Abstract
OBJECTIVE
To determine whether glargine is noninferior to detemir regarding the percentage of patients reaching A1C <7% without symptomatic hypoglycemia ≤3.1 mmol/l.
RESEARCH DESIGN AND METHODS
In this 24-week trial, 973 insulin-naive type 2 diabetic patients on stable oral glucose-lowering drugs with A1C 7.0–10.5% were randomized to glargine once daily or detemir twice daily. Insulin doses were systematically titrated.
RESULTS
27.5 and 25.6% of patients reached the primary outcome with glargine and detemir, respectively, demonstrating the noninferiority of glargine. Improvements in A1C were −1.46 ± 1.09% for glargine and −1.54 ± 1.11% for detemir (P = 0.149), with similar proportions of patients achieving A1C <7% (P = 0.254) but more detemir-treated patients reaching A1C <6.5% (P = 0.017). Hypoglycemia risk was similar. Weight gain was higher for glargine (difference: 0.77 kg, P < 0.001). Glargine doses were lower than detemir doses: 43.5 ± 29.0 vs. 76.5 ± 50.5 units/day (P < 0.001).
CONCLUSIONS
In insulin-naive type 2 diabetic patients, glargine reached similar control as detemir, with more weight gain, but required significantly lower doses.
The “treat-to-target” clinical trials have demonstrated that the addition of systematically titrated basal insulin to existing oral therapy results in adequate glycemic control in the majority of patients with type 2 diabetes (1–3). The basal insulin analogs, insulin glargine and insulin detemir, achieve this with a reduced risk of hypoglycemia compared with the conventional NPH insulin (1,2). The aim of this study was to compare the efficacy, safety, and the effect on quality of life of once-daily glargine with twice-daily detemir in insulin-naive patients with type 2 diabetes inadequately controlled on oral glucose-lowering drugs (OGLDs), including metformin. The primary objective was to determine whether glargine was noninferior to detemir regarding the percentage of patients reaching A1C <7% without symptomatic hypoglycemia with plasma glucose (PG) ≤3.1 mmol/l.
RESEARCH DESIGN AND METHODS
The rationale for dosing detemir twice daily and the study methods we used have been detailed before (4). In brief, this multinational, open-label trial randomized insulin-naive type 2 diabetic subjects treated for ≥3 months with stable OGLDs (including metformin ≥1 g/day) and with A1C of 7.0–10.5%, to 24-week treatment with glargine in the evening or detemir at breakfast and dinner. Glargine doses were increased every 2 days by 2 units until fasting PG reached <5.6 mmol/l, while the systematic titration of detemir involved three steps to obtain both fasting and predinner PG of <5.6 mmol/l (4).
The primary outcome was the percentage of patients reaching A1C <7% without symptomatic hypoglycemia confirmed by PG ≤3.1 mmol/l. Secondary outcomes included proportions of patients achieving A1C <7% and <6.5%, hypoglycemia, weight, insulin doses, and quality of life (5–8).
Noninferiority of glargine to detemir was accepted if the lower limit of the two-sided 95% CI for the difference in the proportions of patients reaching the primary outcome was ≥30% of the percentage of detemir-treated patients achieving this outcome (4).
RESULTS
Of 1,230 patients screened, 973 were randomized, and 478 were treated with glargine and 486 with detemir. More patients on glargine than on detemir completed the study (95.4 and 89.9%, respectively, P = 0.001). The main reason for study discontinuation was an adverse event: 7 patients on glargine (1 event possibly related to study drug) and 22 on detemir (20 possibly related) dropped out of the study due to adverse events (P = 0.005) (online Fig. A, available in an online appendix at http://care.diabetesjournals.org/cgi/content/full/dc09-2294). Online Table A shows the population's baseline characteristics. Of 865 patients using insulin secretagogs at study entry, 42.4% stopped these at randomization (43.5 and 41.4% in the glargine and detemir groups, respectively).
In the glargine and detemir groups, 27.5 and 25.6% of patients, respectively, reached A1C <7% without symptomatic hypoglycemia with PG ≤3.1 mmol/l (difference: 1.85% [95% CI −3.78 to 7.48%]), demonstrating noninferiority of glargine to detemir (noninferiority margin: −7.68%).
Secondary outcomes
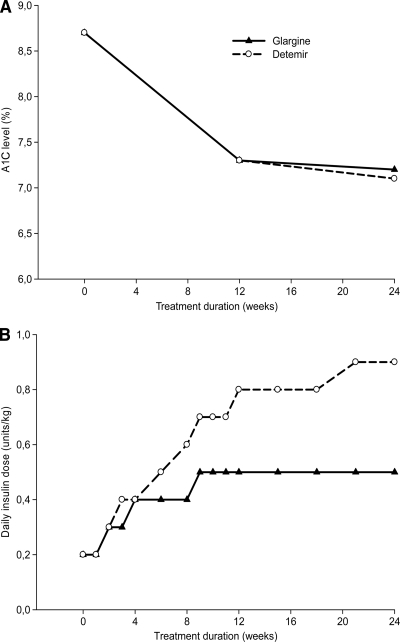
Fig. 1A illustrates that the mean improvements in A1C were similar: −1.46 ± 1.09% for glargine and −1.54 ± 1.11% for detemir (P = 0.149). The proportions of patients achieving A1C <7% were also similar (44.1 and 47.8%, respectively, P = 0.254), but significantly fewer glargine- than detemir-treated patients reached A1C <6.5% (16.5 and 22.7%, respectively, P = 0.017). The 8-point PG profiles at baseline and end-of-study show that while the decrease in fasting PG was significantly greater for glargine (P < 0.001), detemir resulted in significantly larger reductions in PG before and after lunch, before and after dinner, and at bedtime (all P < 0.001) (online Fig. B).
Figure 1.
A1C levels (A) and daily insulin doses (B) during the 24-week treatment period.
Risk of hypoglycemia was comparable between treatments with ∼30% of patients experiencing symptomatic hypoglycemia with PG ≤3.1 mmol/l in either group (online Table B). Weight gain was significantly higher with glargine versus detemir: 1.4 ± 3.2 and 0.6 ± 2.9 kg (P < 0.001). Insulin doses, however, were significantly lower for glargine: 43.5 ± 29.0 versus 76.5 ± 50.5 units/day (P < 0.001) (Fig. 1B). Quality of life improved during the study with no differences between groups, except for a discrepancy in treatment satisfaction in favor of glargine (online Table C).
CONCLUSIONS
This “treat-to-target” comparison between glargine and detemir in insulin-naive patients with type 2 diabetes demonstrated that glargine and detemir result in similar improvements in A1C and similar risk of hypoglycemia. In addition, our study confirms the higher weight gain, lower daily insulin doses, and fewer drop-outs (because of adverse events) for glargine versus detemir, found in the previous comparison of the two basal analogues in this patient group (3). Finally, our findings suggest that initiating glargine or detemir in patients not achieving adequate control on OGLDs positively affects quality of life.
Our study indicates that higher detemir doses may be needed to obtain the same level of glycemic control as with other basal insulins. This difference has been attributed to the twice-daily dosing of detemir (9), but NPH dosed twice-daily does not lead to dose escalation (1). Moreover, trial data suggest that, although insulin doses are indeed higher in patients using detemir twice- versus once-daily, once-daily detemir doses are still higher than once-daily NPH and glargine doses (3,10,11). At present there is no clear explanation for the increased dose requirements for detemir (12).
A limitation of our study was its open-label design. This design was necessary, however, as detemir was dosed twice-daily with a separate titration target before dinner. As explained elsewhere (4), we deliberately chose to dose detemir twice-daily. Trial data available at the time of the current study's design suggested that twice-daily detemir reached superior A1C compared with once-daily dosing (1,13). The difference in dosing schedule for the two insulins does, however, affect the interpretation of some of our findings. Advantages of glargine over detemir, such as the greater increase in treatment satisfaction, may be explained by its once-daily dosing and less complex titration. Also, since the design of our study, the current recommendation has become to initiate detemir once-daily (based on a more recent once-daily detemir versus NPH trial showing noninferior A1C reductions for detemir (11). Thus, with advancing knowledge, it is now clear that another “treat-to-target” trial comparing both basal analogues using an identical, once-daily dosing regimen is desirable.
In conclusion, we demonstrated that in insulin-naive patients with type 2 diabetes glargine once-daily is noninferior to detemir twice-daily regarding the percentage of patients reaching target A1C without hypoglycemia. Detemir-treated patients had less weight gain and more often achieved A1C <6.5%, but the drop-out rate and daily insulin doses were lower in the glargine group.
Supplementary Material
Acknowledgments
The study was sponsored by sanofi-aventis, Paris, France. S.G.S. is employed by the Department of Internal Medicine of the Academic Medical Center, partly through funding from Novo Nordisk and sanofi-aventis for the conduct of clinical trials. M-P.D. is employed by sanofi-aventis. R.A., A.F.P., and J.B.H. have acted as a scientific consultants and speakers for sanofi-aventis. M.D., H.C.G., F.J.S., and J.H.D. have received honoraria for consulting and speaking, as well as grants for research from Novo Nordisk and sanofi-aventis. F.H. has acted as a consultant for sanofi-aventis and received a study grant from Novo Nordisk.
No other potential conflicts of interest relevant to this article were reported.
Additional acknowledgments can be found in the online appendix at at http://care.diabetesjournals.org/cgi/content/full/dc09-2294.
Parts of this study were presented in poster form at the 45th annual meeting of the European Association for the Study of Diabetes, Vienna, Austria, 29 September to 2 October 2009, and at the 12th annual European congress of the International Society for Pharmacoeconomics and Outcomes Research, Paris, France, 24–27 October 2009.
Footnotes
Clinical trial reg. no.: NCT00405418, clinicaltrials.gov.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1. Hermansen K, Davies M, Derezinski T, Martinez Ravn G, Clauson P, Home P: A 26-week, randomized, parallel, treat-to-target trial comparing insulin detemir with NPH insulin as add-on therapy to oral glucose-lowering drugs in insulin-naive people with type 2 diabetes. Diabetes Care 2006;29:1269–1274 [DOI] [PubMed] [Google Scholar]
- 2. Riddle MC, Rosenstock J, Gerich J: The treat-to-target trial: randomized addition of glargine or human NPH insulin to oral therapy of type 2 diabetic patients. Diabetes Care 2003;26:3080–3086 [DOI] [PubMed] [Google Scholar]
- 3. Rosenstock J, Davies M, Home PD, Larsen J, Koenen C, Schernthaner G: A randomised, 52-week, treat-to-target trial comparing insulin detemir with insulin glargine when administered as add-on to glucose-lowering drugs in insulin-naive people with type 2 diabetes. Diabetologia 2008;51:408–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Swinnen SG, Snoek FJ, Dain MP, DeVries JH, Hoekstra JB, Holleman F: Rationale, design, and baseline data of the insulin glargine (Lantus) versus insulin detemir (Levemir) Treat-To-Target (L2T3) study: a multinational, randomized noninferiority trial of basal insulin initiation in type 2 diabetes. Diabetes Technol Ther 2009;11:739–743 [DOI] [PubMed] [Google Scholar]
- 5. Bonsignore M, Barkow K, Jessen F, Heun R: Validity of the five-item WHO Well-Being Index (WHO-5) in an elderly population. Eur Arch Psychiatry Clin Neurosci 2001;251(Suppl. 2):II27–II31 [DOI] [PubMed] [Google Scholar]
- 6. Bradley C: Diabetes Treatment Satisfaction Questionnaire (DTSQ). In Handbook of Psychology and Diabetes. Chur, Switzerland, Harwood Academic Publishers; 1994, p. 111–132 [Google Scholar]
- 7. Cox DJ, Irvine A, Gonder-Frederick L, Nowacek G, Butterfield J: Fear of hypoglycemia: quantification, validation, and utilization. Diabetes Care 1987;10:617–621 [DOI] [PubMed] [Google Scholar]
- 8. Grootenhuis PA, Snoek FJ, Heine RJ, Bouter LM: Development of a type 2 diabetes symptom checklist: a measure of symptom severity. Diabet Med 1994;11:253–261 [DOI] [PubMed] [Google Scholar]
- 9. Koenen C: How do detemir and glargine compare when added to oral agents in insulin-naive patients with type 2 diabetes mellitus? Nat Clin Pract Endocrinol Met 2008;4:E1 [DOI] [PubMed] [Google Scholar]
- 10. Hollander P, Cooper J, Bregnhøj J, Pedersen CB: A 52-week, multinational, open-label, parallel-group, noninferiority, treat-to-target trial comparing insulin detemir with insulin glargine in a basal-bolus regimen with mealtime insulin aspart in patients with type 2 diabetes. Clin Ther 2008;30:1976–1987 [DOI] [PubMed] [Google Scholar]
- 11. Philis-Tsimikas A, Charpentier G, Clauson P, Ravn GM, Roberts VL, Thorsteinsson B: Comparison of once-daily insulin detemir with NPH insulin added to a regimen of oral antidiabetic drugs in poorly controlled type 2 diabetes. Clin Ther 2006;28:1569–1581 [DOI] [PubMed] [Google Scholar]
- 12. Swinnen SG, DeVries JH: Higher dose requirements with insulin detemir in type 2 diabetes—three cases and a review of the literature. Diabetes Res Clin Pract 2009;84:e24–e26 [DOI] [PubMed] [Google Scholar]
- 13. European Medicines Agency. Levemir European Public Assessment Report. Available from http://www.emea.europa.eu/humandocs/Humans/EPAR/levemir/levemir.htm. Accessed 13 February 2009
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.