Abstract
OBJECTIVE
We determined the relationships between glycemia at randomization, concurrent antidiabetic therapy, and change in A1C and fasting plasma glucose (FPG) in patients with diabetes receiving standard treatment for diabetes and randomized to ranolazine or placebo within the MERLIN-TIMI-36 (MERLIN) study. Ranolazine is a novel first-in-class drug approved for treating angina pectoris.
RESEARCH DESIGN AND METHODS
Randomization and 4-month glycemic and antidiabetes drug usage data from MERLIN were analyzed using Spotfire and SAS version 9.1 software.
RESULTS
In patients with diabetes and A1C of ≥8–10% at randomization (n = 171), there was an absolute A1C reduction in the ranolazine group of 1.2% (95% CI −1.4 to −1.0), and the placebo-adjusted (n = 182) decrease in A1C by ranolazine was 0.59% (95% CI −0.99 to −0.20, P < 0.001). In patients with FPG of 150–400 mg/dl at randomization, ranolazine (n = 131) compared with placebo (n = 147) reduced FPG by 25.7 mg/dl (95% CI −43.3 to −8.1, P = 0.001). When changes in either A1C or FPG were correlated to A1C or FPG at randomization, the slopes were significantly steeper for ranolazine than placebo (A1C, P = 0.046; FPG, P < 0.001), indicating that lowering of A1C and FPG by ranolazine is related to hyperglycemia at randomization. Ranolazine, compared with placebo, was not associated with serious hypoglycemic events, associated with significant changes in concurrent antidiabetic therapy, or dependent on a history of angina.
CONCLUSIONS
Ranolazine, when added to concurrent antidiabetes treatment, lowers FPG and A1C in patients with cardiovascular disease and poorly controlled diabetes.
Diabetes is an established risk factor for cardiovascular disease, and the risk of cardiovascular disease increases with worsening hyperglycemia (1–3). Furthermore, coronary artery disease is the most common cause of death in patients with diabetes (4). Patients with coronary artery disease and a recent myocardial infarction or acute coronary syndrome (ACS) have an increased incidence of impaired fasting plasma glucose (FPG) and new-onset diabetes (5–7). Management of diabetes in patients with cardiovascular disease is complicated by the fact that the cardiovascular safety of some oral glucose–lowering agents has been questioned, and outcome data are lacking (8).
Ranolazine is a first-in-class anti-anginal drug with cardioprotective properties without effects on heart rate or blood pressure (9). The drug inhibits the cardiac late sodium current (10,11). The late sodium current is enhanced during ischemia and in the failing heart and contributes to the Na+-dependent cellular calcium overload associated with these pathological conditions (10,11). Ranolazine has been shown effective in treating chronic angina both as a monotherapy (MARISA trial) and in combination with commonly prescribed cardiovascular drugs (CARISA and ERICA trials) (12–14), with no increase in mortality in patients with established coronary artery disease, including those with diabetes (15,16).
Post hoc analysis of data from the CARISA study demonstrated that ranolazine lowered A1C, a long-term biomarker of glucose control, in patients with chronic angina and diabetes, in a dose-dependent manner (17). While the mechanism of glycemic improvement remains incompletely understood, preliminary studies using isolated rat and human pancreatic islets suggest ranolazine may promote glucose-stimulated insulin secretion (18).
In the MERLIN-TIMI-36 (MERLIN) study, the effects of ranolazine to lower A1C and glucose were confirmed using prespecified glycemic end points (16). In this study, patients with diabetes were receiving standard of care treatment for diabetes with mean A1C levels of 7.5% at randomization. Despite the relatively low mean A1C at randomization, ranolazine was found to significantly reduce A1C in patients with diabetes and to reduce the incidence of newly elevated A1C in initially normoglycemic patients (16). The mean placebo-corrected reductions in A1C with ranolazine treatment at 4 months were 0.42% (P < 0.001) and 0.18% (P < 0.001) for patients with and without diabetes, respectively. There were no differences in the reported incidence of hypoglycemia between placebo and ranolazine.
The glucose-lowering response to multiple antidiabetic therapies is greater in patients with higher baseline A1C and glucose values (19). Therefore, the current analysis of the MERLIN data was undertaken to evaluate the effects of ranolazine on FPG and A1C in diabetic patients with moderate or severe hyperglycemia, defined as an A1C of 6 to <8% or ≥8–10%, or FPG <150 or ≥150–400 mg/dl, respectively, at randomization. Additionally, MERLIN data were assessed as to whether effects of ranolazine on glycemia were influenced by concurrent antidiabetic therapy.
RESEARCH DESIGN AND METHODS
Study overview
In the MERLIN trial, 6,560 patients with non-ST elevation ACS with at least one marker of moderate-to-high risk of recurrent ischemic events (including diabetes) were randomized at 440 sites in 17 countries. The study design, investigators, primary results of the trial, and prespecified end points of glycemic control have been reported (15,16,20,21). As previously described (20), eligible patients were randomly assigned by a central interactive voice response system in a 1:1 ratio to receive either ranolazine or placebo, which was initiated as an intravenous infusion and followed by oral administration at a dose of 1,000 mg twice daily until the end of the study. Randomization was stratified by “intention to manage the patient with early invasive strategy (angiography within 48 h and revascularization if necessary).” There was no stratification based on the presence of new or established diabetes. Patients returned for study visits at 14 days, 4 months, and every 4 months thereafter until the end of the study at 16 months.
After patient interviews and review of medical records, investigators recorded a history of diabetes and its treatment on study case record forms. Patients with diabetes, regardless of treatment group, received standard of care treatment for diabetes, and there was no prespecified glycemic goal. The protocol stipulated that A1C and plasma glucose were to be measured locally at randomization (median 24 h after symptom onset), 4 months, 8 months, 16 months, and the final study visit, with data recorded in the case report form as to whether the patient was fasting or not.
Statistical analysis
Patients with A1C values at randomization and month 4 were included in this retrospective exploratory analysis performed using Spotfire Software (Tibco Software, Somerville, MA). In addition, FPG was assessed in patients with fasting measures. Spotfire filtering and visualization tools were used specifically to search the dataset for relationships between A1C and glucose at different levels of glycemic control. For each analysis, the studied population is described in the corresponding figure legend and the analysis was reproduced in SAS Version 9.1 (SAS, Cary, NC) as described below.
Analyses of change from randomization in A1C, and separately FPG, were estimated by two models. The first model used ANCOVA including factors for treatment, the randomization covariate of interest, and the randomization stratification variable. The randomization covariate categories for A1C and FPG were defined to include all nonmissing randomization values. The second model, a detailed analysis of treatment differences within covariate levels, was prepared with a cell means version of the linear model. The cell means model included an intercept and one factor for each combination of treatment and randomization covariate category and a single factor for the randomization stratification variable. The estimates of the treatment effects from the analyses of covariance are least square means. A Tukey-Kramer procedure was used to estimate the confidence limits and P value. The analyses were performed using proc mixed, in SAS Version 9.1 (SAS, Cary, NC).
All medications and dosages were recorded at each study visit by the investigator. Changes in antidiabetic therapy were described as changes in the number or dosage of hypoglycemic agents. Patients were categorized as having an increase, decrease, or no change in concurrent antidiabetes therapy. In complex cases, the records were evaluated by expert review while blinded to treatment. A small number of patient records could not be categorized because of nonsensical data and were omitted from the frequency analysis. Differences in intensification and de-intensification (decrease in antidiabetic therapy) frequencies between treatment groups were determined by χ2 analysis.
RESULTS
Study population
The current analysis includes patients with A1C data and/or fasting glucose data and a history of diabetes measured at both randomization and 4 months. Of the patients (placebo 2,679, ranolazine 2,565) reaching month 4 (supplementary Table A1, available in an online appendix at http://care.diabetesjournals.org/cgi/content/full/dc09-2334/DC1), one-third had a history of diabetes (placebo 892, ranolazine 842). Eighty-five percent of the patients with diabetes had A1C measurements at both randomization and month 4 (placebo 770, ranolazine 707), and 35% had FPG measurements at both times (placebo 328, ranolazine 310). All patients had access to standard of care antidiabetes treatment, and baseline characteristics were similar for the placebo- and ranolazine-treated groups (Table 1). The mean A1C and FPG values at randomization for patients included and excluded from this analysis because of missing data at randomization or month 4 were not different.
Table 1.
Patient characterization at randomization and antidiabetic drug usage data at 4 months for patients with a history of diabetes
| Patient characteristics at randomization | Placebo | Ranolazine |
|---|---|---|
| n | 770 | 707 |
| Age (years) [median (25th to 75th percentile)] | 64 (57–71)* | 65 (57–72)* |
| Female sex (%) | 39.6* | 43.2* |
| Weight (kg/m2) [median (25th to 75th percentile)] | 85 (75–96)* | 84 (74–95)* |
| BMI (kg/m2) [median (25th to 75th percentile)] | 30 (27–33)* | 30 (27–33)* |
| Hypertension (%) | 84.4* | 84.5* |
| Hyperlipidemia (%) | 73.8* | 75.9* |
| Current smoker (%) | 19.5* | 17.4* |
| Antidiabetes drug usage [% (n)] | ||
| Sulfonylurea | 39.4 (303) | 42.9 (303) |
| Biguanide (metformin) | 37.4 (288) | 36.4 (257) |
| Insulin | 28.2 (217) | 27.3 (193) |
| Thiazolidinedione | 5.5 (42) | 4.2 (30) |
| α-Glucosidase inhibitor | 2.9 (22) | 3.0 (21) |
| Meglitinide | 2.3 (18) | 1.4 (10) |
| No antidiabetic drug | 16.6 (128) | 19.0 (134) |
| Monotherapy | 50.0 (385) | 47.4 (335) |
| Dual therapy | 28.3 (218) | 28.9 (204) |
| Three or more drugs | 5.0 (38) | 4.8 (34) |
*Patient characterization data are reproduced from Morrow et al. (16).
Effect of ranolazine on A1C and FPG
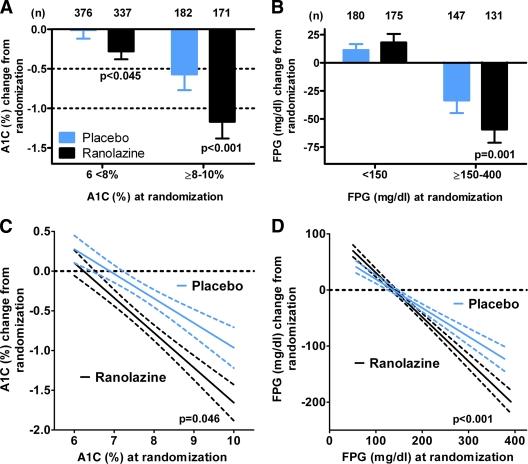
The diabetes study population was divided into good/moderate and poor glycemic control groups defined as A1C 6 to <8% or ≥8–10%. There was a significant reduction in A1C with ranolazine in addition to standard of care antidiabetes treatment for both the A1C 6 to <8% and A1C ≥8–10% groups (Fig. 1A). The absolute reduction in A1C in the ranolazine-treated patients with better glycemic control (A1C 6 to <8%) was 0.28% (95% CI −0.38 to −0.19), and for individuals with poorer glycemic control (A1C ≥8–10%), A1C was reduced 1.2% (95% CI −1.4 to −1.0). The placebo-corrected decrease in A1C with ranolazine was 0.28% (95% CI −0.55 to 0.00, P = 0.045) for the A1C 6 to <8% group and 0.59% (95% CI −0.99 to −0.20, P < 0.001) for patients with A1C 8–10%.
Figure 1.
Relationship between glycemia at randomization and lowering of A1C and FPG by ranolazine in patients with a history of diabetes. A: In a cell means model, with parameters for combinations of treatment, A1C category, and diabetes, the placebo-adjusted effect of ranolazine on A1C was −0.28% (95% CI −0.55 to 0.003, P = 0.045) for patients with A1C 6 to <8% and −0.59% (−0.99 to −0.20, P < 0.001) for patients with A1C ≥8–10%. B: In a cell means model, with parameters for combinations of treatment, FPG category, and diabetes, the placebo-adjusted effect on FPG for these patients was 6.8 mg/dl (95% CI −8.8 to 22.3, P = 0.677) for patients with FPG <150 mg/dl and −25.7 mg/dl (−43.3 to −8.1, P = 0.001) for patients with FPG ≥150–400 mg/dl. Changes in A1C and FPG at month 4 are summarized by mean, associated 95% CI, and number of patients (n). C: Relationship between A1C at randomization and the change in A1C at month 4. The slope for placebo was −0.31 (95% CI −0.41 to −0.21), R = 0.26 and n = 558. For ranolazine, the slope was −0.44 (−0.53 to −0.36), R = 0.41 and n = 508. The slopes were significantly different (P = 0.045). D: Relationship between FPG at randomization and the change in FPG at month 4. The slope for placebo was −0.55 (95% CI −0.64 to −0.46), R = 0.54 and n = 328. For ranolazine, the slope was −0.81 (−0.89 to −0.73), R = 0.76 and n = 310. The slopes were significantly different (P < 0.001). Least squares regression was performed by Graphpad Prism 5.0, and the best-fit line and 95% CI for the fit are shown for each group. Similar results were obtained using an ANCOVA model with a term for treatment, A1C or FPG at randomization, and the interaction of treatment.
In this study, the FPG level at randomization corresponding to an A1C of 8% was ∼150 mg/dl (data not shown). As a result, patients (placebo n = 327, ranolazine n = 306) were similarly divided into two groups: good/moderate glycemic control (<150 mg/dl) and poor glycemic control (≥150–400 mg/dl, Fig. 1B). The FPG ≥150 mg/dl group was limited to 400 mg/dl to exclude five patients with very high initial FPG values and potential confounding concomitant illnesses. There was a significant placebo-corrected reduction in FPG of 25.7 mg/dl (95% CI −43.3 to −8.1, P = 0.001) by ranolazine for patients with marked hyperglycemia (FPG ≥150–400 mg/dl), whereas there was no change in FPG (6.8 mg/dl, 95% CI −8.8 to 22.3, P = 0.68) in those patients with normal to moderate fasting hyperglycemia (FPG <150 mg/dl).
The finding that both A1C and FPG lowering by ranolazine were greater in hyperglycemic patients suggested a relationship between glycemia at randomization and A1C or FPG lowering by ranolazine. As shown in Fig. 1C, there was a linear relationship between A1C at randomization and decrease in A1C with both placebo and ranolazine. However, the inverse relationship was stronger for ranolazine (R = 0.41 vs. R = 0.26 for placebo), and the slope was significantly steeper for ranolazine (slope = −0.44 [95% CI −0.53 to −0.36]) compared with placebo (slope = −0.31 [95% CI −0.41 to −0.21], P = 0.046). Similarly, when FPG at randomization was correlated with the change in FPG at 4 months, there was a linear relationship between FPG at randomization and decrease in FPG with treatment (Fig. 1D). The inverse relationship was stronger for ranolazine (R = 0.72 vs. R = 0.48 for placebo), and the slope was significantly steeper for ranolazine (slope = −0.79 [95% CI −0.88 to −0.70]) compared with placebo (slope = −0.51 [95% CI −0.6 to −0.42], P < 0.001). The linear regression lines intersected at an FPG value of 141 mg/dl (Fig. 1D), indicating a greater effect for ranolazine than placebo in lowering FPG in patients with FPG >141 mg/dl. This finding is consistent with the previous analysis (Fig. 1B) showing that ranolazine did not lower mean FPG in patients with more moderate dysglycemia (FPG <150 mg/dl), whereas it did lower FPG in individuals with more marked hyperglycemia (FPG >150 mg/dl). In patients without a history of diabetes, but with new or undiagnosed diabetes, as defined by an A1C of ≥6.5–10% or FPG of ≥126–400 mg/dl, the effect of ranolazine to reduce FPG and A1C was similar to that observed patients with a history of diabetes at comparable mean baseline A1C and FPG levels (supplementary Table A2).
Angina history and glycemic lowering by ranolazine
All patients (n = 1,477) in the present analysis had ACS, and 65% also had a history of angina. Thus, the effect of angina status on glucose lowering by ranolazine was determined. The placebo-corrected change in A1C by ranolazine was independent of angina history (P = 0.213; angina: −0.5%, 95% CI −0.9 to −0.1, P = 0.014; no angina: −0.8%, 95% CI −1.2 to −0.3, P < 0.001; supplementary Fig. 1A). The absolute A1C reduction in ranolazine-treated patients with and without angina was 1.1% (95% CI −1.4 to −0.9) and 1.2% (−1.5 to −0.9), respectively. In patients having an FPG of ≥150–400 mg/dl, the placebo-corrected effect of ranolazine (angina: −26.2 mg/dl, 95% CI −48.2 to −4.3, P = 0.02; no angina: −25.1 mg/dl, −49.5 to −0.7, P = 0.04) on FPG was independent of a history of angina (P = 0.408; supplementary Fig. 1B). Therefore, ranolazine improves glycemia equally in patients with or without history of angina pectoris.
Concurrent antidiabetic therapy
The usage rate and type of antidiabetic therapy for placebo- and ranolazine-treated patients with a history of diabetes was similar between both ranolazine- and placebo-treated groups at month 4 (Table 1). The majority of patients were taking either a biguanide (metformin) (placebo 37.4%; ranolazine 36.4%) and/or a sulfonylurea (placebo 39.4%; ranolazine 42.9%). There were no major differences in insulin, thiazolidinedione, α-glucosidase inhibitor, or meglitinide usage. Additionally, the frequency of patients receiving monotherapy (placebo 50.0%; ranolazine 47.4%) and dual therapy (placebo 28.3%; ranolazine 28.9%) for diabetes was similar between placebo and ranolazine groups. When changes to antidiabetic therapy (new or intensified hypoglycemic therapy) were evaluated between 0 and 4 months, there were no significant differences between the placebo and ranolazine groups within any of the study populations. As a result, the effect of ranolazine to lower FPG and A1C does not appear to be attributable to intensification of concurrent antidiabetic therapy in ranolazine-treated patients. Similarly, a reduction in antidiabetic therapy could underestimate the effect of ranolazine to lower A1C and FPG; however, there were no significant differences in the frequency of reductions in hypoglycemic agents between placebo and ranolazine in any of the study populations.
Furthermore, there were no significant differences in the reported number of severe hypoglycemic adverse events between placebo- and ranolazine-treated patients between randomization and month 4 (placebo 16; ranolazine 19, P = 0.69).
Probability of ranolazine-treated patients achieving an A1C goal of ≤7% by antidiabetic treatment
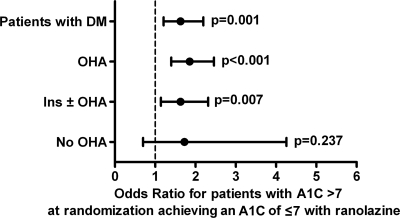
Patients with a history of diabetes were divided into three groups based on the type of antidiabetic treatment (supplementary Table A3): no antidiabetes drugs (placebo 16.6%, ranolazine 19.0%), oral hypoglycemic agents (OHAs) but no insulin (placebo 55.2%, ranolazine 53.7%), and insulin with or without any combination of other diabetes drugs (insulin ± OHA, placebo 28.2%, ranolazine 27.3%). To determine whether the effect of A1C lowering by ranolazine was influenced by concurrent antidiabetic therapy, the odds ratio (OR) for a patient with an A1C >7% at randomization to achieve an A1C of ≤7% after 4 months on ranolazine, compared with placebo, was calculated (Fig. 2). The OR for all patients with a history of diabetes and an A1C >7% (placebo 399, ranolazine 378) was 1.6 (95% CI 1.2–2.2, P < 0.001). The ORs for the respective subgroups were as follows: no antidiabetic drugs (placebo 34, ranolazine 37), OR 1.7 (95% CI 0.7–4.3, P = 0.24); OHA (placebo 219, ranolazine 203), OR 1.9 (1.4–2.5, P < 0.001); and insulin ± OHA (placebo 146, ranolazine 138), OR 1.6 (1.1–2.3, P < 0.007). Whereas there were few patients not receiving antidiabetes pharmacologic therapy, the OR favored ranolazine and indicated that patients taking ranolazine had a 60–90% greater probability of achieving an A1C ≤7% than did patients not taking ranolazine. Furthermore, the effect of ranolazine to lower A1C does not appear to be modulated by the type of concurrent antidiabetic therapy. Consistent with this observation, the placebo-corrected effect of ranolazine on A1C in patients with an A1C of ≥6–10% categorized by concomitant diabetes medication was similar among groups (no diabetes drugs, metformin only, sulfonylureas only, metformin plus sulfonylurea, and insulin only; supplementary Fig. 2).
Figure 2.
Effect of concurrent antidiabetes drug treatment on patients treated with ranolazine reaching an A1C goal of ≤7%. Patients with a history of diabetes who were hyperglycemic at randomization (A1C >7%) were grouped by drug treatment. These patients were reexamined at 4 months and categorized as responders if A1C was ≤7%. OR for hyperglycemic patients in each group to reach an A1C ≤7% was calculated using a logistics analysis model (SAS software, Cary, NC). Data are plotted as OR (95% CI). Ins, insulin.
CONCLUSIONS
Findings from the MERLIN trial demonstrate that ranolazine reduces A1C in patients with a history of diabetes (13,16,17). The current analysis of the MERLIN data extends these observations by examining the relationships between A1C and FPG concentrations at randomization and the magnitude of the glycemic-lowering effect of ranolazine when added to standard of care. Consistent with the efficacy of other antidiabetic drugs, A1C lowering by ranolazine was greater in patients with more marked hyperglycemia (A1C ≥8–10% or FPG ≥150–400 mg/dl at randomization). While the magnitude of A1C lowering with ranolazine compared with placebo may appear small, it is important to recognize that effects were assessed as add-on to established therapies with dose adjustments of concomitant medications permitted. This is not typical for studies examining glycemia as the primary end point. Ranolazine was not associated with increased rates of severe hypoglycemic adverse events. These findings are particularly noteworthy, since ranolazine has established cardiovascular safety in patients with ACS, a particularly vulnerable population that has been infrequently investigated during early development of diabetes-specific therapies.
Furthermore, ranolazine appears more effective than placebo for glycemic improvement regardless of background antidiabetes therapy. For patients with A1C >7%, above the current treatment goal recommended by the American Diabetes Association and the European Association for the Study of Diabetes (22), the effect of ranolazine to lower A1C to ≤7% appears independent of the concurrent antidiabetic therapy. The lack of significant effect in patients with diabetes not receiving antidiabetes drugs may be explained by the very low number of patients in this group with an initial A1C >7% (placebo 34, ranolazine 37). Additionally, both treatment groups had very large improvements in A1C at 4 months (placebo −0.9%, ranolazine −1.3%), perhaps representing more newly diagnosed disease or increased physician or patient attentiveness.
Ranolazine significantly reduced placebo-adjusted FPG by −25.7 mg/dl in patients with elevated FPG (≥150–400 mg/dl), but not in patients with milder dysglycemia (FPG >150 mg/dl) at randomization. This finding is in agreement with data from adverse event reporting, indicating that ranolazine is not associated with excess hypoglycemia compared with placebo. In the subgroup of diabetes patients with better glycemic control (FPG <150 mg/dl) treated with a sulfonylurea, changes in FPG were similar between placebo and ranolazine, suggesting that the risk of hypoglycemia with a sulfonylurea is not increased by ranolazine. It is noteworthy that ranolazine previously has been shown to reduce incident diabetes by 32% (16).
Patients in the study with a history of diabetes generally had good/moderate glycemic control (placebo A1C 7.4%; ranolazine A1C 7.5%) at study entry, and all patients received regular medical and antidiabetic care. As a result, both the placebo and ranolazine treatment groups had improvements in glycemic control. There was no stabilization period possible in the MERLIN trial, as the enrollment eligibility was based on having an ACS event followed by immediate stratified randomization and initiation of treatment. Furthermore, the study design limits the direct comparison of the antidiabetic effects of ranolazine with other drugs evaluated in conventional antidiabetic drug trials.
These results indicate that the previously reported lowering of A1C by ranolazine is positively correlated with A1C levels at randomization and is associated with a reduction in FPG in patients with hyperglycemia (13,16). These findings address the previous concern of a potential lack of correlation between A1C and glucose changes with ranolazine (13,16).
We have focused exclusively on the patients who had glycemic data available at both randomization and 4 months postrandomization for the following reasons. The average duration of treatment in the trial was 8 months (16); however, 4 months is sufficient time to evaluate changes in A1C, and the number of patients with a history of diabetes and glycemic data was greater at 4 (n = 1,477) than at 8 (n = 1,133) and 16 (n = 234) months. Moreover, because this was an intent-to-treat trial, the 4-month time minimizes the impact of the patients in the ranolazine group who were no longer taking ranolazine. The discontinuation rate for the placebo and ranolazine groups during the entire study period was 22 and 28%, respectively (15). While nearly all patients had glucose measurements at 0 and 4 months, fasting was not strictly enforced, and only ∼50% of individuals with A1C data had true FPG measurements based on case report forms. We have not attempted to divide the treatment populations by sex, ethnicity, individual antidiabetic drug treatment, cardiovascular drug treatment, or study site, since the number of patients with glycemic data would have been limiting.
In conclusion, ranolazine in addition to its anti-anginal and anti-ischemic action has clinically meaningful effects on glucose and A1C in coronary artery disease patients receiving standard of care diabetes treatment. The magnitude of the effect on glycemic control is increased in patients with elevated FPG or A1C. In patients with normal glucose, ranolazine does not lower FPG compared to placebo. Although there are insufficient data to conclusively state that ranolazine does not cause hypoglycemia, there is no evidence that patients treated with ranolazine were more likely than those in the placebo group to develop hypoglycemia. The mechanism of action of ranolazine to lower FPG and A1C is currently being investigated, however preliminary data from studies using rat and human pancreatic islets suggests that ranolazine may promote glucose-stimulated insulin secretion (18).
Supplementary Material
Acknowledgments
J.W.C., A.K.D., E.K.P., and L.B. are employees and shareholders of Gilead Sciences Inc., and D.M. and E.B. have received grant support from CV Therapeutics Inc. (Gilead Sciences Inc.).
No other potential conflicts of interest relevant to this article were reported.
We thank Drs. C. Wang, C. Barker, A. DeVault, and W. Wang of the Gilead Sciences Department of Biostatistics for carefully reviewing and validating the statistical analysis in this manuscript.
Footnotes
Clinical trial reg. no. NCT00099788, clinicaltrials.gov.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1. Selvin E, Coresh J, Golden SH, Boland LL, Brancati FL, Steffes MW: Atherosclerosis Risk in Communities Study. Glycemic control, atherosclerosis, and risk factors for cardiovascular disease in individuals with diabetes: the Atherosclerosis Risk in Communities Study. Diabetes Care 2005;28:1965–1973 [DOI] [PubMed] [Google Scholar]
- 2. Selvin E, Marinopoulos S, Berkenblit G, Rami T, Brancati FL, Powe NR, Golden SH: Meta-analysis: glycosylated hemoglobin and cardiovascular disease in diabetes mellitus. Ann Intern Med 2004;141:421–431 [DOI] [PubMed] [Google Scholar]
- 3. National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 2002;106:3143–3421 [PubMed] [Google Scholar]
- 4. Rydén L, Standl E, Bartnik M, Van den Berghe G, Betteridge J, de Boer MJ, Cosentino F, Jönsson B, Laakso M, Malmberg K, Priori S, Ostergren J, Tuomilehto J, Thrainsdottir I, Vanhorebeek I, Stramba-Badiale M, Lindgren P, Qiao Q, Priori SG, Blanc JJ, Budaj A, Camm J, Dean V, Deckers J, Dickstein K, Lekakis J, McGregor K, Metra M, Morais J, Osterspey A, Tamargo J, Zamorano JL, Deckers JW, Bertrand M, Charbonnel B, Erdmann E, Ferrannini E, Flyvbjerg A, Gohlke H, Juanatey JR, Graham I, Monteiro PF, Parhofer K, Pyörälä K, Raz I, Schernthaner G, Volpe M, Wood D: Task Force on Diabetes and Cardiovascular Diseases of the European Society of Cardiology (ESC), European Association for the Study of Diabetes (EASD). Guidelines on diabetes, pre-diabetes, and cardiovascular diseases: executive summary. The Task Force on Diabetes and Cardiovascular Diseases of the European Society of Cardiology (ESC) and of the European Association for the Study of Diabetes (EASD). Eur Heart J 2007;28:88–136 [DOI] [PubMed] [Google Scholar]
- 5. Hashimoto K, Ikewaki K, Yagi H, Nagasawa H, Imamoto S, Shibata T, Mochizuki S: Glucose intolerance is common in Japanese patients with acute coronary syndrome who were not previously diagnosed with diabetes. Diabetes Care 2005;28:1182–1186 [DOI] [PubMed] [Google Scholar]
- 6. Norhammar A, Tenerz A, Nilsson G, Hamsten A, Efendíc S, Rydén L, Malmberg K: Glucose metabolism in patients with acute myocardial infarction and no previous diagnosis of diabetes mellitus: a prospective study. Lancet 2002;359:2140–2144 [DOI] [PubMed] [Google Scholar]
- 7. Mozaffarian D, Marfisi R, Levantesi G, Silletta MG, Tavazzi L, Tognoni G, Valagussa F, Marchioli R: Incidence of new-onset diabetes and impaired fasting glucose in patients with recent myocardial infarction and the effect of clinical and lifestyle risk factors. Lancet 2007;370:667–675 [DOI] [PubMed] [Google Scholar]
- 8. Fisman EZ, Motro M, Tenenbaum A: Non-insulin antidiabetic therapy in cardiac patients: current problems and future prospects. Adv Cardiol 2008;45:154–170 [DOI] [PubMed] [Google Scholar]
- 9. Hale SL, Shryock JC, Belardinelli L, Sweeney M, Kloner RA: Late sodium current inhibition as a new cardioprotective approach. J Mol Cell Cardiol 2008;44:954–967 [DOI] [PubMed] [Google Scholar]
- 10. Belardinelli L, Shryock JC, Fraser H: Inhibition of the late sodium current as a potential cardioprotective principle: effects of the late sodium current inhibitor ranolazine. Heart 2006;92(Suppl. 4):iv6–iv14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zaza A, Belardinelli L, Shryock JC: Pathophysiology and pharmacology of the cardiac “late sodium current.” Pharmacol Ther 2008;119:326–339 [DOI] [PubMed] [Google Scholar]
- 12. Chaitman BR, Skettino SL, Parker JO, Hanley P, Meluzin J, Kuch J, Pepine CJ, Wang W, Nelson JJ, Hebert DA, Wolff AA: MARISA Investigators. Anti-ischemic effects and long-term survival during ranolazine monotherapy in patients with chronic severe angina. J Am Coll Cardiol 2004;43:1375–1382 [DOI] [PubMed] [Google Scholar]
- 13. Chaitman BR, Pepine CJ, Parker JO, Skopal J, Chumakova G, Kuch J, Wang W, Skettino SL, Wolff AA: Combination Assessment of Ranolazine In Stable Angina (CARISA) Investigators. Effects of ranolazine with atenolol, amlodipine, or diltiazem on exercise tolerance and angina frequency in patients with severe chronic angina: a randomized controlled trial. JAMA 2004;291:309–316 [DOI] [PubMed] [Google Scholar]
- 14. Stone PH, Gratsiansky NA, Blokhin A, Huang IZ, Meng L: ERICA Investigators. Antianginal efficacy of ranolazine when added to treatment with amlodipine: the ERICA (Efficacy of Ranolazine in Chronic Angina) trial. J Am Coll Cardiol 2006;48:566–575 [DOI] [PubMed] [Google Scholar]
- 15. Morrow DA, Scirica BM, Karwatowska-Prokopczuk E, Murphy SA, Budaj A, Varshavsky S, Wolff AA, Skene A, McCabe CH, Braunwald E: MERLIN-TIMI 36 Trial Investigators. Effects of ranolazine on recurrent cardiovascular events in patients with non-ST-elevation acute coronary syndromes: the MERLIN-TIMI 36 randomized trial. JAMA 2007;297:1775–1783 [DOI] [PubMed] [Google Scholar]
- 16. Morrow DA, Scirica BM, Chaitman BR, McGuire DK, Murphy SA, Karwatowska-Prokopczuk E, McCabe CH, Braunwald E: MERLIN-TIMI 36 Investigators. Evaluation of the glycometabolic effects of ranolazine in patients with and without diabetes mellitus in the MERLIN-TIMI 36 randomized controlled trial. Circulation 2009;119:2032–2039 [DOI] [PubMed] [Google Scholar]
- 17. Timmis AD, Chaitman BR, Crager M: Effects of ranolazine on exercise tolerance and HbA1c in patients with chronic angina and diabetes. Eur Heart J 2006;27:42–48 [DOI] [PubMed] [Google Scholar]
- 18. Dhalla AK, Liu D, Santikul M, Belardinelli L: Ranolazine increases glucose stimulated insulin secretion in rats. J Am Coll Cardiol 2008;51:A321 [Google Scholar]
- 19. Bloomgarden ZT, Dodis R, Viscoli CM, Holmboe ES, Inzucchi SE: Lower baseline glycemia reduces apparent oral agent glucose-lowering efficacy: a meta-regression analysis. Diabetes Care 2006;29:2137–2139 [DOI] [PubMed] [Google Scholar]
- 20. Morrow DA, Scirica BM, Karwatowska-Prokopczuk E, Skene A, McCabe CH, Braunwald E: Evaluation of a novel anti-ischemic agent in acute coronary syndromes: design and rationale for the Metabolic Efficiency with Ranolazine for Less Ischemia in Non-ST-elevation acute coronary syndromes (MERLIN)-TIMI 36 trial. Am Heart J 2006;151:1186–1189 [DOI] [PubMed] [Google Scholar]
- 21. Scirica BM, Morrow DA: Ranolazine in patients with angina and coronary artery disease. Curr Cardiol Rep 2007;9:272–278 [DOI] [PubMed] [Google Scholar]
- 22. Nathan DM, Buse JB, Davidson MB, Ferrannini E, Holman RR, Sherwin R, Zinman B: American Diabetes Association, European Association for Study of Diabetes. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2009;32:193–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.