Abstract
OBJECTIVE
Diabetes during pregnancy is a strong risk factor for obesity in the offspring, but the age at which this association becomes apparent is unknown. The purpose of this study was to examine the relation of glycemia during pregnancy with anthropometry in offspring of nondiabetic pregnant women from the Belfast U.K. center of the multinational Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Study.
RESEARCH DESIGN AND METHODS
Women from the HAPO Study were invited to participate in follow-up of their offspring aged 2 years. Measurements included height, weight, and thickness of triceps, subscapular, and suprailiac skinfolds.
RESULTS
A total of 1,165 offspring (73% of eligible children; 598 boys and 567 girls) were seen from ages 22–30 completed months. The only association that reached statistical significance was between categories of maternal 1-h glucose and BMI Z score ≥85th percentile at 2 years (P = 0.017). Overall the correlations between maternal glucose during pregnancy and BMI Z score at age 2 years were weak (fasting glucose r = 0.05, P = 0.08; 1-h glucose r = 0.04, P = 0.22; 2-h glucose r = 0.03, P = 0.36; and area under the curve for glucose r = 0.04, P = 0.18).
CONCLUSIONS
This study found little association between maternal glucose during pregnancy and obesity in the offspring at this young age. These findings are not unexpected given that study results for young offspring whose mothers had diabetes during pregnancy were indistinguishable from those for normal offspring at this age. It will be interesting to see whether, as these children age, maternal glucose during pregnancy in the ranges included in the HAPO Study will be associated with obesity in their children.
Diabetes during pregnancy, whether gestational diabetes, preexisting type 1 diabetes, or preexisting type 2 diabetes, has long-lasting effects on the offspring, resulting in higher rates of obesity (1–3) and type 2 diabetes (4,5) and lesser degrees of abnormal glucose tolerance (5,6) and an earlier age of onset of maturity-onset diabetes of the young (7) and of type 2 diabetes (8). Type 2 diabetes or impaired glucose tolerance developing at young ages results in a higher rate of pregnancies being complicated by diabetes in the next generation (9). Potential long-term effects of degrees of abnormal glucose tolerance during pregnancy less than what is generally considered to be diabetes have not been well studied. Among nondiabetic Pima Indians of Arizona, a linear relationship was observed between the mother's glucose concentration measured during the third trimester of pregnancy and both the relative weight and the 2-h postload glucose concentration in their offspring after age 5 years (9). Whether this will be a universal finding and, if so, at what age the impact of hyperglycemia during pregnancy will first be identifiable are unknown. A unique opportunity to follow children whose mothers had hyperglycemia during pregnancy, but short of diabetes, as demonstrated on an oral glucose tolerance test (OGTT) is presented by the offspring of women participating in the Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Study (10). This article presents the association between glycemia during pregnancy and anthropometry in children aged 2 years participating in the HAPO Family Study at the Belfast Center.
RESEARCH DESIGN AND METHODS
Details of the HAPO Study have been reported elsewhere (10), and the primary results were published in 2008 (11). In brief, the HAPO study was a 15-center multicultural study designed to examine the association between maternal hyperglycemia and adverse pregnancy outcomes among women with singleton pregnancies whose OGTT results were below the thresholds that have traditionally defined gestational diabetes. Each woman underwent a 75-g OGTT at 24–32 weeks of gestation. Women and their caregivers remained blinded to the results of the OGTT as long as the fasting glucose did not exceed 105 mg/dl, the 2-h glucose concentration did not exceed 200 mg/dl, the woman was not hypoglycemic (glucose <45 mg/dl) at any time, and no random glucose concentration measured between 34 and 37 weeks of gestation was ≥160 mg/dl. The study showed that the four primary outcomes (birth weight >90th percentile, primary cesarean delivery, neonatal hypoglycemia, and cord blood C-peptide >90th percentile) were all significantly positively associated with the mother's glucose concentration during the OGTT (11).
All women from the Belfast U.K. Center who participated in the HAPO Study were invited, along with their 2-year-old offspring, to participate in the HAPO Family Study. Examination of the 2-year-old (range 22–30 completed months) offspring included measurements of weight to the nearest 0.1 kg (SECA model 708; Seca, Birmingham, U.K.), height to the nearest 0.1 cm (Leicester height measure stadiometer; Seca), head circumference and mid-arm circumference to the nearest 0.1 cm and triceps, subscapular, and suprailiac skinfold thicknesses to the nearest 0.1 mm (Holtain skinfold calipers; Holtain, Crymych, U.K.). These skinfold measurements were added to give a summary measure of subcutaneous fat. BMI was calculated as weight in kilograms divided by the square of height in meters and converted to SD or Z score using 1990 standards for British children (12). Z scores were also calculated for height and weight and for head circumference (12). Birth weight Z scores, specific for gestational age, were also calculated based on standards for the U.K. (12) and were used to determine small for gestational age (<10th percentile) and large for gestational age (≥90th percentile).
The HAPO Study stratified the results of each of the three OGTT glucose concentrations (fasting, 1-h, and 2-h) into seven categories and determined the rates of primary outcomes in each stratum (11). For this article, because of the smaller numbers enrolled in a single center, the upper two OGTT glucose strata were combined, leaving six categories of glucose at each of the three time points. In addition, the area under the curve of the maternal OGTT was calculated (13).
All statistical analyses were done using SPSS (SPSS, Chicago, IL). The characteristics of male and female participants were compared using the independent samples Z test and for the categorical variables (BMI Z score ≥85 percentile and ≥95 percentile) a χ2 test. The relationship between the results of the OGTT performed between 24 and 32 weeks of gestation (in six categories) and various pregnancy outcome measures (birth weight ≥90th percentile for gestational age, BMI ≥85th or 95th percentiles at age 2 years) were assessed using the χ2 test for trend. This test was also used to assess the relationship between ordered categories of birth weight, neonatal skinfold thickness, and cord C-peptide results and overweight, obesity, and sum of skinfolds at age 2 years. Correlation coefficients were calculated, and multiple regression analyses were performed to assess the association between pregnancy/neonatal characteristics and the child's BMI Z score and skinfold thickness measurements at 2 years of age. Skinfold thickness measurements were added to give a summary measure. Because the distributions of skinfold thickness were positively skewed, their sum was logarithmically transformed before these analyses.
RESULTS
The HAPO Study enrolled 1,649 Caucasian women in the Belfast Center, 1,612 of whom remained blinded throughout pregnancy. Twenty-eight non-Caucasian subjects, predominantly Asian, are not included in this article. Nine of the Caucasian pregnancies ended in stillbirth or perinatal death. By the time of the 2-year examination, 259 of the children had moved or were lost to follow-up, 51 refused, and 128 were unable to keep their scheduled appointments. Follow-up examinations were therefore performed on 1,165 (72%) of the surviving children of Caucasian HAPO participants who had remained blinded throughout the pregnancy study (Table 1). Boys were taller and heavier than girls with a higher BMI and larger head circumference (P < 0.001 for each) but had slightly smaller skinfold thicknesses (sum of skinfold difference = 1.2 mm, P = 0.001). Z scores for height, weight, BMI, and head circumference were not significantly different between the sexes. The mothers of the nonresponders were younger (27.8 vs. 30.4 years), less likely to be married (49.4% vs. 70.1%), more likely to smoke (37.4% vs. 19.0%), and more likely to drink regularly (30.4% vs. 25.6%, all P < 0.05). Although the 1-h glucose and the A1C were also significantly lower among the nonresponders (132 vs. 135 mg/dl and 4.7 vs. 4.8%, P < 0.05 for each), these differences were clinically small.
Table 1.
Characteristics of subjects examined at age 2 years (range 22–30 months)
| Male | Female | P value | |
|---|---|---|---|
| n | 598 | 567 | |
| Age (years) | 2.15 ± 0.11 | 2.14 ± 0.11 | 0.07 |
| Height (cm) | 88.8 ± 3.6 | 87.5 ± 3.6 | <0.001 |
| Height Z score | 0.19 ± 1.15 | 0.12 ± 1.11 | 0.28 |
| Weight (kg) | 13.9 ± 1.6 | 13.2 ± 1.6 | <0.001 |
| Weight Z score | 0.64 ± 1.01 | 0.57 ± 1.02 | 0.19 |
| BMI (kg/m2) | 17.6 ± 1.5 | 17.2 ± 1.6 | <0.001 |
| BMI Z score | 0.67 ± 1.05 | 0.59 ± 1.09 | 0.18 |
| BMI Z score ≥85% | 219/587 (37.3) | 183/554 (33.0) | 0.13 |
| BMI Z score ≥95% | 104/587 (17.7) | 86/554 (15.5) | 0.32 |
| Head circumference (cm) | 50.3 ± 1.4 | 49.1 ± 1.4 | <0.001 |
| Head circumference Z score | −0.12 ± 1.05 | −0.09 ± 1.18 | 0.64 |
| Arm circumference (cm) | 16.0 ± 1.5 | 15.8 ± 1.3 | 0.02 |
| Triceps skinfold (mm) | 11.4 ± 2.4 | 11.6 ± 2.5 | 0.13 |
| Subscapular skinfold (mm) | 6.3 ± 1.9 | 6.7 ± 2.0 | <0.001 |
| Suprailiac skinfold (mm) | 7.8 ± 3.0 | 8.4 ± 3.0 | 0.002 |
| Sum of skinfolds (mm) | 25.5 ± 6.1 | 26.7 ± 6.1 | 0.001 |
Data are means ± SD or n (%).
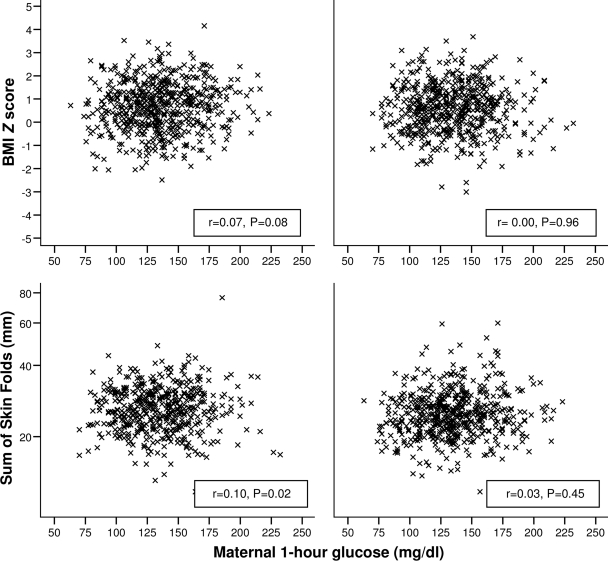
Table 2 shows rates of birth weight ≥90th percentile and, in 2 year olds, rates of overweight (BMI Z score ≥85th percentile) and obesity (BMI Z score ≥95th percentile) according to the six categories of maternal OGTT glucose concentrations and area under the curve for glucose during pregnancy. Maternal glucose concentrations in the Belfast cohort, as in the HAPO Study as a whole (11), were directly associated with risk of birth weight ≥90th percentile among those subjects who were seen subsequently at age 2 years (left column of Table 2). The only other association that reached statistical significance was between categories of maternal 1-h glucose and a BMI Z score at 2 years ≥85th percentile (χ2 for trend = 5.32, df = 1, P = 0.017), but the overall correlation between maternal glucose during pregnancy and BMI Z score at age 2 years was weak (fasting glucose r = 0.05, P = 0.08; 1-h glucose r = 0.04, P = 0.22; 2-h glucose r = 0.03, P = 0.36; and area under the curve r = 0.04, P = 0.18). Weak correlations were found between mother's 1-h glucose and both the (log-transformed) suprailiac skinfold (r = 0.07, P = 0.02) and sum of skinfolds (r = 0.07, P = 0.03) at 2 years, this latter correlation being most notable in boys (r = 0.10, P = 0.02) (Fig. 1).
Table 2.
LGA (birth weight ≥90th percentile for gestational age), overweight, and obesity (BMI Z scores ≥85th and 95th percentiles, respectively) at age 2 years according to strata of maternal OGTT glucose at 24–32 weeks of gestation
| LGA (birth) | Overweight (age 2 years) | Obesity (age 2 years) | |
|---|---|---|---|
| Fasting glucose | |||
| <75 mg/dl | 2/68 (2.9) | 17/66 (25.8) | 11/66 (16.7) |
| 75–79 mg/dl | 14/308 (4.5) | 105/296 (35.5) | 50/296 (16.9) |
| 80–84 mg/dl | 26/394 (6.6) | 134/386 (34.7) | 55/386 (14.2) |
| 85–89 mg/dl | 27/230 (11.7) | 81/229 (35.4) | 36/229 (15.7) |
| 90–94 mg/dl | 13/125 (10.4) | 51/125 (40.8) | 29/125 (23.2) |
| ≥95 mg/dl | 11/40 (27.5) | 14/39 (35.9) | 9/39 (23.1) |
| Ptrend | <0.001 | 0.16 | 0.16 |
| 1-h glucose | |||
| <106 mg/dl | 8/176 (4.5) | 50/171 (29.2) | 24/171 (14.0) |
| 106–132 mg/dl | 17/388 (4.4) | 123/382 (32.2) | 58/382 (15.2) |
| 133–155 mg/dl | 34/332 (10.2) | 127/324 (39.2) | 61/324 (18.8) |
| 156–171 mg/dl | 16/156 (10.3) | 58/153 (37.9) | 30/153 (19.6) |
| 172–193 mg/dl | 12/83 (14.5) | 32/81 (39.5) | 11/81 (13.6) |
| ≥194 mg/dl | 6/30 (20.0) | 12/30 (40.0) | 6/30 (20.0) |
| Ptrend | <0.001 | 0.017 | 0.27 |
| 2-h glucose | |||
| <91 mg/dl | 10/187 (5.3) | 54/183 (29.5) | 26/183 (14.2) |
| 91–108 mg/dl | 28/455 (6.2) | 156/445 (35.1) | 75/445 (16.9) |
| 109–125 mg/dl | 23/299 (7.7) | 105/293 (35.8) | 49/293 (16.7) |
| 126–139 mg/dl | 17/129 (13.2) | 52/127 (40.9) | 26/127 (20.5) |
| 140–157 mg/dl | 6/67 (9.0) | 23/65 (35.4) | 9/65 (13.8) |
| ≥158 mg/dl | 9/28 (32.1) | 12/28 (42.9) | 5/28 (17.9) |
| Ptrend | <0.001 | 0.06 | 0.49 |
| Area under the curve for glucose | |||
| <196 mg/dl · h | 11/200 (5.5) | 59/195 (30.3) | 31/195 (15.9) |
| 196–228 mg/dl · h | 17/389 (4.4) | 132/381 (34.6) | 57/381 (15.0) |
| 229–256 mg/dl · h | 29/308 (9.4) | 109/302 (36.1) | 52/302 (17.2) |
| 257–276 mg/dl · h | 15/137 (10.9) | 54/135 (40.0) | 28/135 (20.7) |
| 277–303 mg/dl · h | 12/96 (12.5) | 35/93 (37.6) | 17/93 (18.3) |
| ≥304 mg/dl · h | 9/35 (25.7) | 13/35 (37.1) | 5/35 (14.3) |
| Ptrend | <0.001 | 0.10 | 0.34 |
Data are n/total n (%). LGA, large for gestational age.
Figure 1.
Correlation coefficients show the relation between 1-h glucose during the pregnancy OGTT and BMI SD score and sum of the skinfolds in the offspring at age 2 years. Left, boys; right, girls.
Table 3 shows the association between overweight, obesity, and sum of skinfolds ≥90th percentile at age 2 years according to categories of weight at birth, sum of skinfolds at birth, and cord blood C-peptide. Both measures of neonatal fatness (birth weight and sum of skinfolds) were associated with overweight and obesity at age 2 (P < 0.01 for each), but C-peptide was not.
Table 3.
Overweight, obesity, and sum of skinfolds ≥90th percentile at age 2 years according to birth parameters
| Overweight (BMI Z scores ≥85th percentile) | Obesity (BMI Z scores ≥95th percentile) | Sum of skinfolds ≥90th percentile | |
|---|---|---|---|
| Birth weight category | |||
| SGA (<10th percentile) | 31/117 (26.5) | 14/117 (12.0) | 9/108 (8.3) |
| Normal | 325/935 (34.8) | 149/935 (15.9) | 82/859 (9.5) |
| LGA (≥90th percentile) | 46/89 (51.7) | 27/89 (30.3) | 13/79 (16.5) |
| Ptrend | <0.001 | <0.001 | 0.09 |
| Sum of skin folds at birth | |||
| <25th percentile | 88/275 (32.0) | 39/275 (14.2) | 20/256 (7.8) |
| 25–74th percentile | 173/547 (31.6) | 77/547 (14.1) | 41/502 (8.2) |
| ≥75th percentile | 116/261 (44.4) | 59/261 (22.6) | 33/234 (14.1) |
| Ptrend | 0.003 | 0.009 | 0.02 |
| Cord C-peptide | |||
| <25th percentile | 97/285 (34.0) | 47/285 (16.5) | 27/254 (10.6) |
| 25–74th percentile | 151/435 (34.7) | 73/435 (16.8) | 30/392 (7.7) |
| ≥75th percentile | 89/247 (36.0) | 38/247 (15.4) | 23/235 (9.8) |
| Ptrend | 0.63 | 0.74 | 0.72 |
Data are n/total n (%). LGA, large for gestational age; SGA, small for gestational age.
Table 4 shows the results of regression models with BMI Z score and sum of skinfolds at age 2 years as dependent variables. Neither outcome was associated with maternal glucose during pregnancy either before or after adjustment for other variables. Age at examination was significantly associated with the BMI Z score, and sex was significantly associated with the sum of skinfolds in both simple and multiple regression analysis. Birth weight Z score, sum of skinfolds at birth, and maternal BMI were associated with both the BMI Z score and sum of skinfolds at age 2 years in simple regression analyses. However, after backward elimination of nonsignificant variables, only birth weight Z score and maternal BMI remained independent predictors of BMI Z score at age 2 years, whereas only sum of skinfolds at birth was an independent predictor of sum of skinfolds at age 2 years.
Table 4.
Summary of regression analyses with BMI Z score and sum of skinfolds as dependent variables showing regression coefficients (95% CI) from simple regression analysis and multiple regression after backward elimination of nonsignificant variables
| Independent variables | BMI Z score at age 2 years |
Sum of skin folds at age 2 years§ |
||
|---|---|---|---|---|
| Simple regression (95% CI) | Multiple regression (95% CI)# | Simple regression (95% CI) | Multiple regression (95% CI)†† | |
| n | 1,082 | 990 | ||
| AUC for maternal glucose (mg/dl · hr)† | 0.001 (−0.001 to 0.003) | −0.001 (−0.002 to 0.001) | 0.0001 (0.0000 to 0.0003) | 0.0001 (−0.0001 to 0.0003) |
| Age at examination (years) | 0.85 (0.25 to 1.44)** | 0.79 (0.22 to 1.37)** | −0.012 (−0.069 to 0.046) | 0.001 (−0.056 to 0.058) |
| Male sex | 0.06 (−0.07 to 0.18) | 0.07 (−0.05 to 0.19) | −0.022 (−0.034 to −0.010)*** | −0.019 (−0.031 to −0.007)** |
| Birth weight Z score | 0.29 (0.22 to 0.35)*** | 0.26 (0.19 to 0.32)*** | 0.013 (0.006 to 0.019)*** | — |
| Sum of skin folds at birth‡ | 1.64 (0.95 to 2.33)*** | — | 0.140 (0.073 to 0.207)*** | 0.120 (0.051 to 0.188)*** |
| Maternal BMI (kg/m2) at 24–30 weeks of gestation | 0.038 (0.024 to 0.052)*** | 0.027 (0.012 to 0.040)** | 0.002 (0.000 to 0.003)** | — |
†AUC, area under the curve for maternal pregnancy OGTT.
‡Sum of triceps, subscapular and suprailiac (flank) skin folds (log10-transformed).
§Sum of triceps, subscapular, and suprailiac skin folds (log10-transformed).
#Maternal glucose, age, and sex all forced into model (R2 = 0.078).
††Maternal glucose, age, and sex all forced into model (R2 = 0.027).
**P < 0.01;
***P < 0.001.
CONCLUSIONS
This article reports the first of periodic examinations planned for the offspring of the women participating in the HAPO Study from the Belfast, Northern Ireland, Center. One of the major strengths of this study is the high rate of follow-up of the two-year-old offspring. This is largely the result of low rates of emigration from the community in Northern Ireland and will facilitate further contact with these subjects at older ages. The fact that this is part of a larger family follow-up further adds to recruitment because the HAPO Study mothers are also participating in the survey rather than just bringing their children for the examinations. Because this study was limited to Caucasian subjects in the Belfast Center, these data will complement previous findings among nondiabetic women in a non-Caucasian group (9).
Both birth weight and sum of skinfolds, as a measure of fetal response to maternal glucose, but not maternal glucose itself, were predictive of BMI Z score at age 2 years. Age at examination was also significantly associated with BMI Z score at 2 years, suggesting that even over a 6-month period at this young age children in Northern Ireland are becoming heavier relative to the U.K. standards. At age 2 years, correlations between the maternal glucose concentrations during the OGTT and anthropometric measurements in the offspring are weak. The aim of this article was to examine the association of maternal glucose and other prenatal and perinatal risk factors with childhood obesity, as has been done in previous studies. Consideration of later environmental risk factors, such as type of infant feeding and activity, are outside the scope of this article. In other studies involving offspring of women with frank diabetes during pregnancy, the obesity associated with elevated maternal glucose concentrations resolved during the 1st year or 2 of life so that the offspring of diabetic women were of normal weight (14,15), even though long-term the associations between maternal diabetes and both obesity and abnormal glucose tolerance recurred at older ages (1–6). It is not surprising, therefore, that in this study of offspring of women with normal glucose tolerance during pregnancy there was little association between maternal glucose concentrations and anthropometric measurements at age 2 years. Whether or not we will see a recurrence of the associations that were so strong at birth as these children age remains to be seen.
Acknowledgments
We would like to acknowledge the HAPO Study Cooperative Group and the staff of the HAPO Family Study. The HAPO study was funded by grants from the National Institute of Child Health and Human Development and the National Institute of Diabetes and Digestive and Kidney Diseases (RO1-HD34242 and RO1-HD34243) and Diabetes UK (RD04/0002756), which supported the enrollment and collection of data on participants. This study was supported by a research grant from the Northern Ireland Research and Development Office.
No potential conflicts of interest relevant to this article were reported.
Parts of this study were presented in abstract form at the 68th annual meeting of the American Diabetes Association, San Francisco, California, 6–10 June 2008.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1. Pettitt DJ, Baird HR, Aleck KA, Bennett PH, Knowler WC: Excessive obesity in offspring of Pima Indian women with diabetes during pregnancy. N Engl J Med 1983;308:242–245 [DOI] [PubMed] [Google Scholar]
- 2. Silverman BL, Landsberg L, Metzger BE: Fetal hyperinsulinism in offspring of diabetic mothers. Association with the subsequent development of childhood obesity. Ann NY Acad Sci 1993. October 29;699:36–45 [DOI] [PubMed] [Google Scholar]
- 3. Pettitt DJ, Knowler WC, Bennett PH, Aleck KA, Baird HR: Obesity in offspring of diabetic Pima Indian women despite normal birthweight. Diabetes Care 1987;10:76–80 [DOI] [PubMed] [Google Scholar]
- 4. Pettitt DJ, Aleck KA, Baird HR, Carraher MJ, Bennett PH, Knowler WC: Congenital susceptibility to NIDDM: role of intrauterine environment. Diabetes 1988;37:622–628 [DOI] [PubMed] [Google Scholar]
- 5. Clausen TD, Mathiesen ER, Hansen T, Pedersen O, Jensen DM, Lauenborg J, Damm P: High prevalence of type 2 diabetes and pre-diabetes in adult offspring of women with gestational diabetes mellitus or type 1 diabetes: the role of intrauterine hyperglycemia. Diabetes Care 2008;31:340–346 [DOI] [PubMed] [Google Scholar]
- 6. Silverman BL, Metzger BE, Cho NH, Loeb CA: Impaired glucose tolerance in adolescent offspring of diabetic mothers. Relationship to fetal hyperinsulinism. Diabetes Care 1995. May;18:611–617 [DOI] [PubMed] [Google Scholar]
- 7. Stride A, Shepherd M, Frayling TM, Bulman MP, Ellard S, Hattersley AT: Intrauterine hyperglycemia is associated with an earlier diagnosis of diabetes in HNF-1α gene mutation carriers. Diabetes Care 2002;25:2287–2291 [DOI] [PubMed] [Google Scholar]
- 8. Pettitt DJ, Lawrence JM, Beyer J, Hillier TA, Liese AD, Mayer-Davis B, Loots B, Imperatore G, Liu L, Dolan LM, Linder B, Dabelea D: Association between maternal diabetes in utero and age of offspring's diagnosis of type 2 diabetes. Diabetes Care 2008;31:2126–2130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pettitt DJ, Bennett PH, Saad MF, Charles MA, Nelson RG, Knowler WC: Abnormal glucose tolerance during pregnancy in Pima Indian women: long-term effects on the offspring. Diabetes 1991;40(Suppl. 2):126–130 [DOI] [PubMed] [Google Scholar]
- 10. HAPO Study Cooperative Research Group. The Hyperglycemia and Adverse Pregnancy Outcome (HAPO) study. Int J Gynecol Obstet 2002;78:69–77 [DOI] [PubMed] [Google Scholar]
- 11. HAPO Study Cooperative Research Group. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med 2008;358:1991–2002 [DOI] [PubMed] [Google Scholar]
- 12. Cole TJ, Freeman JV, Preece MA: British 1990 growth reference centiles for weight, height, body mass index and head circumference fitted by maximum penalized likelihood. Stat Med 1998;17(4):407–429 [PubMed] [Google Scholar]
- 13. Kim S, Min W-K, Chun S, Lee W, Chung H-J, Lee PR, Kim A: Quantitative risk estimation for large for gestational age using the area under the 100-g oral glucose tolerance test curve. J Clin Lab Anal 2009;23:213–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Silverman BL, Rizzo T, Green OC, Cho NH, Winter RJ, Ogata ES, Richards GE, Metzger BE: Long-term prospective evaluation of offspring of diabetic mothers. Diabetes 1991;40:(Suppl. 2):121–125 [DOI] [PubMed] [Google Scholar]
- 15. Pettitt DJ: Summary and comment of: Silverman BL, Rizzo T, Green OC, Cho NH, Winter RJ, Ogata ES, Richards GE, Metzger BE. Long-term prospective evaluation of offspring of diabetic mothers (Diabetes 1991;40:(Suppl. 2):121–25). Diabetes Spectrum 1992;5:39–40 [DOI] [PubMed] [Google Scholar]