Abstract
To elucidate the endocrine regulation of vitellogenin (Vg) synthesis in the red flour beetle, Tribolium castaneum, the titers of juvenile hormone (JH) and ecdysteroids in the whole body of female beetles were measured and compared with Vg mRNA levels. Juvenile hormone levels remained high while the ecdysteroid levels declined steadily during 1–5 days post adult emergence (PAE). The Vg mRNA levels began to increase by the end of 3rd day PAE and peaked by the 4th–5th day PAE. Gene expression profiling by microarray and quantitative real-time PCR analyses of RNA isolated from 1–5 days PAE beetles revealed that the genes coding for proteins involved in JH biosynthesis and action, but not those involved in 20-hydroxyecdysone (20E) biosynthesis and action had similar expression patterns as the genes coding for Vg. RNA interference (RNAi)-aided knock-down in the expression of these genes showed that both JH and 20E were required for Vg gene expression. However, Vg mRNA was induced by the application of JH III but not by the injection of 20E into the previtellogenic females. These data suggest that JH is required for Vg synthesis in the fat body and 20E influences Vg synthesis through its action on oocyte maturation.
Keywords: microarray, RNAi, vitellogenesis, juvenile hormone, 20E, gene expression, Tribolium
1. Introduction
Endocrine regulation of insect vitellogenesis has been investigated for many years (Engelmann, 1968). During vitellogenesis, female-specific proteins, vitellogenins (Vg), are synthesized in the fat body, secreted into the hemolymph and taken up by the developing oocyte. Ecdysteroids (20-hydroxyecdysone, 20E, is the most active form) and juvenile hormones (JH) assume a gonadotrophic role in the adult female insects and regulate vitellogenesis (Hagedorn, 1985; Engelmann, 1986; Bownes, 1989; Bownes et. al., 1996). The hormonal control of vitellogenesis varies widely among insect species, either one of these hormones alone or both together are known to regulate this process (Hegedorn and Kunkel, 1979; Raikhel and Dhadialla, 1992).
In locusts and cockroaches, the hemolymph concentration of JH is a critical factor in the stimulation of the transcription of Vg genes (Engelmann, 1983; Wyatt and Davey, 1996). Moreover, the exogenous JH application can induce Vg production in the fat body of these insects (Keeley and Mckercher, 1985; Zhang et al. 1993; Glinka and Wyatt, 1996; Comas et al. 1999). In the fruit fly, Drosophila melanogaster, ecdysteroids as well as JH control yolk protein production (Bownes, 1989; Richard et al. 2001). In the mosquito, Aedes aegypti, JH plays a priming role in preparing the fat body for Vg synthesis and ecdysteroids regulate expression of Vg gene after a blood meal (Raikhel et al. 2005). Both JH and ecdysteroids regulate reproduction in most of the insects belonging to order Hymenoptera, except for the social insects such as honey bees where the roles of JH and 20E still remain controversial (Pinto et al. 2000; Amdam, et al. 2004; Brent et al. 2006). The lepidopteran insects require either JH or 20E for the initiation of Vg synthesis in the fat body (Ramaswamy et al. 1997). Not much is known about the mechanisms of JH action in the regulation of vitellogenesis. Availability of the complete genome sequence and functioning of systemic RNAi in the red flour beetle, Tribolium castaneum could help in understanding the molecular mechanisms involved in beetle reproduction. As an initial step to study the hormonal regulation of vitellogenesis in T. castaneum, we have determined the titers of JH and 20E and analyzed the expression of a repertoire of genes involved in JH and 20E signaling pathways in the previtellogenic and vitellogenic virgin female beetles by microarray and qRT-PCR. RNAi analysis on genes coding for proteins involved in JH and 20E biosynthesis and action showed that both JH and 20E are required for Vg synthesis. However, topical application of JH but not injection of 20E induced Vg gene expression. These studies suggest that JH regulates Vg synthesis in the fat body and 20E influences Vg synthesis through its action on oocyte maturation.
2. Materials and Methods
2.1. Rearing and staging
Strain GA-1 of T. castaneum was reared on organic wheat flour containing 10% yeast at 30oC under standard conditions (Parthasarathy et al. 2008). The pupae were separated by sex based on the structural differences of genital papillae according to Tribolium rearing protocol (http://bru.gmprc.ksu.edu/proj/tribolium/wrangle.asp). Adult beetles were staged soon after emergence; the adults with untanned cuticle (teneral adults) were designated as 0 h and staged thereafter. The male and female beetles were kept separately under above conditions and only virgin beetles were used for experiments.
2.2. Estimation of JH and ecdysteroid levels
Juvenile hormone levels were estimated following the methods described in Parthasarathy et al. (2009). Juvenile hormone from unknown samples was quantified based on the peak area of internal standard, methoprene (Wellmark International, Dallas, TX). An enzyme immunoassay (EIA) was used to estimate ecdysteroid levels as previously described (Parthasarathy et al. 2009).
2.3. Hormone and hormone analog treatments
Juvenile hormone III (Sigma Chemical Co. St Louis, MO) and its analog hydroprene (Wellmark International, Dallas, TX) were dissolved in acetone. One microliter of 1.0 μg/μl or 10.0 μg/μl JH III was applied topically to day 2 PAE female beetles. One microliter of 10.0 μg/μl hydroprene was applied topically to day 2 PAE female beetles.. The equivalent volume of acetone was applied to control beetles. Twenty hydroxyecdysone (20E, Sigma Chemical Co. St Louis, MO) was dissolved in distilled water. 0.25 μl of 10 mM or 100 mM was injected into each female beetle on day 2 PAE to achieve a final concentration of approximately 1.0 μg and 10.0 μg per insect. The same volume of distilled water was injected into the control beetles.
2.4. Microarray analysis
Total RNA was isolated from the whole body/fat body of staged insects using spin columns (RNeasy, Qiagen, Valencia, CA). The integrity of RNA was verified using an Agilent 2100 Bioanalyzer (Agilent Technologies). 200 ng of total RNA pooled from three beetles in each time-point of 24 h interval from 1 to 4 days PAE was labeled using Agilent Low RNA Input Fluorescent Linear Amplification Kit (Agilent) following the manufacturer’s instructions. Labeled cRNA were purified using RNeasy Mini Elute Kit (Qiagen Valencia, CA). 600 ng of fluorescently labeled cRNA were used for hybridization. The 60-mer-oligonucleotides designed based on 15,208 genes selected from the 16,000 genes predicted by T. castaneum genome annotations. 736 control probe sets were also printed onto glass slides (8 grids of 15K) at Agilent Technologies. The labeling of probes, hybridization, washings, and normalization of the data were performed as described in Parthasarathy et al. (2009). The normalized data were finally subjected to the “t”-test, Bonferroni and Benjamini & Hochberg false discovery rate multiple testing corrections using GeneSpring GX v.9.0.1 software. As Vg gene expression peaked after 3 days PAE, the fold changes in expression levels between day 1 and day 4 were taken into consideration. The differentially-expressed genes of significance were evaluated with the aid of the Volcano plots (p-value versus fold-change).
2.5. Double-stranded RNA (dsRNA) synthesis and injection
The genes for RNAi were selected based on their important roles in hormonal biosynthesis and action. The genes coding for enzymes involved in the biosynthesis of JH (JHAMT, Minakuchi et al, 2008a), biosynthesis of ecdysteroids (Phantom and Shade, Petryk et al. 2003; Niwa et al. 2004; Warren et al. 2004), genes coding for proteins involved in JH action (Met and Kr-h1, Konopova and Jindra, 2007; Minakuchi et al. 2008b) and ecdysteroid action (EcR and USP, Tan and Palli, 2008) were selected.
dsRNAs were synthesized using the Ambion MEGAscript transcription kit (Ambion, Austin, TX). Cognate primers designed based on the sequences available in the Beetlebase (Table 1) with T7 polymerase promoter sequence added at their 5′ ends were used in PCR reactions to amplify 300–500 bp fragments of each gene. The resultant PCR products were used in transcription reaction using the kit following the methods described in the instruction manual. The dsRNAs were injected into the pupae/adult on the ventral side of the first abdominal segment using a aspirator tube assembly (Sigma Chemical Co. St. Louis, MO) fitted with 3.5″ glass capillary tube, pulled by a needle puller (Model P-2000, Sutter Instruments Co.). Injected pupae/adults were reared under standard conditions until use. dsRNA injection of all the candidate genes, except for the genes coding for proteins involved in ecdysteroid biosynthesis and action, were injected into the two-day-old pupae. Since injection of dsRNA of genes coding for proteins involved in ecdysteroid biosynthesis and action blocked pupal-adult metamorphosis, these dsRNAs were injected into adults less than 24 h old. The dsRNA of Escherichia coli malE gene was used as the control.
Table 1.
List of primers used
| Gene | Real-time PCR Forward and Reverse 5′-3′ | dsRNA Forward and Reverse 5′-3′ |
|---|---|---|
| JHAMT | CATCTCGCCCTATCACCATTCG | ATGAACAAAGCCTCACTGTACTCAA |
| CCGCTGAAACCGATTTTGACAA | CTCTGTTCCACCACCCAATGCAA | |
| Met | GGGAAAGCAAAGGATCATCA | TAAGGCGGCAAACTC |
| AAGGCCTTCTTGCTCACTCA | TGGCTCAACCGACTCGTC | |
| Kr-h1 | TGTGACGTTTGCTCGAAGAC | AAGAGCATGGAAGCACACA |
| GCACGAGTAGGGCTTTTCAC | CTTGGCGGAAGACTCAACTC | |
| Phantom | TGATCTCCGACGCCAAACTCATCA | TGTCATCCAAGCGTTTCTTG |
| GCAAATCAGGCCGAAACCCTTCAT | AGCAATTTCCAGCTCTTCCA | |
| Shade | TCGTACTCGAAAGCGTGCGTTACT | CCGGCCTACAAAACACTCAT |
| GCTCCGGTTTGAACTTTGAAGCGT | CAATCCCAAAGTGACACACG | |
| EcR | GATGGATGGCGAAGATCAGT | GCCTCCGGTTACCACTACAA |
| ACTTCGCTGGAACATGCTTT | CTGGTTCAACCTTGATGACG | |
| USP | GATGCAAGCACAGGATGCTA | GAGGGATAAAGTCGGTGCAA |
| CCGACTTTATCCCTCGAACA | GCCCTCCAACATCTCCATTA | |
| TC007765 | TAGCTGCTCTGACTGAAACTGCCA | N/A |
| TGCTAGAAACACGTTCGCGGTAGT | ||
| TC14078 | AGGAAATCAACGCTGCAATCACCG | N/A |
| TATCACCGTGGCACACAACATTGC | ||
| Vg1 | TTGCAAATGCTGGGTGGTGAAGAC | N/A |
| AGCGTGTGCGTTGATAACTTGCTG | ||
| Vg2 | AACGCACACGATTTCGACCAAGTG | N/A |
| ACGGCAGCATTAACTTGGTTGCTC | ||
| HR3 | CCGTGCAAAGTATGTGG | N/A |
| GTCGGCAGTATTGACATC | ||
| JHE | ACTTTACGTGGGGTGTGAGC | N/A |
| TTGATGAGGATCGGGATTTC | ||
| rp49 | TGACCGTTATGGCAAACTCA | N/A |
| TAGCATGTGCTTCGTTTTGG |
2.6. cDNA synthesis and Quantitative real-time reverse-transcriptase PCR (qRT-PCR)
Total RNA was extracted from the whole body of staged adults and from the insects injected with dsRNA for specific genes using TRI reagent (Molecular Research Center Inc., Cincinnati, OH). cDNA was synthesized using 2 μg of DNAse1 (Ambion, Austin, TX) – treated RNA and iScript cDNA synthesis kit (Biorad Laboratories, Hercules, CA) in a 20 μl reaction volume as per the manufacturer’s instructions. Real-time quantitative reverse-transcriptase PCR was performed using MyiQ single-color real-time PCR detection system (Biorad Laboratories). PCR reaction components were: 1 μl of cDNA, 1 μl each of forward and reverse sequence specific primers (Table 1), 7 μl of H2O and 10 μl of supermix (Biorad Laboratories, Hercules, CA). PCR conditions were: 95°C for 3 min followed by 45 cycles of 95°C for 10 seconds, 60°C for 20 seconds, and 72°C for 30 seconds. Both the PCR efficiency and R2 (correlation coefficient) values were taken into account prior to estimating the relative quantities. Relative expression levels of each gene were quantified using ribosomal protein (rp49) mRNA levels as an internal control.
2.7. Statistical analysis
JMP® Start Statistics (SAS institute Inc.) version 5.1 was used for statistical analysis. The mean values of treatments versus mean values of control were compared using one way ANOVA analysis. For comparison of means between two variables, Student’s t-test was used. p-value less than 0.05 is regarded as significant. For comparison of the multiple means, Tukey-Kramer Honestly Significant Difference (HSD) adjustment’s mean separation test was included.
3. Results
3.1. Microarray analysis
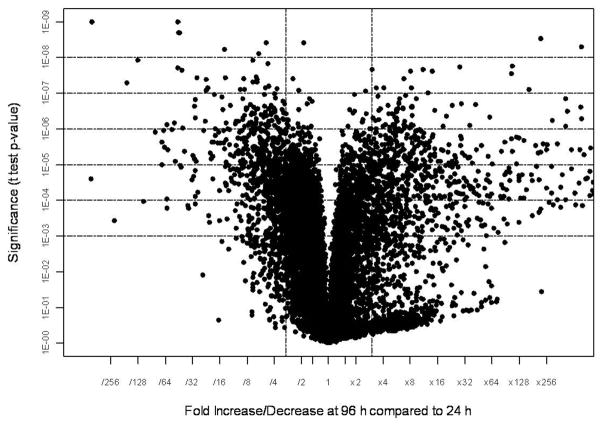
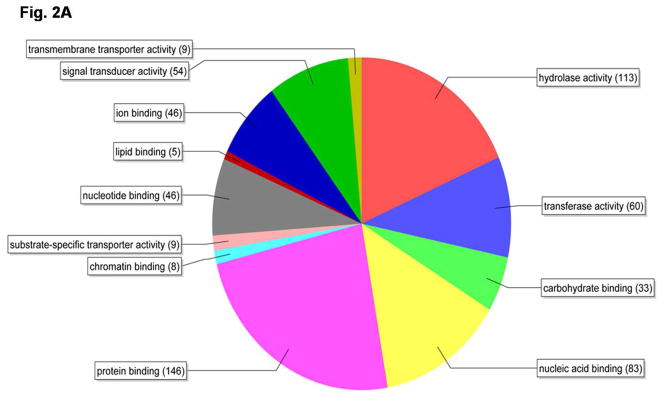
Total RNA samples isolated from the whole body of the female beetles between 1–4 days PAE at 24 h intervals were labeled and hybridized to T. castaneum custom microarrays. Three biological replicates were included for each time-point. Out of the 15,208 probe sets screened, hybridization to 11,534 probe sets was detected. The fold differences in expression (calculated by dividing the mean normalized values for day 1 and day 4 PAE samples) and the significance of difference among means of three replicates (p-value from t-test) for 11,534 probe sets are shown as a volcano plot (Fig. 1). When compared to their expression on day 1 PAE, the expression levels of 866 genes increased on day 4 PAE by 3-fold or more with a p value of <0.01 (Table 2). Upon filtering by false discovery rate multiple testing corrections, the Bonferroni test with high stringency identified 158 genes and the Benjamini-Hochberg test with medium stringency showed 838 genes that showed an increase by 3-fold or more with a p-value of <0.01 on day 4 PAE when compared to their expression on day 1 PAE (Table 2). Among these 838 genes filtered by the Benjamini-Hochberg test, the expression levels of several genes known to be involved in vitellogenesis, JH, and 20E pathways during 1–4 days PAE were compared (Table 3). Some of the genes known to be involved in these pathways with a p-value of 0.01–0.05 filtered by the Benjamini-Hochberg test were also included. The expression of vitellogenin genes (Vg1 represented by TC013602; similar to vitellogenin-1, and Vg2 represented by TC010839; similar to vitellogenin-6, the Beetlebase, http://beetlebase.org) increased by 350–2000-fold from day 1 to day 4 PAE. The genes coding for JHAMT and JH-inducible proteins (TC007665, TC014078); predicted as genes coding for JHIPs based on the sequence homology to JHIPs of Ae. aegypti (XP_001652117.1, 5e-38; XP_001652116.1, 5e-41 respectively) were significantly higher on day 4 PAE, when compared to their expression on day 1 PAE. The genes coding for enzymes known to be involved in ecdysteroid biosynthesis (Phantom) and the genes coding for proteins known to be involved in 20E action (EcR, USP, HR3, HR38, HR4, FTZ-F1) did not show significant increase from day 1 to day 4 PAE. Some of the genes that showed significant changes in their expression from day 1 to day 4 PAE by microarray analysis were selected for confirmation by quantitative real-time qRT-PCR. Besides, the 838 genes filtered by the Benjamini-Hochberg test that showed an increase by 3-fold or more with a p-value of <0.01 on day 4 PAE when compared to their expression on day 1 PAE, were categorized into 12 functional groups based on their annotations on molecular functions (Fig. 2A). The important categories included protein binding (17%), signal transduction (6%), and transporter activities (2%) that might be related to active Vg synthesis.
Fig. 1.
Volcano-plot of differentially-expressed genes identified by microarray analysis. The p-values of the t-test were plotted against the fold-change in gene expression for all genes. The horizontal lines in the plot represent the significance of 0.001–0.00000001 for the t-test, under the assumption that each gene has a unique variance. The vertical bars represent the genes that are a minimum of three-fold up- or down-regulated on day 4 PAE when compared to their expression on day 1 PAE.
Table 2.
Number of genes expressed at 3-fold or higher in the whole body at 4 day PAE when compared to their expression at 1 day PAE
| Statistics | Stringency | p value | Total | ||
|---|---|---|---|---|---|
| < 0.0001 | < 0.001 | <0.01 | |||
| Bonferroni | High | 11 | 33 | 114 | 158 |
| Benjamini-Hochberg | Medium | 177 | 425 | 236 | 838 |
| T-test only | None | 533 | 207 | 126 | 866 |
The total RNA isolated from the whole body of the female beetle on 1 day and 4 day post adult emergence (PAE) were used for microarray analysis. The normalized data filtered using false discovery rate multiple testing corrections with different stringency levels generated by GeneSpring GX v.9.0.1 software were used. The number of up-regulated genes on day 4 PAE when compared to their expression on day 1 PAE at 3-fold or higher levels with different p-value ranges in each statistical analysis are shown.
Table 3.
Genes expressed in the whole body of female beetles during the pre-vitellogenic stage
| Gene ID | 1 day PAE | 2 day PAE | 3 day PAE | 4 day PAE | Fold change | p-value | Annotations |
|---|---|---|---|---|---|---|---|
| JH | |||||||
| TC008469 | 0.415 | 1.469 | 1.212 | 0.945 | 2.277 | 0.00136 | JHAMT |
| TC003908 | 1.299 | 0.879 | 0.968 | 1.014 | 0.780 | 0.04250 | Met |
| TC012990 | 0.017 | 1.731 | 1.195 | 1.083 | 65.519 | 0.00014 | Kr-h1B |
| TC007665 | 0.154 | 1.687 | 0.725 | 64.647 | 421.008 | 0.00002 | JHIP |
| TC014078 | 0.215 | 1.325 | 0.799 | 1.525 | 7.093 | 0.00012 | JHIP |
| Vitellogenin related | |||||||
| TC013602 | 1.044 | 0.784 | 1.144 | 404.733 | 387.530 | 0.00021 | Vg1 |
| TC010839 | 0.191 | 0.589 | 0.556 | 413.365 | 2322.50 | 0.00020 | Vg2 |
| TC004042 | 0.137 | 0.936 | 0.761 | 1.759 | 12.851 | 0.00032 | VgR-1 |
| TC008596 | 0.307 | 0.415 | 0.630 | 1.374 | 4.479 | 0.00825 | Vg R-2 |
| 20E | |||||||
| TC001916 | 1.649 | 0.982 | 1.038 | 0.948 | 0.575 | 0.00986 | Phantom |
| TC012113 | 0.685 | 1.055 | 0.905 | 1.202 | 1.755 | 0.00681 | EcR |
| TC014028 | 0.807 | 1.025 | 0.999 | 1.138 | 1.411 | 0.01180 | USP |
| TC002550 | 1.204 | 0.961 | 1.064 | 0.747 | 0.621 | 0.00550 | FTZ-F1 |
| TC013146 | 1.246 | 1.057 | 1.717 | 0.202 | 0.162 | 0.00015 | HR38 |
| TC014986 | 1.410 | 0.951 | 1.119 | 0.656 | 0.466 | 0.00013 | HR39 |
| TC000543 | 1.770 | 0.851 | 1.1946 | 0.578 | 0.327 | 0.00349 | HR4 |
The total RNA isolated from the whole body of the female beetle collected on days 1–4 post adult emergence (PAE) were used for microarray analysis. The data filtered using Benjamini-Hochberg false discovery rate multiple testing corrections generated by GeneSpring GX v.9.0.1 software were used. The normalized expression levels of selected genes involved in vitellogenesis, JH and 20E biosynthesis and action during 1–4 days PAE showing changes in expression (1 day PAE Vs 4 day PAE) with a p-value of less than 0.05 are shown.
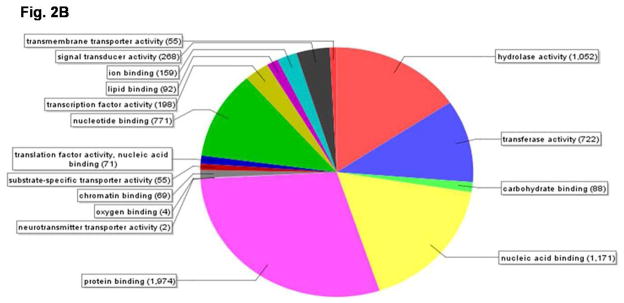
Fig. 2.
Pie-chart showing the distribution of genes based on their annotations on molecular functions using Blast2go software. A) the 838 genes filtered by the Benjamini-Hochberg test that showed an increase by 3-fold or more with a p-value of <0.01 on day 4 PAE when compared to their expression on day 1 PAE by the microarray analysis were categorized using the above software. B) Microarray analysis was performed with RNA samples from day 4 PAE fat body. Normalized data for genes with a value of 1.0 or higher was analyzed by Blast2go software to identify functional classes of genes expressed in the fat body during Vg synthesis.
Total RNA samples isolated from the fat body tissues of the female beetles on day 4 PAE were labeled and hybridized to T. castaneum custom microarrays. Three biological replicates were included. Out of the 15,208 probe sets screened, hybridization to 12,233 probe sets was detected. Out of 12,233 genes, 6108 genes showed a normalized value of 1.0 or higher. These genes were categorized using the Blast2go (http://www.blast2go.org) software based on their annotated molecular functions. These genes were grouped into 16 molecular functional categories (Fig. 2B). Interestingly, on day 4 PAE, in the fat body, the largest numbers of genes were grouped into those coding for protein binding (1,974) and nucleic acid binding (1,171) activities, indicating the peak activity of the machinery involved in Vg synthesis. Also, 198 genes were grouped into those coding for proteins with the function of transcription factor activity in the fat body of beetles on day 4 PAE.
3.2. Hormone titers in young adult females
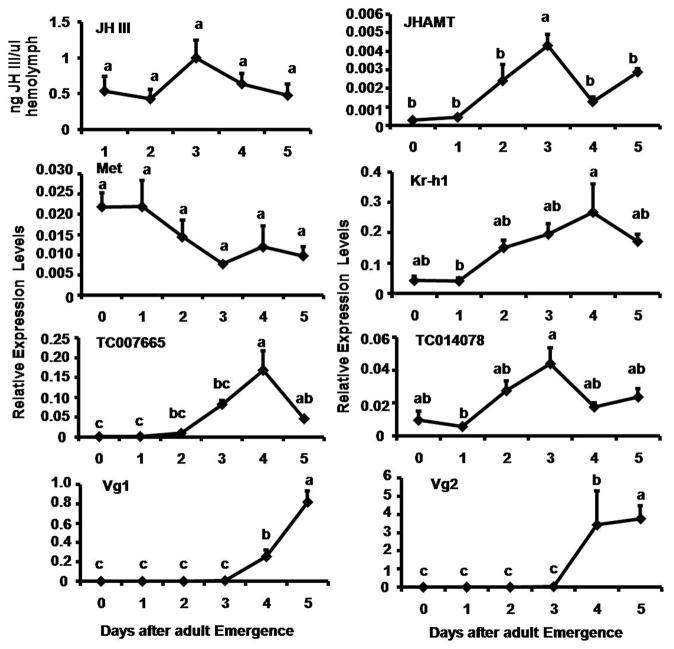
Reverse Phase HPLC/MS/MS Tandem mass spectrometry was used to determine JH titer in the hemolymph of female beetles during 1–5 days PAE (Fig. 3). On day 1 PAE, 0.5 ng of JH III per μl of hemolymph was detected. The JH III levels gradually increased during 2–3 days PAE and reached the maximum of 1.2 ng/μl of hemolymph by day 3 PAE. Then the JH III levels declined to 0.6 ng/μl of hemolymph by day 4 PAE. The JH III levels further declined on day 5 PAE.
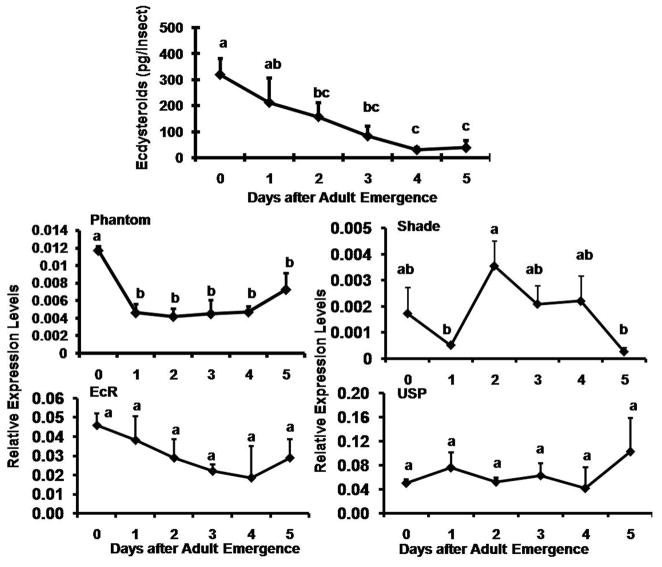
Fig. 3.
JH titer and expression patterns of genes coding for enzymes involved in JH biosynthesis (JHAMT), genes coding for proteins known to be involved in JH action (Met, Kr-h1), and genes coding for JH inducible proteins (JHIP, TC007665, TC014078)) and vitellogenin genes (Vg1 and Vg2) in the whole body of adult females determined by HPLC/MS/MS and qRT-PCR respectively. JH titer is expressed as ng of JH III/μl of hemolymph using the JH analog, methoprene as the standard. Each value is Mean ± S.E. of three replicates. For expression profiles, the X-axis denotes the day-after adult emergence and the Y-axis denotes relative expression with respect to the expression level of internal control, ribosomal protein (rp49). Each time-point mean is the average of three independent biological replicates. Mean + S.E. are shown. The mean expression levels marked with the same alphabetical letter do not differ significantly at p<0.05 by the Tukey-Kramer HSD test.
An enzyme immunoassay (EIA) was used to determine ecdysteroid levels in the female beetles during 0 to 5 days PAE (Fig. 4). Ecdysteroid levels were high (350 pg per insect) on day 0 PAE, which might be the remnants of ecdysteroid surge during the pupal-adult metamorphosis. The ecdysteroid levels gradually decreased during 1–3 days PAE and reached the low levels by 4–5 days PAE.
Fig. 4.
Ecdysteroid titer and mRNA levels of genes known to be involved in ecdysteroid biosynthesis (Phantom and Shade) and ecdysteroid action (EcR and USP) in the whole body of the adult females. Ecdysteroids were estimated by Enzyme immunoassay (EIA) in six independent groups of staged beetles. The mRNA levels were quantified by qRT-PCR. Relative expression in comparison to ribosomal protein (rp49) was determined. Mean ± S.E. of three independent replicates are shown. The mean expression values marked with the same alphabetical letter do not differ significantly at p<0.05 by the Tukey-Kramer HSD test.
3.3. Expression profiles of genes coding for proteins involved in JH and 20E biosynthesis and action in adult females
The mRNA levels of genes selected from the microarray analysis were determined in the total RNA isolated from 0–5 days-old adult females tissues collected at 24 h interval by qRT-PCR. Low levels of JHAMT mRNA were detected at the beginning of the adult stage and the mRNA levels started to increase after day 1 PAE and reached the maximum by day 3 PAE (Fig. 3). The JHAMT mRNA levels then decreased to low levels by day 4 PAE, followed by a small increase on day 5 PAE. High levels of Met mRNA were detected at the beginning of the adult stage and the mRNA levels started to decrease after day 2 PAE and reached the minimum levels by day 2 PAE (Fig. 3). In contrast, the mRNA levels of Kr-h1 were low during the first two days PAE. Then the Kr-h1 mRNA levels gradually increased and reached the maximum levels by day 4 PAE. The Kr-h1 mRNA levels showed a small decline on day 5 PAE (Fig. 3). The expression levels of both JH-inducible genes (TC007665 and TC014078) tested were low at beginning of the adult stage and the mRNA levels started to increase after day 1 PAE and reached the maximum by day 3 or day 4 PAE (Fig. 3). The mRNA of Vg1 and Vg 2 were undetectable until 3 days PAE. Both Vg1 and Vg2 mRNA levels began to increase on day 3 PAE and reached the maximum by day 5 PAE (Fig. 3). Since the Vg2 mRNA levels were higher than the Vg1 mRNA levels, only the Vg2 mRNA levels were quantified in all subsequent experiments.
The mRNA levels of ecdysteroid biosynthesis gene, Phantom were high soon after adult eclosion and declined significantly to low levels by day 1 PAE (Fig. 4). The Phantom mRNA levels remained low until 4 days PAE followed by a small increase on day 5 PAE. The mRNA levels of another ecdysteroid biosynthesis gene, Shade showed a significant increase on day 2 PAE and declined to low levels by day 5 PAE. The expression levels of EcR and USP remained the same without significant differences among the time-points tested during 0–5 days PAE (Fig. 4).
3.4. Effect of RNAi-aided knock-down in the expression of select genes on the vitellogenesis
3.4.1. Knock-down efficiency
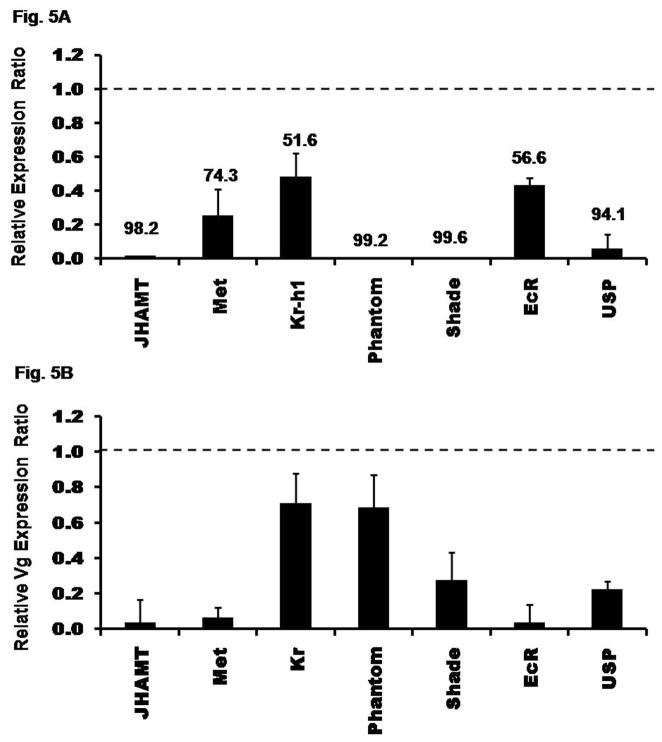
To determine the efficiency of dsRNA in knocking-down the expression of select genes, the mRNA levels of these genes were determined in insects injected with malE or select gene dsRNA. Knock-down efficiency was calculated by comparing the mRNA levels of select genes in these two groups of insects. The expression of genes coding for transcription factors (Met, Kr-h1) and receptor (EcR) were knock-down by 50–70%, while the expression of genes coding for JHAMT, Phantom, Shade, and USP were knock-down by 80–95% (Fig. 5A).
Fig. 5.
Fig. 5A. Knock-down efficiency of genes after injection of dsRNA. dsRNA for genes coding for proteins involved in JH biosynthesis or action (JHAMT, Met, Kr-h1) and malE (control) were injected into two-day-old female pupae. Genes coding for proteins involved in 20E biosynthesis or action (Phantom, Shade, EcR, USP) and malE (control) dsRNA were injected into female adults on day 0 PAE (within 24 h of emergence). At day 4 PAE, RNA was extracted and the relative expression in comparison to ribosomal protein (rp49) was determined by qRT-PCR. The expression levels of these respective genes in control insects were set to 1 and the relative expression levels with respect to control was determined for each treatment. The numbers above each bar show the percent knock-down efficiency for each gene. Mean ± S.E. of three independent replicates are shown.
Fig. 5B. Effect of RNAi on the expression of vitellogenin gene (Vg2). dsRNA were injected as mentioned in figure legend 5. RNA was extracted on day 4 PAE. Relative expressions of Vg2 mRNA levels for individual genes were determined in comparison to ribosomal protein (rp49) expression levels by qRT-PCR. The expression ratio of Vg2 mRNA levels in each of the RNAi insects was calculated by setting the respective control insect Vg2 mRNA levels at 1.0. Mean ± S.E. of three independent replicates are shown.
3.4.2. RNAi effect on vitellogenin gene expression
Knocking-down the expression of gene coding for JHAMT or Met caused a drastic reduction in the Vg2 mRNA levels (80–90%). In contrast, knocking-down the expression of gene coding for transcription factor known to be involved in JH action, Kr-h1, caused only 30% reduction in the Vg2 mRNA levels (Fig. 5B). These data suggest that JH biosynthesis and action are required for Vg gene expression. Knocking-down the expression of gene coding for Phantom did not affect the Vg2 mRNA levels. In contrast, knocking-down the expression of gene coding for Shade lowered the Vg2 mRNA levels by 75%. Also, knocking-down the expression of genes coding for ecdysone receptors, EcR and USP showed 80–90% reduction in the Vg2 mRNA levels (Fig. 5B). These data suggest that ecdysteroid biosynthesis is not required but the conversion of ecdysteroids to 20E as well as 20E action are required for Vg synthesis.
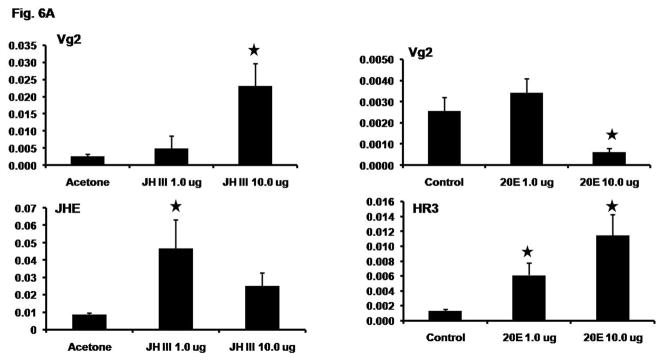
3.5. Effect of application of JH and 20E on Vg gene expression
As knocking-down the expression of genes coding for proteins involved in both JH and 20E signaling affected Vg gene expression, we determined the roles of JH and 20E in the regulation of Vg gene expression by exogenous application of JH III or 20E during the previllogenic stage. Application of 10 μg JH III induced Vg2 mRNA levels by 9-fold, while the application of 1.0 μg JH III showed an induction of Vg2 mRNA levels by 2-fold when compared to the Vg2 mRNA levels in the acetone applied beetles (Fig. 6A). As a positive control for JH induction, the known JH inducible gene coding for juvenile hormone esterase (JHE) was used. The JHE mRNA levels were induced by 5- and 3-folds in beetles applied with 1.0- and 10.0 μg JH III respectively, when compared to the JHE mRNA levels in the acetone applied beetles (Fig. 6A).
Fig. 6.
Fig. 6A. Exogenous JH application induces Vg gene expression. JH III in 1 μl acetone (1.0 μg/μl and 10.0 μg/μl) or acetone alone (1 μl, control) per insect were applied topically to day 2 female beetles and RNA was extracted from the whole body at 6 h after application. Vg2 and JHE (positive control) mRNA levels were determined by qRT-PCR. In another experiment, 1.0 μg or 10.0 μg 20E in 0.25 μl of DDH2O per insect was injected. 0.25 μl of DDH2O alone was injected into control beetles. RNA was extracted from the whole body at 6 h after injection. Vg2 and HR3 (positive control) mRNA levels were determined by qRT-PCR. In both experiments, five replications with two beetles in each replication were performed. Mean ± S.E. are shown. The mean expression levels of treatments marked with star differ significantly from the mean expression levels of respective control at p<0.05 by the Student’s t-test.
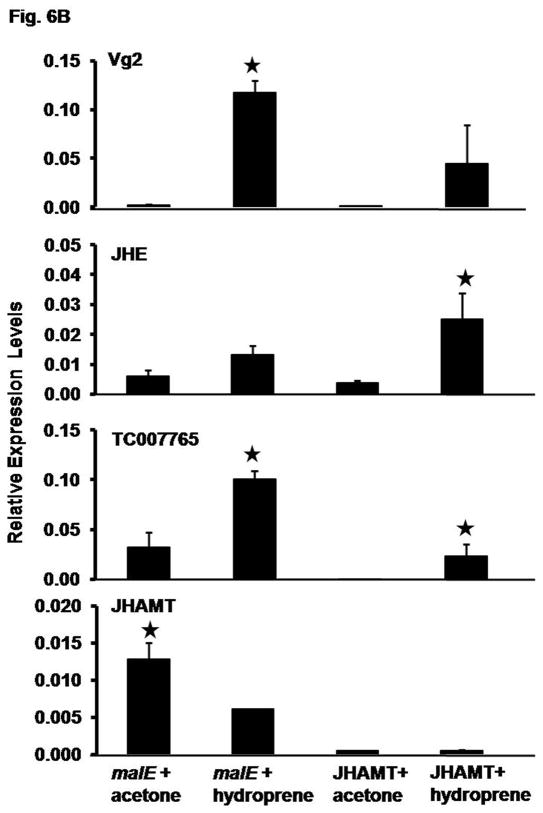
Fig. 6B. Induction of vitellogenin gene expression by hydroprene in the presence and absence of endogenous JH. dsRNA for JHAMT or malE (control) were injected into female pupae on day 2 of the pupal stage. The adults were staged upon emergence. On day 2 PAE, 10.0 μg hydroprene was topically applied per insect. Acetone treated insects served as a control. RNA was extracted at 24 h after treatment. JHAMT, Vg2, JHIP (TC007665), and JHE mRNA levels were determined by qRT-PCR. Relative expression in comparison to ribosomal protein (rp49) expression was determined. Mean ± S.E. of three independent replicates are shown. The mean expression levels of treatments marked with star differ significantly from the mean expression levels of respective control at p<0.05 by the Student’s t-test.
Injection of 1.0 μg of 20E did not cause a significant change in the Vg2 mRNA levels while the injection of 10.0 μg of 20E caused a significant decrease (4-fold) in theVg2 mRNA levels when compared to its levels in control beetles injected with water alone (Fig. 6A). As a positive control for 20E response, a known ecdysone inducible gene, HR3, was used. Injection of 1.0- and 10.0 μg of 20E induced the HR3 mRNA levels by 6- and 9-fold respectively when compared to the HR3 mRNA levels in the control beetles injected with water alone (Fig. 6A).
We also tested JH analog hydroprene to determine whether it is able to induce Vg gene expression. Preliminary studies showed that hydroprene induces Vg gene expression and it works much better than the natural JH III, most likely due to its stability in vivo. Therefore, we used hydroprene to determine whether it could induce Vg gene expression in the absence of endogenous JH. JHAMT dsRNA was used for injections to create JH deficient conditions. JHAMT or malE (control) dsRNA was injected into the two-day-old female pupae and the adults emerged from these pupae were treated with hydroprene on day 2 PAE.
As shown in Figure 6B, the expression of gene coding for JHAMT was knock-down by more than 90% in the beetles injected with JHAMT dsRNA when compared to its expression in malE dsRNA injected beetles. Interestingly, application of hydroprene suppressed the expression of gene coding for JHAMT by 2-fold in malE dsRNA injected beetles (Fig. 6B). In contrast, the Vg2 mRNA levels increased by 49-fold in beetles injected with malE dsRNA and treated with hydroprene when compared to its expression in beetles injected with malE dsRNA and treated with acetone. Hydroprene treatment induced Vg2 mRNA levels in JHAMT RNAi beetles by 8–37-fold when compared to its expression in acetone treated JHAMT RNAi beetles. Control genes coding for JH inducible proteins, JHE and TC007765 were also induced by hydroprene in both malE and JHAMT RNAi beetles (Fig. 6B). These data suggest that hydroprene could induce the expression of genes coding for Vg and other JH inducible proteins in the absence of endogenous JH.
4. Discussion
The microarray analysis of gene expression in the whole body of female beetles during 1–4 days PAE showed up-regulation of several genes. Among them, Vg mRNA levels increased dramatically after day 3 PAE, indicating that the previtellogenic phase covers 0–3 days PAE and the vitellogenic cycle starts after day 3 PAE. Also, the microarray analysis showed up-regulation of several genes coding for proteins such as JHAMT, Kr-h1, JHE, JHEH, and JHIPs involved in the JH biosynthesis, metabolism and signaling. However, the expression levels of genes coding for proteins involved in ecdysteroid signaling did not change significantly during this stage. Based on the data from the microarray and qRT-PCR analyses and measurement of hormone titers the following conclusions could be drawn: 1) JH levels are high during the previtellogenic stage in the female beetles while ecdysteroid titers decline to low levels; 2) the expression of genes involved in JH biosynthesis and action increase during the previtellogenic stage while the expression of genes involved in ecdysteroid biosynthesis and action decrease or do not change during 1–5 days PAE; and 3) the expression levels of the selected genes determined by qRT-PCR confirmed the changes among the time-points observed in the microarray analysis. Hormone titers and expression profiles of genes involved in biosynthesis and action of these two hormones showed that JH but not ecdysteroids plays key roles in preparing the fat body for Vg synthesis.
Juvenile hormones are shown to regulate female reproduction including development of the genital gland and vitellogenesis (Wigglesworth, 1970; Goodman and Granger, 2005). In this study, we determined JH titers during the previtellogenic phase. We used JHAMT, a terminal enzyme involved in the JH biosynthesis (Minakuchi et al. 2008a) and RNAi to create JH deficient conditions. The over-expression of JHAMT caused pharate adult lethal phenotype similar to that obtained with the application of JH analogs in D. melanogaster (Niwa et al. 2008). JHAMT RNAi studies in T. castaneum by Minakuchi et al. (2008a) showed a critical role for this enzyme in JH biosynthesis. Our previous studies showed that JHAMT RNAi caused JH deficiency in the male beetles of T. castaneum (Parthasarathy et al. 2009). In the present study, JHAMT RNAi blocked the expression of the Vg gene. These data suggest that JH plays a key role in Vg synthesis in this insect. RNAi effect of Met gene involved in JH action confirmed this role of JH in Vg synthesis. However, RNAi effects of another gene known to be involved in JH action, Kr-h1, did not yield conclusive results. This might be due to the poor knock-down efficiency of the gene coding for this transcription factor, leaving sufficient molecules to achieve its natural function. Interestingly, several JHIPs were up-regulated during the Vg synthesis in the female beetle. These JHIPs are induced by JH analogues and are rescued by hydroprene treatment in JH deficient insects. However, the knock-down in the expression of genes coding for these JHIPs did not affect the Vg synthesis (data not shown). The precise roles of Kr-h1 and JHIPs in the regulation of Vg synthesis needs further investigation.
We further confirmed the significant role of JH in Vg gene regulation by exogenous hormone applications. We selected two- day-old female beetles when Vg mRNA levels are very low. Application of JH III showed induction of Vg2 mRNA levels by nearly 10-fold, while the injection of 20E reduced Vg2 mRNA levels (4-fold less) when compared to the Vg2 mRNA levels in the respective control beetles. Similar opposite action of 20E and JH has been reported in an endoparasitic wasp, Pteromalus puparum (Dong et al. 2009). In this wasp, Vg gene regulation is under the control of 20E; injection of 20E induced Vg gene expression while exogenous application of JH III suppressed the Vg gene expression. In the present study, we also used a JH analog, hydroprene, to confirm the effects of JH III. The application of Hydroprene induced Vg2 expression levels and also partially rescued Vg2 mRNA levels in hydroprene treated JHAMT RNAi beetles during the previtellogenic stage. The regulation of Vg gene expression by JH or JH analogues has been described in many insect species including some beetles (Wyatt and Davey, 1996; Belles 1998, 2004; Taub-Montemayor and Rankin, 1997; Zhai et al. 1984; Sakurai et al. 1987). However, hydroprene application to JHAMT RNAi insects did not restore Vg2 mRNA levels similar to those observed in hydroprene-treated control insects injected with malE dsRNA. This may be due to the function of JH during previtellogenic stage to prepare fat body for Vg synthesis during vitellogenic stage. Since JHAMT RNAi insects are depleted of JH during previtellogenic stage, the fat body would have not become completely competent and therefore hydroprene was not able to induce Vg synthesis to the maximum levels observed in control insects.
In the fat body of B. germanica, JH directly induces the synthesis of Vg (Belles, 2004), while JH makes fat body competent in Ae. aegypti to synthesize Vg (Klowden, 1997). JH action on beetle vitellogenesis could be direct or indirect. The direct effect of hormones demands in vitro documentation of the effect of JH on Vg synthesis. This has been accomplished in only a few systems (Wyatt et al. 1976; Bohm, et al., 1978; Raikhel et al., 1997). For direct effect, it is thought that the transcription of the Vg gene depends on the binding of a hormone-receptor complex to hormone response elements (HREs) which are usually located in the promoter region of the genes (Segraves, 1994). Molecular characterization of insect vitellogenins showed the presence of hormone response elements (HRE) in the Vg promoter regions of insects (Raikhel et al. 2005, Locke et al. 1987; Wyatt, 1988; Tufail and Takeda, 2008). In T. castaneum, the in vitro culture of abdominal fat bodies did increase the Vg mRNA levels but not to the significant levels observed in vivo. de Loof and de Wilde (1970) showed that two hormonal factors, a brain factor and JH are necessary for Vg synthesis in the Colorado potato beetle. It is likely that such a brain factor is also required for Vg synthesis in T. castaneum. Our data clearly show the requirement of JH and its action for synthesis of Vg in the fat body. The role of other factors including the hormones released from the brain or ovary is under investigation.
Regarding the role of ecdysteroids in the Vg synthesis, it is interesting that the expression pattern of genes involved in 20E biosynthesis (Phantom) and action (EcR, USP, HR) did not change significantly during 1–4 days PAE (microarray data). Since ecdysteroid levels decrease from day 0 to day 5 PAE, it appears that the presence of ecdysteroid is not a requisite for Vg synthesis in the fat body. Also, the knock-down in the expression of Phantom, an enzyme involved in the biosynthesis of the ecdysteroids did not affect the Vg mRNA levels suggesting that synthesis of ecdysteroids is not necessary for Vg gene expression. However, the role of existing 20E action on the Vg regulation could not be ruled out, since Shade, EcR, and USP RNAi insects showed complete block in Vg synthesis. Studies on the detailed analysis of 20E signaling during previtellogenesis and vitellogenesis showed that the action of 20E on Vg gene expression is an indirect effect caused by block in maturation of oocytes. Detailed discussion on this subject is covered in a manuscript submitted for publication (Parthasarathy et al., 2010). We hypothesize that Vg synthesis in the fat body is regulated by JH as well as hormones released by brain or ovary after the maturing oocytes are ready for uptake of Vg. 20E regulates maturation of oocytes and hence indirectly affects Vg synthesis in the fat body.
Taken together these studies suggest that: 1) JH is required for Vg gene expression in the fat body; 2) 20E and its receptors are required for maturation of oocytes in the ovary, and matured oocytes likely signal the initiation of Vg gene expression in the fat body (Parthasarathy et al., 2010 submitted). Though the molecular mechanisms of Vg gene expression need further experimentation, the present study in conjunction with the study on ovarian growth and oocyte maturation (Parthasarathy et al., 2010 submitted) provides the baseline data on tissue-specific physiological roles of ecdysteroids and JH in the regulation of the less-explored coleopteran insect reproduction.
Acknowledgments
We thank Dr. Nigel Cooper and Ms. Xiahong Li of University of Louisville for help with microarray analysis. We also thank Dr. Chen and Dr. Rankin of Integrative Biology Department of University of Texas at Austin, Austin, TX, for the help with the estimation of JH titer. This work was supported by National Institutes of Health (GM070559-06). The University of Louisville microarray facility is supported by NCRR IDeA Awards INBRE-P20 RR016481 and COBRE-P20RR018733. This is contribution number 09-08-084 from the Kentucky Agricultural Experimental Station.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amdam GV, Norberg K, Fondrik MK, Page RE. Reproductive ground plan may mediate colony-level selection effects on individual foraging behavior in honey bees. Proc Natl Acad Sci USA. 2004;101:11350–11355. doi: 10.1073/pnas.0403073101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belles X. Endocrine effectors in insect vitellogenesis. In: Coast GM, Webster SG, editors. Recent advances in arthropod endocrinology. Cambridge University Press; Cambridge: 1998. pp. 71–88. [Google Scholar]
- Belles X. Vitellogenesis directed by juvenile hormone. In: Raikhel AS, editor. Reproductive biology of invertebrate: Progress in vitellogenesis. Science Publishers Inc; 2004. pp. 157–197. [Google Scholar]
- Bohm MK, Behan M, Hagedorn HH. Termination of vitellogenin synthesis by mosquito fat body, a programmed response to ecdysterone. Physiol Entomol. 1978;3:17–25. [Google Scholar]
- Bownes M. The roles of juvenile hormone, ecdysone, and the ovary in the control of Drosophila vitellogenesis. J Insect Physiol. 1989;35:409–413. [Google Scholar]
- Bownes M, Ronaldson E, Mauchline D. 20-hydroxyecdysone, but not juvenile hormone, regulation of yolk protein gene expression can be mapped to cis-acting DNA sequences. Dev Biol. 1996;173:475–489. doi: 10.1006/dbio.1996.0041. [DOI] [PubMed] [Google Scholar]
- Brent C, Peters C, Dietemann V, Crewe RM, Vargo EL. Hormonal correlates of reproductive status in the queenless ponerine ant, Streblognathus peetersi. J Compar Physiol. 2006;192:315–320. doi: 10.1007/s00359-005-0065-6. [DOI] [PubMed] [Google Scholar]
- Comas D, Piulachs MD, Belles X. Fast induction of vitellogenin gene expression by juvenile hormone III in the cockroach, Blatella germanica (L.) (Dictyoptera, Blattellidae) Insect Mol Biol. 1999;29:821–827. doi: 10.1016/s0965-1748(99)00058-2. [DOI] [PubMed] [Google Scholar]
- de Loof A, de wide J. Hormonal control of synthesis of vitellogenic female protein in the Colorado potato beetle, Leptinotarsa decemlineata. J Insect Physiol. 1970;16:1455–1466. doi: 10.1016/0022-1910(70)90144-7. [DOI] [PubMed] [Google Scholar]
- Dong S, Ye G, Guo J, Hu C. Roles of ecdysteroids and juvenile hormone in vitellogenesis in an endoparasitic wasp, Pteromalus puparum (Hymenoptera: Pteromalidae) Gen Compar Endocrinol. 2009;160:102–108. doi: 10.1016/j.ygcen.2008.11.007. [DOI] [PubMed] [Google Scholar]
- Engelmann F. Endocrine control of reproduction in insects. Annu Rev Entomol. 1968;13:1–26. [Google Scholar]
- Engelmann F. Endocrine regulated insect vitellogenesis: A synthesis. Adv Invertebr Reproduction. 1986;4:31–37. [Google Scholar]
- Engelmann F. Vitellogenesis controlled by juvenile hormone. In: Downer RGH, Laufer H, editors. Endocrinology of insects. Alan R Liss; New York: 1983. pp. 259–270. [Google Scholar]
- Glinka AV, Wyatt GR. Juvenile hormone activation of gene transcription in locust fat body. Insect Biochem Mol Biol. 1996;26:13–18. [Google Scholar]
- Goodman WG, Granger NA. The juvenile hormone. In: Gilbert LI, Iatrou K, Gill SS, editors. Comprehensive Molecular Insect Science. Elsevier; Oxford: 2005. pp. 319–408. [Google Scholar]
- Hagedorn HH. The role of ecdysteroids in reproduction. In: Kerket GA, Gilbert LI, editors. Comprehensive Insect Physiology, Biochemistry and Pharmocology. Pergamon Press; Oxford: 1985. pp. 205–262. [Google Scholar]
- Hagedorn HH, Kunkel JG. Vitellogenin and vitellin in insects. Annu Rev Entomol. 1979;24:475–505. [Google Scholar]
- Keeley LL, McKercher SR. Endocrine regulations of ovarian maturations in the cockroach, Blaberus discoidalis. Compar Biochem Physiol. 1985;80:115–121. doi: 10.1016/0300-9629(85)90688-7. [DOI] [PubMed] [Google Scholar]
- Klowden MJ. Endocrine aspects of mosquito reproduction. Arch Insect Biochem Physiol. 1997;35:491–512. [Google Scholar]
- Konopova B, Jindra M. Juvenile hormone resistance gene Methoprene-tolerant controls entry into metamorphosis in the beetle Tribolium castaneum. Proc Natl Acad Sci U S A. 2007;104:10488–93. doi: 10.1073/pnas.0703719104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locke J, White BN, Wyatt GR. Cloning and 5′ end nucleotide sequences of two juvenile hormone inducible vitellogenin genes of the African migratory locust. DNA. 1987;6:331–342. doi: 10.1089/dna.1987.6.331. [DOI] [PubMed] [Google Scholar]
- Minakuchi C, Namiki T, Yoshiyama M, Shinoda T. RNAi-mediated knockdown of juvenile hormone acid O-methyltransferase gene causes precocious metamorphosis in the red flour beetle Tribolium castaneum. FEBS J. 2008a;275:2919–31. doi: 10.1111/j.1742-4658.2008.06428.x. [DOI] [PubMed] [Google Scholar]
- Minakuchi C, Zhou X, Riddiford LM. Kruppel homolog 1 (Kr-h1) mediates juvenile hormone action during metamorphosis of Drosophila melanogaster. Mech Dev. 2008b;125:91–105. doi: 10.1016/j.mod.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwa R, Matsuda T, Yoshiyama T, Mita K, Kujimoto Y, Katoaka H. CYP306A, a cytochrome P450 enzyme is essential for ecdysone biosynthesis in the prothoracic glands of Bombyx and Drosophila. J Biol Chem. 2004;279:35942–49. doi: 10.1074/jbc.M404514200. [DOI] [PubMed] [Google Scholar]
- Niwa R, Niimi T, Honda N, Yoshiyama M, Itoyama K, Kataoka H, Shinoda T. Juvenile hormone acid o-methyl transferase in Drosophila melanogaster. Insect Biochem Mol Biol. 2008;38:714–20. doi: 10.1016/j.ibmb.2008.04.003. [DOI] [PubMed] [Google Scholar]
- Parthasarathy R, Tan A, Bai H, Palli SR. Transcription factor broad suppresses precocious development of adult structures during larval-pupal metamorphosis in the red flour beetle, Tribolium castaneum. Mech Dev. 2008;125:299–313. doi: 10.1016/j.mod.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parthasarathy R, Tan A, Sun Z, Chen Z, Rankin M, Palli SR. Juvenile hormone regulation of male accessory gland activity in the red flour beetle, Tribolium castaneum. Mech Dev. 2009;126:563–579. doi: 10.1016/j.mod.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parthasarathy R, Sheng Z, Sun Z, Palli SR. Ecdysteroid regulation of ovarian growth and oocyte maturation in the red flour beetle, Tribolium castaneum. Insect Biochem Mol Biol. 2010 doi: 10.1016/j.ibmb.2010.04.002. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petryk A, Warren JT, Marques G, Jarcho MP, Gilbert LI, Kahler J, Parvy JP, Li Y, Dauphin-Villemant C, O’Connor MB. Shade is the Drosophila p450 enzyme that mediates the hydroxylation of ecdysone to the steroid insect moting hormone 20E-hydroxyecdysone. Proc Natl Acad Sci U S A. 2003;111:13773–78. doi: 10.1073/pnas.2336088100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto LZ, Bitondi MMG, Simoes ZI. Inhibition of vitellogenin synthesis in Apis mellifera workers by a juvenile hormone analogue, pyripoxyfen. J Insect Physiol. 2000;46:153–160. doi: 10.1016/s0022-1910(99)00111-0. [DOI] [PubMed] [Google Scholar]
- Raikhel AS, Dhadialla TS. Accumulation of yolk proteins in insect oocytes. Annu Rev Entomol. 1992;37:217–251. doi: 10.1146/annurev.en.37.010192.001245. [DOI] [PubMed] [Google Scholar]
- Raikhel AS, Deitsch KW, Sappington TW. Culture and analysis of the insect fat body. In: Crampton JM, Beard CB, Louis C, editors. Molecular Biology of Insect Disease Vectors. Chapman and Hall; 1997. pp. 507–521. [Google Scholar]
- Raikhel AS, Brown MR, Belles X. Hormonal control of reproductive process. In: Gilbert LI, Iatrou K, Gill SS, editors. Comprehensive Molecular Insect Science. Boston: Elsevier Ltd; 2005. pp. 433–491. [Google Scholar]
- Ramaswamy SB, Shu S, Park YI, Zeng F. Dynamics of juvenile hormone-mediated gonadotropism in the Lepidoptera. Arch Insect Biochem Physiol. 1997;35:539–558. [Google Scholar]
- Richard DS, Jones JM, Barbarito MR, Cerula S, Detweiler JP, Fisher SJ, Brannigan DM, Scheswohl DM. Vitellogenesis in diapausing and mutant Drosophila melanogaster: further evidence for the relative roles of ecdysteroids and juvenile hormones. J Insect Physiol. 2001;47:905–913. [Google Scholar]
- Sakurai H, Hirano T, Takeda S. Change in electrophoretic pattern of hemolymph protein in diapause regulation of the lady beetle Coccinella septempunctata (Coleoptera: Coccinellidae) Appl Entomol Zool. 1987;22:286–291. [Google Scholar]
- Segraves WA. Steroid receptors and other transcription factors in ecdysone response. Recent progress in Hormone Research. 1994;49:167–195. doi: 10.1016/b978-0-12-571149-4.50013-1. [DOI] [PubMed] [Google Scholar]
- Tan A, Palli SR. Edysone receptor isoforms play distinct roles in controlling molting and metamorphosis in the red flour beetle, Tribolium castaneum. Mol Cell Endocrinol. 2008;291:42–49. doi: 10.1016/j.mce.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taub-Montemayor TE, Rankin MA. Regulation of vitellogenin synthesis and uptake in the boll weevil, Anthonomas grandis. Physiol Entomol. 1997;22:261–268. [Google Scholar]
- Tufail M, Takeda M. Molecular characteristics of insect vitellogenesis. J Insect Physiol. 2008;54:1447–1458. doi: 10.1016/j.jinsphys.2008.08.007. [DOI] [PubMed] [Google Scholar]
- Warren JT, Petryk A, Marques G, Parvy JP, Shinoda T, Itoyama K, Kobayashi J, Jarcho M, Li Y, O’Connor MB, Dauphin-Villemant C, Gilbert LI. Phantom encodes the 25-hydroxylase of Drosophila melanogaster and Bombyx mori: a P450 enzyme critical in ecdysone biosynthesis. Insect Biochem Mol Biol. 2004;34:991–1010. doi: 10.1016/j.ibmb.2004.06.009. [DOI] [PubMed] [Google Scholar]
- Wigglesworth VB. Insect hormones. WH Freeman; San Franscisco: 1970. p. 159. [Google Scholar]
- Wyatt GR. Vitellogenin synthesis and the analysis of juvenile hormone action in locust fat body. Can J Zool. 1988;66:2601–2610. [Google Scholar]
- Wyatt GR, Chen TT, Couble P. Juvenile hormone induced vitellogenin synthesis in locust fat body in vitro. In: Kurstack E, Maramorosch K, editors. Invertebrate Tissue Culture. Academic Press; New York: 1976. p. 195. [Google Scholar]
- Wyatt GR, Davey KG. Cellular amd molecular actions of juvenile hormone. II roles of juvenile hormones in adult insects. Adv Insect Physiol. 1996;26:1–155. [Google Scholar]
- Zhai QH, Zhang JZ. Studies on the vitellogenesis of Coccinella septempunctata synthesis of vitellogenin by fat body in vitro. Acta Entomol Sin. 1984;27:361–367. [Google Scholar]
- Zhang J, McCracken A, Wyatt GR. Properties and sequence of a female-specific juvenile hormone-induced protein from locust hemolymph. J Biol Chem. 1993;268:3282–3288. [PubMed] [Google Scholar]