Abstract
Individuals on methadone maintenance for the treatment of addiction (MM) are demonstrated to be hyperalgesic to cold-pressor pain in comparison to matched controls and ex-opioid addicts, a finding described as clinical evidence of opioid-induced hyperalgesia (OIH). Interestingly, opioids induce hyperalgesia via many of the same neuro-inflammatory and central sensitization processes that occur with the development of neuropathic pain. Evaluated in this study was the efficacy of a key pharmacotherapy for neuropathic pain, gabapentin (GPN), to reverse OIH in MM patients. Utilizing a clinical trial design and double blind conditions, changes in cold-pressor pain threshold and tolerance following a five-week trial of GPN (titrated to 2400mg/day) were evaluated at peak and trough methadone plasma levels in a well-characterized MM sample. Drug abstinence was encouraged via an escalating payment schedule, and compliance monitored via pill counts and GPN plasma levels; entered into the analyses were only those subjects compliant and abstinent throughout the study (approx 45%). Utilizing change scores from baseline, significant improvements in cold-pressor pain threshold and pain tolerance were observed at both peak and trough methadone levels (p < 0.05). Notably, drop-out rates due to medication side effects were low (2%) and the medication was well-tolerated. These results support that GPN, as prescribed for the treatment of neuropathic pain, is effective in decreasing OIH in patients who are abstinent and stable in methadone treatment.
Keywords: gabapentin, hyperalgesia, methadone, treatment
1. Introduction
Abundant evidence exists to support that individuals on the opioid maintenance agent methadone for the treatment of opioid addiction are relatively hyperalgesic to experimental pain. First noted in the literature 40 years ago, Ho and Dole (1979) observed that methadone-maintained heroin addicts were significantly more sensitive to cold pressor-induced pain as compared to drug-free controls. Since that time, significantly diminished tolerance to experimental (electrical stimulation, cold-pressor [CP]) pain has been reliably demonstrated in methadone patients’ in comparison to matched drug-free addicts (Compton, 1994) and controls (Athanasos et al., 2006; Compton et al., 2000; 2001; Doverty et al., 2001; Pud et al., 2006), at both peak and trough methadone blood levels. Cross-sectional data suggest a large effect size, indicating that methadone-maintained patients (MM) are 42% – 76% less tolerant of CP pain than are normal controls matched on age, gender and ethnicity. These findings have implications for the management of pain in methadone patients, and support increased analgesic need in patients receiving this addiction pharmacotherapy (Alford et al., 2006; Newshan, 2000; Scimeca et al., 2000).
It has been suggested that the relative pain intolerance noted in these opioid maintained individuals is the result of an increasingly appreciated consequence of ongoing opioid exposure, opioid-induced hyperalgesia (OIH) (see reviews Angst and Clark, 2006; Chu et al., 2008; Fishbain et al., 2009; Mao, 2006; Ossipov et al., 2005). Convergent lines of preclinical and clinical evidence indicate that opioid administration not only provides a rapid and powerful analgesia, but concurrently sets into motion certain anti-analgesic or hyperalgesic opponent processes, which can be observed both during opioid activity and withdrawal (Angst et al., 2003; Chu et al., 2006; Koppert et al., 2003; Li et al., 2001; Mao et al., 2002; Simonnet, 2005; Vanderah et al 2001a), and which have been suggested to contribute to opioid tolerance (Colpaert, 1996; Gardell et al., 2006; Laulin et al., 1999; Mao, 2006). The implications of this altered pain state have become of interest to investigators and clinicians who prescribe opioid analgesics for chronic pain (Ballantyne and Shin, 2008; Chang et al., 2007; Koppert and Schmelz, 2007; Wilder-Smith and Arendt-Nielsen, 2006).
Phenomenological similarities between OIH and the hyperalgesia associated with neuropathic pain have been appreciated. In both, central ascending hyperexcitability and/or diminished descending supraspinal inhibition have been posited to underlie the hypersensitivity to noxious stimuli (see reviews, Chu et al, 2008; Finnerup, 2008; Harvey and Dickinson, 2008; Taylor, 2009). More recent work has demonstrated a common immune mechanism underlying the development of both opioid-induced and neuropathic hyperalgesia, and related to glial activation (Hutchinson et al., 2007; 2008; Mika, 2008; Watkins et al., 2007). Due to the overlap between the central neuroplastic processes underlying OIH and neuropathic pain, it is reasonable to suspect that an effective pharmacotherapy for the treatment of the latter might generalize to treatment of the former.
Gabapentin (GPN), a γ-aminobutyric acid (GABA) agonist anticonvulsant, is increasingly considered a first-line medication for the treatment of chronic neuropathic pain (Dobecki et al., 2006; Dworkin et al., 2007; Knotkova and Pappagallo, 2007; Moulin et al., 2007; Vadalouca et al., 2006). Attributed to its ability to hyperpolarize neurons via activity at voltage-gated Ca2+ channels (see Cheng and Chiou, 2006; Rose and Kam, 2002), the analgesic effect of GPN has been demonstrated in animal models of nerve injury (Blackburn-Munro and Erichsen, 2005), and in clinical trials of patients with diabetic neuropathy, post-herpetic neuralgia, and trigeminal neuralgia (Backonja and Glanzman, 2003; Sindrup and Jensen, 1999; Mellegers et al., 2001; Wiffin et al., 2009). Detailed evaluation of its ability to decrease neuropathic pain in animal models shows that GPN acts primarily by diminishing the hyperalgesia associated with nerve injury (Cesena and Calcutt, 1999; De la O-Arciniega et el., 2009; Gustafsson et al., 2003; Jones and Sorkin, 1998; Jun and Yaksh, 1998; Patel et al., 2001; Yasuda et al., 2005); similar findings have been demonstrated in humans under experimental (Dirks et al., 2002; Gottrup et al., 2004; Gustorff et al., 2004; Iannetti et al., 2005; Segerdahl, 2006; Wallace and Schulteis, 2008) and clinical pain conditions (Attal et al., 1998; Berry and Petersen, 2005; Cuignet et al., 2006). This anti-hyperalgesic effect is proposed to account for accumulating clinical observations that preoperative GPN administration results in decreased post-operative opioid requirement (Giron, 2007; Hayashida et al., 2007; Kong and Irwin, 2007; Tiippana et al., 2007).
That GPN can treat the mechanistically similar hyperalgesia associated with opioid use has been recently supported by animal data collected in the laboratories of Van Elstraete (2008), which showed that fentanyl-induced hyperalgesia could be prevented by the administration of GPN in a dose-dependent manner. The apparent large effect size of GPN on OIH warrants translation of these findings to the clinical setting for patients on opioid therapy. The aim of this study was to evaluate the ability of GPN to diminish OIH to experimental pain in a well-characterized sample of MM individuals. It was hypothesized that chronic GPN therapy would effectively increase tolerance for experimental pain in this sample, and thus support a gabaminergic mechanism by which to treat their notable hyperalgesia.
2. Methods
2.1 Design
The ability of the GPN to decrease OIH in MM patients was tested using a placebo-controlled, randomized clinical trial design. Threshold and tolerance to cold-pressor (CP) pain were measured prior to and following five weeks of continuous GPN vs. placebo therapy. To control for the effects of methadone dosing on pain responses, pre- and post-GPN pain measures were collected at peak and trough methadone blood levels.
2.2 Sample
A convenience sample was recruited from a single methadone clinic affiliated with UCLA Integrated Substance Abuse Programs, providing a recruitment pool of approximately 300 patients. Enrolled participants were selected so as to be between the ages of 18 and 55, in good general physical and psychological health, compliant in MM treatment, and on a stable dose of methadone for at least 6 weeks. Being in MM therapy, all met DSM-IV criteria for opioid dependence. Based upon previous demonstrations in comparison to controls (Athanasos et al., 2006; Compton et al., 2000; Doverty et al., 2001; Pud et al., 2006), it was anticipated that all subjects would demonstrate some degree of OIH by virtue of their prolonged and ongoing exposure to opioids.
Individuals were excluded from study participation if they met DSM-IV dependence criteria for alcohol, benzodiazepine, CNS stimulant, marijuana or other drug of abuse; had a neurologic or psychiatric condition (i.e., peripheral neuropathy, schizophrenia, neuropathic pain, Raynaud’s disease, urticaria) known to affect pain responses; or were currently taking analgesic medication (opioid or otherwise) for a painful condition on a regular basis. Prior to participation, all potential subjects provided informed consent in strict adherence with UCLA Institutional Review Board standards. Subjects were compensated for their time and compliance.
2.3 Gabapentin (GPN)
GPN was titrated to a total daily dose of 2400mg PO over a one week period (120mg QID × 2 days, 200mg QID × 2 days, 320mg QID × 2 days). As suggested by the meta-analyses of Dworkin and colleagues (2007) and Wiffen and colleagues (2009), GPN dose was selected so as to be safe and tolerable, and the five-week dosing period used to capture the effective dosing period for the treatment of neuropathic pain
2.4 Cold Pressor (CP) Pain
Prior to and 5 weeks following medication administration, pain responses to a standardized cold-pressor (CP) test were observed to evaluate the ability of GPN to diminish OIH in MM patients. Over 60 years of use (Edes and Dallenbach, 1936; Hines and Brown, 1932), this pain induction procedure has been demonstrated to have excellent reliability (Blitz, 1968; Garcia de Jalon et al., 1985; Tassorelli et al., 1995; Wolff et al., 1976) and analogous in nature to various types of clinical pain (Chen et al., 1989).
The CP pain induction procedure as adapted by Eckhardt et al. (1998) was utilized to assess the anti-hyperalgesic effects of GPN. Specifically, study participants were seated in a comfortable chair, asked to roll up their sleeve and remove watches or jewelry from their non-dominant arm and hand, and a blood pressure cuff applied. Eye patches were placed over the eyes to minimize distraction. Participants were instructed to first place their forearm into a bath of room temperature water with fingers wide apart, and no contact with the side or bottom of the container. At 1 minute and 45 seconds, the blood pressure cuff was inflated to 20 mm Hg below the obtained diastolic BP so as to induce mild tissue hypoxia. At exactly 2 minutes, participants were assisted in removing the forearm from the lukewarm bath and placing it immediately in an adjacent circulating ice bath (1.0 +/− 0.5C). Participants were not spoken to during the cold-water immersion to minimize distraction or cues for time
Participants held an event marker button in their dominant hand to indicate when (a) pain was initially detected (threshold), and (b) when pain could be no longer tolerated and the arm was voluntarily removed from the ice bath (tolerance); both were operationalized as time in seconds from initial ice bath immersion. Once the hand was removed from the cold water, eye patches and blood pressure cuff were removed and a warm towel given to gently dry the forearm. All trials were truncated at 5 minutes, so as to avoid the onset of numbness.
2.5 Procedures
Following a screening visit to establish study eligibility, consented participants were familiarized with the CP procedures and baseline pain measures were collected. Subjects were randomized to GPN or matched placebo, and titrated up to the target dose as described above; the placebo group underwent an identical “titration.” Subjects were evaluated weekly by a blinded study clinician for health status, side effect assessment, and concomitant medication use. To encourage abstinence from illicit drug use over the course of the study, subjects received an escalating monetary weekly bonus for submitting a “clean” specimen (free from illicit opioid, cocaine, amphetamine/methamphetamine, benzodiazepine, or marijuana metabolites), beginning at $5 week 1 and increasing by $5/wk for each drug-free sample provided. Medication compliance was evaluated at each visit with pill counts and plasma levels were collected at week 5.
At baseline and immediately following five weeks of medication, CP pain responses were measured on two occasions separated by 72 hours, at both methadone trough (just prior to methadone dosing) and peak (approximately 150min following methadone dosing) blood levels. All pain testing sessions took place in a private setting and the CP administered by one of two trained research assistants. Subjects were instructed to refrain from caffeine and nicotine for one hour prior to testing and throughout the testing session. Prior to each pain testing session, subjects underwent a brief screening to ensure physical and psychological stability, including a measure of subjective opioid withdrawal. First day of menstrual cycle was recorded for all female subjects.
For each pain session, respiration, EKG, pulse oximetry, heart rate and blood pressure were continuously monitored prior to, during, and for at least ten minutes following each pain test to ensure return to baseline. Testing occurred at approximately the same time each morning around clinic methadone dosing hours. Immediately following pain testing, approximately 10cc’s of blood was drawn to enable measurement of methadone and GPN plasma levels at the time of testing.
2.6 Data Analysis
To evaluate the effect of the GPN on putative OIH, difference on pain responses from baseline to after treatment were compared for the treatment group to the control group at peak and trough methadone blood levels separately. Only those subjects who were abstinent (as confirmed by urine toxicology) and compliant (as evidenced by GPN blood level) were entered into the analyses. The outcome variables of interest were change scores in pain tolerance and threshold at both peak and trough blood levels, and measured by the difference in pain responses between week 5 and baseline. Differences in change scores between the experimental and control groups were assessed using t-tests. Chi-square tests were used to assess statistical differences between the two groups for categorical demographic variables and two-sample independent t-tests were conducted to detect significant differences in continuous outcome variables between groups. Assumptions for statistical tests were checked and met. Analyses were performed with SAS/STAT.
3. Results
Characteristics of the overall sample as well as categorized by the experimental and control groups are as described in Table 1. Data are presented as mean ± SE for continuous measures and percentages for categorical variables. Overall there were 26 subjects in the sample that were compliant and abstinent during the entire study (experimental: 10; placebo: 16). Subjects in the treatment group were marginally younger (45.5) compared to the control group (49.5). Half of the sample was Hispanic (50%), and the rest were primarily Caucasian (19%) or African Americans (15%). The majority had at least a high school education, and about half were married and/or had partners. The mean daily dose of methadone was 72mg, and at baseline, peak and trough R-methadone blood levels averaged 177.5ng/ml and 128.8ng/ml respectively.
Table 1.
Sample Demographics of Abstinent and Compliant Study Subjects
| Gabapentin (n = 10) | Placebo (n = 16) | Overall (n = 26) | |
|---|---|---|---|
| Demographic Characteristics | |||
| Continuous Variable | Mean (SD) | Mean (SD) | Mean (SD) |
| Age (yrs)* | 45.5 (4.46) | 49.5 (4.96) | 47.9 (5.05) |
| Methadone Dose (mg) | 73.0 (20.02) | 70.9 (23.16) | 71.7 (21.62) |
| Peak Methadone plasma level (baseline ng/ml) | 191.5 (57.08) | 168.8 (62.32) | 177.5 (60.22) |
| Trough Methadone plasma level (baseline ng/ml) | 125.8 (39.91) | 130.7 (43.16) | 128.8 (41.20) |
| Categorical Variables | % | % | % |
| Gender | |||
| Males | 50.0 | 56.3 | 53.9 |
| Race | |||
| Hispanic | 60.0 | 43.8 | 50.0 |
| American Indian | 10.0 | 0.0 | 3.9 |
| African American | 0.0 | 25.0 | 15.4 |
| Caucasian | 30.0 | 12.5 | 19.2 |
| Other | 0.0 | 18.8 | 11.5 |
| Marital Status | |||
| Married / Living-with-partner | 50.0 | 50.0 | 50.0 |
| Separated / Divorced | 30.0 | 31.3 | 30.1 |
| Widowed / Never-married | 20.0 | 18.8 | 19.2 |
| Education | |||
| < High School | 36.0 | 40.0 | 38.0 |
| High School | 32.0 | 40.0 | 36.0 |
| > High School | 32.0 | 20.0 | 26.0 |
Significant group difference observed at alpha = 0.049 level of significance
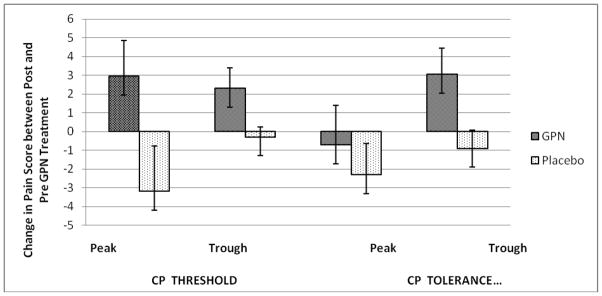
Change scores in pain tolerance and threshold are listed in Table 2 and illustrated in the Figure. As noted, the experimental group showed statistically significant improvements in both pain threshold and tolerance compared to the control group at both peak and trough methadone levels. Using generalized linear modeling, no differences in change scores were noted by age, gender or ethnicity. Change scores were not correlated to methadone dose or its plasma peak or plasma trough levels.
Table 2.
Average Pain Threshold and Tolerance for Cold-Pressor at Baseline and Week 5
| Time | Group | |||
|---|---|---|---|---|
| Mean (SE) | Gabapentin (n = 10) Mean (SE) | Placebo ( n = 14) Mean (SE) | ||
| CP Threshold (Seconds) | Trough Methadone | Baseline | 7.61 (1.23) | 14.23 (1.40) |
| Week 5 | 9.90 (1.56) | 13.78 (1.71) | ||
| Within-group difference in pain between Week 5 and baseline ** | 2.29 (1.58) | −0.45 (2.21) | ||
| Peak Methadone | Baseline | 7.54 (1.54) | 15.82 (2.24) | |
| Week 5 | 10.49 (1.86) | 12.33 (1.77) | ||
| Within-group difference in pain between Week 5 and baseline * | 2.95 (2.41) | −3.49 (2.85) | ||
| CP Tolerance (Seconds) | Trough Methadone | Baseline | 11.15 (1.04) | 20.72 (1.76) |
| Week 5 | 14.19 (1.79) | 19.73 (2.42) | ||
| Within-group difference in pain between Week 5 and baseline *** | 3.04 (2.07) | −0.99 (2.99) | ||
| Peak Methadone | Baseline | 13.43 (3.02) | 21.90 (2.40) | |
| Week 5 | 15.71 (1.90) | 19.63 (2.96) | ||
| Within-group difference in pain between Week 5 and baseline * | −0.72 (3.57) | −2.27 (3.81) | ||
Significant group difference observed at
p = 0.04,
p = 0.02,
p = 0.01
levels of significance using two-sample independent t-tests to test that the change scores are the same between groups versus not.
Figure.
Change in pain threshold and pain tolerance from baseline to week 5 for placebo and gabapentin groups at peak and trough methadone levels.
In general, GPN was well-tolerated by study subjects. Of 149 adverse effects deemed as related to the study medications, 99% were classified as mild to moderate severity and 93% anticipated. Table 3 shows the frequencies for adverse events that were coded as probably or definitely related to the study drug, with the most commonly reported event being nausea.
Table 3.
Adverse events Probably or Definitely related to Gabapentin among abstinent and compliant subjects (n = 26)
| ADVERSE EVENTS* | Overall | GPN | Placebo |
|---|---|---|---|
| NAUSEA | 7 | 6 | 1 |
| GAS | 4 | 3 | 1 |
| CONSTIPATION | 6 | 3 | 3 |
| LETHARGY/TIREDNESS | 7 | 4 | 3 |
| INSOMNIA/TROUBLE SLEEPING | 6 | 1 | 5 |
| HEADACHE | 3 | 1 | 2 |
| DIZZINESS/ LIGHTHEADEDNESS | 5 | 5 | 0 |
: Some subjects experienced more than one AE or the same AE over multiple study visits.
4. Discussion
This study sought to determine if the GABA agonist, gabapentin, would be effective in treating the previously described pain intolerance of MM patients. Hypothesized to be a latent hyperalgesia secondary to chronic opioid exposure, it was hoped that this pharmacotherapy might normalize the pain responses of opioid dependent patients, and thus provide a tool for improving the treatment of clinical pain in this at-risk population. Albeit a small sample, these data provide support for the efficacy of GPN to do so for both measures of pain threshold and pain tolerance at methadone peak and trough blood levels.
Importantly, the focus of this paper is specifically to compare those subjects who were compliant in taking the study medication and who remained abstinent from illicit drug use over the course of the 5 week study to controls. Although subjects were encouraged to provide drug-free urine samples via an escalating payment schedule, less than half were able to do so completely. It is well-known that many drugs of abuse, including the CNS stimulants, illicit opioids, and marijuana, can acutely decrease pain, while withdrawal from the same can make nociceptive stimuli feel more painful. Although no overt opioid withdrawal symptoms were noted prior to pain testing, it is unclear how uncontrolled concomitant drug use might have influenced pain responses and GPN efficacy. For this reason, the focus was kept only on abstinent and compliant subjects.
Similar to previous work (Compton et al, 2008; Doverty et al., 2001), at baseline, subjects tended to be more sensitive to CP stimulation at peak relative to trough methadone plasma levels. It is possible that these non-significant differences reflect a measurable opioid analgesic effect following methadone dosing, enabling subjects to tolerate the ice bath longer. Alternatively, at trough plasma levels, patients may have been experiencing a mild opioid withdrawal hyperalgesia (see Basbaum, 1991; Compton et al., 2003; Kaplan & Fields, 1991) prior to methadone dosing, thus subjects appeared more sensitive to the cold-pressor. Regardless, these differences did not carry over to the post–treatment measures, with significant improvements noted at both peak and trough testing, suggesting that methadone effects on pain responses were not substantively involved in the improvements noted with GPN therapy.
These data are the first to demonstrate that a GABA agonist effectively treats putative opioid-induced hyperalgesia in a clinical sample of methadone patients. Not unlike the considerable effect of GPN on chronic neuropathic pain (Mellegers et al., 2001; Wiffin et al., 2009), the general inhibitory effect of GPN on neuronal transmission is evident in central pain systems which have been unregulated secondary to opioid exposure. Singler and colleagues (2007) observation that the GABA agonist, propofol, mitigates remifentanil-induced hyperalgesia in normal human subjects further supports gabaminergic approaches for the treatment of OIH. Further, these data are also among the first published to demonstrate the effectiveness of GPN to treat OIH in a human model of opioid exposure. Van Elstraete and colleagues (2008) recently demonstrated that both intrathecal and intraperitoneal GPN administration dose-dependently prevents the hyperalgesia induced by repeated fentanyl administration in uninjured rats; the current findings translate these preclinical observations to a clinical population of humans who receive opioid therapy in the absence of injury or pain.
Pharmacotherapies with activity at non-GABA sites have also been suggested as treatment for OIH. Probably best studied is the relatively weak NMDA-antagonist dextromethorphan, although evidence for its efficacy to offset OIH in clinical samples with pain has been mixed (Compton et al., 2008; Dudgeon et al., 2007; Galer et al., 2005; Haugan et al., 2008; Heiskanen et al., 2002; Helmy and Bali, 2001; Weinbroum et al., 2001). Other potential agents include CCK antagonists to block descending pain facilitory processes (Gardell et al., 2002; Vanderah et al., 2001), and the α2-receptor agonists which attenuated OIH in a small sample of healthy human subjects (Koppert et al., 2003). Of increasing interest in the literature is the use of low-dose opioid antagonists in conjunction with opioid agonists to counteract the development of OIH (Cepeda et al. 2004; Chindalore et al., 2005; and Terner, 2006; Wang et al., 2005; Webster, 2007; Webster et al., 2006), and the use of glial antagonists (i.e., ibudilast) to mitigate the neuroimmune activation associated with opioid administration (Hutchinson et al., 2007; 2008; Watkins et al., 2007).
Absolute improvements in cold-pressor responses to GPN were small (2 – 3 seconds), calling into question the clinical significance of these findings. Relative to the short pain threshold and tolerance responses noted at baseline (approx. 7 – 21 seconds), a 3-second change represents a 14% to 43% increase in cold-pressor immersion, thus is clearly significant in this context. Yet, the extrapolation of magnitude of analgesic responses from experimental pain to the clinical pain experience remains an ongoing issue in pain research, (Arendt-Nielsen et al., 2007; Petersen-Felix and Arendt-Nielsen, 2002), and one which may never fully be resolved. To the authors’ knowledge there is not a clear cut method for determining the clinical equivalence of changes in cold-pressor pain responses. To provide perspective, CP pain threshold improvement in normal healthy controls to an analgesic dose of morphine (10mg PO) is 5 seconds (Jones et al., 1988), while improvements in CP tolerance to a 0.5mg/kg dose of morphine PO averaged 10 seconds (Cleeland et al., 1996; Grach et al., 2004), thus lengthy changes in cold-pressor pain responses are not associated with clinically potent analgesics.
Several limitations are evident in this work. Firstly, baseline hyperalgesia was not established in this group of subjects, although their average CP pain threshold and tolerance times were consistent with those reported in previous work on this population (Athanasos et al., 2006; Compton, 1994; Compton et al., 2000; 2001; Doverty et al., 2001). Although these data support changes in CP pain threshold and tolerance on GPN, conclusions about changes to OIH specifically must be drawn cautiously. Also, the trial lasted for five weeks, and then subjects were titrated off study mediation; future studies should evaluate the longer-term effects of GPN therapy in this population. Finally, these findings can only be generalized to those MM who are able to abstain from illicit drug use over an extended period of time. In that complete and ongoing drug abstinence is a relatively uncommon outcome of methadone treatment (Johansson et al., 2007; Maremmani et al., 2007), the clinical utility of GPN may be limited.
In conclusion, these data support that ongoing GABA agonist therapy, as provided by GPN and under the dosing and clinical conditions evaluated, reduces or mitigates OIH in MM patients. Although the analyzable sample size was small, impressive is the large effect of GPN on CP pain responses in this sample (Cohen’s d = 1.01 [threshold peak methadone]; 1.73 [threshold trough methadone]; 1.11 [tolerance peak methadone]; 0.30 [tolerance trough methadone]). Like the hyperalgesia of neuropathic pain, OIH appears to respond to GABA-agonist therapy. Gabapentin therapy, in clinically tolerated doses, significantly improved cold-pressor pain responses in methadone patients. These findings suggest that gabapentin might be a useful adjuvant for the significant number of methadone patients who also have chronic pain (Clark et al., 2008; Rosenblum et al., 2003; Sheu et al., 2009).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alford DP, Compton P, Samet JH. Acute pain management for patients receiving maintenance methadone or buprenorphine therapy. Ann Intern Med. 2006;17:127–134. doi: 10.7326/0003-4819-144-2-200601170-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angst MS, Koppert W, Pahl I, Clark DJ, Schmelz M. Short-term infusion of the mu-opioid agonist remifentanil in humans causes hyperalgesia during withdrawal. Pain. 2003;106:49–57. doi: 10.1016/s0304-3959(03)00276-8. [DOI] [PubMed] [Google Scholar]
- Angst MS, Clark JD. Opioid-induced hyperalgesia; a qualitative systematic review. Anesthesiology. 2006;104:570–587. doi: 10.1097/00000542-200603000-00025. [DOI] [PubMed] [Google Scholar]
- Arendt-Nielsen L, Curatolo M, Drewes A. Human experimental pain models in drug development: translational pain research. Curr Opin Investig Drugs. 2007;8(1):41–53. [PubMed] [Google Scholar]
- Attal N, Brasseur L, Parker F, Chauvin M, Bouhassira D. Effects of gabapentin on the different components of peripheral and central neuropathic pain syndromes: a pilot study. Eur Neurol. 1998;40:191–200. doi: 10.1159/000007979. [DOI] [PubMed] [Google Scholar]
- Athanasos P, Smith CS, White JM, Somogyi AA, Bochner F, Ling W. Methadone maintenance patients are cross tolerant to the antinociceptive effects of very high plasma morphine concentrations. Pain. 2006;120:267–275. doi: 10.1016/j.pain.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Backonja M, Glanzman RL. Gabapentin Dosing for Neuropathic Pain: Evidence from Randomized, Placebo-Controlled Clinical Trials. Clinical Therapeutics. 2003:81–104. doi: 10.1016/s0149-2918(03)90011-7. [DOI] [PubMed] [Google Scholar]
- Ballantyne JC, Shin NS. Efficacy of opiods for chronic pain: a review of the evidence. Clin J Pain. 2008;24:469–78. doi: 10.1097/AJP.0b013e31816b2f26. [DOI] [PubMed] [Google Scholar]
- Basbaum AI. Insights into the development of opioid tolerance. Pain. 1995 Jun;61(3):349–52. doi: 10.1016/0304-3959(95)00009-H. [DOI] [PubMed] [Google Scholar]
- Berry JD, Peterson KL. A single dose of gabapentin reduces acute pain and allodynia in patients with herpes zoster. Neurology. 2005;65:444–447. doi: 10.1212/01.wnl.0000168259.94991.8a. [DOI] [PubMed] [Google Scholar]
- Blackburn-Munro G, Erichsen HK. Antiepileptics and the treatment of neuropathic pain: evidence from animal models. Curr Pharm Des. 2005;11:2961–76. doi: 10.2174/1381612054865000. [DOI] [PubMed] [Google Scholar]
- Blitz B, Dinnerstein AJ, Lowenthal M. Performance on the pain apperception test and tolerance for experimental pain: a lack of relationship. J Clin Psychol. 1968;24(1):73. doi: 10.1002/1097-4679(196801)24:1<73::aid-jclp2270240121>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Cepeda MS, Alvarez H, Morales O, Carr DB. Addition of ultralow dose naloxone to postoperative morphine PCA: unchanged analgesia and opioid requirement but decreased incidence of opioid side effects. Pain. 2004;107:41–6. doi: 10.1016/j.pain.2003.09.011. [DOI] [PubMed] [Google Scholar]
- Ceseña RM, Calcutt NA. Gabapentin prevents hyperalgesia during the formalin test in diabetic rats. Neuroscience letters. 1999;262:101–104. doi: 10.1016/s0304-3940(99)00057-9. [DOI] [PubMed] [Google Scholar]
- Chang G, Chen L, Mao J. Opioid tolerance and hyperalgesia. Med Clin North Am. 2007;91:199–211. doi: 10.1016/j.mcna.2006.10.003. [DOI] [PubMed] [Google Scholar]
- Chen AC, Dworkin SF, Haug J, Gehrig J. Human pain responsivity in a tonic pain model: psychological determinants. Pain. 1989;37(2):143–60. doi: 10.1016/0304-3959(89)90126-7. [DOI] [PubMed] [Google Scholar]
- Cheng JK, Chiou LC. Mechanisms of the antinociceptive action of gabapentin. J Pharmacol Sci. 2006;100:471–86. doi: 10.1254/jphs.cr0050020. [DOI] [PubMed] [Google Scholar]
- Chindalore VL, Craven RA, Yu KP, Butera PG, Burns LH, Friedmann N. Adding ultralow-dose naltrexone to oxycodone enhances and prolongs analgesia: a randomized, controlled trial of Oxytrex. J Pain. 2005;6:392–9. doi: 10.1016/j.jpain.2005.01.356. [DOI] [PubMed] [Google Scholar]
- Chu LF, Angst MS, Clark D. Opiod-induced hyperalgesia in humans: molecular mechanisms and clinical considerations. Clin J Pain. 2008;24:479–96. doi: 10.1097/AJP.0b013e31816b2f43. [DOI] [PubMed] [Google Scholar]
- Chu LF, Clark DJ, Angst MS. Opioid tolerance and hyperalgesia in chronic pain patients after one month of oral morphine therapy: a preliminary prospective study. J Pain. 2006;7:43–48. doi: 10.1016/j.jpain.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Clark MR, Stoller KB, Brooner RK. Assessment and management of chronic pain in individuals seeking treatment for opioid dependence disorder. Can J Psychiatry. 2008;53(8):496–508. doi: 10.1177/070674370805300804. [DOI] [PubMed] [Google Scholar]
- Cleeland CS, Nakamura Y, Howland EW, Morgan NR, Edwards BA, Backonja M. Effects of Oral Morphine on Cold Pressor Tolerance Time and Neuropsychological Performance. Neuropsychopharmacology. 1996;15:252–262. doi: 10.1016/0893-133X(95)00205-R. [DOI] [PubMed] [Google Scholar]
- Colpaert FC. System theory of pain and of opiate analgesia: no tolerance to opiates. Pharmacol Rev. 1996;48:355–408. [PubMed] [Google Scholar]
- Compton MA. Cold pressor pain tolerance in opiate and cocaine abusers: Correlates of drug type and use status. J Pain Symptom Manage. 1994;9:462–73. doi: 10.1016/0885-3924(94)90203-8. [DOI] [PubMed] [Google Scholar]
- Compton P, Athanasos P, Elashoff D. Withdrawal Hyperalgesia After Acute Opioid Physical Dependence in Nonaddicted Humans: A Preliminary Study. J of Pain. 2003;4(9):511–519. doi: 10.1016/j.jpain.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Compton P, Charuvastra C, Kintaudi K, Ling W. Pain responses in methadone-maintained opioid abusers. J Pain Symptom Manage. 2000;20:237–245. doi: 10.1016/s0885-3924(00)00191-3. [DOI] [PubMed] [Google Scholar]
- Compton P, Charuvastra VC, Ling W. Pain intolerance in opioid-maintained former opiate addicts: Effect of long-acting maintenance agent. Drug Alcohol Dep. 2001;3:139–46. doi: 10.1016/s0376-8716(00)00200-3. [DOI] [PubMed] [Google Scholar]
- Compton PA, Ling W, Torrington MA. Lack of effect of chronic dextromethorphan on experimental pain tolerance in methadone-maintained patients. Addiction Biology. 2008;13:393–402. doi: 10.1111/j.1369-1600.2008.00112.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuignet O, Pirson J, Soudon O, Zizi M. Effects of gabapentin on morphine consumption and pain in severely burned patients. Burns. 2006;33:81–86. doi: 10.1016/j.burns.2006.04.020. [DOI] [PubMed] [Google Scholar]
- De La O-Arciniega M, Díaz-Reval IM, Cortés-Arroyo AR, Domínguez-Ramírez AM, López-Muñoz FJ. Anti-nociceptive synergism of morphine and gabapentin in neuropathic pain induced by chronic constriction injury. Pharmacology, Biochemistry and Behavior. 2009;92:457–464. doi: 10.1016/j.pbb.2009.01.013. [DOI] [PubMed] [Google Scholar]
- Dirks J, Petersen KL, Rowbotham MC, Dahl JB. Gabepentin suppresses cutaneous hyperalgesia following heat-capsaicin sensitization. Anesthesiology. 2002;97:102–7. doi: 10.1097/00000542-200207000-00015. [DOI] [PubMed] [Google Scholar]
- Dobecki DA, Schocket SM, Wallace MS. Update on Pharmacotherapy Guidelines for the Treatment of Neuropathic Pain. Current Pain and Headache Reports. 2006;10:185–190. doi: 10.1007/s11916-006-0044-9. [DOI] [PubMed] [Google Scholar]
- Doverty M, White JM, Somogyi AA, Bochner F, Ali R, Ling W. Hyperalgesic responses in methadone maintenance patients. Pain. 2001;90:91–6. doi: 10.1016/s0304-3959(00)00391-2. [DOI] [PubMed] [Google Scholar]
- Dworkin RH, O’Connor AB, Backonja M, Farrar JT, Finnerup NB, Jensen TS, Kalso EA, Loeser JD, Miaskowski C, Nurmikko TJ, Portenoy RK, Rice AS, Stacey BR, Treede RD, Turk DC, Wallace MS. Pharmacologic management of neuropathic pain: Evidence–based recommendations. Pain. 2007;132:237–251. doi: 10.1016/j.pain.2007.08.033. [DOI] [PubMed] [Google Scholar]
- Dudgeon DJ, Bruera E, Gagnon B, Watanabe SM, Allan SJ, Warr DG, MacDonald SM, Savage C, Tu D, Pater JL. A phase III randomized, double-blind, placebo-controlled study evaluating dextromethorphan plus slow-release morphine for chronic cancer pain relief in terminally ill patients. J Pain Symptom Manage. 2007;33:365–71. doi: 10.1016/j.jpainsymman.2006.09.017. [DOI] [PubMed] [Google Scholar]
- Eckhardt K, Li S, Ammon S, Schanzle G, Mikus G, Eichelbaum M. Same incidence of adverse drug events after codeine administration irrespective of the genetically determined differences in morphine formation. Pain. 1998;76:27–33. doi: 10.1016/s0304-3959(98)00021-9. [DOI] [PubMed] [Google Scholar]
- Edes B, Dallenbach KM. The adaptation of pain aroused by cold. Am J Psychol. 1936;48:307–315. [Google Scholar]
- Finnerup NB. A review of central neuropathic pain states. Curr Opin Anaesthiesiol. 2008;21:596–9. doi: 10.1097/ACO.0b013e32830a4c11. [DOI] [PubMed] [Google Scholar]
- Fishbain DA, Cole B, Lewis JE, Gao J, Rosomoff RS. Do Opiods Induce Hyperalgesia in Humans? An Evidence-Based Structured Review. Pain Med. 2009 doi: 10.1111/j.1526-4637.2009.00653.x. [DOI] [PubMed] [Google Scholar]
- Galer BS, Lee D, Ma T, Nagle B, Schlagheck TG. MorphiDex (morphine sulfate/dextromethorphan hydrobromide combination) in the treatment of chronic pain: three multicenter, randomized, double-blind, controlled clinical trials fail to demonstrate enhanced opioid analgesia or reduction in tolerance. Pain. 2005;115:284–95. doi: 10.1016/j.pain.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Garcia de Jalon PD, Harrison FJ, Johnson KI, Kozma C, Schnelle K. A modified cold stimulation technique for the evaluation of analgesic activity in human volunteers. Pain. 1985;22(2):183–189. doi: 10.1016/0304-3959(85)90178-2. [DOI] [PubMed] [Google Scholar]
- Gardell LR, Wang R, Burgess SE, Ossipov MH, Vanderah TW, Malan TP, Jr, Lai J, Porreca F. Sustained morphine exposure induces a spinal dynorphin-dependent enhancement of excitatory transmitter release from primary afferent fibers. J Neurosci. 2002;22:6747–6755. doi: 10.1523/JNEUROSCI.22-15-06747.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardell LR, King T, Ossipov MH, Rice KC, Lai J, Vanderah TW, Porreca F. Opioid receptor-mediated hyperalgesia and antinociceptive tolerance induced by sustained opiate delivery. Neurosci Lett. 2006;396:44–9. doi: 10.1016/j.neulet.2005.11.009. [DOI] [PubMed] [Google Scholar]
- Gilron I. Gabapentin and pregabalin for chronic neuropathic and early postsurgical pain: current evidence and future directions. Curr Opin Anaesthesiol. 2007 Oct;20(5):456–72. doi: 10.1097/ACO.0b013e3282effaa7. [DOI] [PubMed] [Google Scholar]
- Gottrup H, Juhl G, Kristensen AD, Lai R, Chizh BA, Brown J, Bach FW, Jensen TS. Chronic oral gabapentin reduces elements of central sensitization in human experimental hyperalgesia. Anesthesiology. 2004;101:1400–8. doi: 10.1097/00000542-200412000-00021. [DOI] [PubMed] [Google Scholar]
- Grach M, Massalha W, Pud D, Adler R, Eisenberg E. Can coadministration of oxycodone and morphine produce analgesic synergy in humans? An experimental cold pain study. Br J Clin Pharmacol. 2004;58(3):235–42. doi: 10.1111/j.1365-2125.2004.02141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson H, Flood K, Berge O, Brodin E, Olgart L, Stiller C. Gabapentin reverses mechanical allodynia induced by sciatic nerve ischemia and formalin-induced nociception in mice. Experimental Neurology. 2003;182:427–434. doi: 10.1016/s0014-4886(03)00097-9. [DOI] [PubMed] [Google Scholar]
- Gustorff B, Hoechtl K, Sycha T, Felouzis E, Lehr S, Kress HG. The effects of remifentanil and gabapentin on hyperalgesia in a new extended inflammatory skin pain model in healthy volunteers. Anesth Analg. 2004;98:401–7. doi: 10.1213/01.ANE.0000095150.76735.5D. [DOI] [PubMed] [Google Scholar]
- Harvey VL, Dickenson AH. Mechanisms of pain in nonmalignant disease. Curr Opin Support Palliat Care. 2008;2:133–9. doi: 10.1097/SPC.0b013e328300eb24. [DOI] [PubMed] [Google Scholar]
- Haugan F, Rygh LJ, Tjølsen A. Ketamine blocks enhancement of spinal long-term potentiation in chronic opioid treated rats. Acta Anaesthesiol Scand. 2008;52(5):681–7. doi: 10.1111/j.1399-6576.2008.01637.x. [DOI] [PubMed] [Google Scholar]
- Hayashida K, Parker R, Eisenach JC. Oral gabapentin activates spinal cholinergic circuits to reduce hypersensitivity after peripheral nerve injury and interacts synergistically with oral donepezil. Anesthesiology. 2007;106(6):1213–9. doi: 10.1097/01.anes.0000267605.40258.98. [DOI] [PubMed] [Google Scholar]
- Heiskanen T, Hartel B, Dahl ML, Seppala T, Kalso E. Analgesic effects of dextromethorphan and morphine in patients with chronic pain. Pain. 2002;96:261–267. doi: 10.1016/S0304-3959(01)00455-9. [DOI] [PubMed] [Google Scholar]
- Helmy SA, Bali A. The effect of the preemptive use of the NMDA receptor antagonist dextromethorphan on postoperative analgesic requirements. Anesth Analg. 2001;92:739–44. doi: 10.1097/00000539-200103000-00035. [DOI] [PubMed] [Google Scholar]
- Hines EA, Brown GE. A standard stimulus for measuring vasometer reactions: its application in the study of hypertension. Proceed Staff Meeting Mayo Clinic. 1932;7:332–340. [Google Scholar]
- Ho A, Dole V. Pain perception in drug-free and in methadone-maintained human ex-addicts. Proc Society Exp Bio Med. 1979;162:392–5. doi: 10.3181/00379727-162-40689. [DOI] [PubMed] [Google Scholar]
- Hutchinson MR, Bland ST, Johnson KW, Rice KC, Maier SF, Watkins LR. Opiod-induced glial activation: mechanisms of activation and implications for opiod analgesia, dependence, and reward. Scientific World Journal. 2007;27:98–111. doi: 10.1100/tsw.2007.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson MR, Coats BD, Lewis SS, Zhang Y, Sprunger DB, Rezvani N, Baker EM, Jekich BM, Wieseler JL, Somogyi AA, Martin D, Poole S, Judd CM, Maier SF, Watkins LR. Proinflammatory cytokines oppose opioid-induced acute and chronic analgesia. Brain Behav Immun. 2008:1. doi: 10.1016/j.bbi.2008.05.004. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iannetti GD, Zambreanu L, Wise RG, Buchanan TJ, Huggins JP, Smart TS, Vennart W, Tracey I. Pharmacological modulation of pain-related brain activity during normal and central sensitization states in humans. PNAS. 2005;102:18195–18200. doi: 10.1073/pnas.0506624102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson BA, Berglund M, Lindgren A. Efficacy of maintenance treatment with methadone for opioid dependence: a meta-analytical study. Nord J Psychiatry. 2007;61(4):288–95. doi: 10.1080/08039480701415251. [DOI] [PubMed] [Google Scholar]
- Jones DL, Sorkin LS. Systemic gabapentin and S(+)-3isobutyl-γ-aminobutyric acid block secondary hyperalgesia. Brain Research. 1998;810:93–99. doi: 10.1016/s0006-8993(98)00890-7. [DOI] [PubMed] [Google Scholar]
- Jones SF, McQuay HJ, Moore RA, Hand CW. Morphine and ibuprofen compared using the cold pressor test. Pain. 1988;34(2):117–22. doi: 10.1016/0304-3959(88)90156-X. [DOI] [PubMed] [Google Scholar]
- Jun JH, Yaksh TL. The effect of intrathecal gabapentin and 3-isobutyl-γ-aminobutyric acid on the hyperalgesia observed after thermal injury in the rat. Anesth Analg. 1998;86:348–54. doi: 10.1097/00000539-199802000-00025. [DOI] [PubMed] [Google Scholar]
- Kaplan H, Fields HL. Hyperalgesia during acute opioid abstinence: evidence for a nociceptive facilitating function of the rostral ventromedial medulla. J Neurosci. 1991 May;11(5):1433–9. doi: 10.1523/JNEUROSCI.11-05-01433.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knotkova H, Pappagallo M. Adjuvant Analgescics. Med Clin N Am. 2007;91:113–124. doi: 10.1016/j.mcna.2006.10.007. [DOI] [PubMed] [Google Scholar]
- Kong VKF, Irwin MG. Gabapentin: a multimodal perioperative drug. BJA. 2007;99:775–86. doi: 10.1093/bja/aem316. [DOI] [PubMed] [Google Scholar]
- Koppert W, Schmelz M. The impact of opioid-induced hyperalgesia for postoperative pain. Best Pract Res Clin Anaesthesiol. 2007;21:65–83. doi: 10.1016/j.bpa.2006.12.004. [DOI] [PubMed] [Google Scholar]
- Koppert W, Sittl R, Scheuber K, Alsheimer M, Schmelz M, Schuttler J. Differential modulation of remifentanil-induced analgesia and postinfusion hyperalgesia by S-ketamine and clonidine in humans. Anesthesiology. 2003;99:152–159. doi: 10.1097/00000542-200307000-00025. [DOI] [PubMed] [Google Scholar]
- Laulin JP, Celerier E, Larcher A, Le Moal M, Simmonet G. Letters to neuroscience: opiate tolerance to daily heroin administration: an apparent phenomenon associated with enhanced pain sensitivity. Neuroscience. 1999;89:631–636. doi: 10.1016/s0306-4522(98)00652-6. [DOI] [PubMed] [Google Scholar]
- Li X, Angst MS, Clark JD. A murine model of opioid-induced hyperalgesia. Brain Res Mol Brain Res. 2001;86:56–62. doi: 10.1016/s0169-328x(00)00260-6. [DOI] [PubMed] [Google Scholar]
- Mao J. Opioid-induced abnormal pain sensitivity. Curr Pain Headache Rep. 2006;10:67–70. doi: 10.1007/s11916-006-0011-5. [DOI] [PubMed] [Google Scholar]
- Mao J, Sung B, Lim G. Chronic morphine induces downregulation of spinal glutamate transporters; Implications in morphine tolerance and abnormal pain sensitivity. J Neuroscience. 2002;22:8312–8323. doi: 10.1523/JNEUROSCI.22-18-08312.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maremmani I, Pani PP, Mellini A, Pacini M, Marini G, Lovrecic M, Perugi G, Shinderman M. Alcohol and cocaine use and abuse among opioid addicts engaged in a methadone maintenance treatment program. J Addict Dis. 2007;26(1):61–70. doi: 10.1300/J069v26n01_08. [DOI] [PubMed] [Google Scholar]
- Mellegers MA, Furlan AD, Mailis A. Gabepntin for Neuropathic Pain: Systematic Review of controlled and Uncontrolled Literature. The Clinical Journal of Pain. 2001;17:284–295. doi: 10.1097/00002508-200112000-00002. [DOI] [PubMed] [Google Scholar]
- Mika J. Modulation of microglia can attenuate neuropathic pain symptoms and enhance morphine effectiveness. Pharmacol Rep. 2008;60:297–307. [PubMed] [Google Scholar]
- Moulin DE, Clark AJ, Gilron I, Ware MA, Watson CP, Sessle BJ, Coderre T, Morley-Forster PK, Stinson J, Boulanger A, Peng P, Finley GA, Talnzer P, Squire P, Dion D, Cholkan A, Gilani A, Gordon A, Henry J, Jovey R, Lynch M, Mailis-Gagnon A, Panju A, Rollman GB, Velly A Canadian Pain Society. Pharmacological management of chronic neuropathic pain – Consensus statement and guidelines from the Canadian Pain Society. Pain Res Manage. 2007;12:13–21. doi: 10.1155/2007/730785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newshan G. Pain management in the addicted patient: Practical considerations. Nurs Outlook. 2000;48:81–85. doi: 10.1016/s0029-6554(00)90007-1. [DOI] [PubMed] [Google Scholar]
- Ossipov MH, Lai J, King T, Vanderah TW, Malan TP, Jr, Hruby VJ, Porreca F. Antinociceptive and nociceptive actions of opioids. J Neurobiol. 2004;61:6–148. doi: 10.1002/neu.20091. [DOI] [PubMed] [Google Scholar]
- Ossipov MH, Lai J, King T, Vanderah TW, Porreca F. Underlying mechanisms of pronociceptive consequences of prolonged morphine exposure. Biopolymers. 2005;80:319–324. doi: 10.1002/bip.20254. [DOI] [PubMed] [Google Scholar]
- Patel S, Naeem S, Kesingland A, Froestl W, Capogna M, Urban L, Fox A. The effects of Gaba agonists and gabapentin on mechanical hyperalgesia in models of neuropathic and inflammatory pain in the rat. Pain. 2001;90:217–226. doi: 10.1016/S0304-3959(00)00404-8. [DOI] [PubMed] [Google Scholar]
- Petersen-Felix S, Arendt-Nielsen L. From pain research to pain treatment: the role of human experimental pain models. Best Pract Res Clin Anaesthesiol. 2002;16(4):667–80. doi: 10.1053/bean.2002.0258. [DOI] [PubMed] [Google Scholar]
- Pud D, Cohen D, Lawental E, Eisenberg E. Opioids and abnormal pain perception: New evidence from a study of chronic opioid addicts and healthy subjects. Drug Alcohol Depend. 2006;82:218–223. doi: 10.1016/j.drugalcdep.2005.09.007. [DOI] [PubMed] [Google Scholar]
- Rose MA, Kam PCA. Gabapentin: pharmacology and its use in pain management. Anaesthesia. 2002;57:451–462. doi: 10.1046/j.0003-2409.2001.02399.x. [DOI] [PubMed] [Google Scholar]
- Rosenblum A, Joseph H, Fong C, Kipnis S, Cleland C, Portenoy RK. Prevalence and characteristics of chronic pain among chemically dependent patients in methadone maintenance and residential treatment facilities. JAMA. 2003;14;289(18):2370–8. doi: 10.1001/jama.289.18.2370. [DOI] [PubMed] [Google Scholar]
- Segerdahl M. Multiple dose gabapentin attenuates cutaneous pain and central sensitation but not muscle pain in healthy volunteers. Pain. 2006;125:158–164. doi: 10.1016/j.pain.2006.05.008. [DOI] [PubMed] [Google Scholar]
- Scimeca MM, Savage SR, Portenoy R, Lowinson J. Treatment of pain in methadone-maintained patients. Mt Sinai J Med. 2000;67:412–422. [PubMed] [Google Scholar]
- Sheu R, Lussier D, Rosenblum A, Fong C, Portenoy J, Joseph H, Portenoy RK. Prevalence and characteristics of chronic pain in patients admitted to an outpatient drug and alcohol treatment program. Pain Med. 2008;9(7):911–7. doi: 10.1111/j.1526-4637.2008.00420.x. [DOI] [PubMed] [Google Scholar]
- Simonnet G. Opioids: from analgesia to anti-hyperalgesia? Pain. 2005;118:8–9. doi: 10.1016/j.pain.2005.07.021. [DOI] [PubMed] [Google Scholar]
- Singler B, Tröster A, Manering N, Schüttler J, Koppert W. Modulation of remifentanil-induced postinfusion hyperalgesia by propofol. Anesth Analg. 2007 Jun;104(6):1397–403. doi: 10.1213/01.ane.0000261305.22324.f3. [DOI] [PubMed] [Google Scholar]
- Tassorelli C, Micieli G, Osipova V, Rossi F, Nappi G. Pupillary and cardiovascular responses to the cold-pressor test. J Auton Nerv Syst. 1995;55(1–2):45–9. doi: 10.1016/0165-1838(95)00026-t. [DOI] [PubMed] [Google Scholar]
- Taylor BK. Spinal inhibitory neurotransmission in neuropathic pain. Current Pain and Headache Reports. 2009;13:208–214. doi: 10.1007/s11916-009-0035-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terner JM, Barrett AC, Lomas LM, Negus SS, Picker MJ. Influence of low doses of naltrexone on morphine antinociception and morphine tolerance in male and female rats of four strains. Pain. 2006;122:90–101. doi: 10.1016/j.pain.2006.01.019. [DOI] [PubMed] [Google Scholar]
- Tiippana EM, Hamunen K, Kontinen VK, Kalso E. Do Surgical Pateints Benefit from Perioperative Gabapentin/Pregabalin? A systematic Review of Efficacy. Anesth Analg. 2007;104:1545–56. doi: 10.1213/01.ane.0000261517.27532.80. [DOI] [PubMed] [Google Scholar]
- Vadalouca A, Siafaka J, Argyra E, Vrachnou E, Moka E. Therapeutic Management of Chronic Neuropathic Pain. Ann NYAcad Sci. 2006;1088:164–186. doi: 10.1196/annals.1366.016. [DOI] [PubMed] [Google Scholar]
- Van Elstraete AC, Sitbon P, Mazoit JX, Benhamou D. Gabapentin Prevents Delayed and Long-Lasting Hyperalgesia Induced by Fentanyl in Rats. Anesthesiology. 2008;108:484–94. doi: 10.1097/ALN.0b013e318164cf85. [DOI] [PubMed] [Google Scholar]
- Vanderah TW, Ossipov MH, Lai J, Malan TP, Jr, Porreca F. Mechanisms of opioid–induced pain and antinociceptive tolerance: descending facilitation and spinal dynorphin. Pain. 2001;92:5–9. doi: 10.1016/s0304-3959(01)00311-6. [DOI] [PubMed] [Google Scholar]
- Wallace MS, Schulteis G. Effect of chronic oral gabapentin on capsaicin-induced pain and hyperalgesia. Clin J Pain. 2008;24:544–549. doi: 10.1097/AJP.0b013e3181673b93. [DOI] [PubMed] [Google Scholar]
- Wang HY, Friedman E, Olmstead MC, Burns LH. Ultra-low-dose naloxone suppresses opioid tolerance, dependence and associated changes in mu opioid receptor-G protein coupling and Gbetagamma signaling. Neuroscience. 2005;135:247–61. doi: 10.1016/j.neuroscience.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Watkins LR, Hutchinson MR, Ledeboer A, Wieseler-Frank J, Milligan ED, Maier SF. Norman Cousins Lecture. Glia as the “bad guys”: implications for improving clinical pain control and the clinical utility of opiods. Brain Behav Immun. 2007;21:131–46. doi: 10.1016/j.bbi.2006.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster LR. Oxytrex: An oxycodone and ultra-low-dose naltrexone formulation. Expert Opin Investig Drugs. 2007;16:1277–83. doi: 10.1517/13543784.16.8.1277. [DOI] [PubMed] [Google Scholar]
- Webster LR, Butera PG, Moran LV, Wu N, Burns LH, Friedmann N. Oxytrex minimizes physical dependence while providing effective analgesia: A randomized controlled trial in low back pain. J Pain. 2006;7:937–46. doi: 10.1016/j.jpain.2006.05.005. [DOI] [PubMed] [Google Scholar]
- Weinbroum AA, Gorodezky A, Niv D, Ben-Abraham R, Rudick V, Szold A. Dextromethorphan attenuation of postoperative pain and primary and secondary thermal hyperalgesia. Can J Anaesth. 2001;48:167–174. doi: 10.1007/BF03019730. [DOI] [PubMed] [Google Scholar]
- Wiffen PJ, McQuay HJ, Moore RA, Rees J. Gabapentin for actue and chronic pain. The Cochrane Collaboration. 2009;(2):1–24. [Google Scholar]
- Wilder-Smith OH, Arendt-Nielsen L. Postoperative hyperalgesia: its clinical importance and relevance. Anesthesiology. 2006;104:601–607. doi: 10.1097/00000542-200603000-00028. [DOI] [PubMed] [Google Scholar]
- Wolff BB, Kantor TG, Cohen P. Laboratory pain induction methods for human analgesic assays. In: Bonica JJ, Albe-Fessard D, editors. Advances in pain research and therapeutics. Vol. 1. New York: Raven Press; 1976. pp. 363–367. [Google Scholar]
- Yasuda T, Miki S, Yoshinga N, Senba E. Effects of amitriptyline and gabapentin on bilateral hyperalgesia observed in an animal model of unilateral axotomy. Pain. 2005;115:161–170. doi: 10.1016/j.pain.2005.02.026. [DOI] [PubMed] [Google Scholar]