Abstract
Background
In recent years, there has been an increasing interest in targeting human prostate tumor-associated antigens (TAAs) for prostate cancer immunotherapy as an alternative to other therapeutic modalities. However, immunologic tolerance to TAA poses a significant obstacle to effective, TAA-targeted immunotherapy.
We sought to investigate whether androgen deprivation would result in circumventing immune tolerance to prostate TAA by impacting CD8 cell responses.
Methods
To this end, we generated a transgenic mouse that expresses the human prostate specific antigen (PSA) specifically in the prostate, and crossed it to the HLA-A2.1 transgenic mouse.
Results
Our PSA transgenic mouse showed restricted expression of PSA in the prostate and detectable circulating PSA levels. Additionally, PSA expression was androgen-dependent with reduced PSA expression in the prostate within one week of castration, and undetectable PSA by day 42 after castration as evaluated by ELISA. Castration of the PSA/A2.1 hybrid mouse prior to immunization with a PSA-expressing recombinant vaccinia virus resulted in a significant augmentation of PSA-specific cytotoxic lymphocytes.
Conclusions
This humanized hybrid mouse model provides a well defined system to gain additional insight into the mechanisms of immune tolerance to PSA and to test novel strategies aiming at circumventing immune tolerance to PSA and other TAA for targeted prostate cancer immunotherapy.
Keywords: Prostate cancer, immune tolerance, immunotherapy
INTRODUCTION
To date, clinical trials of PCa immunotherapy included immunization with defined antigenic preparations such as synthetic peptides (1-3), antigen- (4, 5) or mRNA-loaded dendritic cells (6), manipulated tumor cells (7), or with plasmid DNA (8) or viral vectors engineered to express immunogenic genes (9). In particular, recombinant vaccinia virus expressing prostate specific antigen (PSA) was tested in clinical trials in combination with recombinant PSA-expressing fowlpox virus (10, 11), demonstrating its ability to trigger a specific immune response (11) and an 8.5 month improvement in median overall survival in men with metastatic castration-resistant prostate cancer (10). However, the survival benefit remains modest, suggesting an opportunity and need for significant improvement of efficacy in PCa immunotherapy.
In 1999, a study reported combined immunization with androgen deprivation to modulate antigen expression as a means of circumventing tolerance to a prostate TAA-specific in the setting of a human PCa clinical trial that targeted PSA as a prototype PCa TAA (12). The scientific basis for combining androgen deprivation and PCa TAA-specific immunization was subsequently validated in mouse models that focused on MHC class II-mediated helper T cell responses (13, 14). Androgen ablation results in a rapid involution of benign and neoplastic prostate tissue at both primary and metastatic sites, seemingly due to apoptosis of androgen-dependent epithelial cells (15-17). This treatment has been shown to induce infiltration of lymphocytes, macrophages and dendritic cells into the prostate and trigger inflammation (18). Such infiltration proved to be beneficial for PCa immunotherapy as it provides a synergistic help by increasing the number of tumor antigen-specific lymphocytes (13, 19, 20). Additional supporting evidence for this concept was provided by the demonstration that testosterone can be immunosuppressive by stimulating tumors to secrete TGF-β, a cytokine that promotes the expansion of Treg (21). Androgen deprivation has been shown to result in a decrease in expression of these genes in both patients undergoing androgen ablation therapy and in human PCa cell lines (22). In mice, androgen deprivation-induced TAA gene downregulation has been shown to circumvent immune tolerance and enhance CD4 T cell responses to prostate TAA (13). However, the effect of androgen deprivation on the generation of TAA-specific CD8 T cells has not been addressed before.
In this report, we describe the initial characterization of transgenic mice expressing the PSA transgene in an androgen-regulated and prostate-specific manner. To further refine current prostate cancer models, we developed a unique double-transgenic mouse model co-expressing PSA and HLA-A2.1 to facilitate the investigation of PSA-specific tolerance in the context of human MHC. Finally, we sought to determine whether castration of male mice prior to immunization to PSA improves class I MCH-restricted T cell responses to this clinically relevant target antigen.
MATERIALS AND METHODS
Peptides, proteins and viruses
Previously described HLA-A2.1-restricted, PSA-derived peptide PSA-3 (VISNDVCAQV) and its agonist PSA-3A (YISNDVCAQV) (23) and the H-2Kb restricted-, SV40 Tag-derived Tag-IV peptide (VVYDFLKC) (24, 25) were purchased from the Macromolecular Resources Facility at Colorado State University (Ft. Collins, CO). Human PSA was purchased from Calbiochem (San Diego, CA). Vac-PSA was obtained from Therion Biologics Corporation (Cambridge, MA). Vac-mTag was generated in our laboratory (26-28).
Generation of transgenic rPB-PSA mice, construction of PSA transgenic map
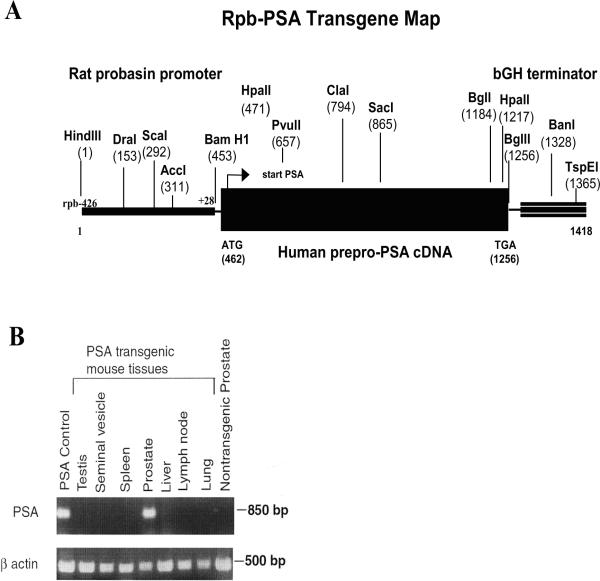
Human prostate-specific antigen (PSA) prepro-cDNA (Accession M26663, NID g618463) was ligated to rat probasin (rPB) promoter cassette (GI:10000942 −426 to +28) (29) at the introduced BamH1 site. The rPB-PSA was ligated to a bovine growth hormone (bGH, GI:2168498 1872 to 2028) terminator sequence at the BglII site. The final rPB-PSA transgene construct was microinjected into oocytes of foster mouse mothers and offspring were evaluated by PCR using PSA primers. The transgene transmission to the progeny of potential founder mice was verified by PCR and Southern blot. From among several candidate founders we have generated, the mouse expressing stably the highest levels of PSA as detected by ELISA was selected as the founder to propagate hybrid transgenic colonies.
The rPB-PSA transgenic map was constructed to confirm boundary sequences between the rat probasin promoter region, PSA open reading frame (ORF) and the bGH terminator. Primer sets located in the promoter-PSA and PSA-terminator flanking regions were designed and utilized to generate overlapping PCR fragments for complete DNA sequencing of the boundary regions.
Generation of PSA/HLA-A2.1 double transgenic mouse
Hybrid PSA and HLA-A2.1double transgenic mice (PSA/A2.1) were generated by cross-breeding PSA mice with HLA A2.1/H2Kb transgenic mice (Harlan Sprague Dawley, Indianapolis, IN). All double transgenic mice were heterozygous for each of their transgenes as confirmed by PCR.
Genotyping of PSA transgenic mice
Tail snippets from three-week old transgenic mice were collected and their DNA isolated. PCR was used to genotype transgenic progeny using the following PSA primers (InVitrogen): PSA -FOR: 5’ ACCATGTGGGTCCCGGTTG 3’and PSA-REV: 5 ’ TCAGGGGTTGGCCACGATG 3’.
DNA quality was tested with a β-globin PCR performed concurrently in each of the PSA reactions using the following primers: FOR: 5’ GCCAATCTGCTCACACAGGATA 3’ and REV: 5’ CATGCAGCTTGTCACAGTGGA 3’.
PCR reactions contained 200-300ng of genomic DNA template, 10mM Tris/HCL pH 8.3, 50mM KCl, 200μM dNTPs, 1μM each of forward and reverse primers, 1.25U Taq DNA polymerase (Applied Biosystems) and 1.5 mM MgCl2 in a 25 μl final reaction volume. PSA-β -globin PCR was performed using PSA and globin primers in 25 μL reaction at 94°C, 3 minutes followed by 30 cycles of 94°C for 1 minute, 55°C for 1 minute, 72°C for 2 minutes, and final elongation at 72°C for 10 minutes. Samples were stored at 4°C until gel electrophoresis was performed using a 1% agarose gel in 1×TAE buffer.
Extraction of RNA and RT-PCR
Total RNA was isolated from the prostate and other tissues using the Nucleospin RNA II Kit (Clontech, Mountain View, CA). RT-PCR was performed using a reverse transcriptase kit (Promega, Madison, WI). PSA primers were as described above, and β-actin primers that were used for control reactions as follows: β-actin Forward: 5’ TGTGATGGTGGGAATGGGTCAG 3’and Reverse: 5’ TTTGATGTCACGCACGATTTCC 3’. A parallel RT-PCR reaction without reverse transcriptase served as a negative control. PCR products were visualized on a 1% agarose gel.
Castration of PSA transgenic mice
Mice were anesthetized with a ketamine-xylazine mixture (ratio 90 mg/kg ketamine to 4.5 mg/kg xylazine) administered i.p. A lower abdominal incision was made, the testicular blood supply isolated and ligated with electrocautery. The testes were removed and the vasa cauterized. Mice were re-explored at various time intervals (e.g. 7, 14, 21, 28, and 42 days) for harvesting of tissues.
Quantitaion of PSA levels by ELISA
Tissues were homogenized by mincing with surgical blade, resuspend in equal volume of Buffer A (50mM Tris, KCl 1.15% pH 7.5) and Buffer C (10mM KPO4, 0.25 mM Sucrose pH 7.4) (max volume of 500 ul), and passed through a 21G needle with 1ml of syringe. Cells were sonicated at 30% output (180 watt) for 10 sec pulse (three times). The lysate was centrifuged at 13,000 rpm for 10 minutes at room temperature. The protein concentration in the supernatant was calculated using the Bio-Rad protein assay kit (BioRad Laboratories, Hercules, CA). 120 μl of lysate containing 50 μg of protein was used for PSA ELISA. Serum PSA concentration was measured by AxSYM PSA assay (AxSym system, Abbott Diagnostics, Abbott Park, IL), a Microparticle Enzyme Immunoassay designed to quantitate PSA levels in human serum.
One hundred micrograms of total protein was placed in 250μl of clear Hanks balanced salt solution and samples were submitted to the University of Michigan clinical pathology laboratory for PSA ELISA. Samples consisting of purified PSA from human semen or protein from wild type C57BL/6 mice were submitted to serve as positive and negative controls, respectively.
Mouse whole blood processing and HLA A2.1/Kb FACS analysis
One hundred microliters of blood was obtained from each mouse via orbital puncture and placed in a heparinized microfuge tube. Blood was placed in 2ml lysis buffer (Mouse Erythrocyte Lysing Kit, R&D Systems Inc.) for 10 minutes at room temperature. Samples were centrifuged for 5 minutes at 250×g, the supernatant was discarded, and the pellets were washed with 2ml of 1×PBS. One million peripheral blood cells were incubated with 0.5 μg anti-mouse FcγR antibody (Mouse Fc Block, BD PharMingen) for 10 minutes at 4°C. 0.5 μg of FITC-conjugated anti-HLA A2 antibody (One Lambda, Inc., Canoga Park, CA) or isotype control was then added and the reaction mixture was incubated in a final volume of 20 μl at 4°C for 1 hour. Cells labeled with isotype control were used to assess background fluorescence, and 10,000 viable cells were analyzed in a FACScan microflourometer (Becton Dickinson, Sunnyvale, CA).
Immunization of castrated PSA/A2.1 transgenic mice
PSA/A2.1 mice were anesthetized with a ketamine-xylazine mixture (ratio 90 mg/kg ketamine to 4.5 mg/kg xylazine) administered i.p. A lower abdominal incision was made, the testicular blood supply isolated, and ligated with electrocautery. The testes were removed and the vasa cauterized. Four weeks post-castration, mice were immunized intravenously with 106 PFU of a recombinant vaccinia virus expressing either the entire sequence of PSA (vac-PSA) (30) or the modified SV40 T antigen (vac-mTag) we previously generated in our laboratory (26-28).
Generation of bone-marrow-derived dendritic cells
Dendritic cells (DC) were generated from bone marrow using IL-4 and GM-CSF (PeproTech, Rocky Hill, New Jersey), and purified on a metrizamide gradient. DC maturation was evaluated by measuring MHC Class II, CD11c, CD40, CD80 and CD86 surface expression. Peptides (final 20μg/ml) or proteins (50 μg /ml) were loaded on DC at 37°C for 4 hrs or overnight, respecitvely. Loaded DC were washed and used as antigen-presenting cells in the ELISPOT assay.
IFN-γ ELISPOT assay
MultiScreen 96-well plates were first coated with purified anti-mouse IFN-γ antibody (capture antibody) (4 μg/ml in 1X PBS; PharMingen) overnight at 4°C. Plates were blocked with PBS/1% BSA (PBS-BSA) at room temperature for 90 min and then washed 3 times with 1X PBS before seeding the cells. CD8+ T cells were isolated from mice three weeks after immunization with 106 pfu/mouse vac-PSA or 107 vac-mTag. One million CD8+ T cells were seeded into each well (E/S = 10) and incubated at 37°C/5% CO2 for 24 hours with irradiated (5000 rads) peptide-or protein-loaded DC. Plates were washed 3 times with 1X PBS and then 4 times with 1X PBS/0.025% Tween-20 (PBS-TW20) before adding biotin rat anti-mouse IFN-γ antibody (2 μg/ml in PBS-BSA; PharMingen) overnight at 4°C. The plates were washed 4 times with PBS-TW20 and incubated with anti-biotin antibody (1:1000 dilution; Vector; Burtingame, CA) at room temperature for 90 min, followed by washing 4 times with PBS. Plates were then developed with NBT/BCIP before subjecting to an ELISPOT reader (Cellular Technology Laboratories, Ltd.; Cleveland, OH) to count spots.
RESULTS
Prostate-specific, androgen-dependent expression of PSA in a transgenic mouse
The rat probasin gene promoter has been demonstrated to be both developmentally and hormonally regulated in the mouse and demonstrates a high ability to direct transgene expression specifically to the prostate tissue (31). The promoter has been previously utilized to generate transgenic mice that express viral (29) and human oncogenes (32, 33) in the prostate to study prostate cancer development. We adopted a similar strategy to generate a transgenic mouse that exhibits prostate-specific expression of human PSA. To this end, an expression cassette containing human PSA cDNA, the rat probasin promoter, and the bovine growth hormone terminator sequence (Figure 1A) served to generate transgenic founders. The founder that produced progeny that had stable and high levels of PSA expression was selected to propagate the colony.
Figure 1. Generation of Rat probasin-regulated PSA transgenic mice.
(A) schematic map of the rpb-PSA transgene shows unique restriction enzyme sites. The transgene map was generated by sequencing PCR products using primer pairs that span the boundaries between the rat probasin (rpb) promoter, the PSA prepro-cDNA and the bovine growth hormone (bGH) terminator. Restriction map was generated using the BCM Search Launcher WebCutter Search Utility. (B) PSA expression in rpb-PSA transgenic mice was confirmed by RT-PCR. RNAs isolated from various tissues show prostate-specific transcription. RT-PCR was performed in the presence or absence (not shown) of Reverse Transcriptase. The amplification of actin transcripts was used as internal control. Only samples isolated from prostate tissues displayed significant amplification of PSA transcripts by RT-PCR.
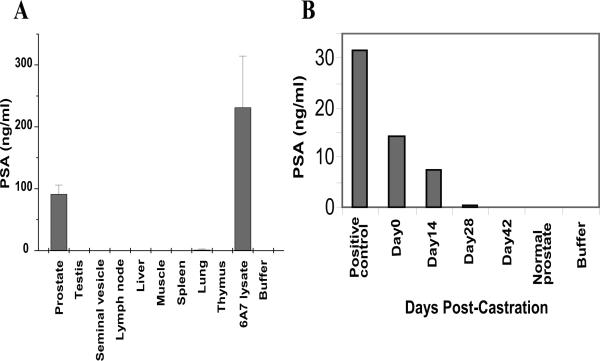
To address the specificity of the PSA expression, we isolated RNA from prostate, testis, seminal vesicle, spleen, pancreas, liver, lung, and nodal tissue from multiple progeny. Using RT-PCR, we confirmed that the presence of RNA transcript for PSA was restricted to the prostate tissue (Figure 1B). In line with this finding, analysis of protein extracts from these tissues by ELISA detected PSA in the prostate but not in any of the other tissues (Figure 2A).
Figure 2. Androgen-regulated, prostate-specific expression of PSA.
(A) Prostate lysates from three different mice (P719, P722, P748) showed significant PSA levels as compared to lysates from other tissues/organs or prostate from non-transgenic mice. Tissue PSA levels were determined using PSA-specific ELISA. Data shown as Mean ± SD from 3 animals. (B) Expression of the PSA transgene is androgen dependent and tissue levels of the protein decrease with time following surgical castration. PSA mice exhibit high levels of PSA expression and are similar to PSA positive controls. Following castration, prostate levels of PSA fall and at 28 days are similar to non-transgenic mouse prostates. One representative mouse is shown.
To test androgen-responsiveness of the PSA transgene, mice were castrated and sacrificed at various time points, and the presence of PSA mRNA was tested by RT-PCR. As shown in figure2B, expression of the transgene decreased in a time-dependent fashion after castration. Only a minimal expression was present at day 28 post-castration, and no transcripts were detected at day 42.
Generation of a PSA/HLA-A2.1 hybrid mouse
To generate a hybrid mouse that expresses both human HLA-A2.1 and PSA, PSA and HLA A2.1/Kb transgenic mice were crossed and the progeny tested by PCR of genomic DNA (data not shown). Progeny that possessed copies of both transgenes (data not shown) were further tested for HLA-A2.1 expression by flow cytometry. These double positive mice demonstrated expression of the HLA A2.1/Kb chimeric receptor on peripheral blood lymphocytes as demonstrated by FACS analysis. Some of these mice were sacrificed and protein extracts from mouse prostate tissue contained high levels of PSA as demonstrated by ELISA (data not shown).
Androgen deprivation augments CD8 T cell responses to prostate TAA
Consistent with the known immunosuppressive effects of androgens, castration of prostate tumor-bearing mice has been shown to enhance antigen-specific CD4 T cell responses to a model prostate TAA (13). We therefore sought to determine whether CD8 responses are affected by androgen deprivation using the PSA/A2.1 as a model and PSA or PSA-derived peptides as immunogens. Male A2.1 and female PSA/A2.1 mice were used as controls.
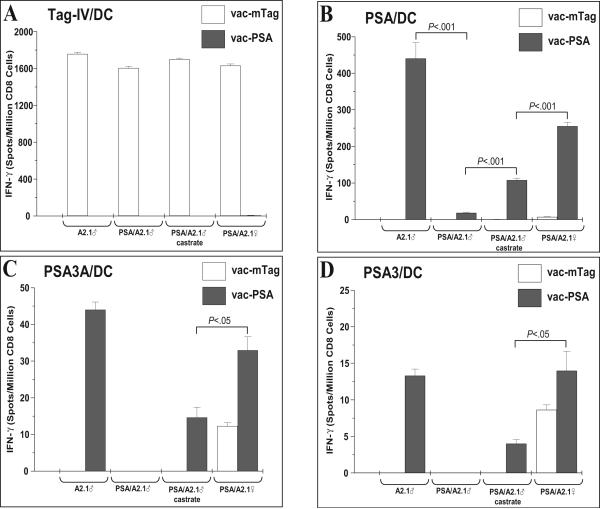
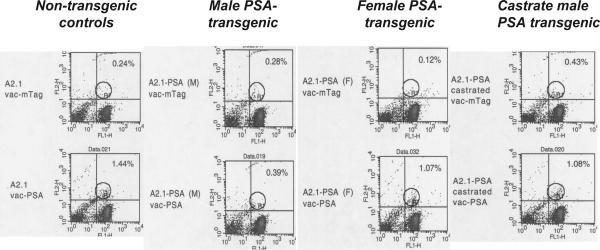
Mice were immunized with recombinant vaccinia virus constructs that express either PSA (vac-PSA) or SV40 Tag (vac-mTag). Splenocytes from immunized mice were re-stimulated in vitro with peptide- or full protein-loaded dendritic cells and IFN-γ-releasing CD8 T cells were quantitated by ELISPOT and tetramer staining. Our data show that splenocytes from sham-castrated male, castrated male, and female PSA/A2.1 mice immunized with vac-mTag responded in a similar fashion to restimulation with Tag-IV, while no response was observed with splenocytes from vac-PSA immunized mice. A similar response to Tag IV was obtained in control vac-mTag-immunized A2.1 mice, indicating a complete absence of tolerance to exogenous antigen in these mice (Figure 3A). Immunization with vac-PSA resulted in a strong and specific response in A2.1 mice upon re-stimulation with full PSA-loaded DC. However, a very weak response was observed in PSA/A2.1. Castrated mice responded better (P<.001), although this response was still significantly lower than male A2.1 and female PSA/A2.1 mice (P<.001) (Figure 3B). Splenocytes from vac-PSA-immunized mice showed a much lower number of IFN-γ-releasing, PSA-specific CD8 T cells upon re-stimulation with DC loaded with the peptides PSA3A (Figure 3C) and PSA3 (Figure 3D). Such a weak response to PSA peptides as compared to full PSA protein is possibly due to the contribution of H2-Db to the response to potential H2-Db-restricted, PSA-derived epitopes in this mouse model. Sham-castrated male PSA/A2.1 showed no response, while castrated mice showed a weak response. Due to the overall signal to noise window for ELISPOT evaluation of A2.1-restricted PSA peptide responses (females vac-mTag controls showed low but significant activity of PSA peptides, Figure 3C & 3D), we turned to tetramer assay to ascertain detection of A2.1-restricted, PSA-specific CTL. To this aim, we labeled in vitro-restimulated splenocytes with a PSA-3A-A2.1 tetramer and used flow cytometry to determine the fraction of CD8(+) T cells that bound the tetramer. Consistent with the ELISPOT data, tetramer staining revealed elevated amounts of PSA-specific CD8 T cells in spleens of immunized non-transgenic mice. These amounts were significantly lower (comparable to background level as seen in vac-mTag-immunized mice) in PSA transgenic mice, reflecting a deep immune tolerance to PSA. Splenocytes from castrated immunized male PSA/A2.1 mice exhibited significantly higher numbers of tetramer-positive CD8 cells than did their non-castrated counterparts. The effect of castration was similar to the one observed in A2.1-PSA females, but still lower than in A2.1 male mice (Figure 4).
Figure 3. Castration of male PSA/A2.1 mice augments PSA-specific cellular immune responses.
Castrated male mice were immunized with either vac-mTag or vac-PSA and antigen-specific CD8 responses were evaluated by ELISPOT and tetramer staining. Non-castrated male mice and female mice were used as controls. For splenocyte restimulation in vitro, bone marrow-derived DC were loaded with either H-2Kb restricted-, SV40 Tag-derived epitope IV (Tag-IV/DC) (A), with PSA (PSA/DC) (B) or with HLA-A2.1-restricted, PSA-derived epitopes (PSA3A/DC) (C) and (PSA3/DC) (D). IFN-γ-releasing CD8 T cells were revealed using an ELISPOT assay. Data shown as Mean ± SD, representative of one experiment with from 3 animals/group.
Figure 4. Castration of male PSA/A2.1 mice augments PSA-specific cellular immune responses.
PSA-3A-tetramer staining was performed on splenocytes from the same mice as described under Figure 3 to demonstrate the effect of castration on the elevation of PSA-specific CTL responses. Representative plots corresponding to mice within the same experiment are shown here.
DISCUSSION
A large body of evidence suggests the feasibility of PSA-based vaccines for prostate cancer (2, 3, 12, 34-41). However, because PSA is a self antigen, these vaccines face the challenge of immune tolerance to PSA and are yet to demonstrate clinical efficacy. Hence, immunotherapy for PCa will most likely consist of combined approaches that simultaneously target tumors through TAA and interfere with tolerizing mechanisms that hinder immunity to tumors. These mechanisms include innate and adaptive immune responses, activation/inhibition of co-stimulatory/inhibitory molecules (e.g. CTLA-4), elimination of regulatory/suppressive cells (e.g. regulatory T cells - Treg) and soluble factors, and manipulation of hormonal pathways. So far, interference with the inhibitory co-stimulatory signals mediated by CTLA-4 using monoclonal antibodies has provided the most promising results, although a myriad of adverse autoimmune responses generated by this strategy still represent a sizeable obstacle to its implementation in clinic (reviewed in ref.(42)).
In prostate cancer, androgen deprivation is a therapeutic modality that aims at depriving the prostate from testosterone to suppress its growth. Interestingly, this deprivation also results in strengthening immunity to tumors as shown in mice (13) and humans (12). The mechanisms whereby androgen deprivation affects immunity to tumors are poorly understood, partly due to the lack of appropriate mouse models.
Although the impact of androgen deprivation on prostate tumor immunity has been addressed in previous work, these failed to look at the extent to which CTL responses to a human prostate TAA are affected in vivo in humanized mice.
In this work, we describe a transgenic mouse that expresses human PSA specifically in the prostate. Tissue specific expression was demonstrated at both RNA and protein levels. We additionally show that expression of PSA in this mouse is regulated by androgens, confirming an optimal performance of the rat probasin promoter in this setting. This is evidenced by a time-dependent decrease of PSA levels in castrated male mice. At day 42 post-castration, no PSA was detected.
We crossed the PSA transgenic mouse with HLA-A2.1/Kb transgenic mouse. The resulting male offspring offers a valuable tool that allows investigation of immune tolerance to a human prostate antigen in a way that closely emulates human biology.
As expected, immunization of this hybrid mouse to a non-self antigen (SV40 Tag) resulted in a strong CTL response, whereas only a very weak response was generated towards a self antigen (PSA). In contrast, castration of male mice 4 weeks prior to immunization resulted in a significant augmentation of CTL response to PSA, while it did not affect the response to SV40 Tag. Interestingly, female hybrid mice showed a stronger response to PSA than did their castrated male counterparts.
Although interference of castration with immune tolerance to prostate specific antigens has been reported in two previous studies (13, 14), these studies either focused on CD4 responses (13) or did not show any effect of castration when applied before immunization (14). In the latter study, a prime-boost immunization protocol was applied and immune responses following primary immunization were not evaluated.
Overall, our present work describes a PSA/A2.1 transgenic mouse that might represent an attractive animal model to investigate immune tolerance to human prostate TAA and for preclinical development and refinement of PSA-targeted vaccines. It also provides compelling evidence supporting the use of androgen deprivation as a modality to circumvent immune tolerance to prostate TAA.
Acknowledgments
We thank Priyanka Priyadarsiny, PhD and Robert Vessella, PhD for their help with this work.
This work is supported by grants NIH P50 DK065313-1 (M Sanda, PI) and NIH R01 CA8241901-A1 (M Sanda, PI)
References
- 1.Vieweg J, Dannull J. Technology Insight: vaccine therapy for prostate cancer. Nat Clin Pract Urol. 2005;2:44–51. doi: 10.1038/ncpuro0079. [DOI] [PubMed] [Google Scholar]
- 2.Noguchi M, Kobayashi K, Suetsugu N, Tomiyasu K, Suekane S, Yamada A, Itoh K, Noda S. Induction of cellular and humoral immune responses to tumor cells and peptides in HLA-A24 positive hormone-refractory prostate cancer patients by peptide vaccination. Prostate. 2003;57:80–92. doi: 10.1002/pros.10276. [DOI] [PubMed] [Google Scholar]
- 3.Hildenbrand B, Sauer B, Kalis O, Stoll C, Freudenberg MA, Niedermann G, Giesler JM, Juttner E, Peters JH, Haring B, Leo R, Unger C, Azemar M. Immunotherapy of patients with hormone-refractory prostate carcinoma pre-treated with interferon-gamma and vaccinated with autologous PSA-peptide loaded dendritic cells--a pilot study. Prostate. 2007;67:500–508. doi: 10.1002/pros.20539. [DOI] [PubMed] [Google Scholar]
- 4.Pandha HS, John RJ, Hutchinson J, James N, Whelan M, Corbishley C, Dalgleish AG. Dendritic cell immunotherapy for urological cancers using cryopreserved allogeneic tumour lysate-pulsed cells: a phase I/II study. BJU Int. 2004;94:412–418. doi: 10.1111/j.1464-410X.2004.04922.x. [DOI] [PubMed] [Google Scholar]
- 5.Small EJ, Schellhammer PF, Higano CS, Redfern CH, Nemunaitis JJ, Valone FH, Verjee SS, Jones LA, Hershberg RM. Placebo-controlled phase III trial of immunologic therapy with sipuleucel-T (APC8015) in patients with metastatic, asymptomatic hormone refractory prostate cancer. J Clin Oncol. 2006;24:3089–3094. doi: 10.1200/JCO.2005.04.5252. [DOI] [PubMed] [Google Scholar]
- 6.Gilboa E, Vieweg J. Cancer immunotherapy with mRNA-transfected dendritic cells. Immunol Rev. 2004;199:251–263. doi: 10.1111/j.0105-2896.2004.00139.x. [DOI] [PubMed] [Google Scholar]
- 7.Simons JW, Mikhak B, Chang JF, DeMarzo AM, Carducci MA, Lim M, Weber CE, Baccala AA, Goemann MA, Clift SM, Ando DG, Levitsky HI, Cohen LK, Sanda MG, Mulligan RC, Partin AW, Carter HB, Piantadosi S, Marshall FF, Nelson WG. Induction of immunity to prostate cancer antigens: results of a clinical trial of vaccination with irradiated autologous prostate tumor cells engineered to secrete granulocyte-macrophage colony-stimulating factor using ex vivo gene transfer. Cancer Res. 1999;59:5160–5168. [PubMed] [Google Scholar]
- 8.Pavlenko M, Roos AK, Lundqvist A, Palmborg A, Miller AM, Ozenci V, Bergman B, Egevad L, Hellstrom M, Kiessling R, Masucci G, Wersall P, Nilsson S, Pisa P. A phase I trial of DNA vaccination with a plasmid expressing prostate-specific antigen in patients with hormone-refractory prostate cancer. Br J Cancer. 2004;91:688–694. doi: 10.1038/sj.bjc.6602019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eder JP, Kantoff PW, Roper K, Xu GX, Bubley GJ, Boyden J, Gritz L, Mazzara G, Oh WK, Arlen P, Tsang KY, Panicali D, Schlom J, Kufe DW. A phase I trial of a recombinant vaccinia virus expressing prostate-specific antigen in advanced prostate cancer. Clin Cancer Res. 2000;6:1632–1638. [PubMed] [Google Scholar]
- 10.Kantoff PW, Schuetz T, Blumenstein BA, Glode MM, Bilhartz D, Gulley J, Schlom J, Laus R, Godfrey W. Overall survival (OS) analysis of a phase II randomized controlled trial (RCT) of a poxviral-based PSA targeted immunotherapy in metastatic castration-resistant prostate cancer (mCRPC). J Clin Oncol. 2009;27 doi: 10.1200/JCO.2009.25.0597. abstr 5013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaufman HL, Wang W, Manola J, DiPaola RS, Ko YJ, Sweeney C, Whiteside TL, Schlom J, Wilding G, Weiner LM. Phase II randomized study of vaccine treatment of advanced prostate cancer (E7897): a trial of the Eastern Cooperative Oncology Group. J Clin Oncol. 2004;22:2122–2132. doi: 10.1200/JCO.2004.08.083. [DOI] [PubMed] [Google Scholar]
- 12.Sanda MG, Smith DC, Charles LG, Hwang C, Pienta KJ, Schlom J, Milenic D, Panicali D, Montie JE. Recombinant vaccinia-PSA (PROSTVAC) can induce a prostate-specific immune response in androgen-modulated human prostate cancer. Urology. 1999;53:260–266. doi: 10.1016/s0090-4295(98)00539-1. [DOI] [PubMed] [Google Scholar]
- 13.Drake CG, Doody AD, Mihalyo MA, Huang CT, Kelleher E, Ravi S, Hipkiss EL, Flies DB, Kennedy EP, Long M, McGary PW, Coryell L, Nelson WG, Pardoll DM, Adler AJ. Androgen ablation mitigates tolerance to a prostate/prostate cancer-restricted antigen. Cancer Cell. 2005;7:239–249. doi: 10.1016/j.ccr.2005.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koh YT, Gray A, Higgins SA, Hubby B, Kast WM. Androgen ablation augments prostate cancer vaccine immunogenicity only when applied after immunization. Prostate. 2009 doi: 10.1002/pros.20906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eng MH, Charles LG, Ross BD, Chrisp CE, Pienta KJ, Greenberg NM, Hsu CX, Sanda MG. Early castration reduces prostatic carcinogenesis in transgenic mice. Urology. 1999;54:1112–1119. doi: 10.1016/s0090-4295(99)00297-6. [DOI] [PubMed] [Google Scholar]
- 16.Isaacs JT, Furuya Y, Berges R. The role of androgen in the regulation of programmed cell death/apoptosis in normal and malignant prostatic tissue. Semin Cancer Biol. 1994;5:391–400. [PubMed] [Google Scholar]
- 17.Feldman BJ, Feldman D. The development of androgen-independent prostate cancer. Nat Rev Cancer. 2001;1:34–45. doi: 10.1038/35094009. [DOI] [PubMed] [Google Scholar]
- 18.Mercader M, Bodner BK, Moser MT, Kwon PS, Park ES, Manecke RG, Ellis TM, Wojcik EM, Yang D, Flanigan RC, Waters WB, Kast WM, Kwon ED. T cell infiltration of the prostate induced by androgen withdrawal in patients with prostate cancer. Proc Natl Acad Sci U S A. 2001;98:14565–14570. doi: 10.1073/pnas.251140998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kwon ED, Hurwitz AA, Foster BA, Madias C, Feldhaus AL, Greenberg NM, Burg MB, Allison JP. Manipulation of T cell costimulatory and inhibitory signals for immunotherapy of prostate cancer. Proc Natl Acad Sci U S A. 1997;94:8099–8103. doi: 10.1073/pnas.94.15.8099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roden AC, Moser MT, Tri SD, Mercader M, Kuntz SM, Dong H, Hurwitz AA, McKean DJ, Celis E, Leibovich BC, Allison JP, Kwon ED. Augmentation of T cell levels and responses induced by androgen deprivation. J Immunol. 2004;173:6098–6108. doi: 10.4049/jimmunol.173.10.6098. [DOI] [PubMed] [Google Scholar]
- 21.Coles AJ, Thompson S, Cox AL, Curran S, Gurnell EM, Chatterjee VK. Dehydroepiandrosterone replacement in patients with Addison's disease has a bimodal effect on regulatory (CD4+CD25hi and CD4+FoxP3+) T cells. Eur J Immunol. 2005;35:3694–3703. doi: 10.1002/eji.200526128. [DOI] [PubMed] [Google Scholar]
- 22.Holzbeierlein J, Lal P, LaTulippe E, Smith A, Satagopan J, Zhang L, Ryan C, Smith S, Scher H, Scardino P, Reuter V, Gerald WL. Gene expression analysis of human prostate carcinoma during hormonal therapy identifies androgen-responsive genes and mechanisms of therapy resistance. Am J Pathol. 2004;164:217–227. doi: 10.1016/S0002-9440(10)63112-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Terasawa H, Tsang KY, Gulley J, Arlen P, Schlom J. Identification and characterization of a human agonist cytotoxic T-lymphocyte epitope of human prostate-specific antigen. Clin Cancer Res. 2002;8:41–53. [PubMed] [Google Scholar]
- 24.Mylin LM, Deckhut AM, Bonneau RH, Kierstead TD, Tevethia MJ, Simmons DT, Tevethi SS. Cytotoxic T lymphocyte escape variants, induced mutations, and synthetic peptides define a dominant H-2Kb-restricted determinant in simian virus 40 tumor antigen. Virology. 1995;208:159–172. doi: 10.1006/viro.1995.1139. [DOI] [PubMed] [Google Scholar]
- 25.Mylin LM, Bonneau RH, Lippolis JD, Tevethia SS. Hierarchy among multiple H-2b-restricted cytotoxic T-lymphocyte epitopes within simian virus 40 T antigen. J Virol. 1995;69:6665–6677. doi: 10.1128/jvi.69.11.6665-6677.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tseng-Rogenski SS, Arredouani MS, Escara-Wilke JF, Neeley YC, Imperiale MJ, Sanda MG. A safety-modified SV40 Tag developed for human cancer immunotherapy. Drug Design, Development and Therapy. 2008;2:17–24. [PMC free article] [PubMed] [Google Scholar]
- 27.Tseng-Rogenski SS, Arredouani MS, Neeley YC, Lu B, Chinnaiyan AM, Sanda MG. Fas-mediated T cell deletion potentiates tumor antigen-specific tolerance in a mouse model of prostate cancer. Cancer Immunol Immunother. 2008 doi: 10.1007/s00262-008-0471-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xie YC, Hwang C, Overwijk W, Zeng Z, Eng MH, Mule JJ, Imperiale MJ, Restifo NP, Sanda MG. Induction of tumor antigen-specific immunity in vivo by a novel vaccinia vector encoding safety-modified simian virus 40 T antigen. Journal of the National Cancer Institute. 1999;91:169–175. doi: 10.1093/jnci/91.2.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Greenberg NM, DeMayo F, Finegold MJ, Medina D, Tilley WD, Aspinall JO, Cunha GR, Donjacour AA, Matusik RJ, Rosen JM. Prostate cancer in a transgenic mouse. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:3439–3443. doi: 10.1073/pnas.92.8.3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hodge JW, Schlom J, Donohue SJ, Tomaszewski JE, Wheeler CW, Levine BS, Gritz L, Panicali D, Kantor JA. A recombinant vaccinia virus expressing human prostate-specific antigen (PSA): safety and immunogenicity in a non-human primate. Int J Cancer. 1995;63:231–237. doi: 10.1002/ijc.2910630215. [DOI] [PubMed] [Google Scholar]
- 31.Greenberg NM, DeMayo FJ, Sheppard PC, Barrios R, Lebovitz R, Finegold M, Angelopoulou R, Dodd JG, Duckworth ML, Rosen JM, et al. The rat probasin gene promoter directs hormonally and developmentally regulated expression of a heterologous gene specifically to the prostate in transgenic mice. Mol Endocrinol. 1994;8:230–239. doi: 10.1210/mend.8.2.8170479. [DOI] [PubMed] [Google Scholar]
- 32.Tomlins SA, Laxman B, Varambally S, Cao X, Yu J, Helgeson BE, Cao Q, Prensner JR, Rubin MA, Shah RB, Mehra R, Chinnaiyan AM. Role of the TMPRSS2-ERG gene fusion in prostate cancer. Neoplasia. 2008;10:177–188. doi: 10.1593/neo.07822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carver BS, Tran J, Chen Z, Varmeh S, Carracedo-Perez A, Alimonti A, Nardella C, Gopalan A, Scardino PT, Cordon-Cardo C, Gerald w., Pandolfi PP. ETS genetic rearrangements are progression events in prostate tumorigenesis. Nature (submitted) 2008 [Google Scholar]
- 34.Kim JJ, Trivedi NN, Wilson DM, Mahalingam S, Morrison L, Tsai A, Chattergoon MA, Dang K, Patel M, Ahn L, Boyer JD, Chalian AA, Schoemaker H, Kieber-Emmons T, Agadjanyan MA, Weiner DB. Molecular and immunological analysis of genetic prostate specific antigen (PSA) vaccine. Oncogene. 1998;17:3125–3135. doi: 10.1038/sj.onc.1201736. [DOI] [PubMed] [Google Scholar]
- 35.Correale P, Walmsley K, Nieroda C, Zaremba S, Zhu M, Schlom J, Tsang KY. In vitro generation of human cytotoxic T lymphocytes specific for peptides derived from prostate-specific antigen. J Natl Cancer Inst. 1997;89:293–300. doi: 10.1093/jnci/89.4.293. [DOI] [PubMed] [Google Scholar]
- 36.Barrou B, Benoit G, Ouldkaci M, Cussenot O, Salcedo M, Agrawal S, Massicard S, Bercovici N, Ericson ML, Thiounn N. Vaccination of prostatectomized prostate cancer patients in biochemical relapse, with autologous dendritic cells pulsed with recombinant human PSA. Cancer Immunol Immunother. 2004;53:453–460. doi: 10.1007/s00262-003-0451-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arlen PM, Gulley JL, Parker C, Skarupa L, Pazdur M, Panicali D, Beetham P, Tsang KY, Grosenbach DW, Feldman J, Steinberg SM, Jones E, Chen C, Marte J, Schlom J, Dahut W. A randomized phase II study of concurrent docetaxel plus vaccine versus vaccine alone in metastatic androgen-independent prostate cancer. Clin Cancer Res. 2006;12:1260–1269. doi: 10.1158/1078-0432.CCR-05-2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Heiser A, Coleman D, Dannull J, Yancey D, Maurice MA, Lallas CD, Dahm P, Niedzwiecki D, Gilboa E, Vieweg J. Autologous dendritic cells transfected with prostate-specific antigen RNA stimulate CTL responses against metastatic prostate tumors. J Clin Invest. 2002;109:409–417. doi: 10.1172/JCI14364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marshall DJ, San Mateo LR, Rudnick KA, McCarthy SG, Harris MC, McCauley C, Schantz A, Geng D, Cawood P, Snyder LA. Induction of Th1-type immunity and tumor protection with a prostate-specific antigen DNA vaccine. Cancer Immunol Immunother. 2005;54:1082–1094. doi: 10.1007/s00262-005-0687-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roos AK, Pavlenko M, Charo J, Egevad L, Pisa P. Induction of PSA-specific CTLs and anti-tumor immunity by a genetic prostate cancer vaccine. Prostate. 2005;62:217–223. doi: 10.1002/pros.20135. [DOI] [PubMed] [Google Scholar]
- 41.Wallecha A, Maciag PC, Rivera S, Paterson Y, Shahabi V. Construction and characterization of an attenuated Listeria monocytogenes strain for clinical use in cancer immunotherapy. Clin Vaccine Immunol. 2009;16:96–103. doi: 10.1128/CVI.00274-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Langer LF, Clay TM, Morse MA. Update on anti-CTLA-4 antibodies in clinical trials. Expert Opin Biol Ther. 2007;7:1245–1256. doi: 10.1517/14712598.7.8.1245. [DOI] [PubMed] [Google Scholar]