Abstract
OBJECTIVE
Sulfonylureas have historically been analyzed as a medication class, which may be inappropriate given the differences in properties inherent to the individual sulfonylureas (hypoglycemic risk, sulfonylurea receptor selectivity, and effects on myocardial ischemic preconditioning). The purpose of this study was to assess the relationship of individual sulfonylureas and the risk of overall mortality in a large cohort of patients with type 2 diabetes.
RESEARCH DESIGN AND METHODS
A retrospective cohort study was conducted using an academic health center enterprise-wide electronic health record (EHR) system to identify 11,141 patients with type 2 diabetes (4,279 initiators of monotherapy with glyburide, 4,325 initiators of monotherapy with glipizide, and 2,537 initiators of monotherapy with glimepiride), ≥18 years of age with and without a history of coronary artery disease (CAD) and not on insulin or a noninsulin injectable at baseline. The patients were followed for mortality by documentation in the EHR and Social Security Death Index. Multivariable Cox models were used to compare cohorts.
RESULTS
No statistically significant difference in the risk of overall mortality was observed among these agents in the entire cohort, but we did find evidence of a trend toward an increased overall mortality risk with glyburide versus glimepiride (hazard ratio 1.36 [95% CI 0.96–1.91]) and glipizide versus glimepiride (1.39 [0.99–1.96]) in those with documented CAD.
CONCLUSIONS
Our results did not identify an increased mortality risk among the individual sulfonylureas but did suggest that glimepiride may be the preferred sulfonylurea in those with underlying CAD.
The University Group Diabetes Program (UGDP) raised concern that the administration of tolbutamide, a first-generation sulfonylurea, may increase the risk of cardiovascular death (1). It was largely this uncertainty surrounding sulfonylureas that prompted the UK Prospective Diabetes Study (UKPDS), which itself did not support the suggestion by the UGDP that sulfonylurea therapy increased the risk of cardiovascular mortality (2).
The proposed increased risk of cardiovascular death largely went unexplained until reports surfaced suggesting deleterious effects of some sulfonylureas (glyburide), specifically on the ischemic myocardium (impairment of ischemic preconditioning and/or increased infarct size) (3,4). Interestingly, this has not been observed to be a class effect of the sulfonylureas but an important difference among individual sulfonylureas based largely on their affinity for the three isoforms of the sulfonylurea receptor (SUR1, SUR2A, and SUR2B). SUR1 is largely found in the ATP-dependent K+ channels (KATP channels) of β-cells, whereas SUR2A and SUR2B are largely found in the KATP channels of cardiac and vascular smooth muscle (5,6). Sulfonylureas specific for SUR1, so-called pancreatic-specific sulfonylureas (tolbutamide, chlorpropamide, gliclazide, and glipizide), are specific for the pancreatic β-cells, and thus their effect is largely on potentiating insulin secretion (5,7). Non–pancreatic-specific sulfonylureas (glibenclamide [glyburide] and glimepiride), in addition to potentiating insulin secretion via the β-cells, also exhibit their effects on cardiovascular and vascular smooth muscle (7,8).
Although both glibenclamide (glyburide) and glimepiride have affinity for the SUR2 receptor (non–pancreatic specific), as determined by receptor interaction studies, glimepiride was found not to impair ischemic preconditioning in rats or in human experiments, whereas glibenclamide (glyburide) has been shown to prevent ischemic preconditioning in humans (9–11). A recent cohort analysis by Evans et al. (12) found no difference in mortality between users of pancreatic and non–pancreatic-specific sulfonylureas; however, grouping non–pancreatic-specific sulfonylureas (glimepiride and glibenclamide [glyburide]) together into the same cohort, given their differing effects on ischemic preconditioning, as well as their differing risk of hypoglycemia, may be inappropriate (13).
We have previously reported an increased risk of overall mortality with sulfonylurea monotherapy (14); however, sulfonylureas were analyzed as a class (as they have been historically). It is possible that meaningful clinical differences could exist between the different specific sulfonylureas given their differences in pharmacologic characteristics. Through our enterprise-wide electronic health record (EHR), we were able to identify users of a pancreatic-specific sulfonylurea, glipizide, and two non–pancreatic-specific sulfonylureas, glimepiride and glyburide (glibenclamide), with different effects on the ischemic myocardium (as well as differing risks of hypoglycemia), to determine whether differences in overall mortality risk are present, as this would have important implications when picking a sulfonylurea agent to control glycemia in patients with type 2 diabetes, especially those with documented coronary artery disease (CAD).
RESEARCH DESIGN AND METHODS
The methods of data collection and analysis utilized in this study are similar to those used in our previously published analysis investigating adverse cardiovascular outcomes and overall mortality risk with oral antidiabetes anti-hyperglycemic monotherapy (14). The source population was obtained from an EHR-derived clinical data repository at the Cleveland Clinic. This study was approved by the institutional review board.
For the period 24 October 1998 to 12 October 2006, we identified all newly and previously diagnosed patients with type 2 diabetes using documented ICD-9 and by identifying patients with at least two encounters for diabetes after visiting the Cleveland Clinic main campus or family health centers and who had a prescription for glyburide, glipizide, or glimepiride entered into the EHR. Patients were stratified into three medication cohorts according to the initial prescription entered in the EHR at baseline. All patients were ≥18 years of age and had no history of dialysis at baseline. Patients prescribed insulin or other injectable diabetes medications (as monotherapy or in conjunction with oral agents) and those on multiple oral agents at baseline were excluded.
Follow-up
Follow-up began on the day after the first prescription of the qualifying study drug was entered in the EHR. Patients entered the cohort in a staggered fashion at any time point between 24 October 1998 and 12 October 2006 and from that time were followed until the date of mortality or censoring. Patients with no observed mortality were censored on the last clinic encounter or the date of extraction of vital status from the Social Security Death Index (SSDI) minus a 6-month lag, whichever came last.
Multivariable analysis
A multivariable analysis was utilized to compare patients in each cohort, which allowed us to adjust for differences in baseline characteristics. Variables were chosen and derived based on prior considerations of their clinical relevance with respect to the risk of mortality. The baseline medical history variables chosen for the overall mortality model were as follows: age, sex, race (Caucasian versus non-Caucasian), Modification of Diet in Renal Disease estimated glomerular filtration rate, hemoglobin A1C (A1C), BMI, systolic blood pressure, diastolic blood pressure, HDL cholesterol, LDL cholesterol, triglycerides, smoking status, ACE therapy, or angiotensin receptor blocker therapy, aspirin therapy, clopidogrel therapy, cholesterol-lowering medication, new diabetes, CAD, congestive heart failure, and median household income.
We were unable to use family history or alcohol use as predictor variables due to inconsistent documentation in the EHR. The baseline variables were derived from the EHR on the date closest to the date of the first sulfonylurea prescription up to 21 days after baseline. Missing baseline values were imputed by Chained Equations (Mice) Package, version 1.16 for R, without regard to the outcomes, using regression techniques that included all patients and all baseline values to predict the missing value.
Outcomes
Mortality was defined by documentation of death in the EHR or by being listed as deceased in the SSDI, which allowed us to identify those deceased individuals who were lost to follow-up in the EHR.
Analysis
Analyses were performed using the statistical package R for Windows, version 2.8.1 (R Development Core Team 2008). Survival curves for mortality were estimated with the Kaplan-Meier procedure. Multivariable Cox proportional hazards models were used to derive hazard ratios for the three baseline medication group comparisons. Restricted cubic splines were used to relax linearity assumptions for the continuous variables. After adjustments were made for the baseline covariates, the following comparisons were made in all patients and restricted to patients with a history of CAD:
Glipizide versus glyburide
Glipizide versus glimepiride
Glyburide versus glimepiride
RESULTS
Using the EHR, we were able to identify 4,279 initiators of monotherapy with glyburide, 4,325 initiators of monotherapy with glipizide, and 2,537 initiators of monotherapy with glimepiride, with and without a history of CAD, ≥18 years of age and not on insulin or a noninsulin injectable at baseline. Table 1 shows the distribution of the baseline categorical variables for the entire cohort as well as the subgroup of patients with a history of CAD. The baseline continuous variables for both groups are displayed in Table 2.
Table 1.
Baseline characteristics of the entire cohort and the subgroup of patients with CAD: categorical variables
| Variable | Entire cohort |
Patients with CAD |
||||
|---|---|---|---|---|---|---|
| Glimepiride | Glipizide | Glyburide | Glimepiride | Glipizide | Glyburide | |
| n | 2,537 | 4,325 | 4,279 | 341 | 584 | 580 |
| Male | 1,370 (54.0) | 2,422 (56.0) | 2,408 (56.3) | 233 (68.3) | 400 (68.5) | 419 (72.2) |
| Caucasian | 2,044 (80.6) | 3,237 (74.8) | 3,207 (74.9) | 285 (83.6) | 488 (83.6) | 477 (82.2) |
| Missing | 86 (3.4) | 129 (3.0) | 131 (3.1) | 10 (2.9) | 10 (2.5) | 11 (2.5) |
| Current smoker | 254 (10.0) | 459 (10.6) | 425 (9.9) | 34 (10.0) | 49 (8.4) | 50 (8.6) |
| Never | 836 (33.0) | 1,329 (30.7) | 1,326 (31.0) | 97 (28.4) | 145 (24.8) | 147 (25.3) |
| Passive | 4 (0.2) | 10 (0.2) | 9 (0.2) | 0 (0.0) | 2 (0.3) | 0 (0.0) |
| Quit | 739 (29.1) | 1,241 (28.7) | 1,209 (28.3) | 157 (46.0) | 272 (46.6) | 240 (41.4) |
| Missing | 704 (27.7) | 1,286 (29.7) | 1,310 (30.6) | 53 (15.5) | 116 (19.9) | 143 (24.7) |
| ACE/angiotensin receptor blocker inhibitors | 1,344 (53.0) | 2,213 (51.2) | 2,220 (51.9) | 245 (71.8) | 378 (64.7) | 382 (65.9) |
| Cholesterol-lowering medication | 1,158 (45.6) | 1,922 (44.4) | 1,787 (41.8) | 264 (77.4) | 422 (72.3) | 401 (69.1) |
| Plavix | 221 (8.7) | 333 (7.7) | 322 (7.5) | 90 (26.4) | 101 (17.3) | 113 (19.5) |
| Aspirin | 669 (26.4) | 1,029 (23.8) | 1,017 (23.8) | 178 (52.2) | 277 (47.4) | 263 (45.3) |
| CAD | 341 (13.4) | 584 (13.5) | 580 (13.6) | 341 (100) | 584 (100) | 580 (100) |
| Heart failure | 197 (7.8) | 319 (7.4) | 326 (7.6) | 82 (24.0) | 141 (24.1) | 150 (25.9) |
| New diabetes | 249 (9.8) | 411 (9.5) | 280 (6.5) | 47 (13.8) | 71 (12.2) | 43 (7.4) |
Data are n (%).
Table 2.
Baseline characteristics of the entire cohort and the subgroup of patients with CAD: continuous variables
| Characteristic | Glimepiride | Glipizide | Glyburide | Missing |
|---|---|---|---|---|
| Entire cohort | ||||
| Age (years) | 65.6 ± 13.1 | 66.1 ± 13.3 | 67.8 ± 13.1 | 0.0 |
| BMI (kg/m2) | 31.1 ± 6.5 | 30.8 ± 6.8 | 30.8 ± 6.7 | 49.7 |
| Systolic blood pressure (mmHg) | 134.8 ± 20.8 | 135.1 ± 21.8 | 135.9 ± 22.1 | 24.5 |
| Diastolic blood pressure (mmHg) | 75.8 ± 11.6 | 75.4 ± 11.8 | 74.9 ± 11.8 | 24.5 |
| HDL (mg/dl) | 45.4 ± 14.4 | 45.6 ± 14.2 | 45.9 ± 15.4 | 56.0 |
| LDL (mg/dl) | 105.3 ± 36.4 | 107.0 ± 39.5 | 106.7 ± 39.4 | 57.5 |
| Triglycerides (mg/dl) | 205.8 ± 225.1 | 204.4 ± 193.5 | 192.0 ± 170.9 | 56.5 |
| A1C (%) | 7.5 ± 1.8 | 7.7 ± 1.9 | 7.6 ± 1.8 | 54.5 |
| MDRD eGFR truncated at 90 | 71.2 ± 20.1 | 70.5 ± 20.9 | 69.8 ± 20.3 | 32.1 |
| Zip median income ($) | 46,216.0 ± 14,888.5 | 43,786.1 ± 14,737.8 | 43,477.7 ± 14,583.6 | 0.1 |
| Patients with CAD | ||||
| Age (years) | 68.8 ± 11.2 | 70.3 ± 10.8 | 71.2 ± 10.3 | 0 |
| BMI (kg/m2) | 30.2 ± 6.0 | 29.9 ± 6.0 | 30.3 ± 6.4 | 41.2 |
| Systolic blood pressure (mmHg) | 133.4 ± 21.4 | 130.6 ± 22.1 | 132 ± 23.7 | 12.6 |
| Diastolic blood pressure (mmHg) | 73.3 ± 11.7 | 71.6 ± 11.6 | 71.9 ± 11.7 | 12.7 |
| HDL (mg/dl) | 43.7 ± 12.9 | 43.4 ± 13.2 | 44.2 ± 14.4 | 34.0 |
| LDL (mg/dl) | 90.2 ± 35.6 | 93.9 ± 39.8 | 96.0 ± 36.7 | 35.0 |
| Triglycerides (mg/dl) | 194.8 ± 256.2 | 203.6 ± 195.0 | 191.5 ± 191.0 | 35.0 |
| A1C (%) | 7.3 ± 1.4 | 7.5 ± 1.6 | 7.3 ± 1.5 | 44.1 |
| MDRD eGFR truncated at 90 | 64.9 ± 20.9 | 66.5 ± 20.9 | 62.7 ± 21.1 | 17.9 |
| Zip median income ($) | 47,551.4 ± 15,925.4 | 44,756.9 ± 15,225.5 | 44,871.9 ± 15,716.3 | 0.1 |
Data are means ± SD or percent. eGFR, estimated glomerular filtration rate; MDRD, Modification of Diet in Renal Disease.
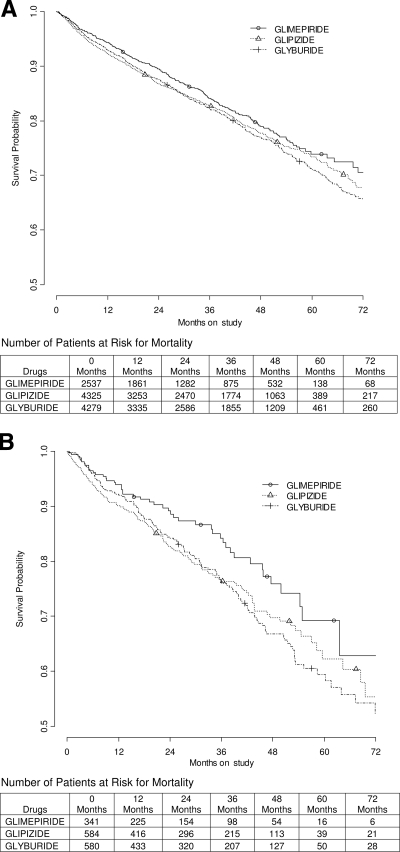
The cohorts contained a total of 1,921 mortality events in the entire cohort (n = 11,141) and 322 in the subgroup with a history of documented CAD (n = 1,505). The survival curves for mortality, for both the entire cohort and for the subgroup with a documented history of CAD, can be seen in Fig. 1. There were 1,753 patients lost to follow-up in the EHR but with vital status from the SSDI. The median follow-up was 2.4 years. The hazard ratios with 95% CIs for the sulfonylurea monotherapy comparisons for mortality in the entire cohort, and the subgroup with documented CAD, can be seen in Table 3, after adjusting for baseline variables.
Figure 1.
Overall mortality in the entire cohort (A) and subgroup with a documented history of CAD (B), treated with sulfonylurea monotherapy. The decreasing numbers of patients at risk for mortality are secondary to the staggered entry of the study subjects, not loss to follow-up. The final status of all patients was ascertained via the SSDI.
Table 3.
Hazard ratio (95% CI) for the sulfonylurea monotherapy comparisons for mortality in the entire cohort and the subgroup with documented CAD
| Hazard ratio (95% CI) | P value | |
|---|---|---|
| Entire cohort contrast | ||
| Glyburide vs. glimepiride | 1.00 (0.89–1.14) | 0.952 |
| Glipizide vs. glyburide | 1.04 (0.94–1.15) | 0.430 |
| Glipizide vs. glimepiride | 1.05 (0.92–1.19) | 0.499 |
| CAD subgroup contrast | ||
| Glyburide vs. glimepiride | 1.36 (0.96–1.91) | 0.081 |
| Glipizide vs. glyburide | 1.03 (0.80–1.31) | 0.838 |
| Glipizide vs. glimepiride | 1.39 (0.99–1.96) | 0.059 |
Mortality model adjusted for baseline covariates: age, sex, race (Caucasian vs. non-Caucasian), Modification of Diet in Renal Disease estimated glomerular filtration rate, A1C, BMI, systolic blood pressure, diastolic blood pressure, HDL cholesterol, LDL cholesterol, triglycerides, smoking status, ACE or angiotensin receptor blocker therapy, aspirin therapy, clopidogrel therapy, cholesterol-lowering medication, new diabetes, CAD, congestive heart failure, and median household income.
For the period 24 October 1998 to 12 October 2006, no difference in overall mortality risk was found with glipizide versus glyburide (hazard ratio 1.04 [95% CI 0.94–1.15]), glipizide versus glimepiride (1.05 [0.92–1.19]), or with glyburide versus glimepiride (1.00 [0.89–1.14]). The subanalysis on patients with documented CAD revealed a trend toward an increased overall mortality risk with glyburide versus glimepiride (1.36 [0.96–1.91]) and glipizide versus glimepiride (1.39 [0.99–1.96]). No difference (or trend) in overall mortality within the subgroup was appreciated with glipizide versus glyburide (1.03 [0.80–1.31]).
CONCLUSIONS
The present study did not find a statistically significant difference in the risk of overall mortality among the various treatment options, suggesting that overall mortality is not substantially influenced by the choice of sulfonylurea. However, in the subanalysis of patients with documented CAD, a trend toward an increased overall mortality risk with glyburide versus glimepiride (hazard ratio 1.36 [95% CI 0.96–1.91]), and surprisingly a trend toward an increased risk of mortality with the SUR1-specific sulfonylurea glipizide versus glimepiride (1.39 [0.99–1.96]), were observed, suggesting that glimepiride may be the preferred sulfonylurea in those with underlying CAD.
Although the study did not find any obvious difference in mortality risk between patients treated with specific sulfonylureas, it is still possible that some differences in mortality may truly exist. There were significantly fewer patients in the CAD subanalysis, and the results showed a strong trend toward a reduced risk with glimepiride. It is quite possible that a larger sample size would have detected a significant difference. However, it would not be appropriate to perform a post hoc power calculation since nonsignificant P values will tend to be associated with low power even if the sample size was adequate (15). A clinically meaningful difference in mortality would seem unlikely in the main analysis of all patients given the large sample size. The point estimates (hazard ratios) were all very close to 1.
Substantial multicollinearity in a regression model can cause erroneous conclusions about the association between individual variables (e.g., sulfonylurea type) and the outcome of interest. We calculated the variance inflation factors for the sulfonylurea comparisons. The variance inflation factors ranged from 1.93 to 1.95 in the entire cohort and 2.35 to 2.40 in the subset of patients with documented CAD, which, according to Snee (16), suggests substantial multicollinearity is unlikely to be present.
There is a discrepancy within the literature regarding the risk of mortality (overall or cardiovascular mortality) with specific sulfonylureas. A recent report found no substantial (statistically significant) differences in either 30-day or 1-year mortality in users of various sulfonylureas after myocardial infarction (although use of gliclazide monotherapy showed a trend toward lower mortality [hazard ratio 0.70 {95% CI 0.48–1.0}]), suggesting that mortality is not substantially influenced by the choice of sulfonylurea (17). However, Khalangot et al. (18) found that total mortality was lower for gliclazide and glimepiride versus glibenclamide (glyburide) treatment (0.33 [0.26–0.41], P < 0.001, and 0.605 [0.41–0.89], P < 0.01, respectively) as well as a reduced cardiovascular mortality with gliclazide versus glibenclamide (glyburide) (0.29 [0.21–0.38], P < 0.001). The point estimates (hazard ratios) differ greatly between the analyses conducted by Horsdal et al. (17) and ourselves when compared with the analysis by Khalangot et al. (18), likely because Khalangot et al. adjusted for few variables, many of which may have caused confounding.
There are a variety of proposed mechanisms for an increased mortality risk with specific sulfonylureas. Despite the differing effects of individual sulfonylureas on the SUR receptors and myocardial ischemic preconditioning, there are also differing effects regarding the risk of hypoglycemia, independent of their SUR-binding characteristics, which may be influencing mortality (13). Among the sulfonylureas studied in our analysis, glyburide is the most common agent associated with documented hypoglycemia (19). Glyburide has been shown to continue to stimulate insulin secretion in the setting of profound hypoglycemia to a greater extent when compared with glimepiride (20), in part because glyburide accumulates within the β-cell (21), unlike other sulfonylureas, prolonging insulin secretion. Thus, hypoglycemia could be playing a dominant role in increasing the risk of mortality (more so than differing selectivity and effects on the SUR receptors and ischemic preconditioning, respectively), which has previously been reported with sulfonylureas, specifically when compared with metformin (14,22–24). Other than the increased risk of hypoglycemia documented with glyburide (and the differences in other pharmacologic properties inherent to the individual sulfonylureas: SUR specificity and effects on ischemic myocardium), glipizide, glimepiride, and glyburide generally have very similar side-effect profiles.
The current study has limitations inherent to most retrospective studies. The analysis was based on exposure to a medication based on the initial prescription entered in the EHR; however, there is no documentation of compliance with the prescribed medication. The prescribed medication at baseline defined which medication group the patient belonged; however, the medication exposure times after baseline are unknown. Current clinical practice procedures suggest that it is more likely for additional agents to be added to a baseline medication than to switch from one class of medication to another or from one sulfonylurea to another. Approximately 70% of the cohort remained on a single drug (baseline medication) throughout their time in the cohort.
The medication groups in our study were not balanced with respect to baseline variables and risk factors; however, the multivariable analysis adjusted for the differences in baseline variables and risk factors that had the most relevance with respect to the risk of mortality. Although some covariates may have changed over time, we would not anticipate these changes to favor one specific sulfonylurea versus another (besides the inherent characteristics of the individual agents). Nonetheless, we could not adjust for differences in unmeasured variables or characteristics.
Sulfonylurea monotherapy was not randomized in the present study, so selection bias may be present. It is possible that one sulfonylurea may have been chosen over another because of cost (the Food and Drug Administration did not approve first-time generic formulations of glimepiride until November 2005), patient age, reduced glomerular filtration rate, risk of hypoglycemia, or perceived differing effects on myocardial ischemic preconditioning. However, although age and renal insufficiency are associated with an increased risk of death, the multivariable analysis adjusted for differences in baseline age and renal function, so this should not explain the results. To take into account the fact that generic glimepiride was not available throughout the entire duration of our study, we adjusted for socioeconomic status by including the median household income estimated from zip code data from the 2000 census in the multivariable analysis.
The strengths of the study include a large cohort of patients followed up to 8 years and real-world effect of the medications in a diverse patient population. In addition, we adjusted for many baseline variables (accurately captured by the EHR) that have substantial effects on mortality. Furthermore, linking our outcome to the SSDI allowed us to capture mortality in those patients lost to follow-up in the EHR.
Our results did not identify an increased mortality risk among the individual sulfonylureas (glyburide, glipizide, or glimepiride) in the entire cohort but did find evidence of a trend toward an overall mortality reduction with glimepiride in those with documented CAD, suggesting that glimepiride may be the preferred sulfonylurea in those with underlying CAD. The literature contains conflicting results regarding whether an increased overall mortality (or cardiovascular mortality) risk accompanies the various sulfonylureas (12,17,18). This discrepancy would support prospective studies to determine whether the difference in pharmacologic properties inherent to individual sulfonylureas translates into differences in the risk of adverse cardiovascular outcomes and overall mortality, especially in patients with preexisting CAD.
Acknowledgments
The study was supported through a research grant from Astra Zeneca (to M.W.K.).
R.S.Z. has provided consulting and lecture services for Daiichi Sankyo, Merck, Pfizer, Novo Nordisk, and Glaxo-SmithKline within the past 12 months. M.W.K., A.J., and A.A. have received grant support from Astra Zeneca. K.P., B.W., C.Y., and S.A. report no potential conflicts of interest.
Parts of this study were submitted in abstract form for presentation at The Endocrine Society National Meeting, June 2010.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1. The University Group Diabetes Program. A study of the effects of hypoglycemic agents on vascular complications in patients with adult-onset diabetes: V. evaluation of pheniformin therapy. Diabetes 1975;24(Suppl. 1):65–184 [PubMed] [Google Scholar]
- 2. UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998;352:837–853 [PubMed] [Google Scholar]
- 3. Gross GJ, Auchampach JA: Blockade of ATP-sensitive potassium channels prevents myocardial preconditioning in dogs. Circ Res 1992;70:223–233 [DOI] [PubMed] [Google Scholar]
- 4. Grover GJ, Sleph PG, Dzwonczyk S: Role of myocardial ATP-sensitive potassium channels in mediating preconditioning in the dog heart and their possible interaction with adenosine A1-receptors. Circulation 1992;86:1310–1316 [DOI] [PubMed] [Google Scholar]
- 5. Gribble FM, Tucker SJ, Seino S, Ashcroft FM: Tissue specificity of sulfonylureas: studies on cloned cardiac and β-cell K(ATP) channels. Diabetes 1998;47:1412–1418 [DOI] [PubMed] [Google Scholar]
- 6. Ashcroft FM, Gribble FM: ATP-sensitive K+ channels and insulin secretion: their role in health and disease. Diabetologia 1999;42:903–919 [DOI] [PubMed] [Google Scholar]
- 7. Gribble FM, Reimann F: Sulphonylurea action revisited: the post-cloning era. Diabetologia 2003;46:875–891 [DOI] [PubMed] [Google Scholar]
- 8. Song DK, Ashcroft FM: Glimepiride block of cloned beta-cell, cardiac and smooth muscle K(ATP) channels. Br J Pharmacol 2001;133:193–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mocanu MM, Maddock HL, Baxter GF, Lawrence CL, Standen NB, Yellon DM: Glimepiride, a novel sulfonylurea, does not abolish myocardial protection afforded by either ischemic preconditioning or diazoxide. Circulation 2001;103:3111–3116 [DOI] [PubMed] [Google Scholar]
- 10. Klepzig H, Kober G, Matter C, Luus H, Schneider H, Boedeker KH, Kiowski W, Amann FW, Gruber D, Harris S, Burger W: Sulfonylureas and ischaemic preconditioning; a double-blind, placebo-controlled evaluation of glimepiride and glibenclamide. Eur Heart J 1999;20:439–446 [DOI] [PubMed] [Google Scholar]
- 11. Tomai F, Crea F, Gaspardone A, Versaci F, De Paulis R, Penta de Peppo A, Chiariello L, Gioffre PA: Ischemic preconditioning during coronary angioplasty is prevented by glibenclamide, a selective ATP-sensitive K+ channel blocker. Circulation 1994;90:700–705 [DOI] [PubMed] [Google Scholar]
- 12. Evans JM, Ogston SA, Reimann F, Gribble FM, Morris AD, Pearson ER: No differences in mortality between users of pancreatic-specific and non-pancreatic-specific sulphonylureas: a cohort analysis. Diabetes Obes Metab 2008;10:350–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tayek J: SUR receptor activity vs. incidence of hypoglycaemia and cardiovascular mortality with sulphonylurea therapy for diabetics. Diabetes Obes Metab. 2008;10:1128–1129; author reply 1129–30 [DOI] [PubMed] [Google Scholar]
- 14. Pantalone KM, Kattan MW, Yu C, Wells BJ, Arrigain S, Jain A, Atreja A, Zimmerman RS: The risk of developing coronary artery disease or congestive heart failure, and overall mortality, in type 2 diabetic patients receiving rosiglitazone, pioglitazone, metformin, or sulfonylureas: a retrospective analysis. Acta Diabetol 2009;46:145–154 [DOI] [PubMed] [Google Scholar]
- 15. Hoenig JM, Heisey DM: The abuse of power: the pervasive fallacy of power calculations for data analysis. American Stat 2001;55:19–24 [Google Scholar]
- 16. Snee RD: Validation of regression models: methods and examples. Technometrics 1977;19:415–428 [Google Scholar]
- 17. Horsdal HT, Johnsen SP, Sondergaard F, Jacobsen J, Thomsen RW, Schmitz O, Sorensen HT, Rungby J: Sulfonylureas and prognosis after myocardial infarction in patients with diabetes: a population-based follow-up study. Diabetes Metab Res Rev 2009;25:515–522 [DOI] [PubMed] [Google Scholar]
- 18. Khalangot M, Tronko M, Kravchenko V, Kovtun V: Glibenclamide-related excess in total and cardiovascular mortality risks: data from a large Ukrainian observational cohort study. Diabetes Res Clin Pract 2009;86:247–253 [DOI] [PubMed] [Google Scholar]
- 19. Gangji AS, Cukierman T, Gerstein HC, Goldsmith CH, Clase CM: A systematic review and meta-analysis of hypoglycemia and cardiovascular events: a comparison of glyburide with other secretagogues and with insulin. Diabetes Care 2007;30:389–394 [DOI] [PubMed] [Google Scholar]
- 20. Szoke E, Gosmanov NR, Sinkin JC, Nihalani A, Fender AB, Cryer PE, Meyer C, Gerich JE: Effects of glimepiride and glyburide on glucose counterregulation and recovery from hypoglycemia. Metabolism 2006;55:78–83 [DOI] [PubMed] [Google Scholar]
- 21. Hellman B, Sehlin J, Taljedal IB: Glibenclamide is exceptional among hypoglycaemic sulphonylureas in accumulating progressively in beta-cell-rich pancreatic islets. Acta Endocrinol (Copenh) 1984;105:385–390 [DOI] [PubMed] [Google Scholar]
- 22. Johnson JA, Majumdar SR, Simpson SH, Toth EL: Decreased mortality associated with the use of metformin compared with sulfonylurea monotherapy in type 2 diabetes. Diabetes Care 2002;25:2244–2248 [DOI] [PubMed] [Google Scholar]
- 23. Johnson JA, Simpson SH, Toth EL, Majumdar SR: Reduced cardiovascular morbidity and mortality associated with metformin use in subjects with type 2 diabetes. Diabet Med 2005;22:497–502 [DOI] [PubMed] [Google Scholar]
- 24. Evans JM, Ogston SA, Emslie-Smith A, Morris AD: Risk of mortality and adverse cardiovascular outcomes in type 2 diabetes: a comparison of patients treated with sulfonylureas and metformin. Diabetologia 2006;49:930–936 [DOI] [PubMed] [Google Scholar]