Abstract
OBJECTIVE
To assess the effects of exenatide on body weight and glucose tolerance in nondiabetic obese subjects with normal or impaired glucose tolerance (IGT) or impaired fasting glucose (IFG).
RESEARCH DESIGN AND METHODS
Obese subjects (n = 152; age 46 ± 12 years, female 82%, weight 108.6 ± 23.0 kg, BMI 39.6 ± 7.0 kg/m2, IGT or IFG 25%) were randomized to receive exenatide (n = 73) or placebo (n = 79), along with lifestyle intervention, for 24 weeks.
RESULTS
Exenatide-treated subjects lost 5.1 ± 0.5 kg from baseline versus 1.6 ± 0.5 kg with placebo (exenatide − placebo, P < 0.001). Placebo-subtracted difference in percent weight reduction was −3.3 ± 0.5% (P < 0.001). Both groups reduced their daily calorie intake (exenatide, −449 cal; placebo, −387 cal). IGT or IFG normalized at end point in 77 and 56% of exenatide and placebo subjects, respectively.
CONCLUSIONS
Exenatide plus lifestyle modification decreased caloric intake and resulted in weight loss in nondiabetic obesity with improved glucose tolerance in subjects with IGT and IFG.
Several well-designed trials have demonstrated that weight reduction can reduce diabetes risk (1–4). However, with only lifestyle modification, even modest weight loss is difficult to achieve over time (5,6); therefore, optimal pharmacologic strategies for treating obesity are being developed. This study explored exenatide in combination with lifestyle modification as treatment for weight loss in nondiabetic obese subjects with normal glucose tolerance (NGT), impaired glucose tolerance (IGT), or impaired fasting glucose (IFG).
RESEARCH DESIGN AND METHODS
Obese adult subjects with a BMI ≥30 kg/m2 were included. Subjects with type 2 diabetes, previous use of glucose-lowering medications for >3 months, or unstable body weight before screening were excluded. At screening, subjects received an oral glucose tolerance test to stratify into subgroups: NGT, IGT (fasting glucose <7 mmol/l and 2-h postprandial glucose ≥7.8 and <11.1 mmol/l), or IFG (fasting glucose 6.1–6.9 mmol/l and 2-h postprandial glucose <7.8 mmol/l). Subjects then participated in a 1-week single-blind placebo lead-in period before randomization to exenatide (10 μg with a 4-week 5-μg dose-initiation period) or placebo administered before morning and evening meals. A structured program of diet and physical activity was implemented through week 24. Subjects fasted overnight, and study drug was withheld before oral glucose tolerance test assessments. A follow-up visit was conducted 4 weeks after study completion.
The primary end point was the effect of exenatide on body weight. Secondary end points included assessment of change in glucose and lipid concentrations, A1C, blood pressure, physical activity, and food intake. Primary analyses were based on the intent-to-treat sample and a mixed-model repeated-measures ANCOVA. Least squares means were produced to estimate the magnitudes of treatment effects.
RESULTS
Of 322 participants screened, 163 were randomized, 152 received at least one dose of study drug (intent-to-treat sample), 102 completed treatment through 24 weeks, and 96 completed a follow-up visit after being off the study drug for ∼4 weeks. Average BMI was 39.6 ± 7.0 kg/m2, and 38 subjects had IGT or IFG at baseline. The withdrawal rate was 34% for exenatide and 32% for placebo; baseline characteristics were comparable to completers. Adverse events accounted for nine withdrawals (five related to nausea) with exenatide and three with placebo.
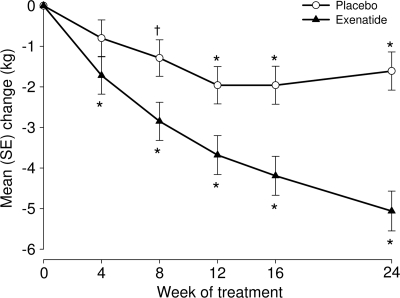
Mean baseline body weight was 109.5 ± 2.7 and 107.6 ± 2.6 kg with exenatide and placebo, respectively. Exenatide-treated subjects lost 5.1 ± 0.5 kg from baseline versus 1.6 ± 0.5 kg with placebo (Figure 1: exenatide − placebo, P < 0.001). A placebo-subtracted difference in percentage weight reduction was first observed at week 4 (−0.9 ± 0.4%, P = 0.03) and remained at week 24 (−3.3 ± 0.5%, P < 0.001). Subjects who returned after being off the study drug for ∼4 weeks sustained weight loss with increases of only 0.5 kg in both groups. A greater percentage of exenatide-treated subjects experienced ≥5% body weight reduction at 24 weeks compared with placebo (32 vs. 17%, respectively; P = 0.039). Exenatide-treated subjects who did (n = 18) or did not (n = 55) experience nausea had mean body weight reductions at 24 weeks (nausea: −3.8 ± 1.2 kg; no nausea: −4.1 ± 0.8 kg).
Figure 1.
Changes in body weight over 24 weeks in nondiabetic obese subjects treated with lifestyle intervention and randomized to exenatide or placebo. ○, Placebo (n = 78); ▲, exenatide (n = 73). Results derived from mixed-model repeated-measures analysis and presented as least squares means ± SE. Change from baseline: *P < 0.001, †P < 0.05.
Most subjects with IGT or IFG normalized glucose tolerance at end point (exenatide 77%, placebo 56%). Five participants (three exenatide, two placebo) developed type 2 diabetes during the study, three of which (two exenatide, one placebo) had IGT or IFG at baseline. Both groups significantly (P < 0.05) reduced their daily calorie intake (exenatide −449 ± 64 calories; placebo −387 ± 63 calories). Significant baseline–to–end point changes were not observed for A1C, fasting glucose, oral glucose tolerance test, and physical activity. Similar changes in lipid concentrations and blood pressure were observed in both treatment groups.
No deaths, serious adverse events, or hypoglycemia were observed during the study. Nausea was experienced by 25 and 4% and diarrhea by 14 and 3% of exenatide- and placebo-treated subjects, respectively. The majority of adverse events were mild or moderate in severity.
CONCLUSIONS
GLP-1 receptor agonists are among the few treatments for type 2 diabetes in which significant weight loss was recognized as an added value. This study of exenatide, combined with a pragmatic lifestyle intervention, was designed to evaluate the potential for weight loss in obese (mean baseline BMI 39.6 kg/m2) nondiabetic subjects in clear need of therapeutic intervention, as recommended by current guidelines (7,8). Exenatide treatment plus lifestyle modification was associated with significantly greater mean percent reduction in body weight (treatment difference, −3.3%) than lifestyle modification alone. The placebo-subtracted change in weight (−3.5 kg) was similar to the change observed with liraglutide at the 2.4-mg dose (9). It is unknown if higher doses of exenatide might achieve greater weight reductions than those observed in the present study.
Although we did not evaluate possible mechanisms to explain the substantial weight loss observed, GLP-1 receptor agonism may activate central pathways mediating satiety- and nausea-regulating mechanisms (10,11). The finding that weight loss in exenatide-treated subjects with type 2 diabetes is sustained in the absence of continued nausea (12) supports a satiety-related mechanism (10,13).
Normalization of glucose tolerance and reduction of caloric intake favored subjects treated with exenatide. The current findings warrant further studies to explore the potential role of GLP-1 receptor agonists for the treatment of obese subjects with IGT or IFG. Exenatide, in addition to lifestyle modification, has potential as a treatment for obesity in subjects at high risk for developing type 2 diabetes. Because of the high recidivism observed with weight loss interventions, sustained weight loss demonstrated in long-term studies is a key issue for future anti-obesity research.
Acknowledgments
This study was supported by Eli Lilly and Company and Amylin Pharmaceuticals. J.R., L.J.K., and S.S. are clinical trial investigators for Eli Lilly and Company and Amylin Pharmaceuticals. M.T., J.H.H., and J.N. are employees of Eli Lilly and Company. K.W. is an employee of Amylin Pharmaceuticals. Eli Lilly and Company and Amylin Pharmaceuticals are involved in the development of exenatide. J.R. has served on advisory boards and received honorarium or consulting fees from Pfizer, Roche, sanofi-aventis, Novo Nordisk, Eli Lilly, MannKind, GlaxoSmithKline, Takeda, Daiichi Sankyo, Forest, Johnson & Johnson, Novartis, Boehringer Ingelheim, and Amylin and has received research grants from Merck, Pfizer, sanofi-aventis, Novo Nordisk, Roche, Bristol-Myers Squibb, Eli Lilly, Forest, GlaxoSmithKline, Takeda, Novartis, AstraZeneca, Amylin, Johnson & Johnson, Daiichi Sankyo, MannKind, and Boehringer Ingelheim. S.S. has conducted clinical trials and/or provided consultation for Abbott, Amgen, Astra-Zeneca, Aventis, Becton-Dickson, Bristol-Myers, Dexcom, Forest Lab, Genentech, GlaxoSmithKline, Johnson & Johnson, LifeScan, Medisense, Medtronic, Merck, Novartis, Novo Nordisk, Proctor & Gamble, Pfizer, Roche, Solvay, and Takeda. L.J.K. has received research/grant support from Eli Lilly, Novo Nordisk, GlaxoSmithKline, Boehringer Ingelheim, Forest Research, and Merck. No other potential conflicts of interest relevant to this article were reported.
Parts of this study were presented in abstract form at the 3rd International Congress on Pre-diabetes and Metabolic Syndrome, Nice, France, 1–4 April 2009, and the Endocrine Society's Annual Meeting, Washington, D.C., 10–13 June 2009.
We thank the subjects, investigators, and their staff for participating in this study. We also thank Dr. Dachuang Cao for providing statistical support. Additional study investigators were as follows: L. Thurman, P. Campbell, K. Charani, W. Figueroa, C. Fogarty, A. Gupta, R. Khairi, L. Kirby, E. Ross, T. Smith, M. Cox, and K. Hoppock.
Footnotes
Clinical trial reg. no. NCT00500370, clinicaltrials.gov.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1. Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM: Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lindström J, Louheranta A, Mannelin M, Rastas M, Salminen V, Eriksson J, Uusitupa M, Tuomilehto J: Finnish Diabetes Prevention Study Group. Lifestyle intervention and 3-year results on diet and physical activity. Diabetes Care 2003;26:3230–3236 [DOI] [PubMed] [Google Scholar]
- 3. Lindström J, Ilanne-Parikka P, Peltonen M, Aunola S, Eriksson JG, Hemiö K, Hämäläinen H, Härkönen P, Keinänen-Kiukaanniemi S, Laakso M, Louheranta A, Mannelin M, Paturi M, Sundvall J, Valle TT, Uusitupa M, Tuomilehto J: Finnish Diabetes Prevention Study Group. Sustained reduction in the incidence of type 2 diabetes by lifestyle intervention: follow-up of the Finnish Diabetes Prevention Study. Lancet 2006;368:1673–1679 [DOI] [PubMed] [Google Scholar]
- 4. Pan XR, Li GW, Hu YH, Wang JX, Yang WY, An ZX, Hu ZX, Lin J, Xiao JZ, Cao HB, Liu PA, Jiang XG, Jiang YY, Wang JP, Zheng H, Zhang H, Bennett PH, Howard BV: Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. The Da Qing IGT and Diabetes Study. Diabetes Care 1997;20:537–544 [DOI] [PubMed] [Google Scholar]
- 5. Nathan DM, Buse JB, Davidson MB, Ferrannini E, Holman RR, Sherwin R, Zinman B: American Diabetes Association, European Association for Study of Diabetes. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2009;32:193–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Norris SL, Zhang X, Avenell A, Gregg E, Schmid CH, Kim C, Lau J: Efficacy of pharmacotherapy for weight loss in adults with type 2 diabetes mellitus: a meta-analysis. Arch Intern Med 2004;164:1395–1404 [DOI] [PubMed] [Google Scholar]
- 7. Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults: the evidence report. National Institutes of Health. Obes Res 1998;6:51S–209S [PubMed] [Google Scholar]
- 8. Snow V, Barry P, Fitterman N, Qaseem A, Weiss K: Clinical Efficacy Assessment Subcommittee of the American College of Physicians. Pharmacologic and surgical management of obesity in primary care: a clinical practice guideline from the American College of Physicians. Ann Intern Med 2005;142:525–531 [DOI] [PubMed] [Google Scholar]
- 9. Astrup A, Rössner S, Van Gaal L, Rissanen A, Niskanen L, Al Hakim M, Madsen J, Rasmussen MF, Lean ME: NN8022-1807 Study Group. Effects of liraglutide in the treatment of obesity: a randomised, double-blind, placebo-controlled study. Lancet 2009;374:1606–1616 [DOI] [PubMed] [Google Scholar]
- 10. Mack CM, Moore CX, Jodka CM, Bhavsar S, Wilson JK, Hoyt JA, Roan JL, Vu C, Laugero KD, Parkes DG, Young AA: Antiobesity action of peripheral exenatide (exendin-4) in rodents: effects on food intake, body weight, metabolic status and side-effect measures. Int J Obes (Lond) 2006;30:1332–1340 [DOI] [PubMed] [Google Scholar]
- 11. Nauck MA: Unraveling the science of incretin biology. Am J Med 2009;122:S3–S10 [DOI] [PubMed] [Google Scholar]
- 12. Klonoff DC, Buse JB, Nielsen LL, Guan X, Bowlus CL, Holcombe JH, Wintle ME, Maggs DG: Exenatide effects on diabetes, obesity, cardiovascular risk factors and hepatic biomarkers in patients with type 2 diabetes treated for at least 3 years. Curr Med Res Opin 2008;24:275–286 [DOI] [PubMed] [Google Scholar]
- 13. Edwards CM, Stanley SA, Davis R, Brynes AE, Frost GS, Seal LJ, Ghatei MA, Bloom SR: Exendin-4 reduces fasting and postprandial glucose and decreases energy intake in healthy volunteers. Am J Physiol Endocrinol Metab 2001;281:E155–E161 [DOI] [PubMed] [Google Scholar]