Abstract
OBJECTIVE
To determine the prevalence of subnormal testosterone concentrations in patients with obesity and with type 2 diabetes in a primary care clinic population.
RESEARCH DESIGN AND METHODS
Free testosterone concentrations of 1,849 men (1,451 nondiabetic and 398 diabetic) in the Hypogonadism In Males (HIM) study were analyzed. The HIM study was a U.S.-based cross-sectional study designed to define the prevalence of hypogonadism in men aged >45 years. Free testosterone was measured by equilibrium dialysis.
RESULTS
The prevalence of subnormal free testosterone concentrations in lean, overweight, and obese nondiabetic men was 26% (n = 275), 29% (n = 687), and 40% (n = 489), respectively (P < 0.001 for trend), and 44% (n = 36), 44% (n = 135), and 50% (n = 227), respectively, in diabetic men (P = 0.46 for trend within group and P < 0.05 compared with nondiabetic men). The mean free testosterone concentration of diabetic men was significantly lower than that of nondiabetic men. Free testosterone concentrations were negatively and significantly (P < 0.001) related to age (r = −0.37), BMI (r = −0.18), and sex hormone–binding globulin (r = −0.11) in multiple regression analysis. The average decline of free testosterone concentrations was 7.8 pg/ml per decade in nondiabetic men and 8.4 pg/ml per decade in diabetic men.
CONCLUSIONS
Forty percent of obese nondiabetic men and 50% of obese diabetic men aged ≥45 years have subnormal free testosterone concentrations. In view of its high prevalence, obesity is probably the condition most frequently associated with subnormal free testosterone concentrations in males. The concomitant presence of diabetes is associated with an additional increase in the prevalence of subnormal free testosterone concentrations.
We have previously shown that men with type 2 diabetes have a high prevalence of hypogonadotropic hypogonadism (1). In these patients, there was a significant inverse relationship of BMI with total and free testosterone concentrations (1,2). Our observations on the inverse relationship of BMI and testosterone concentrations in patients with type 2 diabetes have been confirmed in other studies (3,4). In another study we found that BMI and total testosterone and free testosterone concentrations were also inversely related in type 1 diabetic men who rarely had hypogonadism (5). These observations raise the question whether obesity itself is associated with low free testosterone.
Studies in the past have suggested that total testosterone concentrations are lower in obese men than in men with normal BMI (6). The Hypogonadism In Males (HIM) study, which confirmed the presence of frequent low testosterone concentrations in men with diabetes (7) also showed that there was a tendency for total testosterone concentrations to be low in patients with higher BMI. However, sex hormone–binding globulin (SHBG) concentrations are also low in obese and diabetic men. According to the Endocrine Society Guidelines, the diagnosis of hypogonadism should be dependent on the measurement of free testosterone or bioavailable testosterone in conditions that can alter SHBG concentrations (8). Free testosterone concentrations should be measured by equilibrium dialysis or calculated using algorithms based on the law of mass action and the methods of Vermeulen et al. (9). Some previous relatively small studies showed that BMI is inversely related to total testosterone, SHBG, and free testosterone but that only morbid obesity (BMI ≥40 kg/m2) was associated with subnormal free testosterone concentrations (10–12). Recent studies have examined the prevalence of hypogonadism in obesity in a much larger number of men. One of these studies is the above-mentioned HIM study. It was designed to determine the prevalence of hypogonadism (defined as low total testosterone) in men in the U.S. aged ≥45 years. This study, which included 2,165 men, showed that 38.7% of men had a subnormal total testosterone concentration and that 52% of the obese men and 50% of diabetic men had subnormal total testosterone concentrations. However, the publication did not address free testosterone concentrations in men with obesity, with or without diabetes. A recently published Dutch study in 149 obese men (mean age 43 years) showed a 36% prevalence of hypogonadotropic hypogonadism (13). Men with diabetes were included in the obese population in both the HIM and the Dutch studies; 23% of the study subjects in the HIM study and 37% in the Dutch study had diabetes. Data on nondiabetic obese men were not reported separately. Large studies on the prevalence of low free testosterone in nondiabetic obese men are thus not available, nor are any data available on the differences in the prevalence of subnormal free testosterone concentrations in nondiabetic obese men compared with those in diabetic men. Although the concentration of free testosterone falls with increasing obesity in diabetic men, 25% of type 2 diabetic men have subnormal free testosterone concentrations even when they have a normal weight (1).
Because of the rising prevalence of obesity in the U.S. and the rest of the world, it is imperative that the prevalence of low free testosterone in obese men be defined. Furthermore, the magnitude of the contribution of obesity to subnormal free testosterone associated with type 2 diabetes needs to be quantitated. To answer these issues, we analyzed free testosterone concentrations in the obese nondiabetic and diabetic men in the HIM study. The hypotheses tested were that 1) obese men have a higher prevalence of subnormal free testosterone concentrations than nonobese men and 2) that men with diabetes have a higher prevalence of subnormal free testosterone concentrations than nondiabetic men, both obese and nonobese.
RESEARCH DESIGN AND METHODS
The HIM study recruited 2,165 men in 95 centers across the U.S. Study criteria have been published previously (7). In brief, all men aged ≥45 years had a blood sample taken in the morning (between 8:00 a.m. and noon). Blood was analyzed for concentrations of total testosterone, free testosterone, bioavailable testosterone, and SHBG. Total testosterone and SHBG were determined by radioimmunoassay, free testosterone by equilibrium dialysis, and bioavailable testosterone by the ammonium sulfate precipitation method (Esoterix, Calabasas Hills, CA). The lower limit for free testosterone of the reference laboratory was 50 pg/ml (0.174 nmol/l). The lower limit for bioavailable testosterone was 90 ng/dl (0.313 nmol/l).
The HIM study had 474 men with self-reported diabetes; 1,691 men did not have diabetes. Judging by their medication lists, we excluded men who were taking antiretroviral drugs (33 men), narcotics (78 men), or testosterone replacement therapy (33 men). We also excluded men currently using steroids, narcotics, antibiotics (to eliminate men with acute illnesses), or drugs that affect the dopaminergic pathway (34 men). The prevalence of low free testosterone in all these excluded men (n = 178) was 50%. Men with missing data on BMI or hormone concentrations were also excluded from analysis (n = 138). We report on the findings from 1,849 men, 398 of whom had diabetes.
Group comparisons were performed by one-way ANOVA, t tests, Mann-Whitney rank sum tests, and χ2 tests as appropriate. Data that were not normally distributed were log-transformed to perform the parametric statistical tests. Total testosterone, free testosterone, bioavailable testosterone, SHBG, age, and BMI were not normally distributed and were log-transformed. Pearson correlation and multiple linear regression analyses between variables were done using SigmaStat software (SPSS, Chicago, IL). Using the statistical program SYSTAT-12, we ran a stepwise regression with a forward and backward stepping generalized linear model analysis to find out the equation that relates the effects of BMI and age in diabetic and nondiabetic men on free testosterone levels. Adjustment for age and BMI in group comparisons was done with ANCOVA and generalized linear model analysis. For purposes of uniformity in comparisons, data were adjusted to the mean age (60.9 years) and BMI (29.7 kg/m2) of the whole population. Data are presented as means ± SD.
RESULTS
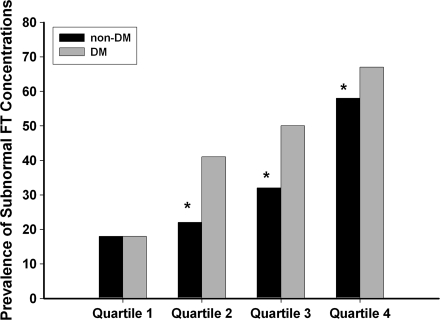
Thirty-five percent of all men had subnormal free testosterone concentrations, irrespective of diabetes status. The mean age, BMI, and hormone concentrations of diabetic and nondiabetic men are shown in Table 1. The crude prevalence of subnormal free testosterone concentrations in diabetic and nondiabetic men was 51 and 31%, respectively. Diabetic men were older and heavier than nondiabetic men. Even after adjustment for age and BMI, the prevalence of subnormal free testosterone concentrations in the diabetic men was higher than that in nondiabetic men (45% vs. 33%). As expected, there was an increase in the prevalence of subnormal free testosterone concentrations with age. Figure 1 shows the prevalence of subnormal free testosterone concentrations in study subjects divided into quartiles of age.
Table 1.
Demographic parameters and hormone concentrations of lean (BMI <25 kg/m2), overweight (BMI 25–29.9 kg/m2), and obese (BMI ≥30 kg/m2) nondiabetic and diabetic men
| All | Nondiabetic | Diabetic | Lean |
Overweight |
Obese |
||||
|---|---|---|---|---|---|---|---|---|---|
| Nondiabetic | Diabetic | Nondiabetic | Diabetic | Nondiabetic | Diabetic | ||||
| n | 1,849 | 1,451 | 398 | 275 | 36 | 687 | 135 | 489 | 227 |
| Age (years) | 60.9 ± 10.2 | 60.2 ± 0.3 | 63.6 ± 9.8† | 62.8 ± 11.5* | 70.7 ± 10.3*b | 60.9 ± 10.1* | 65.9 ± 9.6*b | 57.9 ± 9.1 | 61.0 ± 8.9b |
| BMI (kg/m2) | 29.7 ± 5.6 | 29.0 ± 5.2 | 32.3 ± 6.4† | 23.0 ± 3.2 | 23.2 ± 3.2 | 27.4 ± 3.2 | 27.7 ± 3.2 | 34.6 ± 3.2 | 36.4 ± 3.2b |
| Men with subnormal FT (%) | 35 | 33 | 45† | 26* | 44‡ | 29* | 44† | 40 | 50‡ |
| FT (pg/ml) | 58.1 ± 21.2 | 58.9 ± 19.5 | 55.2 ± 20.0† | 62.0 ± 19.4* | 58.8 ± 19.5* | 60.9 ± 19.3* | 56.9 ± 19.5*‡ | 55.5 ± 19.7 | 50.7 ± 19.4‡ |
| FT (nmol/l) | 0.202 ± 0.074 | 0.205 ± 0.068 | 0.192 ± 0.069† | 0.215 ± 0.067* | 0.204 ± 0.068* | 0.211 ± 0.067* | 0.198 ± 0.068*‡ | 0.193 ± 0.068 | 0.176 ± 0.067a |
| Total T (ng/dl) | 368.5 ± 145.3 | 373.4 ± 140.5 | 351.5 ± 144.2† | 389.5 ± 167.3* | 369.7 ± 149.4* | 388.3 ± 138.0* | 356.4 ± 141.5*† | 350.2 ± 132.7 | 330.4 ± 135.6 a |
| Total T (nmol/l) | 12.8 ± 5.0 | 13.0 ± 4.9 | 12.2 ± 5.0† | 13.5 ± 5.8* | 12.8 ± 5.2* | 13.5 ± 4.8* | 12.4 ± 4.9*† | 12.2 ± 4.6 | 11.5 ± 4.7‡ |
| BT (ng/dl) | 101.0 ± 49.2 | 101.8 ± 44.8 | 97.5 ± 46.0 | 106.7 ± 44.1* | 96.6 ± 44.5 | 106.0 ± 44.2* | 101.3 ± 44.8* | 95.6 ± 45.1 | 89.9 ± 44.4 |
| BT (nmol/l) | 3.5 ± 1.7 | 3.5 ± 1.6 | 3.4 ± 1.6 | 3.7 ± 1.5* | 3.4 ± 1.5 | 3.7 ± 1.5* | 3.5 ± 1.6* | 3.3 ± 1.6 | 3.1 ± 1.5 |
| SHBG (nmol/l) | 58.3 ± 29.9 | 58.1 ± 29.1 | 59.5 ± 32.7 | 71.4 ± 27.6* | 76.0 ± 27.8* | 58.8 ± 27.5* | 57.0 ± 27.8 | 51.8 ± 27.9 | 53.6 ± 27.5 |
Data are means ± SD. Testosterone concentrations and prevalence of subnormal free testosterone in columns for diabetic and nondiabetic men were adjusted to the mean age (60.9 years), BMI (29.7 kg/m2), and SHBG concentration (58.4 nmol/l) of the whole population. Testosterone concentrations and prevalence of subnormal free testosterone in the lean, overweight, and obese columns were adjusted only for age and SHBG. Normal ranges: total testosterone (T) (300–1,000 ng/dl), free testosterone (FT) (50–280 pg/ml), bioavailable testosterone (BT) (90–285 ng/dl), and SHBG (20–60 nmol/l). To convert into nanomoles per liter, total testosterone and bioavailable testosterone were divided by 28.8 and free testosterone by 288.
*P < 0.05 vs. obese men in the same group (diabetic or nondiabetic).
†P < 0.05 vs. nondiabetic men.
‡P < 0.001 vs. nondiabetic men.
Figure 1.
The prevalence of subnormal free testosterone (FT) concentrations in diabetic (DM,  ) and nondiabetic (non-DM, ■) men separated into quartiles of age. Quartile 1 (aged 45–52 years) had 408 nondiabetic and 55 diabetic men. Quartile 2 (aged 53–59 years) had 378 nondiabetic and 82 diabetic men. Quartile 3 (aged 60–68 years) had 326 nondiabetic and 136 diabetic men. Quartile 4 (aged 69–91 years) had 339 nondiabetic and 125 diabetic men. The prevalence of subnormal free testosterone concentrations was calculated in each quartile for nondiabetic and diabetic men. The prevalence was then adjusted to the mean BMI (29.7 kg/m2) of the whole study population. A χ2 test was used to compare the prevalence among groups. A similar percentage of nondiabetic and diabetic men in quartile 1 had subnormal free testosterone (18 vs. 18%, P = 0.97). Nondiabetic men had a lower prevalence of subnormal free testosterone than diabetic men in the other three quartiles (quartile 2, 22 vs. 41%, P < 0.01; quartile 3, 32 vs. 50%, P < 0.01; and quartile 4, 58 vs. 67%, P = 0.05).
) and nondiabetic (non-DM, ■) men separated into quartiles of age. Quartile 1 (aged 45–52 years) had 408 nondiabetic and 55 diabetic men. Quartile 2 (aged 53–59 years) had 378 nondiabetic and 82 diabetic men. Quartile 3 (aged 60–68 years) had 326 nondiabetic and 136 diabetic men. Quartile 4 (aged 69–91 years) had 339 nondiabetic and 125 diabetic men. The prevalence of subnormal free testosterone concentrations was calculated in each quartile for nondiabetic and diabetic men. The prevalence was then adjusted to the mean BMI (29.7 kg/m2) of the whole study population. A χ2 test was used to compare the prevalence among groups. A similar percentage of nondiabetic and diabetic men in quartile 1 had subnormal free testosterone (18 vs. 18%, P = 0.97). Nondiabetic men had a lower prevalence of subnormal free testosterone than diabetic men in the other three quartiles (quartile 2, 22 vs. 41%, P < 0.01; quartile 3, 32 vs. 50%, P < 0.01; and quartile 4, 58 vs. 67%, P = 0.05).
Prevalence of subnormal free testosterone in lean, overweight, and obese men
We analyzed the hormonal concentrations of study subjects after stratifying them in lean (BMI <25 kg/m2), overweight (BMI 25–29.9 kg/m2), and obese (BMI ≥30 kg/m2) categories (Table 1). The data were adjusted for age and SHBG differences. There was a significant decline in free testosterone concentrations with BMI in both nondiabetic and diabetic men. Free testosterone concentrations in lean, overweight, and obese nondiabetic men were 62.0 ± 19.4 pg/ml (0.215 ± 0.067 nmol/l), 60.9 ± 19.3 pg/ml (0.211 ± 0.067 nmol/l), and 55.5 ± 19.7 pg/ml (0.193 ± 0.068 nmol/l), respectively (P < 0.001 by ANOVA). Free testosterone concentrations in lean, overweight, and obese diabetic men were 58.8 ± 19.5 pg/ml (0.204 ± 0.068 nmol/l), 56.9 ± 19.5 pg/ml (0.198 ± 0.068 nmol/l), and 50.7 ± 19.4 pg/ml (0.176 ± 0.068 nmol/l), respectively (P = 0.002 by ANOVA).
The prevalence of subnormal free testosterone concentrations was 26% in lean, 29% in overweight, and 40% in obese nondiabetic men, whereas it was 44, 44, and 50% in lean, overweight, and obese diabetic men, respectively. Diabetic men had a significantly higher prevalence of subnormal free testosterone concentrations in all BMI categories (Table 1). Similar results were obtained when hormonal concentrations were log-transformed and compared (supplementary Table 2, available in an online appendix at http://care.diabetesjournals.org/cgi/content/full/dc09-1649/DC1).
Fifty-one nondiabetic and 57 diabetic men were morbidly obese (BMI ≥40 kg/m2). The mean free testosterone concentrations in morbidly obese nondiabetic and diabetic men were 51.1 ± 19.4 pg/ml (0.177 ± 0.067 nmol/l) and 45.1 ± 19.4 pg/ml (0.157 ± 0.067 nmol/l), respectively (P = 0.15 by t test). The prevalence of subnormal free testosterone in morbidly obese nondiabetic and diabetic men was 49 and 55% (P = 0.09 by χ2 test), respectively.
Relation of free testosterone with age and BMI
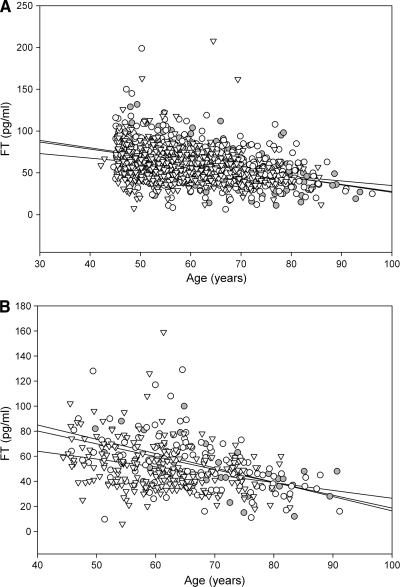
Free testosterone concentrations were significantly (P < 0.001) and negatively related to age (r = −0.38) and BMI (r = −0.10). The final equation that described the decline in free testosterone (FT) concentrations with an increase in age and BMI in nondiabetic men was
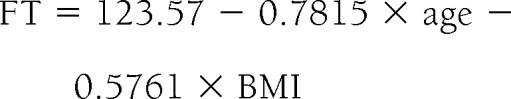 |
where free testosterone is in picograms per milliliter, age is in years, and BMI is weight in kilograms divided by the square of height in meters.
In diabetic men, the equation was
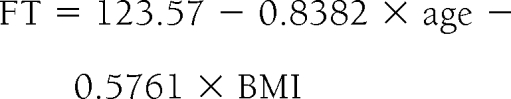 |
Thus, the average reduction in free testosterone concentration with age in nondiabetic and diabetic men was 7.8 and 8.4 pg/ml per decade, respectively. The average reduction in free testosterone concentration if the BMI of the subject increases by 1 kg/m2 was 5.76 pg/ml in both diabetic and nondiabetic men.
Free testosterone concentrations were negatively related to SHBG (r = −0.18, P < 0.001). Age was negatively related to BMI (r = −0.16, P < 0.001) and positively to SHBG (r = 0.34, P < 0.001). BMI was negatively related to SHBG (r = 0.28, P < 0.001). We performed multiple linear regression analysis in a model with free testosterone as the dependent variable and age, BMI, and SHBG as the independent variables. Age, BMI, and SHBG were all independent predictors of serum free testosterone (R2 of the model was 0.18; standardized [β] coefficients for age, BMI, and SHBG were −0.37, −0.18, and −0.11).
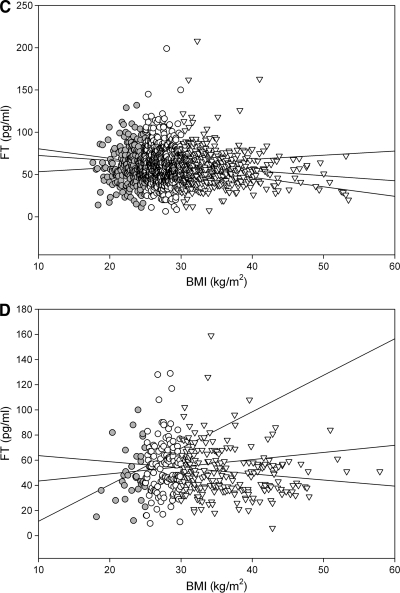
We also analyzed the effect of age and BMI separately in lean, overweight, and obese diabetic and nondiabetic men. The results are presented in Fig. 2. These data show that the relationship of free testosterone with age was stronger in the nonobese men. On the contrary, the relationship of free testosterone with BMI was strongest in obese men.
Figure 2.
A: Inverse relationship of age with free testosterone concentration in nondiabetic (non-DM, ■) lean (r = −0.53, P < 0.001), overweight (r = −0.43, P < 0.001), and obese (r = −0.28, P < 0.001) men. Thus, although age could explain ∼25% of variability (R2) in free testosterone concentrations in nonobese individuals, it accounted for a significantly lower variability (8%) in free testosterone in obese nondiabetic men (P = 0.008). DM,  , diabetic men. B: Inverse relationship of age with free testosterone in lean (
, diabetic men. B: Inverse relationship of age with free testosterone in lean ( , r = −0.57, P < 0.001), overweight (○, r = −0.49, P < 0.001), and obese (▽, r = −0.30, P < 0.001) diabetic men. Thus, although age could explain 25–30% of variability (R2) in free testosterone concentrations in nonobese individuals, it accounted for a significantly lower variability (9%) in free testosterone in obese diabetic men (P = 0.05 compared with nonobese men). C: Relationship of free testosterone with BMI in lean (
, r = −0.57, P < 0.001), overweight (○, r = −0.49, P < 0.001), and obese (▽, r = −0.30, P < 0.001) diabetic men. Thus, although age could explain 25–30% of variability (R2) in free testosterone concentrations in nonobese individuals, it accounted for a significantly lower variability (9%) in free testosterone in obese diabetic men (P = 0.05 compared with nonobese men). C: Relationship of free testosterone with BMI in lean ( , r = 0.01, P = 0.9), overweight (○, r = −0.08, P = 0.04), and obese (▽, r = −0.10, P = 0.03) non-diabetic men. D: Relationship of free testosterone with BMI in lean (
, r = 0.01, P = 0.9), overweight (○, r = −0.08, P = 0.04), and obese (▽, r = −0.10, P = 0.03) non-diabetic men. D: Relationship of free testosterone with BMI in lean ( , r = 0.13, P = 0.5), overweight (○, r = 0.03, P = 0.8), and obese (▽, r = −0.17, P < 0.01) diabetic men.
, r = 0.13, P = 0.5), overweight (○, r = 0.03, P = 0.8), and obese (▽, r = −0.17, P < 0.01) diabetic men.
Prevalence of subnormal free testosterone in diabetic men taking oral hypoglycemics and insulin
Of 398 men with diabetes, 67 were diet controlled with diet, 201 were taking metformin, 204 were taking sulfonylureas, 120 were taking thiazolidinediones, and 60 were taking insulin (insulin monotherapy in 17 and insulin in combination with oral hypoglycemic drugs in 43). The prevalence of subnormal free testosterone was similar (P = 0.40 by χ2) in men treated with diet (45%), metformin (47%), sulfonylureas (54%), thiazolidinediones (48%), and insulin (57%), whether alone or in combination with oral agents.
Bioavailable testosterone
Log-transformed bioavailable testosterone concentrations were lower in diabetic men than in nondiabetic men (supplementary Table 2). Serum bioavailable testosterone concentrations were independently predicted by age, BMI, and SHBG (R2 of the model was 0.31; β coefficients for age, BMI, and SHBG were −0.39, −0.15, and −0.32). The age-, BMI-, and SHBG-adjusted prevalence of subnormal bioavailable testosterone concentrations in diabetic and nondiabetic men was 51 and 43%, respectively (P = 0.004). The prevalence of subnormal bioavailable testosterone concentrations in obese nondiabetic men (52%) was higher than that in lean (37%, P < 0.001) and overweight (38%, P < 0.001) nondiabetic men. The prevalence of subnormal bioavailable testosterone concentrations in obese diabetic men (58%) was higher than that in overweight (49%, P = 0.05) but not lean (51%, P = 0.42) diabetic men.
Prevalence of subnormal total testosterone concentrations
The prevalence of subnormal total testosterone concentrations (<300 ng/dl or 10.4 nmol/l) was 33% in nondiabetic men and 44% in diabetic men (P < 0.001 by χ2 test) after adjustment for age, BMI, and SHBG concentrations. Thiry percent of lean, 29% of overweight, and 39% of obese nondiabetic men had subnormal total testosterone concentrations (P < 0.001 by χ2 test). Thirty-three percent of lean, 44% of overweight, and 46% of obese diabetic men had subnormal total testosterone concentrations (P = 0.56 by χ2 test). Serum total testosterone concentrations were independently predicted by age, BMI, and SHBG (R2 of the model was 0.45; β coefficients for age, BMI, and SHBG were −0.30, −0.16, and 0.65). The age, BMI, and hormonal concentrations of men with subnormal total testosterone, free testosterone, or both are compared in supplementary Table 3 (available in an online appendix).
CONCLUSIONS
In this study, the largest analysis undertaken to define the prevalence of subnormal free testosterone concentrations in obese men, the data show clearly that 40% of all obese nondiabetic men and 49% of morbidly obese nondiabetic men had subnormal free testosterone concentrations. Furthermore, there was an inverse relationship between free testosterone concentrations and BMI. According to the National Health and Nutrition Examination Survey (NHANES) 2003–2004 data, 31% of all adult men in U.S. are obese and 2.8% are morbidly obese (14). Thus, in view of the fact that almost one-third of the U.S. population is obese, these observations have profound pathophysiological, clinical, epidemiological, and public health implications. This study is also the first to comprehensively assess the comparative prevalence of subnormal free testosterone concentrations with obesity and diabetes separately and together when they coexist.
Our study shows that diabetic men with and without obesity have lower free testosterone concentrations than nondiabetic men after adjustment for age or BMI. The effect of having diabetes on the free testosterone concentration in a 60-year-old man was similar to that of an increase in BMI of 6 kg/m2 (equal to weight gain of 25 kg in a 180-cm [6-feet] tall man) in a man without diabetes. The elevated insulin resistance of type 2 diabetic men compared with that of obese men may explain these findings. Cross-sectional analysis of the NHANES III data (101 diabetic and 1,312 nondiabetic men) has shown that low androgens are associated with the presence of diabetes in men (15). The prevalence of low free testosterone concentrations was not mentioned in that analysis. The mean calculated free testosterone concentrations were similar in diabetic and nondiabetic men. We found a small (6%) but statistically significant difference in the age- and BMI-adjusted free testosterone concentrations of diabetic and nondiabetic men. The larger number of diabetic subjects in our study might explain the difference in our data and that from NHANES III. The mean age and BMI of NHANES III subjects (57 years and 29.5 kg/m2) were lower than those of our diabetic study subjects. Consistent with the NHANES III study, we did not find a difference in SHBG concentrations of diabetic and nondiabetic men.
The prevalence of low free testosterone in diabetic men was higher in all BMI categories compared with that in nondiabetic men. Whereas nondiabetic men showed an increase in prevalence of subnormal free testosterone across BMI categories, there was no significant change in the prevalence of low free testosterone with increasing BMI in the diabetic men. This is attributable to the high prevalence of low free testosterone in lean diabetic men (45% after adjustment for age). Nevertheless, we found that free testosterone concentrations decreased significantly with increasing BMI in both diabetic and nondiabetic men. In the morbidly obese subjects, we found that there was a nonsignificant trend toward lower free testosterone concentrations and higher prevalence of subnormal free testosterone in diabetic men compared with that in nondiabetic men. This could be due to smaller numbers of morbidly obese men in the study (51 diabetic and 57 nondiabetic).
Free testosterone concentrations were negatively related to age in our study. We found that obese men had a smaller age-related decline of free testosterone concentrations compared with that in nonobese men. This result suggests an independent effect of BMI-related factors on free testosterone concentrations. Although our study cannot answer questions about the causes of low free testosterone in the obese and type 2 diabetic men, several prior studies have addressed this question (10,12,16).
It has been suggested that the increase in adipose tissue mass in obesity may result in increased aromatase activity and thus lead to a greater conversion of testosterone into estradiol (12). An increase in estradiol concentrations would lead to the suppression of hypothalamic gonadotropin-releasing hormone and pituitary gonadotropin secretion. This would result in the reduction of both testosterone secretion by Leydig cells and spermatogenesis in the seminiferous tubules. Young overweight and obese men are indeed known to have a decrease in sperm count (17). However, there hitherto has been no study demonstrating that estradiol concentrations are actually elevated in obese or diabetic patients with subnormal testosterone concentrations. If indeed, this result is confirmed, aromatase inhibition could be a therapeutic strategy in future. Unfortunately, estradiol concentrations are not available in our study.
The other possible mechanism involved in the pathogenesis of obesity-related low free testosterone is insulin resistance. The selective deletion of the insulin receptor gene from neurons results in a syndrome of hypogonadotrophic hypogonadism in mice in addition to a state of systemic insulin resistance (16). It is therefore possible that insulin resistance at the hypothalamic level contributes to the pathogenesis of this syndrome. The concurrent presence of marked inflammation may contribute to insulin resistance because inflammatory mediators such as tumor necrosis factor-α and interleukin-6 may interfere with insulin signal transduction (18). Clearly, further investigation is necessary to define the etiology of this syndrome.
In view of the increasing prevalence of obesity even in younger populations, it would be important to conduct a similar study in young individuals at the prime of their reproductive years. It is relevant that the prevalence of hypogonadotropic hypogonadism is greater than 50% in patients with type 2 diabetes aged between 18 and 35 years (19).
We also found that SHBG concentrations are negatively related to BMI and positively to age. SHBG concentrations decrease with insulin resistance, and low SHBG concentrations are predictive of future development of type 2 diabetes (20). Although this finding has been well established in previous studies, the pathophysiological mechanisms behind these associations are not known and need to be explored in future studies.
One of the limitations of our study is that we could not differentiate between type 1 and type 2 diabetes in our study subjects. The presence of diabetes was recorded by a physician. However, because >90% of diabetic individuals have type 2 diabetes and this number is even higher in those aged >45 years, this issue is not likely to affect the overall conclusions of this study. We have previously shown that the prevalence of hypogonadism in type 1 diabetes is markedly lower than that in type 2 diabetes (5). Only 17 patients were receiving insulin monotherapy, and thus they may have type 1 diabetes. Excluding these men did not change the results of the study.
It is well known that there is a significant day-to-day variability in hormone concentrations, especially testosterone. As for most epidemiological or cross-sectional studies, the testosterone concentrations in the HIM study were measured only once. In view of the variability in testosterone concentrations, this is a limitation. However, it is not likely that the prevalence of low testosterone concentrations would have been altered after repeated measurements because the probability of testosterone concentrations rising or falling with repeated measurements is statistically equal. The issue of repeated measurements is important in the context of diagnosing hypogonadism clinically in the context of a single patient. The fact that our study included a moderately large number of participants also helps to diminish the effect of hormonal variability on study effects and the relationship with BMI.
We did not have a validated questionnaire for erectile dysfunction and symptoms of hypogonadism. Therefore, we cannot comment on the frequency of symptomatic hypogonadism in our study. It has been shown in the past that a high percentage of diabetic men with low testosterone concentrations have symptomatic hypogonadism (3,21). Lower testosterone concentrations are inversely related to visceral adiposity (3,22). However, waist circumference or body composition imaging was not available in our study. Another limitation of our study is that the subjects were not required to be fasting when providing blood samples. It has recently been shown that an oral glucose load of 75 g can acutely lower total testosterone concentrations by 25% (23). However, in a prior analysis of these data, no difference was found in total testosterone concentrations from blood samples drawn between 8 a.m. and 10 a.m. vs. 10 a.m. and 12:00 p.m. (7).
In conclusion, 40% of obese nondiabetic men, aged ≥45 years have subnormal free testosterone concentrations; 26% of normal-weight nondiabetic men and 44% of normal-weight diabetic men had subnormal free testosterone concentrations. The combination of obesity and diabetes increases the prevalence of subnormal free testosterone concentrations to 50%. Thus, both obesity and diabetes appear to exert independent effects on the prevalence of low free testosterone concentrations in addition to age. In view of the high rates of prevalence of subnormal free testosterone in patients with obesity or diabetes, concentrations of free testosterone should be measured in these populations especially when these conditions occur concomitantly.
Supplementary Material
Acknowledgments
The data presented in the manuscript are from the HIM study, which was conducted by Solvay Pharmaceuticals. P.D. is supported by grants from the National Institutes of Health (R01-DK-069805-01A1 and R01-DK-075877-01A2) and the American Diabetes Association (708CR13). He is also supported by grants from sanofi-aventis, Merck, Solvay Pharmaceuticals, GlaxoSmithKline, and Amylin.
No potential conflicts of interest relevant to this article were reported.
We are thankful to Dr. Alan Forrest, PharmD, and Dr. Qusai Al-share, PhD, State University of New York at Buffalo (Buffalo, NY) for their valuable help in statistical analysis.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1. Dhindsa S, Prabhakar S, Sethi M, Bandyopadhyay A, Chaudhuri A, Dandona P: Frequent occurrence of hypogonadotropic hypogonadism in type 2 diabetes. J Clin Endocrinol Metab 2004;89:5462–5468 [DOI] [PubMed] [Google Scholar]
- 2. Dhindsa S, Bhatia V, Dhindsa G, Chaudhuri A, Gollapudi GM, Dandona P: The effects of hypogonadism on body composition and bone mineral density in type 2 diabetic patients. Diabetes Care 2007;30:1860–1861 [DOI] [PubMed] [Google Scholar]
- 3. Kapoor D, Aldred H, Clark S, Channer KS, Jones TH: Clinical and biochemical assessment of hypogonadism in men with type 2 diabetes: correlations with bioavailable testosterone and visceral adiposity. Diabetes Care 2007;30:911–917 [DOI] [PubMed] [Google Scholar]
- 4. Grossmann M, Thomas MC, Panagiotopoulos S, Sharpe K, Macisaac RJ, Clarke S, Zajac JD, Jerums G: Low testosterone levels are common and associated with insulin resistance in men with diabetes. J Clin Endocrinol Metab 2008;93:1834–1840 [DOI] [PubMed] [Google Scholar]
- 5. Tomar R, Dhindsa S, Chaudhuri A, Mohanty P, Garg R, Dandona P: Contrasting testosterone concentrations in type 1 and type 2 diabetes. Diabetes Care 2006;29:1120–1122 [DOI] [PubMed] [Google Scholar]
- 6. Glass AR, Swerdloff RS, Bray GA, Dahms WT, Atkinson RL: Low serum testosterone and sex-hormone-binding-globulin in massively obese men. J Clin Endocrinol Metab 1977;45:1211–1219 [DOI] [PubMed] [Google Scholar]
- 7. Mulligan T, Frick MF, Zuraw QC, Stemhagen A, McWhirter C: Prevalence of hypogonadism in males aged at least 45 years: the HIM study. Int J Clin Pract 2006;60:762–769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bhasin S, Cunningham GR, Hayes FJ, Matsumoto AM, Snyder PJ, Swerdloff RS, Montori VM: Testosterone therapy in adult men with androgen deficiency syndromes: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 2006;91:1995–2010 [DOI] [PubMed] [Google Scholar]
- 9. Vermeulen A, Verdonck L, Kaufman JM: A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab 1999;84:3666–3672 [DOI] [PubMed] [Google Scholar]
- 10. Vermeulen A, Kaufman JM, Deslypere JP, Thomas G: Attenuated luteinizing hormone (LH) pulse amplitude but normal LH pulse frequency, and its relation to plasma androgens in hypogonadism of obese men. J Clin Endocrinol Metab 1993;76:1140–1146 [DOI] [PubMed] [Google Scholar]
- 11. Zumoff B, Strain GW, Miller LK, Rosner W, Senie R, Seres DS, Rosenfeld RS: Plasma free and non-sex-hormone-binding-globulin-bound testosterone are decreased in obese men in proportion to their degree of obesity. J Clin Endocrinol Metab 1990;71:929–931 [DOI] [PubMed] [Google Scholar]
- 12. Giagulli VA, Kaufman JM, Vermeulen A: Pathogenesis of the decreased androgen levels in obese men. J Clin Endocrinol Metab 1994;79:997–1000 [DOI] [PubMed] [Google Scholar]
- 13. Hofstra J, Loves S, van Wageningen B, Ruinemans-Koerts J, Jansen I, de Boer H: High prevalence of hypogonadotropic hypogonadism in men referred for obesity treatment. Neth J Med 2008;66:103–109 [PubMed] [Google Scholar]
- 14. Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM: Prevalence of overweight and obesity in the United States, 1999–2004. JAMA 2006;295:1549–1555 [DOI] [PubMed] [Google Scholar]
- 15. Selvin E, Feinleib M, Zhang L, Rohrmann S, Rifai N, Nelson WG, Dobs A, Basaria S, Golden SH, Platz EA: Androgens and diabetes in men: results from the Third National Health and Nutrition Examination Survey (NHANES III). Diabetes Care 2007;30:234–238 [DOI] [PubMed] [Google Scholar]
- 16. Brüning JC, Gautam D, Burks DJ, Gillette J, Schubert M, Orban PC, Klein R, Krone W, Müller-Wieland D, Kahn CR: Role of brain insulin receptor in control of body weight and reproduction. Science 2000;289:2122–2125 [DOI] [PubMed] [Google Scholar]
- 17. Jensen TK, Andersson AM, Jørgensen N, Andersen AG, Carlsen E, Petersen JH, Skakkebaek NE: Body mass index in relation to semen quality and reproductive hormones among 1,558 Danish men. Fertil Steril 2004;82:863–870 [DOI] [PubMed] [Google Scholar]
- 18. Dandona P, Aljada A, Bandyopadhyay A: Inflammation: the link between insulin resistance, obesity and diabetes. Trends Immunol 2004;25:4–7 [DOI] [PubMed] [Google Scholar]
- 19. Chandel A, Dhindsa S, Topiwala S, Chaudhuri A, Dandona P: Testosterone concentration in young patients with diabetes. Diabetes Care 2008;31:2013–2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ding EL, Song Y, Manson JE, Hunter DJ, Lee CC, Rifai N, Buring JE, Gaziano JM, Liu S: Sex hormone-binding globulin and risk of type 2 diabetes in women and men. N Engl J Med 2009;361:1152–1163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kapoor D, Clarke S, Channer KS, Jones TH: Erectile dysfunction is associated with low bioactive testosterone levels and visceral adiposity in men with type 2 diabetes. Int J Androl 2007;30:500–507 [DOI] [PubMed] [Google Scholar]
- 22. Nielsen TL, Hagen C, Wraae K, Brixen K, Petersen PH, Haug E, Larsen R, Andersen M: Visceral and subcutaneous adipose tissue assessed by magnetic resonance imaging in relation to circulating androgens, sex hormone-binding globulin, and luteinizing hormone in young men. J Clin Endocrinol Metab 2007;92:2696–2705 [DOI] [PubMed] [Google Scholar]
- 23. Caronia L, Dwyer A, Hayden D, Pitteloud N, Hayes F: Abrupt decrease in testosterone following an oral glucose load in men. Abstract presented at ENDO 09, 10–13 June 2009, Washington, DC, OR42-2 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.