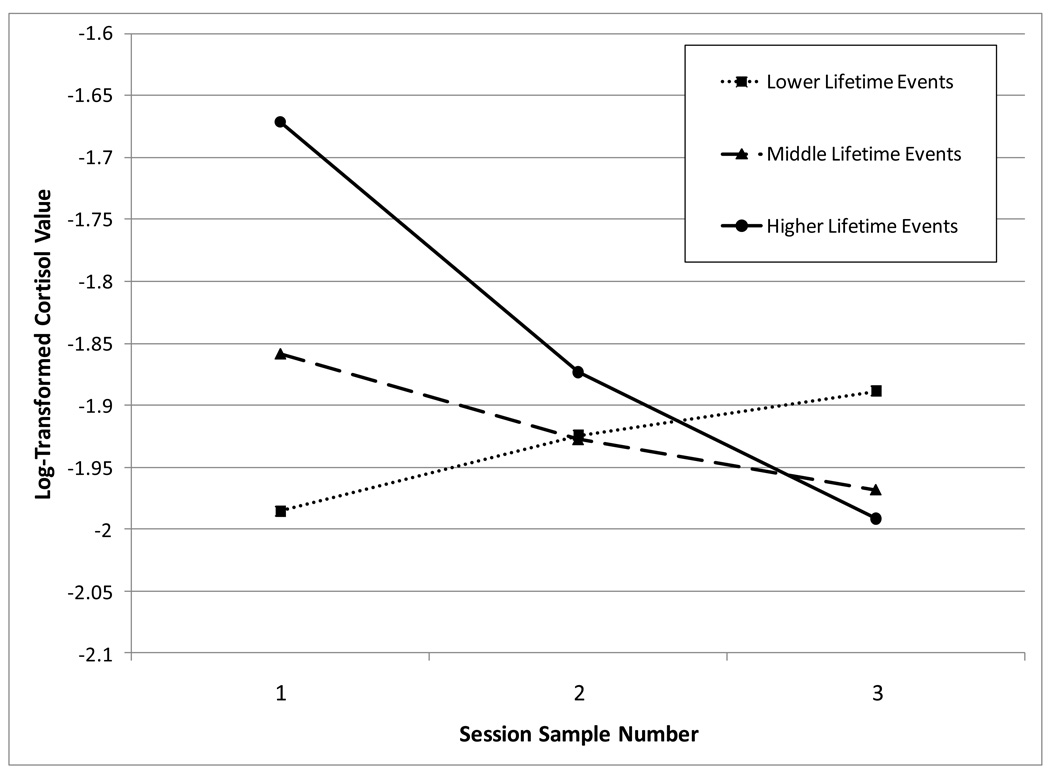
Results of the LMM are provided in Table 6. Only participants with at least one plausible cortisol value during the session were included (n = 63). Significant fixed effects for intercept emerged for two variables: the time of day the session occurred and lifetime event scores. A greater number of negative lifetime events was related to higher cortisol values at the start of the session. The only significant fixed effect for slope was the lifetime events score. This estimate suggests a negative relationship in which an increased number of negative lifetime events was related to a relatively more negative slope (see Figure 1). Socioeconomic risk scores were not significant for either intercept or slope. Again, this held when considering the full model that included socioeconomic risk and lifetime events, as well as each separately.
Table 6.
Parameter estimates (standard errors) for linear mixed model predicting cortisol response to session.
| Full Model | Without Socioeconomic Risk |
Without Lifetime Events |
|
|---|---|---|---|
| Term |
Estimate (Std Error) |
Estimate (Std Error) |
Estimate (Std Error) |
| Unconditional Intercept | −0.51 (.69) | −.36 (.68) | −0.30 (.71) |
| Unconditional Slope | −0.09 (.88) | −0.15 (.86) | −0.34 (.90) |
| Fixed Effects, Intercept Estimates | |||
| Age | 0.00 (.10) | 0.00 (.10) | −0.00 (.11) |
| Sex | 0.08 (.14) | 0.07 (.14) | 0.01 (.14) |
| Time of Session | −0.13 (.03) *** | −0.13 (.03) *** | −0.13 (.03) *** |
| Socioeconomic Risk | 0.06 (.06) | -- | 0.05 (.06) |
| Lifetime Events | 0.07 (.03) * | 0.07 (.03) * | -- |
| Fixed Effects, Slope Estimates | |||
| Age | 0.03 (.13) | 0.03 (.13) | 0.04 (.14) |
| Sex | −0.10 (.18) | −0.09 (.17) | −0.02 (.18) |
| Time of Session | 0.01 (.04) | 0.01 (.04) | 0.00 (.04) |
| Socioeconomic Risk | −0.02 (.08) | -- | −0.01 (.08) |
| Lifetime Events | −0.09 (.04) * | −0.09 (.04) * | -- |
| Random Effect Estimates | |||
| Intercept | 0.21 (.05) *** | 0.21 (.05) *** | 0.23 (.05) *** |
| Slope | 0.32 (.08) *** | 0.32 (.08) *** | 0.35 (.09) *** |
| Intercept*Slope | −0.08 (.05) | −0.08 (.05) | −0.10 (.05) |
| Residual | 0.06 (.01)*** | 0.06 (.01)*** | 0.06 (.01)*** |
p < .05;
p < .001
Figure 1.
Observed lines for children with lower, middle, and higher levels of lifetime events representing trajectories of log-transformed Cortisol levels over the course of the assessment session.
Figure 1 illustrates the results of the linear mixed model. For interpretation, participants were divided into three groups based on their lifetime events score for depiction in this figure. These groups represented thirds: lower, middle, and higher lifetime event scores. Those with the highest lifetime event scores have higher cortisol levels at the start of the session and, on average, decline over the course of the session. In contrast, those with the lowest number of lifetime events not only have lower intercept values at the start of the session, but also tend to show either no response or a rising pattern of cortisol response over the course of the session.
DISCUSSION
A history of many negative life experiences in this sample of young homeless children was linked to higher cortisol levels in the morning, higher levels at the start of an assessment session, and falling levels across a session of challenging cognitive tasks. Negative lifetime event scores primarily reflected reports of family level disruptions and problems, such as being exposed to violence or conflict in the home, having a parent impaired by substance use or psychological disorder, or loss or separation from a caregiver. The association of cortisol with lifetime events was unique in relation to cumulative socioeconomic risk scores based on the family’s level of resources, which were not related to cortisol in this sample.
It is consistent that both the morning and initial session sample show a ‘more lifetime events–high cortisol’ association. However, it is unclear if the differences in slope during the cognitive testing session were indexing a different stress response for children, or if the children all experienced comparable stress responses during the session once the initial difference is taken into account. In other words, the difference in slope reflects a decrease over the session from an initial elevation for higher-adversity children. All children engaged in about 20 minutes of calm interaction and rapport building prior to the first sample collection with the goal of obtaining a more controlled initial value. Yet, differences in slope over the session may reflect a recovery from a previous stress response (including stressors immediately prior to the session or in anticipation of the session) that may be more common in children who experience more negative life events. Alternatively, the difference in slope may be a qualitatively distinct response from the basal levels which are also tied to differences in life event history. Future work that includes measurement of children’s basal levels or a longer calming period before the first sample may clarify the meaning of these slope differences.
A cumulative socioeconomic risk index of physical resources was not related to morning cortisol levels or cortisol in response to the session. This does not appear to be related to any restriction of range in the cumulative risk score. There was substantial variance in risk and heterogeneity among families with respect to the six cumulative risk indicators (see Table 1). While it is conceivable that socioeconomic risk levels were all above a threshold where variation in cortisol could not be observed, it is also possible that the lifetime event score assessed more important proximal conditions in the child’s life. In the current analyses, indicators of cumulative risk were specifically selected to reflect the family’s physical and socioeconomic resources in a broad sense. In contrast, the lifetime events score was mainly comprised of stressful experiences arising in the family and likely reflecting proximal threats to security and the function of parents in the family. Therefore, the cumulative socioeconomic risk score may not be as influential in predicting homeless children’s HPA axis functioning as the family-based lifetime events score.
The emphasis on family level factors beyond socioeconomic risk is supported elsewhere in the literature. For example, maternal depression has been shown to have moderating and mediating effects on the link between SES and child cortisol, a mechanism which likely operates through exposure to stress, less adequate caregiving environments, shared genetic factors, and their combination over time (e.g., Lupien et al., 2000; Essex et al., 2002; Fernald et al., 2008). Blair and colleagues (2008) found that low income infants with lower levels of maternal engagement evidenced higher levels of cortisol overall and in response to an emotionally arousing stimulus. Flinn and England (1997) demonstrated that family relationship factors predict children’s diurnal cortisol levels, and family change or conflict (such as arguments or residential change) was the most common reason for large increases in cortisol.
The link between low SES and higher morning cortisol does not seem to operate through limited family resources, but rather through disruptions in and threats to the family. While changes in income or improvements in financial resources can impact HPA axis functioning (Fernald & Gunnar, 2009), the family’s SES and level of physical resources are undoubtedly risk markers for a number of developmental contexts, childhood adversity, and family problems that account for the differences in child cortisol functioning found in the current study. This is consonant with developmental theory emphasizing the central role of family function for children (e.g., Bronfenbrenner, 1979; Sroufe, 1996; Masten & Shaffer, 2006), as well as specific findings from families experiencing adversity and crisis that illustrate how children can be either protected from or exposed to deleterious psychosocial agents depending on family relationship factors (see, e.g., Miliotis et al., 1999; Scheeringa & Zeanah, 2001;Luthar, 2006). Family functioning and relationships appear to be more central when it comes to child HPA axis functioning, just as it is for many other child outcomes. Future work should include direct measures of family relationship quality and family functioning.
There is growing recognition that developmental history influences the sequelae of later traumatic or highly stressful life events, and that problems earlier in life can be particularly deleterious. Experiencing significant childhood adversity increases the risk for adult psychopathology following a new episode of trauma or high stress (Bremner et al., 1993; Heim & Nemeroff, 2001; Heim et al., 2008). Relatedly, resilience in development is the product of past competence that has resulted in more robust adaptive systems (Yates et al., 2003). Under this view, development is cumulative with the child acquiring increasingly differentiated abilities (Gottlieb, 1991; Gottlieb & Halpern, 2002; Gottesman & Gould, 2003; Sroufe, 2007). The cumulative nature of development suggests that adversity may have a greater impact earlier in life when adaptive systems are less differentiated.
Consideration of normative development also helps explain why threats to the family are more salient stressors for young children than resource related risks. Younger children are more reliant on caregivers for basic physical needs, as well as important co-regulation of emotional arousal. Stressors that impact family members often also impact the young child through emotional distress in the caregiver and disruption of a parent’s capacity to co-regulate the child’s arousal and experience. Young children rely on signals from caregivers to interpret danger, called social referencing, and experience distal threats through their proximal effects on the family (see Scheeringa & Zeanah, 2001). Developmentally, capabilities for emotional reactions and social referencing emerge earlier than full cognitive understanding of dangers and disruptions. Therefore, many stressors of early childhood appear to reach the lives of children through their impact on parents (Masten et al., 2009; see also maternal depression and the physiological stress response: e.g., Lupien et al., 2000; Essex et al., 2002; Fernald et al., 2008). More attention is needed in future research to better understand how family disruptions and problems may produce higher levels of stress and cortisol production in young children.
The current findings contrast with studies of children experiencing high levels of chronic or repeated trauma who show hypocortisolism, a blunting of morning cortisol levels. Higher levels of adversity as indexed by negative lifetime events were tied to higher levels of cortisol in the current sample, a finding that parallels the literature on SES differences in cortisol. There may be several reasons that the children in the current sample did not evidence hypocortisolism. First, it is possible that these children are all evidencing hypocortisolism which might go undetected because the current study lacks a control group. This is unlikely because the observed cortisol levels are not markedly different from other studies of young children (see Gunnar & Donzella, 2002). However, this issue would be more convincingly settled in studies that include an appropriate control group.
A second possibility is that the high level of deprivation linked to hypocortisolism in other studies may not apply to the current sample. All of the children studied were living in a family and, most likely, did not experience the same sort of deprivation described in children who experienced early and prolonged institutionalization (Kaler & Freeman, 1994). Families in the current sample were not selected for experiencing high levels of neglect and maltreatment. Yet, as noted elsewhere, children in the context of homelessness do have elevated rates of child protection involvement (and presumable maltreatment) compared to the general population (Gewirtz et al., 2008), but these rates are below those of samples that typically evidence hypocortisolism (e.g., foster care samples: Bruce et al., 2009). Finally, while homeless children are more likely to have experienced the multiple and chronic forms of stress associated with hypocortisolism in samples of older children (Bevans et al., 2008), it may be that a developmental difference exists in the pathway from certain forms of chronic stress to hypocortisolism (e.g., Romeo et al., 2006; Tarullo & Gunnar, 2006) or more prolonged exposure may be necessary (e.g., DeBellis, 2001; Lupien et al., 2001).
A third possibility is that methodological constraints may have restricted our ability to detect hypocortisolmic patterns. Our morning saliva sampling was limited to only one sample each day. Morning cortisol levels typically peak within the first 60 minutes after waking up and then decline throughout the day (Sapolsky, 1992). As hypocortisolmic findings tend to focus on attenuated morning levels, and gold-standard assessment of the morning peak requires repeated measures in the first hour after waking, the current study may not have had the sensitivity to detect hypocortisolism as analyses relied on a single morning cortisol value for each child. Furthermore, this value was a composite of three morning saliva samples that were collected across three days at various times relative to child wake up. This methodological constraint should be kept in mind generally when considering the findings reported above. Future research should involve multiple samplings each day across the first hour following wake up.
Finally, despite statistically significant results, the measured variables accounted for a rather small amount of the variance. Along with unmeasured psychological and experiential factors and uncontrollable confounds, it is also likely that genetic factors and epigenetic processes contributed to this variation among children. The development of HPA axis functioning involves an epigenetic process where genes, environments, and the child’s developmental history co-act and interact in complex ways to produce an endophenotype at any given point in time (Wüst et al., 2004; Meaney & Szyf, 2005; Ellis et al., 2006; Kaffman & Meaney, 2007). It will be important to continually increase the sophistication of explanatory models of development to include influences across levels of analysis over time.
Future research must better explicate the most influential factors and processes that produce observed effects of adversity on the developing HPA axis. This includes considering risk and protective factors in a risk/resilience framework. Other research has not necessarily considered both risk/adversity and positive/protective factors, instead documenting group differences in cortisol production based on symptom levels or poor developmental competence, between groups with specific and shared trauma histories, or in conjunction with preexisting differences in stress responsivity (e.g., Gunnar et al., 1992; Fisher et al., 2000; Cicchetti & Rogosch, 2001a, 2001b; Cicchetti, 2003; Heim et al., 2008). Other, more proximal variables may better account for differences in cortisol, such as the child’s cognitive abilities, a history of competent functioning versus psychopathological symptoms, or the presence of other protective or promotive factors (e.g., experiencing high-quality caregiving; lower levels of past stress responsivity or fear) that may buffer negative effects of risk and adversity. Behavioral data clearly indicate that key protective factors, such as receiving better quality parenting or having good cognitive abilities, are linked to resilient outcomes in children experiencing high levels of risk and adversity (Luthar, 2006). Since these protective factors operate to produce resilience at the behavioral level, corresponding processes may be evident in physiological correlates. While cumulative risk and lifetime event scores account for only a portion of children’s cortisol levels in the current study, more nuanced explanations that incorporate known developmental mechanisms would serve to further refine the model and increase explanatory power. Accounts that include processes associated with protective and promotive factors are particularly promising in this regard.
This study had a number of limitations. First, the sample was based on 66 families from a single emergency shelter. A larger sample size would provide statistical power to test more complex models. Furthermore, as noted above, although residents in this shelter and this sample were demographically comparable to national figures on urban homeless families, sampling from a single shelter may raise concerns of the generalizability of the findings. Future efforts should attempt to sample a larger number of families from a number of different shelters to minimize concerns of potential bias.
A prospective, longitudinal design also would further explicate the relationship between stressful life events and HPA axis functioning. For example, the timing and duration of stressful life events may play a key role in producing elevations in cortisol levels. In the adult literature, diurnal cortisol levels are inversely related to the amount of time that has passed since the activating event (see Miller et al., 2007). This could mean that children who recently experienced more highly stressful events might show normative elevations in cortisol levels that could return to pre-stress levels once their situation has stabilized. However, many of these children will continue to experience risk and adversity: an estimated 44% of homeless children with parents have been homeless for at least a year or have experienced homelessness four or more times in the past three years (Wilder Research, 2007). Yet, for children experiencing very high levels of poverty or negative life events or situations, moving into a shelter setting might be viewed positively (Jarvis et al., 2005) and as an improvement over their previous circumstances. Documenting the children and families’ experience over time is a necessary step to better understand the impact of homelessness and poverty on developing physiological systems.
Another consideration involves the impact of stressors and HPA axis functioning over time. Experiencing chronic or repeated stress might have a cumulative effect. Individuals with childhood histories involving traumatic stress or maltreatment are at increased risk for developing PTSD and altered HPA axis functioning after experiencing later traumatic or highly stressful events (Bremner et al., 1993; Resnick et al., 1995). Furthermore, De Bellis (2001) points out that a trauma history has the potential to alter pituitary responsiveness to CRH, as well as adrenal responsiveness to ACTH in the production of cortisol. The result is a diverse pattern of possible endocrine profiles following exposure to adversity. However, this process appears to be protracted and in response to chronic physiological exposure to different levels of endocrine activity. The same profile can change over time in either positively-adaptive or maladaptive ways. A further complication is that healthy adults who experienced significant childhood adversity have also showed attenuated cortisol levels in response to challenging tasks when compared to adults with low or no adversity in childhood (Carpenter et al., 2007; Elzinga et al., 2008). Such findings suggest the link between childhood adversity, cortisol response, and psychopathology in adulthood is likely not a direct correspondence, although additional research is needed to examine these interactions (Resnick et al., 1995; Luthar et al., 2000; Heim et al., 2008). Only prospective longitudinal research will provide a clear understanding of the processes of stress and adaptation that underlie the relationship between adversity and HPA axis functioning.
In conclusion, this study found associations between negative lifetime events and HPA axis functioning, specifically indicating that a greater number of negative lifetime events was related to elevated cortisol levels in the morning and differing patterns of cortisol during a session of cognitive tasks. Results suggest that family related threats often experienced by homeless children may play a role in the development or function of a key stress response system. This finding may help guide future efforts to explain the link between lower SES and higher cortisol, and ultimately the SES gradient with respect to health. Finally, it is worth noting that, to our knowledge, this is the first published study of children’s salivary cortisol in the context of an emergency homeless shelter. This study demonstrates the feasibility of collecting valid and reliable data in this ecologically meaningful setting. With careful attention to methodology, concern for participants who are actively in crisis, and strong relationships with collaborators in the community, sound research can produce findings that will elaborate on models of human adaptation while increasing understanding and awareness of developmental processes of children at very high levels of risk.
ACKNOWLEDGMENTS
This research was supported in part by predoctoral fellowships awarded to J. J. Cutuli from the Center for Neurobehavioral Development (CNBD) at the University of Minnesota and the National Institute of Mental Health (NIMH), grants to Ann S. Masten from the National Science Foundation (NSF No. 0745643) and the Center for Urban and Regional Affairs (CURA) at the University of Minnesota, and support to Megan R. Gunnar from the Experienced-Based Brain Development program of the Canadian Institute for Advanced Research (CIAR) which provided funds for the cortisol assays. Any opinions, findings and conclusions or recommendations expressed here are those of the authors and do not necessarily reflect the views of the CNBD, NIMH, NSF, CURA, or CIAR. The authors wish to acknowledge the extraordinary support of colleagues in the community and University in making this research possible. The following individuals made important contributions to the research reported here: Dan Goodermont, Jim Minor, and Kelly Stillman of People Serving People; Margo Hurrle and Elizabeth Hinz of the Minneapolis Public Schools; Becky Hicks of the St. Paul Public Schools; Bonny Donzella, Jelena Obradović, Danielle Holmes, Theresa Lafavor, and Professor Jeffrey Long of the University of Minnesota
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Results were similar for identical analyses using only observed data without imputation (n = 54). Again, lifetime events were significantly related to morning cortisol levels (β = .34; p < .02), while socioeconomic risk was not (β = .11; ns). This pattern of results again held when socioeconomic risk was not included in the model (lifetime events β = .33; p < .02), and socioeconomic risk was again not significant (β = .06; ns) in a regression that did not include lifetime events using only non-imputed data.
REFERENCES
- Adler NE, Boyce T, Chesney MA, Cohen S, Folkman S, Kahn RL, et al. Socioeconomic status and health: The challenge of the gradient. American Psychologist. 1994;49:15–24. doi: 10.1037//0003-066x.49.1.15. [DOI] [PubMed] [Google Scholar]
- Anderson NB, Armstead CA. Toward understanding the association of socioeconomic status and health: A new challenge for the biopsychosocial approach. Psychosomatic Medicine. 1995;57:213–225. doi: 10.1097/00006842-199505000-00003. [DOI] [PubMed] [Google Scholar]
- Bassuk EL, Weinreb LF, Buckner JC, Browne A, Salomon A, Bassuk SS. The characteristics and needs of sheltered homeless and low-income housed mothers. Journal of the American Medical Association. 1996;276:640–646. [PubMed] [Google Scholar]
- Bassuk EL, Weinreb LF, Dawson R, Perloff JN, Buckner JC. Determinants of behavior in homeless and low-income housed preschool children. Pediatrics. 1997;100:92–100. doi: 10.1542/peds.100.1.92. [DOI] [PubMed] [Google Scholar]
- Berger A, Jones L, Rothbart MK, Posner MI. Computerized games to study the development of attention in children. Behavioral Research Methods and Instrumentation. 2000;32:297–303. doi: 10.3758/bf03207798. [DOI] [PubMed] [Google Scholar]
- Bevans K, Cerbone A, Overstreet S. Relations between recurrent trauma exposure and recent life stress and salivary cortisol among children. Development and Psychopathology. 2008;20:257–272. doi: 10.1017/S0954579408000126. [DOI] [PubMed] [Google Scholar]
- Blair C, Granger DA, Kivlighan KT, Mills-Koonce R, Willoughby M, Greenberg MT, et al. Maternal and child contributions to cortisol response to emotional arousal in young children from low-income, rural communities. Developmental Psychology. 2008;44:1095–1109. doi: 10.1037/0012-1649.44.4.1095. [DOI] [PubMed] [Google Scholar]
- Blair C, Granger D, Razza RP. Cortisol reactivity is positively related to executive function in preschool children attending head start. Child Development. 2005;76:554–567. doi: 10.1111/j.1467-8624.2005.00863.x. [DOI] [PubMed] [Google Scholar]
- Boyce WT, Ellis BJ. Biological sensitivity to context : I. An evolutionary-developmental theory of the origins and functions of stress reactivity. Development and Psychopathology. 2005;17:271–301. doi: 10.1017/s0954579405050145. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Southwick SM, Johnson DR, Yehuda R, Charney DS. Childhood physical abuse and combat-related posttraumatic stress disorder in Vietnam veterans. American Journal of Psychiatry. 1993;150:235–239. doi: 10.1176/ajp.150.2.235. [DOI] [PubMed] [Google Scholar]
- Bronfenbrenner U. The ecology of human development. Cambridge, MA: Harvard University Press; 1979. [Google Scholar]
- Brooks-Gunn J, Duncan GJ. The effects of poverty on children. Children and Poverty. 1997;7:55–71. [PubMed] [Google Scholar]
- Bruce J, Fisher PA, Pears KC, Levine S. Morning cortisol levels in preschool-aged foster children: Differential effects of maltreatment type. Developmental Psychobiology. 2009;51:14–23. doi: 10.1002/dev.20333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner JC, Bassuk EL, Weinreb LF, Brooks LF. Homelessness and its relation to the mental health and behavior of low-income school-age children. Developmental Psychology. 1999;35:246–257. doi: 10.1037//0012-1649.35.1.246. [DOI] [PubMed] [Google Scholar]
- Carpenter LL, Carvalho JP, Tyrka AR, Wier LM, Mello AF, Mello MF, et al. Decreased adrenocorticotropic hormone and cortisol responses to stress in healthy adults reporting significant childhood maltreatment. Biological Psychiatry. 2007;62:1080–1087. doi: 10.1016/j.biopsych.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen E, Matthews KA, Boyce WT. Socioeconomic differences in children’s health: How and why do these relationships change with age? Psychological Bulletin. 2002;128:295–329. doi: 10.1037/0033-2909.128.2.295. [DOI] [PubMed] [Google Scholar]
- Chen E, Paterson LQ. Neighborhood, family, and subjective socioeconomic status: How do they relate to adolescent health? Health Psychology. 2006;25:704–714. doi: 10.1037/0278-6133.25.6.704. [DOI] [PubMed] [Google Scholar]
- Cicchetti D. Neuroendocrine functioning in maltreated children. In: Cicchetti D, Walker E, editors. Neurodevelopmental Mechanisms in Psychopathology. Cambridge: Cambridge University Press; 2003. pp. 345–365. [Google Scholar]
- Cicchetti D, Rogosch FA. Diverse patterns of neuroendocrine activity in maltreated children. Development and Psychopathology. 2001a;13:677–693. doi: 10.1017/s0954579401003145. [DOI] [PubMed] [Google Scholar]
- Cicchetti D, Rogosch FA. The impact of child maltreatment and psychopathology on neuroendocrine functioning. Development and Psychopathology. 2001b;13:783–804. [PubMed] [Google Scholar]
- De Bellis MD. Developmental traumatology: The psychobiological development of maltreated children and its implications for research, treatment, and policy. Development and Psychopathology. 2001;13:539–564. doi: 10.1017/s0954579401003078. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Birmaher B, Keshavan MS, Eccard CH, Boring AM, et al. Developmental traumatology part I: biological stress systems. Biological Psychiatry. 1999;45:1259–1270. doi: 10.1016/s0006-3223(99)00044-x. [DOI] [PubMed] [Google Scholar]
- Dettling AC, Feldon J, Price CR. Repeated parental deprivation in the infact common marmoset (Callithrix jacchus Primates) and analysis of its effects on early development. Biological Psychiatry. 2002;52:1037–1046. doi: 10.1016/s0006-3223(02)01460-9. [DOI] [PubMed] [Google Scholar]
- Diamond A, Taylor C. Development of an aspect of executive control: Development of the abilities to remember what I said and to “do as I say, not as I do.”. Developmental Psychobiology. 1996;29:315–334. doi: 10.1002/(SICI)1098-2302(199605)29:4<315::AID-DEV2>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Dozier M, Manni M, Gordon MK, Peloso E, Gunnar MR, Sovall-McClough KC, et al. Foster children’s diurnal production of cortisol: An exploratory study. Child Maltreatment. 2006;11:189–197. doi: 10.1177/1077559505285779. [DOI] [PubMed] [Google Scholar]
- Duncan GJ, Brooks-Gunn J, editors. Consequences of growing up poor. New York: Russell Sage; 1997. [Google Scholar]
- Ellis BJ, Jackson JJ, Boyce WT. The stress response systems: Universality and adaptive individual differences. Developmental Review. 2006;26:175–212. [Google Scholar]
- Elzinga BM, Roelofs K, Tollenaar MS, Bakvis P, van Pelt J, Spinhoven P. Diminished cortisol responses to psychosocial stress associated with lifetime adverse events: A study among healthy young subjects. Psychoneuroendocrinology. 2008;33:227–237. doi: 10.1016/j.psyneuen.2007.11.004. [DOI] [PubMed] [Google Scholar]
- Essex MJ, Klein MH, Cho E, Kalin NH. Maternal stress beginning in infancy may sensitize children to later stress exposure: Effects on cortisol and behavior. Biological Psychiatry. 2002;52:776–784. doi: 10.1016/s0006-3223(02)01553-6. [DOI] [PubMed] [Google Scholar]
- Evans GW. The environment of childhood poverty. American Psychologist. 2004;59:77–92. doi: 10.1037/0003-066X.59.2.77. [DOI] [PubMed] [Google Scholar]
- Evans GW, English K. The environment of poverty: Multiple stressor exposure, psychophysiological stress, and socioemotional adjustment. Child Development. 2002;73:1238–1248. doi: 10.1111/1467-8624.00469. [DOI] [PubMed] [Google Scholar]
- Evans GW, Kim P. Childhood poverty and health: Cumulative risk exposure and stress dysregulation. Psychological Science. 2007;18:953–957. doi: 10.1111/j.1467-9280.2007.02008.x. [DOI] [PubMed] [Google Scholar]
- Evans GW, Schamberg MA. Childhood poverty, chronic stress, and adult working memory. Proceedings of the National Academy of Sciences. 2009;106:6545–6549. doi: 10.1073/pnas.0811910106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernald LCH, Burke HM, Gunnar MR. Salivary cortisol levels in children of low-income women with high depressive symptomatology. Development and Psychopathology. 2008;20:423–436. doi: 10.1017/S0954579408000205. [DOI] [PubMed] [Google Scholar]
- Fernald LC, Gunnar MR. Poverty-alleviation program participation and salivary cortisol in very low-income children. Social Science and Medicine. 2009;68:2180–2189. doi: 10.1016/j.socscimed.2009.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher PA, Burraston B, Pears K. The early intervention foster care program: Permanent placement outcomes from a randomized trial. Child Maltreatment. 2005;10:61–71. doi: 10.1177/1077559504271561. [DOI] [PubMed] [Google Scholar]
- Fisher PA, Gunnar MR, Chamberlain P, Reid JB. Preventive intervention for maltreated preschool children: Impact on children’s behavior, neuroendocrine activity, and foster parent functioning. Journal of the American Academy of Child and Adolescent Psychiatry. 2000;39:1356–1364. doi: 10.1097/00004583-200011000-00009. [DOI] [PubMed] [Google Scholar]
- Fisher PA, Gunnar MR, Dozier M, Bruce J, Pears KC. Effects of therapeutic interventions for foster children on behavioral problems, caregiver attachment, and stress regulatory neural systems. Annals of the New York Academy of Science. 2006;1094:215–225. doi: 10.1196/annals.1376.023. [DOI] [PubMed] [Google Scholar]
- Fitzmaurice GM, Laird NM, Ware JH. Applied longitudinal analysis. New York: Wiley; 2004. [Google Scholar]
- Flinn MV. Family environment, stress, and health during childhood. In: Panter-Brick C, Worthman C, editors. Hormones, Health, and behavior. Cambridge: Cambridge University Press; 1999. pp. 105–138. [Google Scholar]
- Flinn MV, England BG. Social economics of childhood glucocorticoid stress response and health. American Journal of Physical Anthropology. 1997;102:33–53. doi: 10.1002/(SICI)1096-8644(199701)102:1<33::AID-AJPA4>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Flinn MV, England BG. Childhood stress: Endocrine and immune responses to psychosocial events. In: Wilc JM Jr, editor. Social and Cultural Live of Immune Systems. London: Routledge; 2003. pp. 105–145. [Google Scholar]
- Francis DD, Meaney MJ. Maternal care and the development of stress responses. Current Opinion in Neurobiology. 1999;9:128–134. doi: 10.1016/s0959-4388(99)80016-6. [DOI] [PubMed] [Google Scholar]
- Frye D, Zelazo PD, Palfai T. Theory of mind and rule-based reasoning. Cognitive Development. 1995;10:483–527. [Google Scholar]
- Gewirtz A, Hart-Shegos E, Medhanie A. Psychosocial status of homeless children and youth in family supportive housing. American Behavioral Scientist. 2008;51:810–823. [Google Scholar]
- Gottesman II, Gould TD. The endophenotype concept in psychiatry: Etymology and strategic intentions. American Journal of Psychiatry. 2003;160:636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- Gottlieb G. Experiential canalization of behavioral development: Theory. Developmental Psychology. 1991;27:4–13. [Google Scholar]
- Gottlieb G, Halpern CT. A relational view of causality in normal and abnormal development. Development and Psychopathology. 2002;14:421–435. doi: 10.1017/s0954579402003024. [DOI] [PubMed] [Google Scholar]
- Gunnar MR. Early adversity and the development of stress reactivity and regulation. In: Nelson CA, editor. The Minnesota Symposia on Child Psychology: Vol. 31. The effects of adversity on neurobehavioral development. Mahwah, NJ: Erlbaum; 2000. pp. 163–200. [Google Scholar]
- Gunnar MR, Donzella B. Social regulation of the cortisol levels in early human development. Psychoneuroendocrinology. 2002;27:199–220. doi: 10.1016/s0306-4530(01)00045-2. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Larson MC, Hertsgaard L, Harris ML, Brodersen L. The stressfulness of separation among nine-month-old infants: Effects of social context variables and infant temperament. Child Development. 1992;63:290–303. [PubMed] [Google Scholar]
- Gunnar MR, Vazquez DM. Low cortisol and a flattening of expected daytime rhythm: Potential indices of risk in human development. Development and Psychopathology. 2001;13:515–538. doi: 10.1017/s0954579401003066. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Vasquez DM. Stress neurobiology and developmental psychopathology. In: Cicchetti D, Cohen DJ, editors. Developmental Psychopathology (2nd Ed., Vol. 2) Developmental Neuroscience. Hoboken: Wiley; 2006. pp. 533–577. [Google Scholar]
- Hart J, Gunnar M, Cicchetti D. Salivary cortisol in maltreated children: Evidence of relations between neuroendocrine activity and social competence. Development and Psychopathology. 1995;7:11–26. [Google Scholar]
- Heim C, Nemeroff CB. The role of childhood trauma in the neurobiology of mood and anxiety disorders: Preclinical and clinical studies. Biological Psychiatry. 2001;49:1023–1039. doi: 10.1016/s0006-3223(01)01157-x. [DOI] [PubMed] [Google Scholar]
- Heim C, Newport DJ, Mletzko T, Miller AH, Nemeroff CB. The link between childhood trauma and depression: Insights from HPA axis studies in humans. Psychoneuroendocrinology. 2008;33:693–710. doi: 10.1016/j.psyneuen.2008.03.008. [DOI] [PubMed] [Google Scholar]
- Herbert J, Goodyer IM, Grossman AB, Hastings MH, de Kloet ER, Lightman SL, et al. Do corticosteroids damage the brain? Journal of Neuroendocrinology. 2006;18:393–411. doi: 10.1111/j.1365-2826.2006.01429.x. [DOI] [PubMed] [Google Scholar]
- Herman JP, Cullinan WE. Neurocircuitry of stress: central control of the hypothalamo-pituitary-adrenocortical axis. Trends in Neurosciences. 1997;20:78–84. doi: 10.1016/s0166-2236(96)10069-2. [DOI] [PubMed] [Google Scholar]
- Jarvis KL, Gordon EE, Novaco RW. Psychological distress of children and mothers in domestic violence emergency shelters. Journal of Family Violence. 2005;20:389–402. [Google Scholar]
- Kaffman A, Meaney MJ. Neurodevelopmental sequelae of postnatal maternal care in rodents: clinical and research implication of molecular insights. Journal of Child Psychology and Psychiatry. 2007;48:224–244. doi: 10.1111/j.1469-7610.2007.01730.x. [DOI] [PubMed] [Google Scholar]
- Kaler SR, Freeman BJ. An analysis of environmental deprivation: Cognitive and social development in Romanian orphans. Journal of Child Psychology and Psychiatry. 1994;35:769–781. doi: 10.1111/j.1469-7610.1994.tb01220.x. [DOI] [PubMed] [Google Scholar]
- Kochanska G, Murray KT, Coy KC. Inhibitory control as a contributor to conscience in childhood: From toddler to early school age. Child Development. 1997;68:263–277. [PubMed] [Google Scholar]
- Lupien SJ, King S, Meaney MJ, McEwen BS. Child’s stress hormone levels correlate with mother’s socioeconomic status and depressive state. Biological Psychiatry. 2000;48:976–980. doi: 10.1016/s0006-3223(00)00965-3. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, King S, Meaney MJ, McEwen BS. Can poverty get under your skin? Basal cortisol levels and cognitive function in children from low and high socioeconomic status. Development and Psychopathology. 2001;13:653–676. doi: 10.1017/s0954579401003133. [DOI] [PubMed] [Google Scholar]
- Luthar SS. Resilience in development: A synthesis of research across five decades. In: Cicchetti D, Cohen DJ, editors. Developmental psychopathology, Vol. 3: Risk, disorder, and adaptation. 2nd ed. Vol. 3. New York: Wiley; 2006. [Google Scholar]
- Luthar SS, Becker BE. Privileged but pressured? A study of affluent youth. Child Development. 2002;73:1593–1610. doi: 10.1111/1467-8624.00492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luthar SS, Cicchetti D, Becker B. The construct of resilience: A critical evaluation and guidelines for future work. Child Development. 2000;71:543–562. doi: 10.1111/1467-8624.00164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masten AS, Cutuli JJ, Herbers JE, Reed M-GJ. Resilience in development. In: Snyder CR, Lopez SJ, editors. Handbook of Positive Psychology. 2nd ed. New York: Oxford University Press; 2009. pp. 117–131. [Google Scholar]
- Masten AS, Miliotis D, Graham-Bermann SA, Ramirez ML, Neemann J. Children in homeless families: Risks to mental health and development. Journal of Consulting and Clinical Psychology. 1993;61:335–343. doi: 10.1037//0022-006x.61.2.335. [DOI] [PubMed] [Google Scholar]
- Masten AS, Shaffer A. How families matter in child development: Reflections from research on risk and resilience. In: Clarke-Stewart A, Dunn J, editors. Families count: Effects on child and adolescent development. Cambridge, UK: Cambridge University Press; 2006. pp. 5–25. [Google Scholar]
- McEwen BS. Protective and damaging effects of stress mediators. New England Journal of Medicine. 1998;338:171–179. doi: 10.1056/NEJM199801153380307. [DOI] [PubMed] [Google Scholar]
- McEwen BS. The neurobiology of stress: from serendipity to clinical relevance. Brain Research. 2000;886:172–189. doi: 10.1016/s0006-8993(00)02950-4. [DOI] [PubMed] [Google Scholar]
- McLoyd VC. Socioeconomic disadvantage and child development. American Psychologist. 1998;53:185–204. doi: 10.1037//0003-066x.53.2.185. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Szyf M. Maternal care as a model for experience-dependent chromatin plasticity? Trends in Neuroscience. 2005;28:456–463. doi: 10.1016/j.tins.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Miliotis D, Sesma A, Masten AS. Parenting as a protective process for school success in children from homeless families. Early Education & Development. 1999;10:111–133. [Google Scholar]
- Miller GE, Chen E, Zhou ES. If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychological Bulletin. 2007;133:25–45. doi: 10.1037/0033-2909.133.1.25. [DOI] [PubMed] [Google Scholar]
- Obradović J, Long JD, Cutuli JJ, Chan C-K, Hinz E, Heistad D, et al. Academic achievement of homeless and highly mobile children in an urban school district: Longitudinal evidence on risk, growth, and resilience. Development and Psychopathology. 2009;21:493–518. doi: 10.1017/S0954579409000273. [DOI] [PubMed] [Google Scholar]
- Pardeck JT. An exploration of child maltreatment among homeless families: implication for family policy. Early Child Development and Care. 2005a;175:335–342. [Google Scholar]
- Pardeck JT. An exploration of family violence among the homeless: Implications for policy and practice. Journal of Social Work in Disability and Rehabilitation. 2005b;4:57–64. [Google Scholar]
- Pryce CR, Rüedi-Bettschen D, Dettling AC, Weston A, Russig H, Ferger B, et al. Long-term effects of early-life environmental manipulations in rodents and primates: Potential animal models in depression research. Neuroscience and Biobehavioral Reviews. 2005;29:649–674. doi: 10.1016/j.neubiorev.2005.03.011. [DOI] [PubMed] [Google Scholar]
- Rafferty Y, Shinn M. The impact of homelessness on children. American Psychologist. 1991;46:1170–1179. doi: 10.1037//0003-066x.46.11.1170. [DOI] [PubMed] [Google Scholar]
- Resnick HS, Yehuda R, Pitman RK, Foy DW. Effects of previous trauma on acute plasma corisol level following rape. American Journal of Psychiatry. 1995;152:1675–1677. doi: 10.1176/ajp.152.11.1675. [DOI] [PubMed] [Google Scholar]
- Rog DJ, Buckner JC. Toward Understanding Homelessness: The 2007 National Symposium on Homelessness Research. Washington, DC: Department of Health and Human Services; 2007. Homeless families and children. Available online: http://aspe.hhs.gov/hsp/homelessness/symposium07/rog/index.htm. [Google Scholar]
- Romeo RD, Bellani R, Karatsoreos IN, Chhuna N, Vernov M, Conrad CD, et al. Stress history and pubertal development interact to shape hypothalamic-pituitary-adrenal axis plasticity. Endocrinology. 2006;147:1664–1674. doi: 10.1210/en.2005-1432. [DOI] [PubMed] [Google Scholar]
- Royston R, Ambler G, Sauerbrei W. The use of fractional polynomials to model continuous risk variables in epidemiology. International Journal of Epidemiology. 1999;28:964–974. doi: 10.1093/ije/28.5.964. [DOI] [PubMed] [Google Scholar]
- Rubin DB. Multiple imputation for nonresponse in surveys. New York, NY: John Wiley and Sons; 1987. [Google Scholar]
- Saltzman KM, Holden GW, Holahan CJ. The psychology of children exposed to marital violence. Journal of Clinical Child and Adolescent Psychology. 2005;34:129–139. doi: 10.1207/s15374424jccp3401_12. [DOI] [PubMed] [Google Scholar]
- Sameroff AJ, Gutman L, Peck SC. Adaptation among youth facing multiple risks: Prospective research findings. In: Luthar SS, editor. Resilience and Vulnerability: Adaptation in the context of childhood adversities. New York: Cambridge; 2003. pp. 264–391. [Google Scholar]
- Sameroff AJ, Seifer R, Zax M, Barocas R. Early indicators of developmental risk: The Rochester Longitudinal Study. Schizophrenia Bulletin. 1987;13:383–393. doi: 10.1093/schbul/13.3.383. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. Neuroendocrinology of the Stress Response. In: Becker JB, Breedlove SM, Crews D, editors. Behavioral Endocrinology. Cambridge: The MIT Press; 1992. pp. 287–324. [Google Scholar]
- Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocrine Reviews. 2000;21:55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- Schafer JL, Graham JW. Missing data: Our view of the state of the art. Psychological Methods. 2002 [PubMed] [Google Scholar]
- Scheeringa MS, Zeanah CH. A relational perspective on PTSD in early childhood. Journal of Traumatic Stress. 2001;14:799–815. doi: 10.1023/A:1013002507972. [DOI] [PubMed] [Google Scholar]
- Shonkoff JP, Boyce WT, McEwen BS. Neuroscience, molecular biology, and the childhood roots of health disparities: Building a new framework for health promotion and disease prevention. Journal of the American Medical Association. 2009;301:2252–2259. doi: 10.1001/jama.2009.754. [DOI] [PubMed] [Google Scholar]
- Sroufe LA. Emotional development: The organization of emotional life in the early years. Cambridge: University of Cambridge; 1996. [Google Scholar]
- Sroufe LA. The concept of development in developmental psychopathology; Paper presented at the American Psychological Association annual meeting; San Francisco. 2007. [Google Scholar]
- Talge NM, Donzella B, Kryzer EM, Gierens A, Gunnar MR. It’s not that bad: Error introduced by oral stimulants in salivary cortisol research. Developmental Psychobiology. 2005;47:369–376. doi: 10.1002/dev.20097. [DOI] [PubMed] [Google Scholar]
- Tarullo AR, Gunnar MR. Child maltreatment and the developing HPA axis. Hormones and Behavior. 2006;50:632–639. doi: 10.1016/j.yhbeh.2006.06.010. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Housing and Urban Development. The annual homeless assessment report to congress. 2007 Retrieved October 1, 2008, from http://www.huduser.org/Publications/pdf/ahar.pdf.
- Wechsler D. WPPSI-III administration and scoring manual. San Antonio, TX: Psychological Corporation; 2002. [Google Scholar]
- Whitbeck LB, Hoyt DR. Nowhere to grow: Homeless and runaway adolescents and their families. Hawthorne, NY: Aldine de Gruyter; 1999. [Google Scholar]
- Wilder Research. Overview of homelessness in Minnesota 2006. St. Paul, MN: Wilder Research; 2007. [Google Scholar]
- Willoughby M, Vandergrift N, Blair C, Granger DA. A structural equation modeling approach for the analysis of cortisol data collected using pre-post-post designs. Structural Equation Modeling. 2007;14:125–145. [Google Scholar]
- Wüst S, Federenko IS, van Rossum EFC, Koper JW, Kumsta R, Entringer S, et al. A psychobiological perspective on genetic determinants of hypothalamus-pituitary-adrenal axis activity. Annals of the New York Academy of Sciences. 2004;1032:52–62. doi: 10.1196/annals.1314.005. [DOI] [PubMed] [Google Scholar]
- Yates TM, Egeland B, Sroufe LA. Rethinking resilience: A developmental process perspective. In: Luthar SS, editor. Resilience and vulnerability: Adaptation in the context of childhood adversities. Cambridge: Cambridge University Press; 2003. [Google Scholar]