Abstract
Flavopiridol, a cyclin-dependent kinase inhibitor, is cytotoxic to leukemic blasts. In a Phase II study, flavopiridol 50 mg/m2 was given by 1-hour infusion daily × 3 beginning Day 1 followed by 2 gm/m2/72 hr ara-C beginning Day 6 and 40 mg/m2 mitoxantrone on Day 9 (FLAM) to 45 adults with newly diagnosed acute myelogenous leukemia (AML) with multiple poor risk features. Thirty patients (67%) achieved complete remission (CR) and 4 (9%) died. Twelve (40%) received myeloablative allogeneic bone marrow transplant (BMT) in first CR. Median OS and DFS are not reached (67% alive 12.5–31 months, 58% in CR 11.4–30 months), with median follow-up 22 months. Sixteen received FLAM in CR, with median OS and DFS 9 and 13.1 months, and 36% alive at 21–31 months. Short OS and DFS correlated with adverse cytogenetics, regardless of age or treatment in CR. The addition of allogeneic BMT in CR translates into long OS and DFS in the majority of eligible patients.
Keywords: Flavopiridol, AML, Poor Risk, Timed Sequential Therapy
INTRODUCTION
Adults with newly diagnosed acute myelogenous leukemia (AML) with specific risk features have a poor prognosis in terms of achievement and duration of complete remission (CR). Diverse independent studies have identified secondary AML – i.e., treatment-related or arising from myelodysplasia (MDS) or myeloproliferative disorder (MPD) – and AML presenting with adverse genetics (including chromosome 3 abnormalities, −5/5q, −7/7q, +8, 11q23 abnormalities, 20q−, complex karyotypes, FLT-3 mutations), among others, as particularly poor risk.1–3 For such patients, despite intensive multiagent chemotherapy, CR is achieved in ≤ 30% with 3–5 year survival in <10%, while the CR rate for patients without poor-risk features is ≥ 70% with 3–5 year survival of 30–40%.
CR rate and duration also decrease with increasing age (i.e., ≥ 60), with CR rates <50%, even without overt poor-risk features, and a 3–5 year survival ≤ 10–15%.2,4 Even in a study of non-cross-resistant, response-adapted therapy by van der Jagt, et al,5 where the CR rate was 67% in 42 adults over age 60 with de novo AML, the 5 year overall survival (OS) and disease free survival (DFS) of CR patients were only 9.7% and 8.3%. Mortality during induction therapy in the older age group was 26%.6 Along similar lines, Lowenberg, et al,6 demonstrated that doubling the Daunorubicin dose during induction therapy for “fit”AML patients age 60 and older improved the CR rate from 54% to 64%, with achievement of CR following a single induction cycle in 52% of high-dose vs. 35% of conventional dose group. High dose Daunorubicin yielded improvement in 2 year OS and event free survival (EFS) in the younger patient subgroup (ages 60–65), but did not have a major impact on OS and EFS in patients with adverse cytogenetics, independent of age.6 In contrast, Fernandez’s, et al, study of high-dose Daunorubicin in younger adults under age 60 yielded increases in both CR rate (71% vs. 57%) and OS (23.7 vs. 15.3 months).7 However, there was no apparent benefit for patients age 50–60 or those with unfavorable cytogenetics or FLT-3 mutations.
Flavopiridol 8,9 inhibits growth and induces apoptosis in diverse hematopoietic cell lines.10–12 This apoptosis results at least in part from inhibition of multiple serine-threonine cyclin dependent kinases (CDKs) with cell cycle arrest in G1 and G2.13–15 Inactivation of the CDK9/cyclin T complex (PTEF-b) inhibits phosphorylation of RNA polymerase II, diminishes mRNA synthesis16,17 and blocks production of polypeptides such as cyclin D19,18 and the pro-survival protein MCL-1.12,19
We previously reported on longitudinal clinical-laboratory studies of flavopiridol followed in a timed sequential manner by the cell cycle-dependent, antileukemia drugs cytosine arabinoside (ara-C) and mitoxantrone.20–22 The hypothesis-driven regimen (“FLAM”) was generated based on in vitro modeling where administration of flavopiridol to marrow leukemic blasts followed sequentially by ara-C resulted in synergistic enhancement of ara-C-related blast cell apoptosis.20,23 In a recent Phase II trial of FLAM, 15 patients had newly diagnosed, poor risk AML with multiple poor-risk features including older age (100% > 50 years), secondary AML (100%), and adverse genetic features (53%).22 Twelve (75%) achieved CR, with a 2 year disease free survival (DFS) of 50%. These results compared favorably with historical timed sequential therapy (TST) regimens using sequential ara-C, anthracycline and either amsacrine24 or VP-16,25 in which CR rates are 40–45% for patients ≥ 55 years of age and 30–40% for patients with adverse cytogenetics.
We have now expanded our investigation of FLAM to establish a more accurate estimate of efficacy in inducing durable CRs in this patient population. Further, we evaluated the ability for this regimen to achieve a CR without severe toxicity and, in turn, permit successful allogeneic bone marrow transplantation (BMT) in eligible patients in first CR.26
PATIENTS, MATERIALS AND METHODS
Patient Eligibility and Selection
From December 2006 through June 2008, adults ≥18 years with pathologically confirmed, newly diagnosed, previously untreated AML with poor risk features including age ≥ 50, secondary AML (MDS/AML, MPD/AML, treatment-related AML) and/or known adverse cytogenetics were eligible provided they had ECOG performance status 0–2; normal bilirubin; hepatic enzymes ≤2× normal; serum creatinine ≤1.5× normal; LVEF ≥45%. All patients with MDS/AML or MPD/AML had previous documentation of the original hematologic disorder. Complete history, physical examination, laboratory, imaging and cardiac evaluations (EKG, LVEF) were performed within 3 days of study entry. Patients were ineligible if they had a peripheral blast count ≥50,000/mm3, but cytoreduction with hydroxyurea (HU) was permitted until 24 hours prior to flavopiridol. Prior therapy for MDS or MPD (thalidomide/lenalidomide, 5-azacitidine/decitabine, interferon, low-dose cytoxan, cytokines, HU) was permitted. Additional criteria for ineligibility included disseminated intravascular coagulation (DIC); active uncontrolled infection; active CNS leukemia; prior radiation of >25% of bone marrow; concomitant radiotherapy, chemotherapy or immunotherapy; or coexisting medical or psychiatric conditions that could interfere with study procedures. Pregnant or lactating women were ineligible. All patients provided written informed consent according to The Johns Hopkins Medical Institutional Review Boards and guidelines.
Treatment Schema
All patients were treated as inpatients at the Johns Hopkins Sidney Kimmel Comprehensive Cancer Center. As previously described,21,22 flavopiridol was administered at a dose of 50 mg/m2 over 1 hour daily for 3 days beginning Day 1. A 72 hour continuous infusion of ara-C 2 gms/m2 (667 mg/m2/24 hrs) began Day 6. Mitoxantrone 40 mg/m2 was administered as a single intravenous bolus over 60–120 minutes on Day 9, 12 hrs after completing the ara-C infusion. Patients who achieved CR after cycle 1 were eligible to receive a second cycle of FLAM (consolidation) beginning 30 ± 7 days following hospital discharge from the first cycle. Patients who achieved CR and had a suitable matched related or unrelated donor or a related haploidentical donor were eligible to undergo allogeneic BMT following the first or second cycle of FLAM.
Supportive care
All patients received daily oral allopurinol and the phosphate binder sevelamer until 24 hours after completion of ara-C and mitoxantrone (Day 9). Corticosteroid eyedrops were given Days 6–12 to prevent ara-C related conjunctivitis. Antiemetics were used according to standard practices. Pre-menopausal women received hormonal therapy to suppress menstrual bleeding. Norfloxacin 400 mg orally BID for gastrointestinal (GI) decontamination and Acyclovir prophylaxis against Herpes Simplex virus activation began day 1 and continued until absolute neutrophil count (ANC) >100/mm3 was reached.
Response and Toxicity Evaluations
Bone marrow aspirates and biopsies were performed prior to treatment, on day 14, and at the time of hematologic recovery or when leukemia regrowth was suspected. Hematologic recovery was defined as ANC ≥ 500/mm3 and transfusion-independent platelet count of 50,000/mm3. CR required normal marrow aspirate with absence of identifiable leukemia, ANC ≥1,000/mm3, platelet count ≥100,000/mm3, and absence of blasts in peripheral blood.27 Clearance of cytogenetic abnormalities was not required for CR, but was noted and described separately. No response (NR) was defined as persistent leukemia in marrow and/or blood without significant decrease from pretreatment levels. Adverse events were described and graded based on NCI Common Toxicity Criteria, version 3.0 and treating physician’s assessment.
Statistical Considerations
Overall survival (OS) was calculated from day 1 of FLAM to date of death. DFS was calculated from achievement of CR to date of relapse. CR had to last ≥ 1 month to qualify as a CR.27 Patients who were still alive and/or disease-free were censored at July 1, 2009. OS and DFS probabilities were estimated using the Kaplan-Meier method. Survival curves were compared between groups with a log rank statistic. For the comparisons of OS and DFS by age group, BMT recipients were censored at date of BMT. Cox proportional hazards models compared risks of death and relapse between patients with poor risk vs. non-poor risk cytogenetics and type of treatment in CR (BMT vs. no BMT), while adjusting for age. All analyses were completed using statistical software package R 2.8.1.
RESULTS
Patient Characteristics
A total of 45 adults (median age 61, range 22–72) with newly diagnosed AML with poor risk features were entered on study between December 2006 and June 2008. As depicted in Table 1, 37 patients (82%) had secondary AML and/or prominent trilineage dysplasia (TLD) consistent with preceding MDS and 24 (53%) had adverse cytogenetics. An additional 9 (20%) had FLT-3 mutations consisting of internal tandem duplication (ITD) in 7 (15%) or D835S point mutation in 2 (4%). Eight of the 9 had normal cytogenetics and one had t(8;21) in addition to his FLT-3 ITD. In all, 33 (73%) had AML with adverse genetic features. The majority (31/45, 69%) had AML with ≥2 poor-risk disease features, including all 5 patients under age 50. Only 4 (9%) had no poor risk disease features other than age ≥ 50 (ages 59–63). Age was not considered in calculating poor risk factors.
Table 1.
Pretreatment Demographic and Biologic Characteristics of 45 Adults with Newly Diagnosed AML with Poor Risk Features Treated with FLAM
| Gender | |||
|---|---|---|---|
| Male | 22 (49%) | ||
| Female | 23 (51%) | ||
| Median Age (Range) | 61 (22–72)* | ||
| Biologic Disease Features | |||
| Etiology | |||
| Secondary AML | 37 (82%) | ||
| MDS/AML (including TLD) | 23 | ||
| MPD/AML (including CMML) | 5 | ||
| Treatment-Related AML | 9 | ||
| Prior MDS/MPD Therapy | 6 | ||
| Genetics | |||
| Adverse Cytogenetics | 24 (53%) | ||
| Single −5 or −7 | 3 | ||
| 11q23 translocation | 4** | ||
| Complex (≥ 3 lesions) | 11 | ||
| F LT-3 mutation (normal cytogenetics) | 9 (20%) | ||
| Proliferation | |||
| Peripheral Blood Blasts ≥ 20,000/mm3 | 14 (31%) | ||
| HU (blasts ≥ 50,000/mm3) | 6 (13%) | ||
| No. Patients with Poor Risk AML Features | |||
| None | 4 (9%) | ||
| One | 10 (22%) | ||
| Two or more*** | 31 (69%) | ||
AML, acute myelogenous leukemia; MDS, myelodysplasia; TLD, trilineage dysplasia; MPD, myeloproliferative disorder; CMML, chronic myelomonocytic leukemia; WBC, white blood count; HU, hydroxyurea
5 (11%) younger than age 50 (22–49)
3 of 4 with treatment-related AML
Includes all 5 patients under age 50
Toxicities
Tumor lysis occurred during flavopiridol administration in 19 (42%), manifested by one or more of the following criteria: elevations in serum phosphate (range 4.9–17.8 mg/dl) in 14 (31%), LDH ≥ 5×ULN in 14 (31%), and creatinine (range 1.5–3.5 mg/dl) in 3 (7%). Chemical evidence of lysis began within 12 hours of administering the first flavopiridol dose and peaked on median Day 1 (range 1–4). Two patients developed subclinical DIC. One 59 year old male (acute monoblastic leukemia, FLT-3 ITD positive, WBC 92,000/mm3 before HU cytoreduction over 48 hours to 30,000/mm3) experienced hyperkalemia and DIC with multi-organ failure following the first dose of flavopiridol and succumbed 72 hours later despite rapid intervention with mechanical ventilation and hemodialysis.
Time from initiation of therapy to hematologic recovery was similar to previous TST studies.21,22,24,25 Median time to ANC ≥ 500/mm3 was 32 days (range 23–52) and median time to platelets ≥ 50,000/mm3 was 31 days (range 23–65). Oral mucositis following ara-C and mitoxantrone occurred in 14 (30%) of patients, with 12/14 being grade 2 or less. Flavopiridol-induced diarrhea occurred in 11 (24%) but was grade 3 in only 2 (4%) and self-limited in all cases. Grade 3 GI mucositis following ara-C and mitoxantrone occurred in 5 (11%) and consisted of typhlitis (2), Clostridium dificile colitis (2), and colonic bleeding (1).
Seven patients (16%) experienced cardiac dysfunction during or after FLAM therapy. Five patients developed reversible supraventricular arrhythmias in the setting of sepsis. Two women, ages 60 and 69, with treatment-related AML developed symptomatic cardiomyopathies with decreases in LVEF from 50–65% pretreatment to 15–25% between 1.5–4 months after completing FLAM. Both patients had prior anthracyclines and chest irradiation, but did not reach limiting total anthracycline doses before or after mitoxantrone. A 60 year old gentleman suffered cardiac ischemia with a small troponin leak in the setting of overwhelming fungal infection and did not receive mitoxantrone on day 9. Four patients (9%) died from complications of FLAM induction therapy. Two died within 30 days of beginning therapy (1 grade 5 tumor lysis, 1 fungal infection) and two died days 35 and 50 from sepsis.
Clinical Outcome
Flavopiridol administration was associated with a ≥ 50% decrease in peripheral blood blast counts in 26 (58%) patients following the first drug dose. No patients had sustained increases in peripheral counts during flavopiridol administration. Response to FLAM was assessed initially by day 14 bone marrow aspirates and biopsies in 42 patients. Complete tumor clearance with marrow cellularity ≤ 10% was achieved in 22 (54%), of whom 19 achieved CR. Nine patients (22%) had small numbers of blasts (median 1%, range 0.5–3.5%) with marrow cellularity ≤ 20% and CR was achieved in 7. Two of 4 patients who had ≥ 5% and <10% blasts and variable decreases in marrow cellularity (≤ 5–50%) achieved CR, while none of 7 with ≥ 10% blasts (marrow cellularity 20–70%) on day 14 achieved CR. Three patients did not have day 14 marrow aspirates: 1 grade 5 tumor lysis died day 4, 2 refused (1 CR, 1 NR).
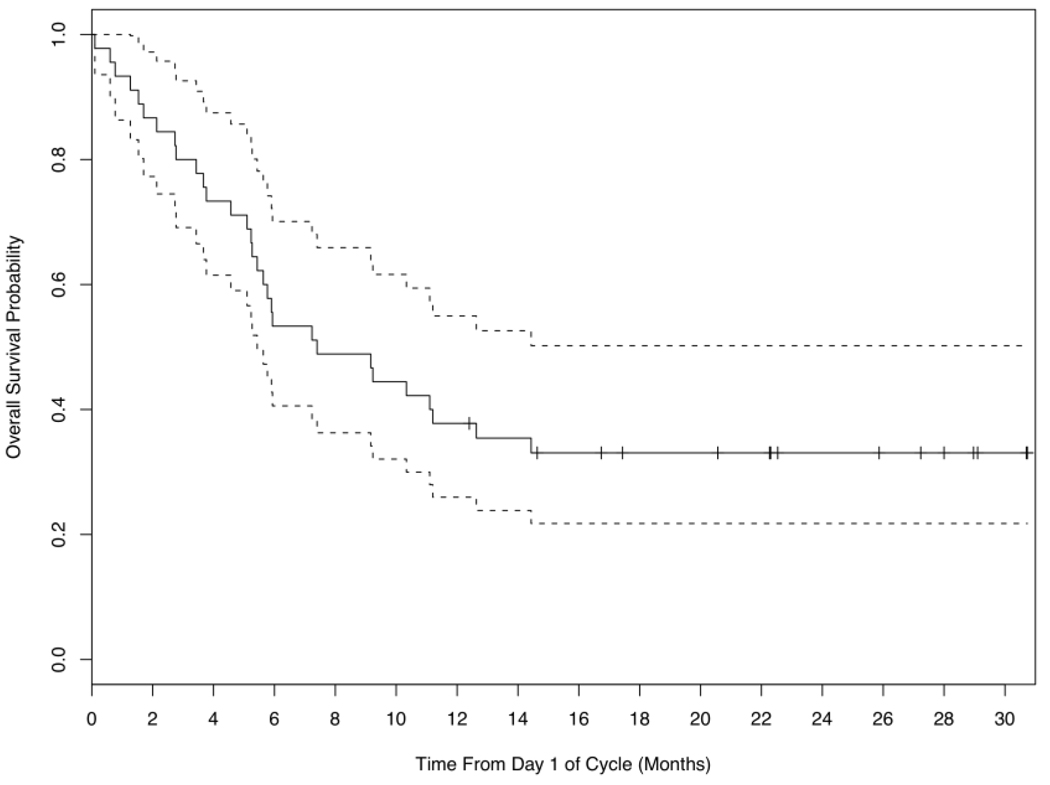
Median OS for all 45 patients was 7.4 months (Figure 1), with some variance among age groups. Median OS was 14.4 months (1.3–31) for the 5 patients under age 50, 18 months for the 16 patients age 50–59 (0.1–29), and 5.8 months (0.6–31) for the 25 patients age 60 and older.
Figure 1.
Overall survival (―) with confidence limits for all 45 patients receiving induction therapy with FLAM. Median overall survival for the entire group is 7.4 months.
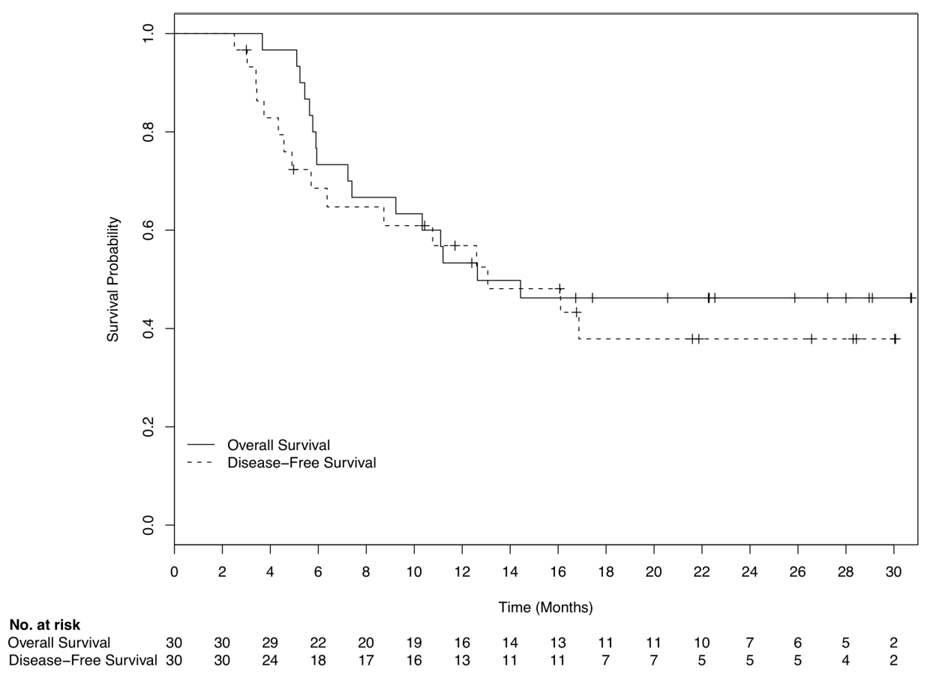
Thirty (67%) of the 45 patients who embarked on FLAM induction therapy achieved CR. As depicted in Table 2, CR varied across disease biologic features, but were similar among all age groups. For the 30 CR patients, median OS and DFS were 12.6 and 13.3 months, respectively, with 10/30 (33%) in CR 11.4–30 months and 14/30 (47%) alive at 12.5–31 months (Figure 2). Median follow-up was 22 months.
Table 2.
Response Following FLAM Induction Therapy
| CR | NR | NE | |
|---|---|---|---|
| All Patients (n=45) | 30 (67%) | 11 (24%) | 4 (9%) |
| Response by Age | |||
| ≤ 50 (n=5) | 3 (60%) | 0 (0%) | 2 (40%) |
| 51–59 (n=16) | 14 (88%) | 1 (6%) | 1 (6%) |
| ≥ 60 (n=24) | 13 (54%) | 10 (42%) | 1 (4%) |
| Response by Poor Risk Biologic Features | |||
| Etiology | |||
| MDS/AML (n=23) | 15 (65%) | 7 (30%) | 1 (4%) |
| MPD/AML (n=5) | 2 (40%) | 3 (60%) | 0 (0%) |
| T-AML (n=9) | 8 (89%) | 0 (0%) | 1 (11%) |
| Genetics | |||
| Adverse Cyto (n=24) | 16 (67%) | 7 (30%) | 1 (3%) |
| FLT-3 (n=9) | 8 (89%) | 0 (0%) | 1 (11%) |
| Proliferation | |||
| Blasts ≥ 20,000/mm3 (n=15) | 9 (60%) | 5 (33%) | 1 (7%) |
| Pretreatment HU (n=6) | 3 (50%) | 2 (33%) | 1 (17%) |
Figure 2.
Overall (―) and disease-free (---) survival for the 30 patients who achieved a complete remission. Median overall survival was 12.6 months and median disease-free survival was 13.3 months.
Table 3 depicts clinical outcome in relation to therapy in CR following FLAM induction. Twelve of 30 CR patients (40%) underwent myeloablative BMT in first CR (7 HLA-matched sibling, 3 matched unrelated donor, 1 syngeneic twin, 1 haploidentical). Eight underwent BMT within 6 weeks of achieving CR, while 4 received a second cycle of FLAM in remission 2.5–6.5 months before BMT. Four (33%) relapsed at 1.5, 2, 9 and 10 months after BMT (CR durations 3.3, 5.9, 11, and 13 months, respectively) and one succumbed to graft-versus-host disease (GVHD) 6 months after transplantation (CR duration 10 months). Median OS and DFS for the 12 post-induction BMT patients have not been reached, with 8/12 (67%) still alive at 12.5–31 months and 7/12 (58%) still in CR at 11.4–30 months.
Table 3.
Clinical Outcome According to Therapy in Complete Remission
| None (4) | Consolidation (14) | BMT (12) | |
|---|---|---|---|
| Median Age (range) | 68 (64–69) | 62 (52–72) | 55 (22–58) |
| Secondary AML | 3/4 (75%) | 11/14 (79%) | 9/12 (75%) |
| MDS | 1 | 8 | 5 |
| MPD | 1 | 0 | 1 |
| T-AML | 1 | 3 | 3 |
| Adverse Genetics | 3/4 (75%) | 9/14 (64%) | 10/12 (83%) |
| Cytogenetics | 2 | 4 | 8 |
| FLT3 | 1 | 5 | 2 |
| Median DFS (mos) | 6.7 | 13.1 | Not Reached |
| (Range) | (2.7–18.5) | (3.2–30) | (3.3–31) |
| Median OS (mos)) | 10.2 | 9 | Not Reached |
| (Range) | (5–20.5) | (3.6–31) | (5.9–31) |
| No. Alive ≥ 12 mos | 1/4 (25%) | 5/14 (36%) | 8/12 (67%) |
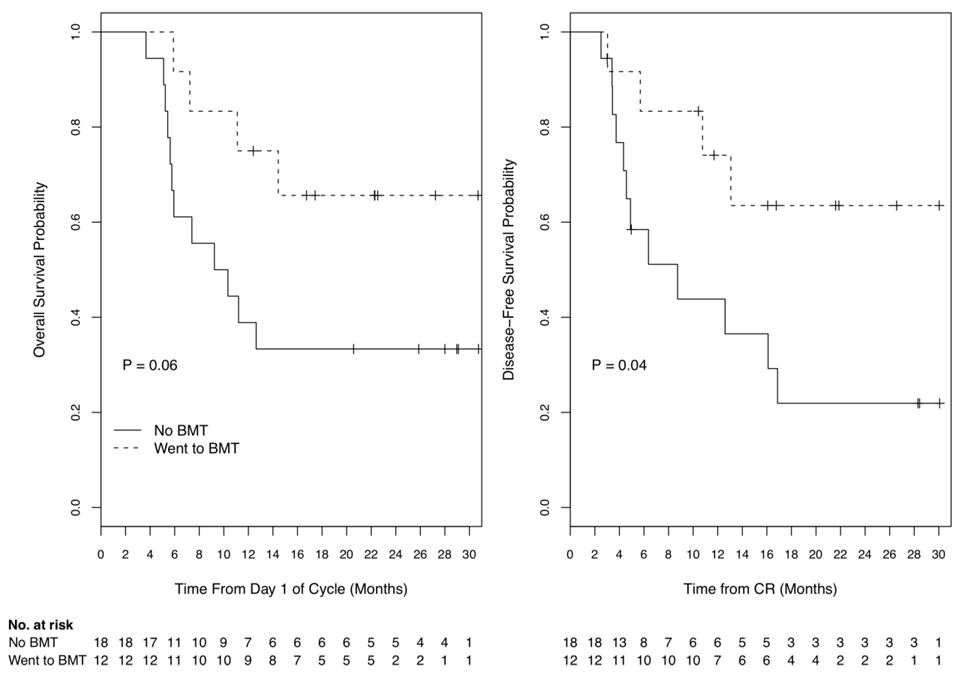
Eighteen patients did not undergo BMT in first CR because of donor unsuitability or unavailability (7), personal decision (8), poor performance status (1) or extensive fungal infection (2) following induction therapy. Fourteen (78%) received a second cycle of FLAM as consolidation therapy beginning 4–6 weeks after count and marrow recovery from induction. Three of 14 (21%) who received the second cycle of FLAM died from overwhelming infection associated with slow marrow recovery ≥ 49 days (2 MDS/AML) or heart failure following marrow recovery (1 treatment-related AML). OS and DFS for patients not receiving BMT in CR was shorter than OS and DFS of those undergoing BMT (Figure 3), with statistically significant differences (log rank p = 0.06 and 0.04, respectively), even with the small sample size.
Figure 3.
Overall and disease-free survival for the 30 complete remission patients, analyzed according to whether or not the patient underwent bone marrow transplantation (BMT) in CR. Median overall survival for 12 BMT patients (---) was not reached and for 18 non-BMT patients (―) was 10 months (p=0.06). Median disease-free survival for BMT patients was not reached and for non-BMT patients was 9 months (p=0.05).
Multivariate analyses showed that CR patients with poor risk cytogenetics demonstrated shorter OS (Hazard ratio = 12.6, 95% C.I. 1.6 to 100.1, p = 0.02) and DFS (Hazard Ratio = 6.1, 95% C.I. 1.5 to 24.6, p = 0.01) relative to patients with non-poor-risk cytogenetics, regardless of age or treatment in CR. Patients who received FLAM consolidation in CR had an increased risk of death (Hazard ratio = 3.9, 95% C.I. 0.9 to 16.6, p = 0.07) and relapse (Hazard ratio = 2.4, 95% C.I. 0.5 to 10.7, p = 0.26) relative to patients undergoing BMT. The same trend was observed for patients receiving no treatment in CR, although the results were not statistically significant, in part due to the small number of patients (n=4) who received no treatment (Hazard ratio for OS = 3.3, 95% C.I. 0.5 to 23.2, p = 0.24; Hazard ratio for DFS = 3.1, 95% C.I. 0.4 to 21.3, p = 0.26). There was no independent association of age with OS or DFS.
DISCUSSION
The results of this Phase II trial of TST with flavopiridol, ara-c and mitoxantrone therapy for adults with newly diagnosed, poor-risk AML expand our initial findings of a salutary CR rate and a sizable fraction of CR patients achieving lengthy DFS and OS. The 67% CR rate following a single cycle of FLAM in the current patient cohort is similar to the 75% CR rate achieved in a previously reported group of 15 newly diagnosed, poor risk patients.22 Moreover, all parameters of response and toxicity (CR, DFS, OS, mortality) compare favorably with other intensive approaches including response-adapted therapy5 and high-dose Daunorubicin,6,7 where ≤ 25% had secondary AML and ≤ 25% had adverse cytogenetics. In contrast, >90% of the patients treated in our present Phase II trial had at least 1 leukemia biologic poor-risk feature and 69% had ≥ 2 such adverse disease features, independent of any age-related contribution to poor risk.
The expanded experience has allowed us to examine in greater detail some issues raised with the original group of patients. Data from the current trial suggest that allogeneic BMT is feasible, tolerable and effective for a substantial proportion of patients achieving CR after FLAM induction therapy, and support the notion that the outcome of BMT in CR may be more salutary than FLAM consolidation (Figure3). While the OS and DFS differences between BMT vs. no BMT (either no therapy or FLAM consolidation) in CR might relate in part to the BMT group being somewhat younger, the proportion of patients with leukemia biology-related poor-risk factors was similar for each treatment group (Table 3).
For the entire patient cohort, independent of age or type of therapy in remission, it appears that the risk of relapse is low once DFS 18 months is reached. Long OS and DFS may be independent of genetics as well, since 7/10 still in CR and 8/14 long term survivors had adverse cytogenetics (6) or FLT3 mutations (2). From our previous study,22 4/12 (33%) CR patients remain alive, with 3 in first CR for 39–55 months (1 treatment-related AML, complex cytogenetics) and one in CR2 16 months following first CR 27 months (FLT3 mutation). A similar pattern may be emerging for the 30 CR patients in the present study.
The death rate for the 45 patients undergoing FLAM induction therapy was relatively low (9%) and was, in fact, only 6% for patients ages 50–59 and 4% for those 60 and older. Nonetheless, two patients (ages 59 and 64) experienced fatal sepsis during prolonged marrow aplasia following the consolidation cycle and 2 additional patients (ages 62 and 70) had incomplete platelet recovery following FLAM consolidation that continued for 12–15 months, at which time both relapsed. Three of these 4 patients had MDS/AML with TLD, normal cytogenetics and no prior MDS therapy, and one had AML-TLD with trisomy 8 without a prior MDS history. It may be that these 4 patients had underlying stem cell defects that were not apparent following induction therapy but prevented full recovery following consolidation therapy. It would be helpful to be able to identify such patients in order to select alternate consolidation approaches, including reduced intensity or alternative donor BMT strategies.
We also noted a delayed decrease in LVEF in 2 women with treatment-related AML who had received previous anthracyclines and chest radiation but whose LVEF was normal prior to beginning FLAM. One of these women died following recovery from FLAM consolidation. Of 7 others with treatment-related AML, none experienced cardiotoxicity despite prior anthracycline (3) or radiation therapy (2). However, none of those 7 had combined anthracycline-radiation therapies. Although the numbers are small, one might speculate that the combination of anthracyclines and chest wall radiation predisposes to mitoxantrone cardiotoxicity, and should be given careful consideration.
Direct leukemia cell cytotoxicity of flavopiridol was confirmed in this expanded cohort of patients, as manifested by rapid-onset drops in peripheral blood blast counts accompanied by metabolic stigmata of tumor lysis. However, while chemical evidence of tumor lysis accompanying flavopiridol-induced leukemia cell death occurred in 42%, clinically significant tumor lysis requiring intervention occurred in only 1 patient. The pattern of tumor lysis that we detected using a 1-hour flavopiridol infusion remains strikingly different from the pattern noted with the pharmacologically-modeled, “hybrid” bolus-infusion schedule of flavopiridol administration developed by Byrd28,29 and Blum.30 The hybrid schedule is designed to overcome the effects of avid flavopiridol binding by human plasma proteins by giving 30–50% of the total flavopiridol dose over 30 minutes, followed by a 4 hour infusion of the remaining flavopiridol dose. Data in refractory chronic lymphocytic leukemia (CLL) demonstrate dramatic clinical responses in ≥ 50% of such patients but also a dose-response acute tumor lysis syndrome characterized by striking hyperkalemia along with increases in phosphate and LDH.28,29 Studies in refractory acute leukemia demonstrate less striking metabolic derangements without relationship to flavopiridol dose.27 Using the bolus administration of flavopiridol, we encountered significant hyperkalemia in only one instance. Interestingly, myeloblasts and especially monoblasts but not lymphoblasts express significant amounts of lysozyme which impedes renal tubular resorption of potassium.31–33 Thus, production of lysozyme may protect against development of hyperkalemia from any cause including cell death, thus distinguishing the AML lysis profile from CLL.
The apparent responsiveness of AMLs exhibiting FLT-3 mutations is noteworthy, with 8 of 9 such patients achieving CR (one succumbed to flavopiridol-induced tumor lysis). It is possible that flavopiridol could overcome FLT3-induced drug resistance by neutralizing the ability of selected FLT3 mutants to upregulate expression of antiapoptotic proteins MCL-134 and/or survivin by activating STAT3.35 Whatever the mechanism, the hypothesis that flavopiridol might augment net cytotoxicity of traditional chemotherapy or possibly small molecular inhibitors against FLT3-positive leukemias could be tested preclinically and clinically.
In summary, TST with flavopiridol followed by ara-C and mitoxantrone exhibits meaningful and reproducible clinical activity in AML with multiple poor-risk biologic features. In turn, the ability to perform BMT in those patients who achieve a CR translates into long OS and DFS in the majority of eligible patients. Ongoing development of this regimen includes comparison of bolus vs. “hybrid” bolus infusion flavopiridol administration with regard to clinical and pharmacologic measurements, aimed at clarifying an optimal delivery strategy for further comparative studies in this newly diagnosed, poor-risk AML patient population.
Acknowledgements
We wish to thanks the Johns Hopkins Department of Medicine house staff and the Johns Hopkins Sidney Kimmel Comprehensive Cancer Center nursing staff for superb medical care, and the patients and families, without whose partnership we could never have conducted the trial and from whom we have learned critical information that will help us improve the treatment of these diseases.
This work was supported in part by the National Cancer Institute (NCI) Cooperative Agreement U01 CA70095 (J.E.K.), NCI Cancer Center support grant P30 CA06973-44, and the National Center for Research Resources (NCRR) grant M01-RR0052.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Byrd JC, Mrózek K, Dodge RK, et al. Pretreatment cytogenetic abnormalities are predictive of induction success, cumulative incidence of relapse, and overall survival in adult patients with de novo acute myeloid leukemia: results from Cancer and Leukemia Group B (CALGB 8461) Blood. 2002;100:4325–4335. doi: 10.1182/blood-2002-03-0772. [DOI] [PubMed] [Google Scholar]
- 2.Estey E. Acute myeloid leukemia and myelodysplastic syndromes in older patients. J Clin Oncol. 2007;25:1908–1915. doi: 10.1200/JCO.2006.10.2731. [DOI] [PubMed] [Google Scholar]
- 3.Schlenk RF, Dohner K, Krauter J, et al. Mutations and treatment outcome in cytogenetically normal acute myeloid leukemia. N Engl J Med. 2008;358:1909–1918. doi: 10.1056/NEJMoa074306. [DOI] [PubMed] [Google Scholar]
- 4.Appelbaum FR, Gundacker H, Head DR, et al. Age and acute myeloid leukemia. Blood. 2006;107:3481–3485. doi: 10.1182/blood-2005-09-3724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van der Jagt R, Robinson KS, Belch A, et al. Sequential reponse-adapted induction and consolidation regimens idarubicin/cytarabine and mitoxantrone/etoposide in adult acute myelogenous leukemia: 10 year follow-up of a study by the Canadian Leukemia Studies Group. Leukemia & Lymphoma. 2006;47:697–706. doi: 10.1080/10428190500467917. [DOI] [PubMed] [Google Scholar]
- 6.Lowenberg B, Ossenkoppele GJ, van Putten W, et al. High-dose Daunorubicin in older pateitns with acute myeloid leukemia. N Engl J Med. 2009;361:1235–1248. doi: 10.1056/NEJMoa0901409. [DOI] [PubMed] [Google Scholar]
- 7.Fernandez HF, Sun Z, Yao X, et al. Anthracycline dose intensification in acute myeloid leukemia. N Engl J Med. 2009;361:1249–1259. doi: 10.1056/NEJMoa0904544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maik RG, Kattice S, Bhat SV, Alreja B, de Souza NJ, Rupp RH. An antiinflammatory cum immunomodulatory piperidinylbenzopyranone from Dysoxylum binectariferum: isolation, structure and total synthesis. Tetrahedron. 1998;44:2081–2086. [Google Scholar]
- 9.Senderowicz AM, Sausville EA. Preclinical and clinical development of cyclin-dependent kinase modulators. J Natl Cancer Inst. 2000;92:376–387. doi: 10.1093/jnci/92.5.376. [DOI] [PubMed] [Google Scholar]
- 10.Bible KC, Kaufmann SH. Flavopiridol: a cytotoxic flavone that induces cell death in noncycling A549 human lung carcinoma cells. Cancer Res. 1995;56:4856–4861. [PubMed] [Google Scholar]
- 11.Decker RH, Dai Y, Grant S. The cyclin-dependent kinase inhibitor flavopiridol induces apoptosis in human leukemia cells (U937) through the mitochondrial rather than the receptor-mediated pathway. Cell Death Diff. 2001;8:715–724. doi: 10.1038/sj.cdd.4400868. [DOI] [PubMed] [Google Scholar]
- 12.Gojo I, Zhang B, Fenton RG. The cyclin-dependent kinase inhibitor Flavopiridol induces apoptosis in multiple myeloma cells through transcriptional repression and down-regulation of Mcl-1. Clin Cancer Res. 2002;8:3527–3538. [PubMed] [Google Scholar]
- 13.Yu C, Rahmani M, Dai Y, Conrad D, Krystal G, Dent P, Grant S. The lethal effects of pharmacological cyclin-dependent kinase inhibitors in human leukemia cells proceed through a phosphatidylinositol 3-kinase/Akt-dependent pathway. Cancer Res. 2003;63:1822–1833. [PubMed] [Google Scholar]
- 14.Carlson BA, Dubay MM, Sausville EA, Brizuela L, Worland PJ. Flavopiridol induces G1 arrest with inhibition of cyclin-dependent kinase (CDK)2 and CDK 4 in human breast carcinoma cells. Cancer Res. 1999;56:2973–2978. [PubMed] [Google Scholar]
- 15.Worland PJ, Kaur G, Stetler-Stevenson M, Sebers S, Sartor O, Sausville EA. Alteration of the phosphorylation state of p34cdc2 kinase by the flavone L86-8275 in breast carcinoma cells. Biochem Pharmacol. 1993;46:1831–1840. doi: 10.1016/0006-2952(93)90590-s. [DOI] [PubMed] [Google Scholar]
- 16.Chao SH, Price DH. Flavopiridol inactivates P-TEFb and blocks most RNA polymerase II transcription in vivo. J Biol Chem. 2001;276:31793–31799. doi: 10.1074/jbc.M102306200. [DOI] [PubMed] [Google Scholar]
- 17.Lam LT, Pickeral OK, Peng AC, et al. Genomic-scale measurement of mRNA turnover and the mechanisms of action of the anti-cancer drug flavopiridol. Genome Biol. 2001;2:1–11. doi: 10.1186/gb-2001-2-10-research0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carlson B, Lahusen T, Loaiza-Perez A, et al. Down-regulation of Cyclin D1 by transcriptional repression in MCF-7 human breast carcinoma cells induced b y flavopiridol. Cancer Res. 1999;59:4534–4643. [PubMed] [Google Scholar]
- 19.Lee YK, Isham CR, Kaufmann SH, Bible KC. Flavopiridol disrupts STAT3/DNA interactions, attenuates STAT3-directed transcription, and combines with the Jak kinase inhibitor AG490 to achieve cytotoxic synergy. Mol Cancer Ther. 2006;5:138–148. doi: 10.1158/1535-7163.MCT-05-0235. [DOI] [PubMed] [Google Scholar]
- 20.Karp JE, Ross DD, Yang W, et al. Timed sequential therapy of acute leukemia with flavopiridol: in vitro model for a Phase I clinical trial. Clin Cancer Res. 2003;9:307–315. [PubMed] [Google Scholar]
- 21.Karp JE, Passaniti A, Gojo I, et al. Phase I and pharmacokinetic study of flavopiridol followed by 1-b-D-arabinofuranosylcytosine and mitoxantrone in relapsed and refractory acute leukemias. Clin Cancer Res. 2005;11:8403–8412. doi: 10.1158/1078-0432.CCR-05-1201. [DOI] [PubMed] [Google Scholar]
- 22.Karp JE, Smith BD, Levis MJ, et al. Sequential flavopiridol, cytosine arabinoside, and mitoxantrone: a Phase II trial in adults with poor-risk acute myelogenous leukemia. Clin Cancer Res. 2007;13:4467–4473. doi: 10.1158/1078-0432.CCR-07-0381. [DOI] [PubMed] [Google Scholar]
- 23.Bible KC, Kaufmann SH. Cytotoxic synergy between Flavopiridol (NSC 649890, L8\278275) and various antineoplastic agents: the importance of sequence of administration. Cancer Res. 1997;57:3375–3380. [PubMed] [Google Scholar]
- 24.Geller RB, Burke PJ, Karp JE, et al. S. A two-step timed sequential treatment for acute myelocytic leukemia. Blood. 1989;4:1499–1506. [PubMed] [Google Scholar]
- 25.Bolaños-Meade J, Karp JE, Guo C, et al. Timed sequential therapy of acute myelogneous leukemia in adults: a phase II study of retinoids in combination with the sequential administration of cytosine arabinoside, idarubicin and etoposide. Leukemia Res. 2003;27:313–321. doi: 10.1016/s0145-2126(02)00177-7. [DOI] [PubMed] [Google Scholar]
- 26.Appelbaum FR, Rosenblum D, Arceci RJ, et al. End points to establish the efficacy of new agents in the treatment of acute leukemia. Blood. 2007;109:1810–1816. doi: 10.1182/blood-2006-08-041152. [DOI] [PubMed] [Google Scholar]
- 27.Cheson BD, Bennett JM, Kopecky KJ, et al. Revised recommendations of the international working group for diagnosis, standardization of response criteria, treatment outcomes and reporting standards for therapeutic trials in acute myeloid leukemia. J Clin Oncol. 2003;21:4642–4649. doi: 10.1200/JCO.2003.04.036. [DOI] [PubMed] [Google Scholar]
- 28.Byrd JC, Lin TS, Dalton JT, et al. Flavopiridol administered using a pharmacologically derived schedule of flavopiridol is associated with marked clinical activity in refractory, genetically high risk chronic lymphocytic leukemia. Blood. 2007;09:399–404. doi: 10.1182/blood-2006-05-020735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Phelps MA, Lin TS, Johnson AJ, et al. Clinical response and pharmacokinetics from a phase I study of an active dosing schedule of flavopiridol in relapsed chronic lymphocytic leukemia. Blood. 2009;113:2637–2645. doi: 10.1182/blood-2008-07-168583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blum W, Klisovic RB, Johnson A, et al. Final results of a dose escalation study of flavopiridol in acute leukemia using a novel treatment schedule. Blood. 2007;110:272a. [Google Scholar]
- 31.Straus DJ, Mertelsman R, Koziner B, et al. The acute monocytic leukemias: multidisciplinary studies in 45 patients. Medicine. 1980;59:409–425. doi: 10.1097/00005792-198011000-00002. [DOI] [PubMed] [Google Scholar]
- 32.Mir MA, Brabin B, Tang OT, Leyland MJ, Delamore IW. Hypokalaemia in acute myeloid leukaemia. Ann Intern Med. 1975;82:54–57. doi: 10.7326/0003-4819-82-1-54. [DOI] [PubMed] [Google Scholar]
- 33.Muggia FM, Heinmann HO, Farhnagi M, Osserman EF. Lysozymuria and renal tubular dysfunction in monocytic and myelomonocytic leukemia. Am J Med. 1969;47:351–366. doi: 10.1016/0002-9343(69)90219-8. [DOI] [PubMed] [Google Scholar]
- 34.Breitenbuecher F, Markova B, Kasper S, et al. A novel molecular mechanism of primary resistance to FLT-3 kinase inhibitors in AML. Blood. 2009;113:4063–4073. doi: 10.1182/blood-2007-11-126664. [DOI] [PubMed] [Google Scholar]
- 35.Zhou J, Bi C, Janakakumara JV, et al. Enhanced activation of STAT pathways and overexpression of survivin confer resistance to FLT3 inhibitors and could be therapeutic targets in AML. Blood. 2009;113:4052–4062. doi: 10.1182/blood-2008-05-156422. [DOI] [PubMed] [Google Scholar]