Abstract
OBJECTIVE
To evaluate the utility of GAD antibodies (GADAs) and islet antigen-2 antibodies (IA-2As) in prediction of type 1 diabetes over 27 years in the general population and to assess the 6-year rates of seroconversion.
RESEARCH DESIGN AND METHODS
A total of 3,475 nondiabetic subjects aged 3–18 years were sampled in 1980, and 2,375 subjects (68.3%) were resampled in 1986. All subjects were observed for development of diabetes to the end of 2007. GADAs and IA-2As were analyzed in all samples obtained in 1980 and 1986.
RESULTS
A total of 34 individuals (1.0%; 9 developed diabetes) initially had GADAs and 22 (0.6%; 9 developed diabetes) IA-2As. Seven subjects (0.2%) tested positive for both autoantibodies. The positive seroconversion rate over 6 years was 0.4% for GADAs and 0.2% for IA-2As, while the inverse seroconversion rates were 33 and 57%, respectively. Eighteen subjects (0.5%) developed type 1 diabetes after a median pre-diabetic period of 8.6 years (range 0.9–20.3). Initial positivity for GADAs and/or IA-2As had a sensitivity of 61% (95% CI 36–83) for type 1 diabetes. Combined positivity for GADAs and IA-2As had both a specificity and a positive predictive value of 100% (95% CI 59–100).
CONCLUSIONS
One-time screening for GADAs and IA-2As in the general childhood population in Finland would identify ∼60% of those individuals who will develop type 1 diabetes over the next 27 years, and those subjects who have both autoantibodies carry an extremely high risk for diabetes. Both positive and inverse seroconversions do occur over time reflecting a dynamic process of β-cell autoimmunity.
Type 1 diabetes is an immune-mediated disease leading to chronic insulin deficiency due to extensive and selective β-cell destruction in subjects with increased genetic disease susceptibility (1). The clinical disease manifestation represents end-stage insulitis, since only 10–20% of the insulin-producing β-cells have been estimated to still be functioning at the time of diagnosis (2). The clinical disease presentation is preceded by an asymptomatic period of variable duration. β-cell autoimmunity is characterized by the presence of autoantibodies, such as islet cell autoantibodies (ICAs), insulin autoantibodies (IAAs), autoantibodies to the 65-kDa isoform of GAD (GADAs), and autoantibodies to the intracellular portion of the protein tyrosine phosphatase–related islet antigen-2 molecule (IA-2As), in the peripheral circulation (3). Many studies (3–5) have shown that these antibodies can be used efficiently for the prediction of type 1 diabetes in first-degree relatives of affected patients. The combination of GADAs and IA-2As has a high sensitivity and specificity for type 1 diabetes in family members, although prospective studies from birth have shown that IAAs are usually the first or among the first antibodies to appear in young children (6–8). In a previous survey, we showed that the predictive characteristics of combined positivity for GADAs and IA-2As were of the same magnitude in the general population as those seen among first-degree relatives over an observation period of 15 years (9). In this study, we set out to assess the predictive characteristics of GADAs and IA-2As over a period of 27 years in a population-based series of 3,475 Finnish subjects initially aged 3–18 years. We also had the opportunity to define in this population the rates of positive and inverse seroconversions over the initial 6 years of follow-up.
RESEARCH DESIGN AND METHODS
A total of 3,596 children and adolescents were initially recruited in 1980 for a population-based study on cardiovascular risk in young Finns (10). Unselected Finnish subjects aged 3, 6, 9, 12, 15, and 18 years at entry of the study were recruited in five university cities in Finland and in 12 rural communities in their vicinity. Data on the predictive value of GADAs and IA-2As in the samples obtained in 1980 have been reported earlier for a follow-period of 15 years (9). All subjects were invited for re-examination in 1986. We tested all available samples of initially nondiabetic subjects taken in 1980 (n = 3,475; girls 50.7%) and 1986 (n = 2,375; girls 52.8%) for GADAs and IA-2As. Subjects who developed type 1 diabetes were asked to report the diagnosis to their study center. All subjects who developed clinical diabetes by the end of year 2007 were identified from the Central Drug Registry of the National Social Insurance Institute as a secondary source. This registry has an ascertainment rate of >99% for new patients with diabetes (11). The diagnosis of type 1 diabetes required that the patient was started on exogenous insulin within 5 days after the diagnosis. All subjects with available serum testing positive for GADAs and/or IA-2As on at least one of the occasions were also analyzed for ICAs and IAAs. The subjects who developed diabetes over the observation period were analyzed for all four autoantibodies in the samples taken in 1980 and 1986, if available. Sera were stored at −20°C until analyzed.
GADAs and IA-2As were quantified with specific radioligand assays based on the same principle as described in detail elsewhere (9). The results were expressed in relative units (RU), and these were transformed into World Health Organization (WHO) units based on the WHO standard serum. The cutoff limit for antibody positivity was set at the 99th percentile in a separate series of >370 nondiabetic children and adolescents being 14.13 WHO units (5.36 RU) for GADAs and 1.91 WHO units (0.43 RU) for IA-2As. The disease sensitivity of the GADA assay was 82% and the specificity 96% and the corresponding characteristics of the IA-2A assay 72 and 100% in the 2005 Centers for Disease Control and Prevention–sponsored Diabetes Autoantibody Standardization Program Workshop. Samples with GADAs or IA-2As levels between the 97th and 99.5th percentiles were reanalyzed to confirm the antibody status. The 1980 and 1986 samples from the subjects seroconverting to antibody positivity or negativity were reanalyzed blindly in the same assay to verify the seroconversion.
ICAs were analyzed by a standard immunofluorescence method from sections of a frozen human group O pancreas as described (9). The threshold for ICA detection was 2.5 Juvenile Diabetes Foundation (JDF) units.
IAA levels were measured with a microassay as described (9). A subject was considered positive for IAAs when the specific binding exceeded 1.55 RU (99th percentile in 371 nondiabetic Finnish subjects). The disease sensitivity of our microassay was 58% and the specificity 98% in the 2005 Diabetes Autoantibody Standardization Program Workshop.
Statistical analysis
The statistical evaluation was performed using the χ2 test when analyzing differences in the frequencies of antibodies and type 1 diabetes between groups unless any expected value was less than five, when Fisher exact test was applied. The Mann-Whitney U test was used for comparisons between the levels of autoantibodies. The Kaplan-Meier method was used to construct life tables of the likelihood of developing type 1 diabetes. The log-rank test was used for comparison of the survival distributions. Hazard ratios (HRs) for factors potentially predictive for type 1 diabetes (sex, age at initial screening, initial GADA and IA-2A positivity, as well as GADA and IA-2A levels) were assessed with the Cox regression model. A two-tailed P value of 0.05 was considered statistically significant. Disease sensitivity and specificity as well as positive (PPV) and negative (NPV) predictive values and likelihood ratios (+LR and −LR) were determined for the autoantibody markers.
RESULTS
Fifteen boys and 19 girls (0.9 vs. 1.1%; P = 0.54) tested positive for GADAs in the samples taken in 1980. There was no difference in GADA titers between sexes. Thirteen boys and nine girls (0.8 vs. 0.5%; P = 0.36) had IA-2As in their initial sample, the boys having higher titers than the girls (median 5.61 vs. 2.47 WHO units; P = 0.05).
Among those with samples available from 1986, 9 of 2,357 subjects negative for GADAs in 1980 (0.4%) had seroconverted to GADA positivity. The positive seroconversion rate did not differ significantly between male and female subjects (0.5 vs. 0.2%, respectively). Six of 18 initially GADA-positive subjects (33%) had experienced an inverse seroconversion. Inverse seroconversions tended to be more common among female than among male subjects (5 of 10 vs. 1 of 8; P = 0.09). Seroconversion to positivity for IA-2As was seen in 4 of 2,361 (0.2%) subjects (boys 0.3%, girls 0.1%) over the 6-year period. The inverse seroconversion rate was somewhat higher for IA-2As (8 of 14; 57%) than that for GADAs, but there was no sex difference. Individual autoantibody data in the subjects who experienced positive seroconversions and corresponding data in subjects with inverse seroconversions are presented in Table 1. One boy who tested positive for IAAs only in 1980, but had GADAs and ICAs in addition to IAAs in 1986, presented with overt diabetes in 1996. Among six participants who experienced an inverse seroconversion for GADAs by 1986, two progressed subsequently to type 1 diabetes. One female progressor had all four autoantibodies in 1980, tested still positive for IA-2As and ICAs in 1986, and presented with diabetes in 1988. The other progressor was a boy who tested positive for GADAs only in 1980 and negative for all four autoantibodies in 1986. He was diagnosed with diabetes in 1992.
Table 1.
Characteristics of the subjects who experienced positive seroconversions for GADAs (GADA+) or IA-2As (IA-2A+) during an observation period of 6 years (1980–1986) and of those who experienced inverse seroconversions for GADAs (GADA−) or IA-2As (IA-2A−) during the same time period
| Autoantibody | Number | Sex | Status in 1980 |
Status in 1986 |
Type 1 diabetes |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age (years) | GADA (WHO units) | IA-2A (WHO units) | ICA (JDF units) | IAA (RU) | Number of positive autoantibodies | Age (years) | GADA (WHO units) | IA-2A (WHO units) | ICA (JDF units) | IAA (RU) | Number of positive autoantibodies | Age (years) | |||
| GADA+ | 1 | Male | 2.9 | 4.25 | 1.64 | 0 | 3.14 | 1 | 8.9 | 249.4 | 1.66 | 219 | 3.20 | 3 | 19.2 |
| GADA+ | 2 | Female | 3.4 | 2.22 | 1.71 | 0 | 1.00 | 0 | 9.4 | 38.7 | 1.67 | 0 | 1.00 | 1 | — |
| GADA+ | 3 | Male | 6.1 | 2.35 | 1.71 | 0 | 1.00 | 0 | 12.0 | 15.7 | 1.60 | 0 | 1.00 | 1 | — |
| GADA+ | 4 | Female | 6.1 | 2.22 | 1.59 | 0 | 1.00 | 0 | 12.1 | 29.7 | 1.57 | 0 | 1.00 | 1 | — |
| GADA+ | 5 | Male | 6.6 | 4.12 | 1.56 | NA | NA | 0 | 12.6 | 39.4 | 1.61 | 0 | 1.00 | 1 | — |
| GADA+ | 6 | Male | 11.9 | 2.49 | 1.69 | 0 | 1.00 | 0 | 17.9 | 24.9 | 1.59 | 0 | 1.00 | 1 | — |
| GADA+ | 7 | Female | 12.1 | 2.10 | 1.53 | 0 | 1.00 | 0 | 18.1 | 81.5 | 1.59 | 0 | 1.00 | 1 | — |
| GADA+ | 8 | Male | 12.8 | 2.54 | 1.58 | 0 | 1.00 | 0 | 18.8 | 14.6 | 1.64 | 0 | 1.00 | 1 | — |
| GADA+ | 9 | Male | 18.2 | 4.99 | 1.59 | 5 | 1.00 | 1 | 24.2 | 16.8 | 1.53 | 5 | 1.00 | 2 | — |
| IA-2A+ | 1 | Female | 3.5 | 2.57 | 1.50 | 0 | 1.00 | 0 | 9.5 | 2.15 | 3.09 | 0 | 1.00 | 1 | — |
| IA-2A+ | 2 | Male | 6.3 | 1.60 | 1.53 | 0 | 1.00 | 0 | 12.3 | 1.46 | 2.22 | 0 | 1.00 | 1 | — |
| IA-2A+ | 3 | Male | 6.8 | 1.85 | 1.56 | 0 | 1.00 | 0 | 12.7 | 2.69 | 2.32 | NA | 1.00 | 1 | — |
| IA-2A+ | 4 | Male | 15.2 | 1.16 | 1.70 | 9 | 1.05 | 0 | 21.2 | 1.83 | 2.15 | 0 | 1.00 | 1 | — |
| GADA− | 1 | Female | 3.0 | 338.9 | 124.8 | 110 | 6.75 | 4 | 8.9 | 9.51 | 181.0 | 110 | 1.00 | 2 | 10.7 |
| GADA− | 2 | Female | 3.5 | 22.3 | 1.66 | NA | 1.00 | 1 | 9.6 | 3.33 | 1.56 | 0 | 1.00 | 0 | — |
| GADA− | 3 | Male | 6.6 | 27.3 | 1.59 | 0 | 1.00 | 1 | 12.6 | 12.3 | 1.52 | 0 | 1.00 | 0 | 18.6 |
| GADA− | 4 | Female | 9.1 | 52.3 | 1.61 | 0 | 1.00 | 1 | 15.0 | 1.90 | 1.56 | 0 | 1.00 | 0 | — |
| GADA− | 5 | Female | 12.6 | 35.0 | 1.58 | NA | NA | 1 | 18.5 | 7.63 | 1.60 | 0 | 1.00 | 0 | — |
| GADA− | 6 | Female | 12.7 | 28.7 | 1.60 | NA | 1.00 | 1 | 18.7 | 1.80 | 1.61 | 0 | 1.00 | 0 | — |
| IA-2A− | 1 | Female | 2.9 | 3.82 | 2.14 | 0 | 1.00 | 1 | 8.8 | 10.9 | 1.67 | 0 | 1.00 | 0 | — |
| IA-2A− | 2 | Female | 6.0 | 3.43 | 2.47 | 5 | 1.00 | 2 | 12.0 | 1.78 | 1.66 | 5 | 1.00 | 1 | — |
| IA-2A− | 3 | Male | 6.0 | 1.75 | 5.42 | 0 | 1.00 | 1 | 12.0 | 1.58 | 1.54 | 0 | 1.00 | 0 | — |
| IA-2A− | 4 | Male | 6.1 | 4.45 | 5.61 | NA | 1.00 | 1 | 12.0 | 2.62 | 1.58 | 0 | 1.00 | 0 | — |
| IA-2A− | 5 | Male | 6.7 | 3.53 | 2.97 | 0 | 1.00 | 1 | 12.7 | 3.70 | 1.70 | 0 | 1.00 | 0 | — |
| IA-2A− | 6 | Male | 9.6 | 2.25 | 4.23 | NA | NA | 1 | 15.6 | 2.59 | 1.69 | 0 | 1.00 | 0 | — |
| IA-2A− | 7 | Female | 15.1 | 1.80 | 2.20 | 0 | 1.00 | 1 | 21.0 | 1.68 | 1.69 | 0 | 1.00 | 0 | — |
| IA-2A− | 8 | Male | 18.0 | 2.54 | 2.39 | 0 | 1.00 | 1 | 24.0 | 3.21 | 1.56 | 0 | 1.00 | 0 | — |
GADAs and IA-2As were analyzed in 3,475 Finnish subjects 3–18 years of age in 1980, out of whom 2,375 ( 68.3%) were resampled in 1986. Positive autoantibody titers appear in bold. NA, not available.
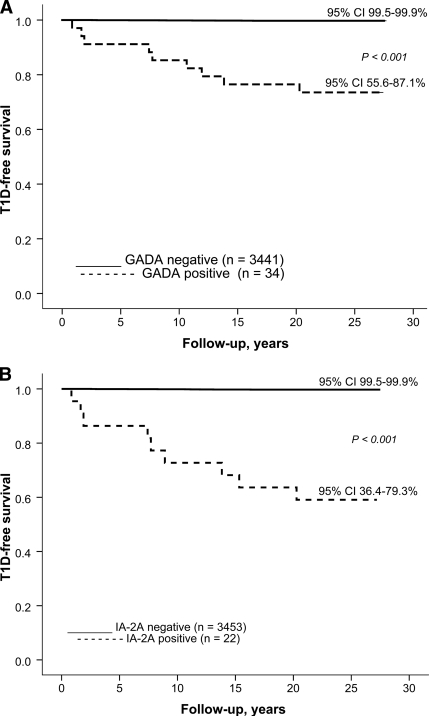
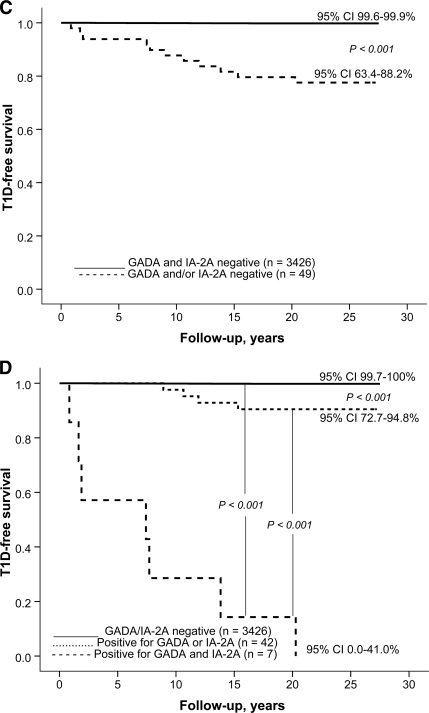
Over the observation period of 27 years 12 male and 6 female subjects developed clinical type 1 diabetes (0.7 vs. 0.3%; P = 0.16). The progressors were initially ∼1.7 years younger than the nonprogressors (aged 9.1 years [range 2.9–18.9] vs. aged 10.8 years [2.7–18.9]; P = 0.14). The diabetes-free survival over time is presented in relation to initial autoantibody status in Fig. 1. Both GADA and IA-2A positivity were associated with relatively high probability (9 of 34 = 26% and 9 of 22 = 41%, respectively) of type 1 diabetes (Table 2). Similarly positivity for GADAs and/or IA-2As was related to an increased likelihood of progression to overt diabetes (Fig. 1C). All seven double-positive individuals developed clinical diabetes during the follow-up (Fig. 1D). The GADA titers were of the same magnitude in the progressors and nonprogressors positive for GADAs. The antibody-positive progressors had higher titers of IA-2As than the nonprogressors both among male subjects (median 141.8 vs. 3.84 WHO units; P = 0.005) and female subjects (median 95.3 vs. 2.20 WHO units; P = 0.04). More than 60% of those (11 of 18; 61%) who later presented with type 1 diabetes tested positive for GADAs and/or IA-2As, whereas the proportion was ∼1.1% (38 of 3,438) among the nonprogressors. Close to 40% of the progressors tested initially positive for both GADAs and IA-2As, while none of the 3,457 nonprogressors had both antibodies in 1980. Individual autoantibody data in the progressors are presented in Table 2. The predictive characteristics of GADAs and IA-2As and their combinations are shown in Table 3. Five progressors (28%) tested negative for all four autoantibodies in 1980. The autoantibody-positive progressors tended to be older at diagnosis (mean age 20.2 years [range 10.7–32.8]) than the antibody-negative subjects (mean age 12.5 years [5.5–20.3]; P = 0.06). One of the progressors (case 7) tested positive for ICAs only, the observed positivity preceding clinical disease by 13.1 years.
Figure 1.
A: Probability of remaining nondiabetic in Finnish subjects initially positive (n = 34, 9 developed type 1 diabetes) or negative for GADAs (n = 3,441, 9 developed type 1 diabetes); B: positive (n = 22, 9 developed type 1 diabetes) or negative (n = 3453, 9 developed type 1 diabetes) for IA-2As; C: positive for GADAs and/or IA-2As (n = 49; 11 developed type 1 diabetes) or negative for both autoantibodies (n = 3426, 7 developed type 1 diabetes); and D: in those initially positive for two (n = 7, all developed type 1 diabetes), one (n = 42, 4 developed diabetes), or no (n = 3,426, 7 developed type 1 diabetes) antibodies.
Table 2.
Characteristics of the participants who developed clinical type 1 diabetes over an observation period of 27 years among 3,475 Finnish subjects representing the general population and initially aged 3–18 years
| Number | Sex | Status in 1980 |
Status in 1986 |
Type 1 diabetes |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age (years) | GADA (WHO units) | IA-2A (WHO units) | ICA (JDF units) | IAA (RU) | Number of positive autoantibodies | Age (years) | GADA (WHO units) | IA-2A (WHO units) | ICA (JDF units) | IAA (RU) | Number of positive autoantibodies | Age at diagnosis | Interval from first sample to diagnosis (years) | ||
| 1 | Male | 2.9 | 4.25 | 1.64 | 0 | 3.14 | 1 | 8.9 | 249.4 | 1.66 | 219 | 3,20 | 3 | 19.2 | 16.3 |
| 2 | Male | 2.9 | 2.79 | 1.60 | 0 | 1.00 | 0 | NA | NA | NA | NA | NA | NA | 5.5 | 2.6 |
| 3 | Female | 3.0 | 339.9 | 124.8 | 110 | 6.75 | 4 | 8.9 | 9.51 | 181.0 | 110 | 1.00 | 2 | 10.7 | 7.7 |
| 4 | Male | 3.0 | 4.79 | 1.52 | 0 | 1.00 | 0 | NA | NA | NA | NA | NA | NA | 8.8 | 5.8 |
| 5 | Female | 3.6 | 1.48 | 1.53 | 0 | 1.00 | 0 | 9.6 | 1.50 | 1.51 | 0 | 1.00 | 0 | 11.9 | 8.3 |
| 6 | Male | 6.1 | 5.04 | 228.1 | 110 | 4.24 | 3 | 12.1 | 3.73 | 207.3 | 55 | 2.54 | 3 | 21.4 | 15.3 |
| 7 | Male | 6.3 | 3.14 | 1.67 | 3 | 1.00 | 1 | 12.2 | 3.48 | 1.60 | 5 | 1.00 | 1 | 19.4 | 13.1 |
| 8 | Male | 6.6 | 27.3 | 1.59 | 0 | 1.00 | 1 | 12.6 | 12.3 | 1.52 | 0 | 1.00 | 0 | 18.6 | 12.0 |
| 9 | Female | 8.9 | 116.9 | 68.2 | 55 | 1.61 | 4 | NA | NA | NA | NA | NA | NA | 10.8 | 1,9 |
| 10 | Female | 9.5 | 1.95 | 1.57 | 0 | 1.00 | 0 | NA | NA | NA | NA | NA | NA | 12.5 | 3.0 |
| 11 | Male | 9.7 | 39.5 | 1.56 | 0 | 1.00 | 1 | NA | NA | NA | NA | NA | NA | 20.3 | 10.7 |
| 12 | Male | 9.8 | 265.4 | 141.8 | 55 | 1.00 | 3 | 15.7 | 108.3 | 83.7 | 8 | 10.91 | 4 | 10.7 | 0.9 |
| 13 | Female | 11.9 | 263.0 | 2.84 | 4 | 6.72 | 4 | NA | NA | NA | NA | NA | NA | 32.2 | 20.3 |
| 14 | Male | 12.1 | 333.3 | 129.3 | 15 | 1.00 | 3 | 18.0 | 118.7 | 157.0 | 8 | 1.00 | 3 | 19.5 | 7.4 |
| 15 | Male | 12.7 | 2.59 | 1.60 | 0 | 1.00 | 0 | NA | NA | NA | NA | NA | NA | 23.9 | 11.2 |
| 16 | Male | 17.9 | 1.51 | 7.53 | 8 | 1.00 | 2 | 13.8 | 1.16 | 4.27 | 5 | 1.00 | 2 | 26.9 | 9.0 |
| 17 | Male | 17.9 | 31.2 | 157.1 | 191 | 1.00 | 3 | NA | NA | NA | NA | NA | NA | 19.6 | 1.7 |
| 18 | Female | 18.9 | 398.7 | 2.08 | 55 | 1.00 | 3 | 24.9 | 320.4 | 2.90 | 28 | 1.00 | 3 | 32.8 | 13.9 |
Positive autoantibody titers appear in bold. NA, not available.
Table 3.
Sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and positive and negative likelihood ratios (+LR and −LR) of GADAs and IA-2As for type 1 diabetes over an observation period of 27 years
| Autoantibody | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | +LR (%) | −LR (%) |
|---|---|---|---|---|---|---|
| GADA | ||||||
| Boys | 42 | 99 | 33 | 99 | 71 | 0.6 |
| Girls | 67 | 99 | 21 | 99 | 78 | 0.3 |
| All | 50 | 99 | 26 | 99 | 69 | 0.5 |
| IA-2A | ||||||
| Boys | 42 | 100 | 38 | 100 | 89 | 0.6 |
| Girls | 67 | 100 | 44 | 100 | 234 | 0.3 |
| All | 50 | 100 | 41 | 100 | 133 | 0.5 |
| GADA and/or IA-2A | ||||||
| Boys | 58 | 99 | 28 | 99 | 55 | 0.4 |
| Girls | 67 | 99 | 17 | 99 | 59 | 0.3 |
| All | 61 | 99 | 22 | 99 | 56 | 0.4 |
| GADA and IA-2A | ||||||
| Boys | 39 | 100 | 100 | 100 | 424 | 0.3 |
| Girls | 67 | 100 | 100 | 100 | 1,170 | 0.7 |
| All | 39 | 100 | 100 | 100 | 1,346 | 0.4 |
In the Cox regression analysis, GADA positivity (HR 37.4 [95% CI 11.0–126.9]), IA-2A positivity (30.7 [6.9–136.8]), and IA-2A level (1.02 [1.00–1.04]) were associated with increased risk for type 1 diabetes. Male sex was associated with a borderline increased HR (3.0 [0.98–9.2]), while age at initial screening was not related to enhanced disease risk.
CONCLUSIONS
As soon the first effective preventive modality becomes available for clinical use, there will be an apparent need to extensively identify individuals at risk for progression to type 1 diabetes from the general population, since the overwhelming majority of the cases with newly diagnosed disease are sporadic (12). The present data show that one-time screening for GADAs and IA-2As is capable of identifying ∼60% of those individuals who will develop type 1 diabetes over the subsequent 27 years. The highest PPV was seen in those testing initially positive for both GADAs and IA-2As, but the sensitivity dropped from 50% for either test alone to 39%.
The first studies focusing on prediction of type 1 diabetes in the general population used ICAs as a marker. Bruining et al. (13) reported that the PPV of ICA positivity was 50% for type 1 diabetes when observing 4,806 schoolchildren for 10 years, while Schatz et al. (14) observed a PPV of 18% associated with ICA positivity in 9,696 school children followed for a maximum period of 8 years. The Washington State Diabetes Prediction Study showed that positivity for at least two autoantibodies out of IAAs, GADAs, and IA-2As was associated with a high sensitivity (100% [95% CI 58–100]) and a reasonable positive predictive value (50% [95% CI 25–75]) in initially 12- to 18-year-old school children over a median time period of 8 years (15). In the Swedish ABIS Study, combined positivity for GADAs and IA-2As at the age of 2.5 years was associated with a PPV of 11% for clinical diabetes by the age of 8 years (16). All the above-referenced studies have been based on a limited number of progressors, and the maximal follow-up time has been 10 years, whereas the present survey is based on the longest observation period ever reported (i.e., 27 years). Unfortunately, no HLA data were available on the participants in our study, which is an apparent limitation. The current survey did not set out to assess whether GADAs and IA-2As are superior to other autoantibodies, since ICAs and IAAs were analyzed only in those children who tested positive for GADAs and/or IA-2As and/or developed clinical type 1 diabetes.
Our observation that both positive and inverse seroconversions occurred during an observation period of 6 years confirms that β-cell autoimmunity represents a dynamic process as previously shown in a Finnish family study (17). Some studies (18,19) have claimed that most positive seroconversions take place before the age of 6 years. The present data do not support such a phenomenon. One-third of the subjects initially positive for GADAs tested negative for GADAs 6 years later, whereas the proportion of inverse seroconverters was close to 60% for IA-2As. A majority of those (12 of 14) who experienced an inverse seroconversion tested positive for one single antibody in their initial sample. The remaining two still tested positive for at least one antibody in the subsequent sample. One of the two children presented with clinical diabetes in 1988. The inverse seroconverters initially positive for a single antibody all had relatively low antibody titers in their first sample. Taken together, these observations suggest that β-cell autoimmunity may be induced at any age, at least before the age of 20 years. Positivity for a single autoantibody seems to reflect transient β-cell autoimmunity in about half of such individuals. Regression to autoantibody negativity was not observed in any of the seven subjects initially positive for both GADAs and IA-2As, suggesting that positivity for at least two diabetes-associated autoantibodies represents, in most cases, a point of no return.
Altogether 18 subjects (0.52% [95% CI 0.31–0.82]) developed type 1 diabetes over the observation period of 27 years, while the expected number of cases would be 20 based on the recorded incidence rate in the age-group from 3 to 35 years in Finland. Available data suggest that the natural history of preclinical type 1 diabetes varies considerably from one individual to another (20). In the present series, the interval from the first auto-antibody-positive sample to clinical presentation of type 1 diabetes ranged from 0.9 to 20.3 years with a median of 8.6 years. When considering those 11 progressors who tested positive for at least one diabetes-associated autoantibody in the pre-diabetic period, the median interval from the first antibody-positive sample to the diagnosis of diabetes was 8.9 years with a minimum of <1 year and a maximum of 20 years. Although we do not know the exact age at seroconversion, the observed range in the duration of the pre-diabetic period supports the view that there is substantial individual variation in the progression rate to clinical diabetes. Family studies have indicated that young age at the appearance of the first autoantibodies, high antibody titers except for GADAs (5), a high-risk HLA genotype, and a reduced first-phase insulin response to intravenous glucose are all factors associated with an accelerated progression to overt type 1 diabetes (21,22). The Cox regression analysis revealed expected predictors of type 1 diabetes, except that age at initial screening turned out to be unrelated to diabetes risk. This may be due to a longer observation time in the current study than in previous reports, as seroconversion at young age has been shown to be associated with rapid progression to clinical disease (23), which may be neutralized by longer follow-up.
There were seven individuals who developed diabetes with no detectable GADAs or IA-2As in their initial sample. Two individuals had positive IAAs or ICAs. In the remaining five subjects, no markers of humoral β-cell autoimmunity were detected in their pre-diabetic period. There might be a series of alternative explanations to this observation. Some pre-diabetic individuals may have seroconverted to autoantibody positivity after the last sample available for testing, whereas a few other may have tested positive for one or more autoantibodies earlier, since there are examples of inverse seroconversions in the pre-diabetic period in some progressors (24). A conspicuous feature in the present series of progressors is the overrepresentation of male subjects (67%). This is, however, consistent with the male predominance among patients with type 1 diabetes diagnosed after puberty (25), as 61% of our cases were diagnosed after the age of 15 years and all but two of these were male subjects.
This study demonstrates that it is possible to identify individuals at high risk for type 1 diabetes from the general population by screening for GADAs and IA-2As. One subject tested positive for four autoantibodies at the age of 11.9 years and presented with clinical diabetes >20 years later, representing, as far as we know, the longest preclinical period so far observed. The occurrence of both positive and inverse seroconversions up to the age of 24 years implies that β-cell autoimmunity represents a dynamic process, and that it can be induced at any age in childhood and adolescence and may be transient in a substantial proportion of subjects testing positive for a single antibody.
Acknowledgments
This study was supported by the Juvenile Diabetes Research Foundation (grant no. 197032), the Academy of Finland, the Diabetes Research Foundation in Finland, the Päivikki and Sakari Sohlberg Foundation, the Novo Nordisk Foundation, and the Helsinki University Hospital Research Fund.
No potential conflicts of interest relevant to this article were reported.
We thank Susanna Heikkilä, Riitta Päkkilä, Päivi Salmijärvi, and Sirpa Anttila for expert technical assistance.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
See accompanying editorial on p. 1403.
References
- 1. Atkinson MA, Eisenbarth GS: Type 1 diabetes: new perspectives on disease pathogenesis and treatment. Lancet 2001;358:221–229 [DOI] [PubMed] [Google Scholar]
- 2. Knip M: Disease-associated autoimmunity and prevention of insulin-dependent diabetes mellitus. Ann Med 1997;29:447–451 [DOI] [PubMed] [Google Scholar]
- 3. Bonifacio E, Genovese S, Braghi S, Bazzigaluppi E, Lampasona V, Bingley PJ, Rogge L, Pastore MR, Bognetti E, Bottazzo GF, Gale EAM, Bosi E: Islet autoantibody markers in IDDM: risk assessment strategies yielding high sensitivity. Diabetologia 1995;38:816–822 [DOI] [PubMed] [Google Scholar]
- 4. Verge C, Gianani R, Kawasaki E, Yu L, Pietropaolo M, Jackson RA, Chase HP, Eisenbarth GS: Prediction of type 1 diabetes in first-degree relatives using a combination of insulin, GAD, and ICA512bdc/IA-2 autoantibodies. Diabetes 1996;45:926–933 [DOI] [PubMed] [Google Scholar]
- 5. Kulmala P, Savola K, Petersen JS, Vähäsalo P, Karjalainen J, Löppönen T, Dyrberg T, Åkerblom HK, Knip M: the Childhood Diabetes in Finland Study Group Prediction of insulin-dependent diabetes mellitus in siblings of children with diabetes: a population-based study. J Clin Invest 1998;101:327–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ziegler A-G, Hummel M, Schenker M, Bonifacio E: Autoantibody appearance and risk for development of childhood diabetes in offspring of parents with type 1 diabetes: the 2-year analysis of the German BABYDIAB Study. Diabetes 1999;48:460–468 [DOI] [PubMed] [Google Scholar]
- 7. Yu Y, Robles DT, Abiru N, Kaur P, Rewers M, Kelemen K, Eisenbarth GS: Early expression of antiinsulin autoantibodies of humans and the NOD mouse: evidence for early determination of subsequent diabetes. Proc Natl Acad Sci U S A 2000;97:1701–1706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kimpimäki T, Kupila A, Hämäläinen A-M, Kukko M, Kulmala P, Savola K, Simell T, Muona P, Ilonen J, Simell O, Knip M: The first signs of ß-cell autoimmunity appear in infancy in genetically susceptible children from the general population: the Finnish Type 1 Diabetes Prediction and Prevention Study. J Clin Endocrinol Metab 2001;86:4782–4788 [DOI] [PubMed] [Google Scholar]
- 9. Siljander H, Veijola R, Reunanen A, Virtanen SM, Åkerblom HK, Knip M: Prediction of type 1 diabetes among siblings of affected children and in the general population. Diabetologia 2007;50:2272–2275 [DOI] [PubMed] [Google Scholar]
- 10. Åkerblom HK, Viikari J, Kouvalainen K: Cardiovascular risk factors in Finnish children and adolescents. Acta Paediatr Scand 1985;318(Suppl.):5–6 [DOI] [PubMed] [Google Scholar]
- 11. Kulmala P, Rahko J, Savola K, Vähäsalo P, Veijola R, Sjöroos M, Reunanen A, Ilonen J, Knip M: Stability of autoantibodies and their relation to genetic and metabolic markers of type I diabetes in initially unaffected schoolchildren. Diabetologia 2000;43:457–464 [DOI] [PubMed] [Google Scholar]
- 12. Veijola R, Reijonen H, Vähäsalo P, Sabbah E, Kulmala P, Ilonen J, Åkerblom HK, Knip M: the Childhood Diabetes in Finland Study Group HLA DQB1 defined genetic susceptibility, beta-cell autoimmunity and metabolic characteristics in familial and non-familial insulin-dependent diabetes mellitus. J Clin Invest 1996;98:2489–2495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bruining GJ, Molenaar JL, Grobbee DE, Hofman A, Sxheffer GJ, Bruining HA, deBruyn AM, Valkenburg HA: Ten-year follow-up study of islet-cell antibodies and childhood diabetes mellitus. Lancet 1989;8647:1100–1103 [DOI] [PubMed] [Google Scholar]
- 14. Schatz D, Krischer J, Horne G, Riley W, Spillar R, Silverstein J, Winter W, Muir A, Derovanesian D, Shah S, Malone J, Maclaren N: Islet cell antibodies predict insulin-dependent diabetes in United States school age children as powerfully as in unaffected relatives. J Clin Invest 1994;93:2403–2407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. LaGasse JM, Brantley MS, Leech NJ, Rowe RE, Monks S, Palmer JP, Nepom GT, McCulloch DK, Hagopian WA: Successful prospective prediction of type 1 diabetes in schoolchildren through multiple defined autoantibodies. Diabetes Care 2002;25:505–511 [DOI] [PubMed] [Google Scholar]
- 16. Gullstrand C, Wahlberg J, Ilonen J, Vaarala O, Ludvigsson J: Progression to type 1 diabetes and autoantibody positivity in relation to HLA-risk genotypes in children participating in the ABIS study. Pediatr Diabetes 2008;9:182–190 [DOI] [PubMed] [Google Scholar]
- 17. Savola K, Läärä E, Vähäsalo P, Kulmala P, Åkerblom HK, Knip M: Dynamic pattern of disease-associated autoantibodies in siblings of children with type 1 diabetes: a population-based study. Diabetes 2001;50:2625–2632 [DOI] [PubMed] [Google Scholar]
- 18. Ziegler AG, Hillebrand B, Rabl W, Mayrhofer M, Hummel M, Mollenhauer U, Vordemann J, Lenz A, Standl E: On the appearance of islet associated autoimmunity in offspring of diabetic mothers: a prospective study from birth. Diabetologia 1993;36:402–408 [DOI] [PubMed] [Google Scholar]
- 19. Leslie RDG, Elliott RB: Early environmental events as a cause of IDDM: evidence and implications. Diabetes 1994;43:843–850 [DOI] [PubMed] [Google Scholar]
- 20. Knip M: Natural course of preclinical type 1 diabetes. Horm Res 2002;57(Suppl. 1):6–11 [DOI] [PubMed] [Google Scholar]
- 21. Veijola R, Vähäsalo P, Tuomilehto-Wolf E, Reijo-nen H, Kulmala P, Ilonen J, Åkerblom HK, Knip M: the Childhood Diabetes in Finland Study Group Human leukocyte antigen identity and DQ risk alleles in autoantibody-positive siblings of children with IDDM are associated with reduced early insulin response. Diabetes 1995;44:1021–1028 [DOI] [PubMed] [Google Scholar]
- 22. Bingley PJ: the ICARUS Group Interactions of age, islet cell antibodies, insulin autoantibodies and first phase insulin response in predicting risk of progression to IDDM in relatives: the ICARUS dataset. Diabetes 1996;45:1720–1728 [DOI] [PubMed] [Google Scholar]
- 23. Siljander H, Simell S, Hekkala A, Lähde J, Simell T, Vähäsalo P, Veijola R, Ilonen J, Simell O, Knip M: Predictive value of diabetes-associated autoantibodies among children with HLA-conferred disease susceptibility recruited from the general population. Diabetes 2009;58:2835–2842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Knip M: Can we predict type 1 diabetes in the general population? Diabetes Care 2002;25:623–625 [DOI] [PubMed] [Google Scholar]
- 25. Gale EA, Gillespie KM: Diabetes and gender. Diabetologia 2001;44:3–15 [DOI] [PubMed] [Google Scholar]