Abstract
Background
Identifying predictors of smoking relapse helps to elucidate the challenges of long-term smoking cessation and provides direction for improved treatment development.
Methods
In this post hoc data analysis, we examined predictors of relapse from end-of-treatment (week 13) through 1-year follow-up (week 52) for treatment-responding participants who achieved the primary efficacy endpoint of 4-week continuous abstinence (weeks 9–12), during two phase III varenicline trials.
Results
Of 626 smokers classified as treatment responders for all treatment groups across both trials, 301 (48%) relapsed during follow-up (weeks 13–52). The odds of relapsing were almost 5 times greater (odds ratio [OR]=4.92, 95% confidence interval [CI]: 2.77–8.97; p<.001) for treatment responders who did not initiate continuous abstinence until the final 4 weeks of the treatment period compared with those who initiated continuous abstinence by their quit date. Participants who reported >30 days of abstinence during the year prior to study entry were significantly more likely to relapse than those who reported 0 days of abstinence (OR=2.38, 95% CI: 1.17–5.04; p=.013).
Conclusion
Results of these analyses suggest that the ability to quit smoking on the initial quit date and maintain abstinence throughout the treatment period is a good prognostic indicator for long-term abstinence. The relationship between post-treatment relapse and longer pretreatment periods of abstinence is counterintuitive, yet not without precedence in the literature.
Keywords: Smoking cessation, Relapse, Predictive factors, Varenicline
1.0 Introduction
Despite the availability of effective tobacco use prevention programs and smoking cessation interventions, cigarette smoking remains a prevalent and intractable addiction with growing casualties worldwide (Mathers and Loncar, 2006). A significant challenge in reducing the burden of tobacco-related morbidity and mortality is helping current smokers to initiate and maintain abstinence. Based on available data, it is the latter of the two change processes (i.e., maintenance of abstinence) that has proven to be most difficult. To quantify the magnitude of this challenge, consider the findings from the 2000 United States National Health Interview Survey (NHIS): of the 15.7 million smokers who stopped smoking for at least 1 day in the prior year in an attempt to quit (41.0% of current smokers), only 4.7% were able to maintain abstinence from smoking for 3 to 12 months (Centers for Disease Control and Prevention, 2002).
The ability to maintain abstinence from smoking, or conversely, to avoid relapse, has been linked to a host of factors that span the biopsychosocial spectrum. Some of the most robust predictors of relapse are smoking after the designated quit date (Kenford et al., 1994; Dale et al., 2001; Higgins et al., 2006), negative affect (Kenford et al., 2002), and urges to smoke (Shiffman et al., 1997) during the post-quit period. A number of other demographic and smoking-related characteristics have been linked to positive cessation outcome, albeit with varying degrees of consistency. These include older age (Murray et al., 2000; Velicer et al.,, 2007), male gender (Ferguson et al., 2003; Dale et al., 2001; Gourlay et al., 1994), being married (Murray et al., 2000; Carlson et al.,, 2000), smoking fewer cigarettes per day (Carlson et al., 2000; Dale et al., 2001), less exposure to smokers in the household (Carlson et al., 2000; Murray et al., 2000), lower severity of nicotine dependence (Ferguson et al., 2003), longer periods of smoking abstinence in the past (Murray et al., 2000; Ward et al., 1997; Garvey et al., 1992), and less alcohol consumption (Garvey et al., 1992; Nides et al., 1995).
Studies examining predictors of relapse are methodologically diverse and have produced heterogeneous findings, perhaps due to differences among the studies in terms of the length of the follow-up over which relapse risk is observed, the statistical methods employed for generating predictive models (e.g., data-driven vs. theoretically-driven selection of predictors), and the method(s) of cessation (e.g., unaided vs. use of a medication and/or counseling). Several other factors also limit the conclusions that can be drawn from this area of research. For example, many studies of relapse predictors do not require that smokers achieve a period of continuous abstinence to be included in analyses examining relapse risk, thereby blurring the distinction between relapse and failure to initiate abstinence. Additionally, smokers who quit with the assistance of non-nicotine pharmacotherapies (i.e., bupropion and varenicline) are not well represented in this body of literature.
The goal of this study was to examine predictors of post-treatment relapse following successful initiation of smoking abstinence (i.e., 4-week continuous abstinence at the end of treatment) using pooled data from two large, multicenter, phase III clinical trials comparing the efficacy of varenicline, bupropion SR (sustained-release), and placebo for smoking cessation (Gonzales et al., 2006; Jorenby et al., 2006). To our knowledge, this is the first study to examine risk for post-treatment relapse among successful quitters in the context of a placebo-controlled pharmacotherapy trial with two different active medication arms. Likewise, prior investigations of relapse predictors have not included smokers who quit while using varenicline. This secondary data analysis adds to the existing literature by expanding the range of quit methods represented in a sample of smokers who successfully initiate abstinence, thereby increasing the generalizability of findings regarding relapse risk.
2.0 Methods
2.1 Study design
Data for these post hoc analyses were derived from two phase III multicenter clinical trials of varenicline as a treatment for smoking cessation (NCT Identifier: NCT00143364 and NCT00141206). A total of 2,052 patients at 33 U.S. centers were enrolled in these identically-designed, randomized, double-blind trials, each of which compared the efficacy of varenicline (titrated to 0.5 mg/d for days 1 to 3, 0.5 mg twice per day for days 4 to 7, then 1 mg twice per day from day 8 through week 12) to both bupropion SR (titrated to 150 mg/d for days 1 to 3, then 150 mg twice per day through week 12) and placebo. A complete description of these studies has been provided previously (see Gonzales et al., 2006; Jorenby et al., 2006). Briefly, each trial included a 12-week treatment phase that included medication and brief individual counseling (i.e., 10 minutes or less per week), with a 40-week follow-up period. The target quit date was day 8 following the start of treatment. Although the medication was discontinued at the conclusion of the 12-week treatment phase, brief smoking cessation counseling was provided at the follow-up clinic visits, which occurred at weeks 13, 24, 36, 44, and 52. There were also telephone contacts at weeks 16, 20, 28, 32, 40, and 48 to assess participants' self-reported smoking status and to provide brief cessation counseling. At each clinic visit, self-report of abstinence from smoking was verified through an expired carbon monoxide (CO) measurement of 10 ppm or less. Continuous abstinence was defined as no smoking (or use of any other tobacco products) and having a CO ≤10 ppm throughout the designated period of evaluation. The primary efficacy endpoint for each study was continuous abstinence during the final 4 weeks of the treatment phase (weeks 9–12). Both studies were reviewed and approved by a human subjects committee and conducted in compliance with the ethical principles of the Declaration of Helsinki and the standards on good clinical practice developed by the International Conference on Harmonization.
In order to be included in the study, participants had to be current smokers between the ages of 18 and 75 who were motivated to quit and smoked at least 10 cigarettes per day for the past year, with no more than 3 consecutive months of abstinence from smoking during that time. Exclusion criteria were: unstable medical conditions or serious physical illness within the past 6 months; a body mass index less than 15 kg/m2 or higher than 38 kg/m2, or body weight less than 45 kg; any history of cancer or severe allergic reactions; an alcohol or other substance use disorder within the past year; a depressive disorder requiring treatment in the past year; lifetime diagnoses of psychosis, panic disorder, bipolar disorder, or eating disorders; prior use of bupropion or varenicline, or any contraindications to bupropion use; use of non-cigarette tobacco products; and use of any other smoking cessation medications within the past 30 days.
2.2 Participants
The 626 randomized participants who were continuously abstinent from smoking during the final 4 weeks of the treatment phase of both trials (weeks 9–12) were classified as successful quitters and included in the present analyses of relapse during the 40-week follow-up period. Of the 626 successful quitters, the distribution by treatment was as follows: 306 (49%) received varenicline, 199 (32%) received bupropion SR, and 121 (19%) received placebo. The mean age of these participants was 45.0 years (standard deviation [SD]=11.3). Approximately 86% (n=541) were Caucasian and 44% (n=278) were female. The average number of cigarettes smoked per day in the month prior to study entry was 20.4 (SD=8.5), and the mean Fagerström Test for Nicotine Dependence (FTND; Heatherton et al.,1991) score was 4.8 (SD=2.1).
2.3 Measures
Self-report data collected at baseline for each of the two phase III trials included demographics, smoking history, alcohol and cigarette consumption, and exposure to socioenvironmental smoking cues (i.e., presence of smokers in household, frequent contact with other smokers). Baseline alcohol consumption was reported in units per week (where 1 unit = 12 oz. of beer, 6 oz. of wine, or 1 oz. [30 ml] of liquor). The FTND was administered to assess severity of nicotine dependence. Serum cotinine levels were also assessed at baseline. The Minnesota Nicotine Withdrawal Scale (MNWS; Hughes and Hatsukami, 1986) was completed at multiple points throughout the trial, including clinic visits at baseline, weeks 1 through 7, and weeks 12 and 13. For the present data analyses, we used only the week 12 MNWS scores, as this was the last measurement period prior to follow-up. Urge to smoke (item 1) and negative affect (average of items 2 through 5) were both extracted from the MNWS data.
2.4 Data analyses
Relapse was defined as any self-report of smoking (even a puff) or use of other tobacco products during the follow-up period. Participants who did not report smoking but had a CO level of >10 ppm at any follow-up visit were classified as relapsers. Logistic regression analysis was used to examine predictors of relapse during weeks 13–52 of the study among the successful quitters. Potential predictors included individual-level demographic and smoking-related characteristics as well as baseline alcohol consumption. Longest period of abstinence from smoking during the year prior to study entry was transformed into a categorical variable (0 days, 1–30 days, or >30 days of abstinence) prior to conducting the regression analyses. Treatment-level predictors were also considered, including urge to smoke and negative affect at week 12 (as measured by the MNWS) and the length of the maximum continuous abstinence period following the quit date during the treatment phase of the trial, which ranged from 4 to 11 weeks. Table 1 provides a complete list of the individual- and treatment-level predictors that were considered.
Table 1.
Demographic and clinical characteristics, by relapse status.
| Characteristic | Relapser (n=301) |
Non-relapser (n=325) |
Difference | 95% CI around difference |
|---|---|---|---|---|
| Demographics | ||||
| Age (years), M (SD) | 44.1 (11.6) | 45.9 (11.0) | -1.8 | -3.53, 0.01 |
| Race (White), no. (%) | 256 (85.0) | 285 (87.7) | -2.7 | -8.03, 2.74 |
| Male, no. (%) | 165 (54.8) | 183 (56.3) | -1.5 | -9.28, 6.30 |
| Body mass index (kg/m2), M (SD) | 27.4 (4.8) | 27.2 (4.6) | 0.2 | -0.52, 0.96 |
| Smoking | ||||
| Age-at-onset of smoking (years), M (SD) | 17.1 (4.5) | 17.4 (4.8) | -0.3 | -1.04, 0.42 |
| Years of smoking, M (SD) | 25.7 (11.7) | 27.3 (11.1) | -1.6 | -3.31, 0.26 |
| Cigarettes/day (past mo.), M (SD) | 20.6 (8.8) | 20.3 (8.2) | 0.3 | -1.01, 1.66 |
| Longest abstinence in prior year, no. (%) | ||||
| 0 days | 152 (50.5) | 198 (60.9) | -10.4 | -18.17, -2.68 |
| 1–30 days | 125 (41.5) | 114 (35.1) | 6.4 | -1.16, 14.06 |
| >30 days | 24 (8.0) | 13 (4.0) | 4.0 | 0.24, 7.70 |
| Lives with a smoker, no. (%) | 98 (32.6) | 96 (29.5) | 3.1 | -4.23, 10.27 |
| Has frequent contact with a smoker, no. (%) | 203 (67.4) | 199 (61.2) | 6.2 | -1.28, 13.70 |
| Made ≥1 serious quit attempt, lifetime, no. (%) | 272 (90.4) | 285 (87.7) | 2.7 | -2.21, 7.56 |
| Pack-years, M (SD) | 26.2 (19.0) | 27.1 (17.9) | -0.9 | -3.81, 1.99 |
| FTND score, M (SD) | 4.8 (2.1) | 4.8 (2.1) | 0.0 | -0.28, 0.38 |
| Baseline serum cotinine (ng/ml), M (SD) | 153.4 (81.7) | 160.5 (88.4) | -7.1 | -20.57, 6.26 |
| Alcohol | ||||
| Baseline weekly alcohol consumption (unit),* M (SD) | 2.9 (4.4) | 3.2 (5.0) | -0.3 | -1.10, 0.38 |
| Urge to smoke, negative affect, and length of abstinence during the treatment phase | ||||
| MNWS urge to smoke, week 12 (no urge), no. (%) | 184 (61.7) | 211 (65.5) | -3.8 | -11.36, 3.77 |
| MNWS negative affect domain, week 12, M (SD) | 0.3 (0.4) | 0.3 (0.5) | 0.0 | -0.09, 0.06 |
| Quit pattern (immediate),† no. (%) | 152 (50.5) | 206 (63.4) | -12.9 | -20.59, -5.18 |
| MaxCA (weeks), M (SD) | 9.2 (2.4) | 10.1 (1.6) | -0.9 | -1.23, -0.59 |
Abbreviations: CI, confidence interval; FTND, Fagerström Test for Nicotine Dependence; MaxCA, maximum length of continuous abstinence during the treatment phase; MNWS, Minnesota Nicotine Withdrawal Scale.
1 unit = 12 oz. of beer, 6 oz. of wine, or 1 oz. (30 ml) of liquor.
Initiated abstinence on or before the target quit date.
Forward entry, backward elimination, and stepwise methods were used to identify robust predictors for inclusion in the final logistic regression model. A “protocol” variable was created to specify the study in which participants were enrolled (either Gonzales et al., 2006 or Jorenby et al., 2006), and this variable was forced into each model to control for differences between the two phase III studies. Each model used a p-value of .10 for the predictor to enter or remain in the resulting model. A reduced model was then created using only the predictors that were consistently significant in the initial models (forward, backward, and stepwise entry). In addition to the variables listed in Table 1, treatment group assignment (varenicline or bupropion SR or placebo) was also considered for inclusion in the initial models as well as in the reduced model as a covariate. Because it did not enter in the initial regression models or affect the relationships between the predictors and the criterion in the reduced models, treatment group assignment was dropped from further consideration as a predictor or covariate. Diagnostics for the reduced model were conducted using Hosmer and Lemeshow's (1989) goodness-of-fit test as well as index plots and influence statistics to identify outliers and influential cases, respectively.
Cox regression analyses were also conducted to examine time to relapse during follow-up (weeks 13–52), taking into account the censored nature of the data. Smoking status was assessed at weeks 13, 16, 20, 24, 28, 32, 36, 40, 44, 48, and 52. Time to relapse was defined as the week corresponding to the first follow-up visit when the subject reported smoking or using other tobacco products (weeks 13–52). For example, if the subject reported not smoking/using other tobacco products at week 16 and smoking/using nicotine at week 20, the time to relapse was set to 20. For subjects who discontinued before relapsing, time to relapse was set to the week corresponding to the first missing visit that occurred after the date of discontinuation. Subjects who did not relapse were assigned a censored time of 52. All the variables included in Table 1 were considered as covariates in the Cox regression analysis.
3.0 Results
Of the 626 smokers who met criteria for successful quitting during the two phase III trials (i.e., continuous abstinence during the final 4 weeks of the treatment phase), 301 (48.1%) relapsed during the post-treatment follow-up period (weeks 13–52). Table 1 characterizes the relapser and abstainer groups on the basis of variables that were considered as predictors in the logistic and Cox regression models of relapse.
On the basis of the initial three logistic regression analyses, the longest period of smoking abstinence in the year prior to study entry and the maximum continuous abstinence during the treatment phase of the study were identified as robust predictors of relapse. These variables were consequently entered in the final model. After controlling for protocol, both the longest period of abstinence in the past year (Wald χ2=8.71, p=.013) and the maximum continuous abstinence during the treatment phase of the trial (Wald χ2=28.40, p<.001) remained significant as predictors of relapse in the 40-week follow-up period. Table 2 provides a summary of the final regression model. Comparing the two groups with the shortest and longest periods of continuous abstinence during the trial (data not shown in table), individuals who did not achieve continuous abstinence until the end of the treatment period (i.e., 4-week continuous abstinence) had almost 5 times the odds to relapse during the follow-up period as those who maintained abstinence from their quit date through the end of the 12-week treatment period (i.e., 11-week continuous abstinence) (odds ratio [OR]=4.92, 95% confidence interval [CI]: 2.77–8.97). Additionally, participants who reported >30 days of smoking abstinence in the past year had more than twice the odds to relapse compared with those who had no days of abstinence in the past year (OR=2.38, 95% CI: 1.17–5.04).
Table 2.
Results of the logistic regression and Cox regression models
| Model 1: Logistic regression to predict relapse | |||||
|---|---|---|---|---|---|
| Variable | β | SE | p | OR | 95% CI |
| Protocol* | 0.05 | 0.08 | 0.511 | 1.12 | 0.81–1.54 |
| Longest abstinence in prior year (LAPY)** | 0.013 | ||||
| LAPY = 1–30 days (vs. 0 days) | 0.38 | 0.17 | 0.028 | 1.47 | 1.04–2.06 |
| LAPY >30 days (vs. 0 days) | 0.87 | 0.37 | 0.019 | 2.38 | 1.15–4.91 |
| MaxCA† | −0.23 | 0.04 | <0.001 | 0.80 | 0.73–0.87 |
| Model 2: Cox regression to predict time to relapse | |||||
| Variable | β | SE | p | HR | 95% CI |
| Protocol* | −0.01 | 0.01 | 0.543 | 0.99 | 0.96–1.02 |
| Abst1yr** | 0.21 | 0.09 | 0.027 | 1.23 | 1.02–1.47 |
| MaxCA† | −0.16 | 0.03 | <0.001 | 0.85 | 0.81–0.89 |
Abbreviations: SE, standard error; p, p-value; OR, odds ratio; HR, hazard ratio; CI, confidence interval.
Protocol 1 (Gonzales et al., 2006) vs. Protocol 2 (Jorenby et al., 2006).
Longest period of abstinence (days) in the year prior to treatment, categorized as: 0, 1–30, and >30.
Maximum length of continuous abstinence (weeks) during the treatment phase.
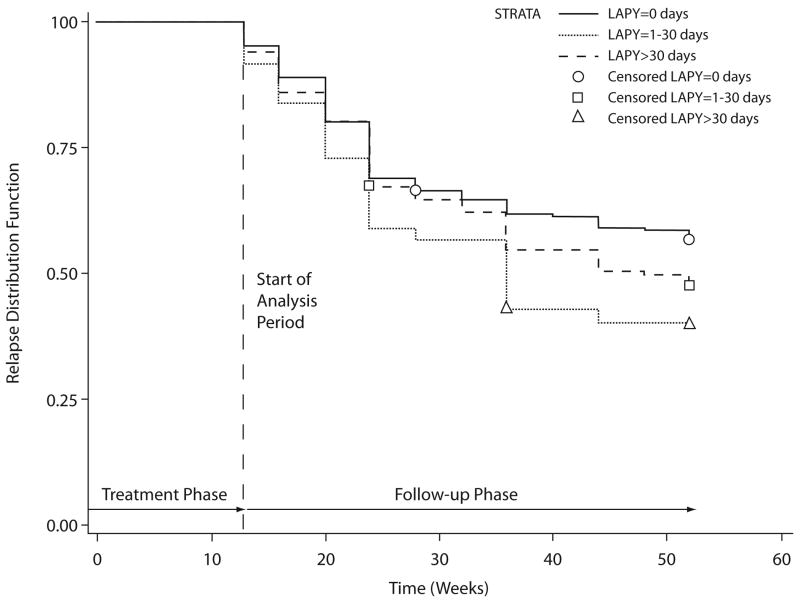
The results of the Cox regression paralleled those of the logistic regression analysis. After controlling for protocol, both the length of maximum continuous abstinence during the treatment phase of the trial (Wald χ2=42.04, p<.001, hazard ratio [HR]=0.85, 95% CI: 0.81–0.89) and the longest period of abstinence during the past year (Wald χ2=4.89, p=.027, HR=1.23, 95% CI: 1.02–1.47) were predictive of relapse risk. Figures 1 and 2 shows the Kaplan–Meier plots of time to relapse as a function of the length of maximum continuous abstinence during the treatment phase of the trial and the longest period of abstinence in the past year, respectively.
Figure 1.
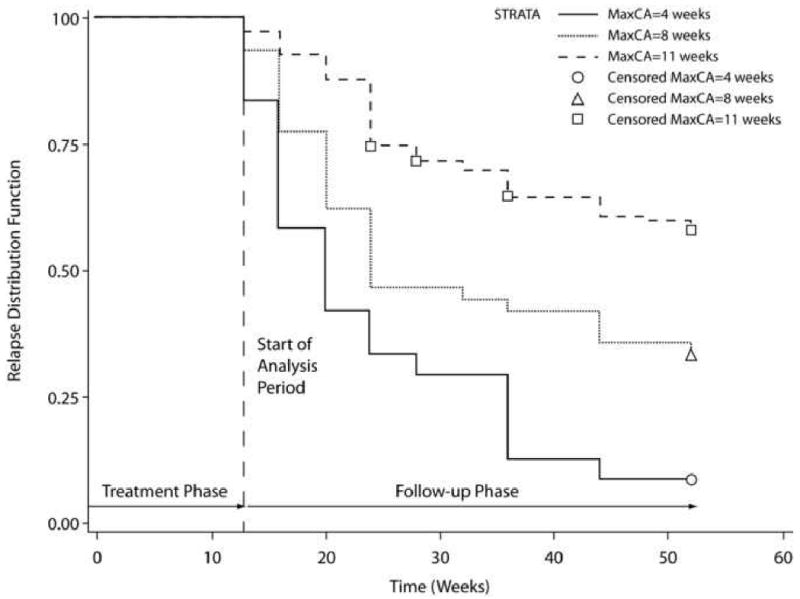
Kaplan–Meier plot of time to relapse by maximum length of continuous abstinence (MaxCA) during the treatment phase of the trial.
Figure 2.
Kaplan–Meier plot of time to relapse by longest abstinence (in days) in the prior year (LAPY), categorized as: 0, 1–30, and >30.
Based on the calculated HRs for these variables (see Table 2), we concluded that: 1) each 1-week increase in continuous abstinence following the target quit date is associated with a 15% reduction in the risk of relapse during follow-up, and 2) each incremental increase in length of abstinence in the year prior to treatment (i.e., 1–30 days, >30 days) is associated with an approximately 23% increase in relapse risk.
4.0 Discussion
In this study, we analyzed pooled data from two large, multicenter smoking cessation trials to identify predictors of post-treatment relapse among smokers who successfully initiated abstinence with the aid of brief counseling in combination with one of three orally administered treatments: varenicline, bupropion, or placebo. Of the 19 demographic, smoking-, alcohol-, and treatment-related events that were considered as potential predictors of relapse, only two were significant: fewer weeks of continuous abstinence during the 12-week treatment phase of the trial and a longer period of abstinence in the year prior to study entry.
Given the robustness of findings from prior research suggesting that any smoking after the target quit date predicts relapse (Dale et al., 2001; Kenford et al., 1994; Hurt et al., 1994; Yudkin et al.,1996; Higgins et al., 2006; Gourlay et al., 1994), our results confirm the prognostic value of early initiation and maintenance of smoking abstinence during the treatment period and suggest that this previously reported relationship is generalizable to smokers who quit with the assistance of varenicline. The roughly 5-fold increase in the odds of relapse associated with quitting late in the course of treatment, as opposed to on or before the quit date, highlights the potential utility of extended treatment for this subset of smokers who are at higher risk for relapse. Although behavioral and pharmacologic relapse prevention interventions have, as a whole, failed to produce improvements in long-term smoking cessation rates (Hajek et al., 2009), there is initial evidence from one study that maintenance therapy with varenicline (Tonstad et al., 2006) may be beneficial in this regard. Extended nicotine replacement therapy (NRT) may also hold promise, but the results of the few studies of extended NRT are mixed (Hajek et al., 2009) and further investigation is necessary before any well-supported conclusions can be stated.
The finding that longer periods of abstinence in the year prior to the quit attempt were associated with increased odds of relapse was contrary to prediction. Where the length of past quit attempts has predicted maintenance of smoking abstinence in prior studies (e.g., Murray et al., 2000; Carlson et al., 2000; Garvey et al., 1992; Velicer et al., 2007), the nature of the relationship has been overwhelmingly in the direction of longer periods of past abstinence predicting better long-term outcomes, or conversely, less likelihood of relapse. However, a U-shaped relationship between length of abstinence and maintenance of smoking abstinence has been observed in several studies, suggesting that either 0 days of abstinence or >30 days of abstinence are associated with better outcomes than 1–30 days of abstinence (Ferguson et al., 2003; Dale et al., 1997; Dale et al., 2001).
Bandura's (1977) theory of self-efficacy has been directly or indirectly invoked by the authors of two of these studies (Dale et al., 1997; Dale et al., 2001) as well as others (e.g., Yzer and van den Putte, 2006) to explain the observed curvilinear relationships between length of past abstinence and success at long-term quitting. In this model, smokers who have not tried to quit or who have quit for longer periods of time in the past would demonstrate greater self-efficacy and have a greater likelihood of persisting and succeeding at this difficult task than those who had relapsed quickly (Yzer and van den Putte, 2006), and failed efforts at quitting should have the strongest effect on self-efficacy when they have occurred in the recent (e.g., past year) versus remote past (Bandura, 1977). In the present study, this could explain the reduced likelihood of smoking relapse among individuals with 0 days of past-year abstinence as opposed to 1–30 days of abstinence, but it does not explain why individuals with >30 days of past-year abstinence had the highest odds of relapse. It could, however, be an artifact of the inclusion/exclusion criteria for the study, which required participants to have no more than 3 consecutive months of smoking abstinence in the prior year. Thus, it is possible that the truncated upper limit of past-year abstinence obscured the potential to detect a curvilinear relationship and that 3 months is a sufficiently short period of abstinence to decrease self-efficacy for quitting.
The lack of an observed relationship between the 17 other potential predictors and smoking relapse is no less noteworthy than the identification of two statistically significant predictors, particularly given that there was an empirical precedent for all of the included variables in relation to relapse. However, considering that relapse propensity is considered a dynamic process (Cui et al., 2006), the relationship between any given predictor and smoking relapse is likely to be complex and to vary as a function of the time at which the predictor is measured (i.e., temporal proximity to the outcome) and the length of the interval over which the outcome is observed (i.e., length of post-treatment follow-up) (Nides et al., 1995). Thus, the presence or absence of observed relationships in the present study may be relatively time-dependent and not generalizable to all points or intervals of measurement. Additionally, the definition of relapse employed in this study (i.e., any smoking during follow-up, even a puff) was fairly stringent, and, for that reason, our results may not be comparable to the results of other studies that define relapse as several consecutive days or episodes of smoking (e.g., Garvey et al., 1992). At the same time, there is reason to believe that the distinction between one-time use and resumption of regular use is negligible given that the vast majority of smokers (88%) who smoke one post-cessation cigarette revert to regular smoking (Brandon et al., 1990).
This study had significant strengths as well as limitations. The sample represents a large, well-defined group of smokers who successfully initiated abstinence with the aid of counseling and one of two medications or placebo, which includes a broader range of quit methods than has been represented in prior studies examining predictors of post-treatment relapse. In terms of limitations, the timing of the assessment of several predictors may have obscured the potential to detect relationships between these variables and smoking relapse. For example, alcohol use was assessed only at baseline, and negative affect and urge to smoke (included in the MNWS) were not continuously assessed throughout the post-treatment follow-up period. Consequently, it is possible that these variables would have been associated with relapse had their assessment occurred closer to the time of relapse. Finally, the exclusion of light smokers and individuals with psychiatric or substance use disorders from study participation limits the generalizability of the findings.
Advancing knowledge of factors that predict relapse to smoking should facilitate the development of novel or improved relapse prevention methods, which are greatly needed considering the formidable challenge of improving long-term abstinence rates. Continued efforts to identify short- and long-term relapse predictors are warranted, particularly in populations of smokers that have traditionally been excluded from smoking cessation treatment research (e.g., individuals with psychiatric disorders, light smokers). Further research is also required to determine the efficacy of tailored interventions for smokers who are at high risk for relapse, such as those who are not able to initiate abstinence until several weeks after their quit date.
Acknowledgments
Data for this study were derived from two phase III clinical trials that were sponsored by Pfizer, Inc. (NCT Identifier: NCT00143364 and NCT00141206). The authors would like to thank Simon Davies, Ph.D., and Carmen Arteaga, Ph.D., of Pfizer, Inc. for assistance in conducting the statistical analyses; Cristina Russ, M.D., and Peter Park, Ph.D., of Pfizer, Inc. for contributing to discussions of the data analysis plan and interpretation of the results; and Ray Beck, Jr., Ph.D., Joanne Cowan Ph.D., and Penny Gorringe, MSc., of UBC Scientific Solutions for editorial assistance in the form of proof reading, collection of authors review comments, formatting the manuscript for submission, and preparation of figures (which was funded by Pfizer, Inc.).
Role of funding source: The studies described in this manuscript were funded by Pfizer, Inc., which provided support with study design; study drug and placebo; monitoring; collection of data and maintenance of the two study databases containing findings of the investigator sites. Statistical analyses were performed by Simon Davies, Ph.D., and Carmen Arteaga, Ph.D., of Pfizer, Inc. and Cristina Russ, M.D., of Pfizer, Inc. contributed towards discussions of the data analysis plan and interpretation of the results. Theodore Lee, M.D., of Pfizer, Inc. provided support with writing and submitting the paper for publication.
Dr. Heffner and Dr. Anthenelli were supported, in part, by National Institute on Alcohol Abuse and Alcoholism grants #AA013307 and #AA013957, National Institute on Drug Abuse/Veterans Affairs CSP #1022, and by the Department of Veterans Affairs.
Footnotes
Contributors: All the authors listed have materially participated in the research and manuscript preparation. Dr. Heffner managed the literature search and summaries of previous related work and wrote the first draft of the manuscript. Dr. Heffner, Dr. Anthenelli, Dr. Arteaga, and Dr. Lee all contributed to subsequent drafts of manuscripts and approved the final manuscript.
Conflict of Interests: Dr. Heffner has no competing interests to disclose. Dr. Anthenelli provides consultancy, advisory, and/or speakers' bureau services to sanofi-aventis and Pfizer, Inc. The Tri-State Tobacco and Alcohol Research Center receives research support from Eli Lilly and Company and Pfizer, Inc. Dr. Lee and Dr. Arteaga are employees of and stockholders in Pfizer, Inc.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bandura A. Self-efficacy: Toward a unifying theory of behavioral change. Psychol Rev. 1977;84:191–215. doi: 10.1037//0033-295x.84.2.191. [DOI] [PubMed] [Google Scholar]
- Brandon TH, Tiffany ST, Obremski KM, Baker TB. Postcessation cigarette use: The process of relapse. Addict Behav. 1990;15:105–114. doi: 10.1016/0306-4603(90)90013-n. [DOI] [PubMed] [Google Scholar]
- Carlson LE, Taenzer P, Koopmans J, Bultz BD. Eight-year follow-up of a community-based large group behavioral smoking cessation intervention. Addict Behav. 2000;25:725–741. doi: 10.1016/s0306-4603(00)00081-2. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Cigarette smoking among adults—United States. MMWR. Morbidity and Mortality Weekly Report. 2000;51:642–645. [PubMed] [Google Scholar]
- Cui Y, Wen W, Moriarty CJ, Levine RS. Risk factors and their effects on the dynamic process of smoking relapse among veteran smokers. Behav Res Ther. 2006;44:967–981. doi: 10.1016/j.brat.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Dale LC, Glover ED, Sachs DPL, Schroeder DR, Offord KP, Croghan IT, Hurt RD. Buproprion for smoking cessation: Predictors of successful outcome. Chest. 2001;119:1357–1364. doi: 10.1378/chest.119.5.1357. [DOI] [PubMed] [Google Scholar]
- Dale LC, Olsen DA, Patten CA, Schroeder DR, Croghan IT, Hurt RD, Offord KP, Wolter TD. Predictors of smoking cessation among elderly smokers treated for nicotine dependence. Tob Control. 1997;6:181–187. doi: 10.1136/tc.6.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson JA, Patten CA, Schroeder DR, Offord KP, Eberman KM, Hurt RD. Predictors of 6-month tobacco abstinence among 1224 cigarette smokers treated for nicotine dependence. Addict Behav. 2003;28:1203–1218. doi: 10.1016/s0306-4603(02)00260-5. [DOI] [PubMed] [Google Scholar]
- Garvey AJ, Bliss RE, Hitchcock JL, Heinold JW, Rosner B. Predictors of smoking relapse among self-quitters: A report from the normative aging study. Addict Behav. 1992;17:367–377. doi: 10.1016/0306-4603(92)90042-t. [DOI] [PubMed] [Google Scholar]
- Gonzales D, Rennard SI, Nides M, Oncken C, Azoulay S, Billing CB, Watsky EJ, Gong J, Williams KE, Reeves KR, Varenicline Phase 3 Study Group Varenicline, an α4β2 nicotinic acetylcholine receptor partial agonist, vs. bupropion and placebo for smoking cessation: A randomized controlled trial. JAMA. 2006;296:47–55. doi: 10.1001/jama.296.1.47. [DOI] [PubMed] [Google Scholar]
- Gourlay SG, Forbes A, Marriner T, Pethica D, McNeil JJ. Prospective study of factors predicting outcome of transdermal nicotine treatment in smoking cessation. BMJ (Clinical research ed) 1994;309:842–846. doi: 10.1136/bmj.309.6958.842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajek P, Stead LF, West R, Jarvis M, Lancaster T. Relapse prevention interventions for smoking cessation. Cochrane Database of Systematic Reviews. 2009 January 21;(1) doi: 10.1002/14651858.CD003999.pub3. CD003999. Retrieved February 1, 2009 from http://mrw.interscience.wiley.com/cochrane/clsysrev/articles/CD003999/frame.html. [DOI] [PubMed]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström test for nicotine dependence: A revision of the Fagerström tolerance questionnaire. British Journal of Addiction. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Heil SH, Dumeer AM, Thomas CS, Solomon LJ, Bernstein IM. Smoking status in the initial weeks of quitting as a predictor of smoking-cessation outcomes in pregnant women. Drug Alcohol Depend. 2006;85:138–141. doi: 10.1016/j.drugalcdep.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Hosmer DW, Lemeshow S. Applied logistic regression. Wiley; New York: 1989. [Google Scholar]
- Hughes JR, Hatsukami D. Signs and symptoms of tobacco withdrawal. Arch Gen Psychiatry. 1986;43:289–294. doi: 10.1001/archpsyc.1986.01800030107013. [DOI] [PubMed] [Google Scholar]
- Hurt RD, Dale LC, Fredrickson PA, Caldwell CC, Lee GA, Offord KP, Lauger GG, Marŭsić Z, Neese LW, Lundberg TG. Nicotine patch therapy for smoking cessation combined with physician advice and nurse follow-up. One-year outcome and percentage of nicotine replacement. JAMA. 1994;271:595–600. [PubMed] [Google Scholar]
- Jorenby DE, Hays JT, Rigotti NA, Azoulay S, Watsky EJ, Williams KE, Billing CB, Gong J, Reeves KR, Varenicline Phase 3 Study Group Efficacy of varenicline, an α4β2 nicotinic acetylcholine receptor partial agonist, vs. placebo or sustained-release bupropion for smoking cessation. JAMA. 2006;296:56–63. doi: 10.1001/jama.296.1.56. [DOI] [PubMed] [Google Scholar]
- Kenford SL, Fiore MC, Jorenby DE, Smith SS, Wetter D, Baker TB. Predicting smoking cessation: Who will quit with and without the nicotine patch. JAMA. 1994;271:589–594. doi: 10.1001/jama.271.8.589. [DOI] [PubMed] [Google Scholar]
- Kenford SL, Smith SS, Wetter DW, Jorenby DE, Fiore MC, Baker TB. Predicting relapse back to smoking: Contrasting affective and physical models of dependence. J Consult Clin Psychol. 2002;70:216–227. [PubMed] [Google Scholar]
- Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Medicine. 2006;3:e442. doi: 10.1371/journal.pmed.0030442. Retrieved July 1, 2008 from http://medicine.plosjournals.org/perlserv/?request=getdocument&doi=10.1371%2Fjournal.pmed.0030442&ct=1. [DOI] [PMC free article] [PubMed]
- Murray RP, Gerald LB, Lindgren PG, Connett JE, Rand CS, Anthonisen NR. Characteristics of participants who stop smoking and sustain abstinence for 1 and 5 years in the Lung Health Study. Prev Med. 2000;30:392–400. doi: 10.1006/pmed.2000.0642. [DOI] [PubMed] [Google Scholar]
- Nides MA, Rakos RF, Gonzales D, Murray RP, Tashkin DP, Bjornson-Benson WM, Lindgren P, Connett JE. Predictors of initial smoking cessation and relapse through the first 2 years of the Lung Health Study. J Consult Clin Psychol. 1995;63:60–69. doi: 10.1037//0022-006x.63.1.60. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Engberg JB, Paty JA, Perz WG, Gnys M, Kassel JD, Hickcox M. A day at a time: Predicting smoking lapse from daily urge. J Abnorm Psychol. 1997;106:104–116. doi: 10.1037//0021-843x.106.1.104. [DOI] [PubMed] [Google Scholar]
- Tonstad S, Tonneson P, Hajek P, Williams KE, Billing CB, Reeves KR. Effect of maintenance therapy with varenicline on smoking cessation. JAMA. 2006;296:64–71. doi: 10.1001/jama.296.1.64. [DOI] [PubMed] [Google Scholar]
- Velicer WF, Redding CA, Sun X, Prochaska JO. Demographic variables, smoking variables, and outcome across five studies. Health Psychol. 2007;26:278–287. doi: 10.1037/0278-6133.26.3.278. [DOI] [PubMed] [Google Scholar]
- Ward KD, Klesges RC, Zbikowski SM, Bliss RE, Garvey AJ. Gender differences in the outcome of an unaided smoking cessation attempt. Addict Behav. 1997;22:521–533. doi: 10.1016/s0306-4603(96)00063-9. [DOI] [PubMed] [Google Scholar]
- Yudkin PL, Jones L, Lancaster T, Fowler GH. Which smokers are helped to give up smoking using transdermal nicotine patches? Results from a randomized, double-blind, placebo-controlled trial. Br J Gen Pract. 1996;46:145–148. [PMC free article] [PubMed] [Google Scholar]
- Yzer MC, van den Putte B. Understanding smoking cessation: The role of smokers' quit history. Psychology of Addict Behav. 2006;20:356–361. doi: 10.1037/0893-164X.20.3.356. [DOI] [PubMed] [Google Scholar]