Abstract
OBJECTIVE
In the Diabetes Therapy Utilization: Researching Changes in A1C, Weight and Other Factors Through Intervention with Exenatide Once Weekly (DURATION-1) study, the safety and efficacy of 30 weeks of treatment with the glucagon-like peptide-1 receptor agonist exenatide once weekly (exenatide QW; 2 mg) was compared with exenatide BID in 295 patients with type 2 diabetes. We now report the safety and efficacy of exenatide QW in 1) patients who continued treatment for an additional 22 weeks (52 weeks total) and 2) patients who switched from exenatide BID to exenatide QW after 30 weeks.
RESEARCH DESIGN AND METHODS
In this randomized, multicenter, comparator-controlled, open-label trial, 258 patients entered the 22-week open-ended assessment phase (n = 128 QW-only; n = 130 BID→QW). A1C, fasting plasma glucose (FPG), body weight, blood pressure, fasting lipids, safety, and tolerability were assessed.
RESULTS
Patients continuing exenatide QW maintained A1C improvements through 52 weeks (least squares mean −2.0% [95% CI −2.1 to −1.8%]). Patients switching from exenatide BID to exenatide QW achieved further A1C improvements; both groups exhibited the same A1C reduction and mean A1C (6.6%) at week 52. At week 52, 71 and 54% of all patients achieved A1C <7.0% and ≤6.5%, respectively. In both treatment arms, FPG was reduced by >40 mg/dl, and body weight was reduced by >4 kg after 52 weeks. Nausea occurred less frequently in this assessment period and was predominantly mild. No major hypoglycemia was observed.
CONCLUSION
Exenatide QW elicited sustained improvements in glycemic control and body weight through 52 weeks of treatment. Patients switching to exenatide QW experienced further improvements in A1C and FPG, with sustained weight loss.
Type 2 diabetes is a complex and increasingly prevalent disease associated with interrelated comorbidities, including obesity, dyslipidemia, and hypertension. The importance of treating not only hyperglycemia, but also the associated comorbidities, is recognized as necessary to reduce the risk of complications, particularly cardiovascular disease (1). Lifestyle modification can improve glycemic control as well as body weight, blood pressure, and lipid profiles; however, behavioral modifications are inherently difficult, and most patients eventually require multiple medications (2–6). Although several classes of antihyperglycemic medications are currently indicated for the treatment of type 2 diabetes, most of them do not improve the comorbidities and several are associated with weight gain.
Exenatide, a glucagon-like peptide-1 receptor (GLP-1R) agonist, improves glycemic control in patients with type 2 diabetes through multiple mechanisms of action: increased glucose-dependent insulin secretion, attenuated postprandial glucagon secretion, slowed gastric emptying, and increased satiety (7,8). The twice-daily formulation of exenatide (exenatide BID) improves both fasting and postprandial glucose control, resulting in A1C reductions of roughly 0.8–1.0% in placebo-controlled trials (9–12) and 1.0–1.4% in open-label trials (13–15). These improvements in glucose control were maintained in patients completing 3 years of treatment (−1.0%) (16). Exenatide therapy is also associated with weight loss and improvement in cardiovascular risk factors, including blood pressure and serum lipid profiles (16). Furthermore, the glucose-dependent mechanisms of action of exenatide minimize the risk of hypoglycemia. GLP-1R agonists have recently been added to the American Diabetes Association and European Association for the Study of Diabetes consensus algorithm for the treatment of type 2 diabetes as an option after the addition of metformin in patients in whom body weight and hypoglycemia risk are concerns (1).
Exenatide BID is administered within the 60-min period before the morning and evening meals and primarily exerts its pharmacodynamic effects on glucose concentrations during the postprandial period. A long-acting once-weekly formulation of exenatide (exenatide QW) has been developed. Weekly administration of 2 mg exenatide QW results in therapeutic plasma exenatide concentrations within 2 weeks and steady-state plasma exenatide concentrations within the therapeutic target range 6–7 weeks after initiation of therapy (17,18).
The Diabetes Therapy Utilization: Researching Changes in A1C, Weight and Other Factors Through Intervention with Exenatide Once Weekly (DURATION-1) trial was designed as a two-stage protocol. We previously reported the first stage, a randomized open-label comparison of exenatide QW to exenatide BID in patients with type 2 diabetes over 30 weeks (17). Both therapies improved glycemic control, and the improvement in A1C observed with exenatide QW treatment was significantly greater than that observed with exenatide BID (−1.9 vs. −1.5%, respectively). Similar improvements in body weight, blood pressure, and fasting lipids were demonstrated with both forms of exenatide therapy. We now describe 52-week results from the second phase of the DURATION-1 trial which examined the safety and efficacy of 1) switching from exenatide BID to exenatide QW after 30 weeks of treatment and 2) continuing exenatide QW treatment for an additional 22 weeks (52 weeks total).
RESEARCH DESIGN AND METHODS
Randomization and interventions
Patients were randomly assigned to one of two open-label treatment groups: weekly subcutaneous injections of 2 mg exenatide QW or 5 μg exenatide BID for the first 28 days followed by a required dose increase to 10 μg BID for the remainder of the 30-week assessment period. The inclusion and exclusion criteria and results from the initial 30 weeks have been reported previously (17). At 30 weeks, participants entered a second, open-ended treatment period in which all patients received exenatide QW. Patients who switched from exenatide BID to exenatide QW treatment and were concomitantly using a sulfonylurea (SFU) were required to reduce their SFU dose to the minimum recommended dose until week 40. Subsequently, the SFU dose was up-titrated based on daily glucose measurements to reach a target fasting plasma glucose (FPG) of ≤110 mg/dl. Patients originally randomly assigned to exenatide QW maintained their treatment regimen.
A common clinical protocol was approved for each site by the appropriate institutional review board. Patients provided written informed consent before participation. The study was conducted in accordance with the principles described in the Declaration of Helsinki, including all amendments through the South Africa revision (19).
Outcomes
For this second phase of the study, glucose control during the transition from exenatide BID to exenatide QW was examined, as well as the safety, tolerability, and efficacy of 52 weeks of exenatide QW treatment. Plasma analytes and A1C were quantitated by Quintiles Laboratories (Smyrna, GA) using standard methods. A1C was measured using high-performance liquid chromatography. Plasma antibodies to exenatide were measured by Millipore Corporation Drug Discovery (St. Charles, MO) using an enzyme-linked immunosorbent assay (20). Titers of antibodies to exenatide were determined by serial 1:5 dilutions after an initial dilution of 1:25 (also expressed as the reciprocal of the highest dilution of sample serum that tests positive in the assay). Blood pressure was measured after ∼5 min of quiet rest in a sitting position, repeated after at least 30 s, and the two measurements were averaged.
Statistical analysis
The intent-to-treat (ITT) population comprised all randomly assigned patients who received at least one injection of exenatide in the previously reported phase of this study (17). The 52-week evaluable population consisted of ITT patients who completed the study visits to at least week 48 in compliance with the protocol. Descriptive statistics on demographics, analysis of primary glycemic end points, body weight, and fasting lipid concentrations are provided for the 52-week evaluable population. All safety analyses, including blood pressure measurements, are provided for the ITT population. The analyses of A1C were based on a general linear model (ANOVA) including original treatment assignment, baseline A1C stratum, and concomitant SFU use at screening. Baseline FPG, body weight, and blood pressure were added in the model (ANCOVA) for FPG, body weight, and blood pressure, respectively. Data for these efficacy end points are expressed as least squares means. There were no substantial differences in A1C (Fig. 2B) or other measures (data not shown) when the evaluable population, the 52-week ITT population, or those who participated after 30 weeks were examined. Therefore, only data for the evaluable population were reported. Statistical analysis was performed using SAS (version 8.2; SAS Institute, Cary, NC).
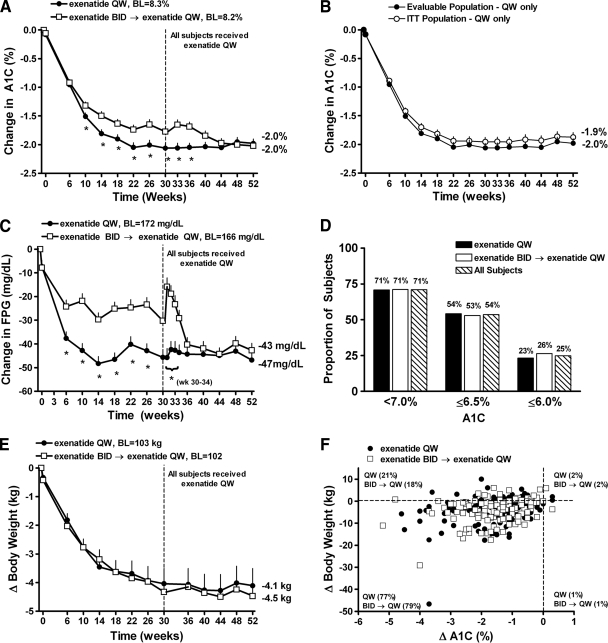
Figure 2.
Glycemic control and body weight over 52 weeks. A: Least squares mean ± SE changes in A1C over 52 weeks for the evaluable population (exenatide QW-only n = 120; exenatide BID→exenatide QW n = 121). B: Least squares mean ± SE change in A1C for ITT (n = 148) and evaluable population patients receiving only exenatide QW for 52 weeks. C: Change in fasting plasma glucose over 52 weeks for the evaluable population. D: Proportion of patients achieving A1C targets of <7.0, ≤6.5, and ≤6.0%. E: Least squares mean ± SE changes in body weight over 52 weeks for the evaluable population. F: Scatterplot of change in A1C vs. change in body weight. *P < 0.05 versus exenatide BID→exenatide QW. BL, baseline.
Treatment-emergent adverse events were defined as those occurring during or after the first injection of exenatide QW in the open-ended assessment period. Treatment-emergent adverse events during the initial 30 weeks of the trial were reported previously (17). Hypoglycemia was categorized as major if the event 1) in the judgment of the investigator or physician, resulted in a loss of consciousness, seizure, or coma and resolved after administration of glucagon or glucose or 2) required third-party assistance to resolve and the subject had a glucose value of <54 mg/dl. Minor hypoglycemia was defined as a report of symptoms consistent with hypoglycemia and a glucose value of <54 mg/dl before treatment of the episode.
RESULTS
Patient disposition and baseline characteristics
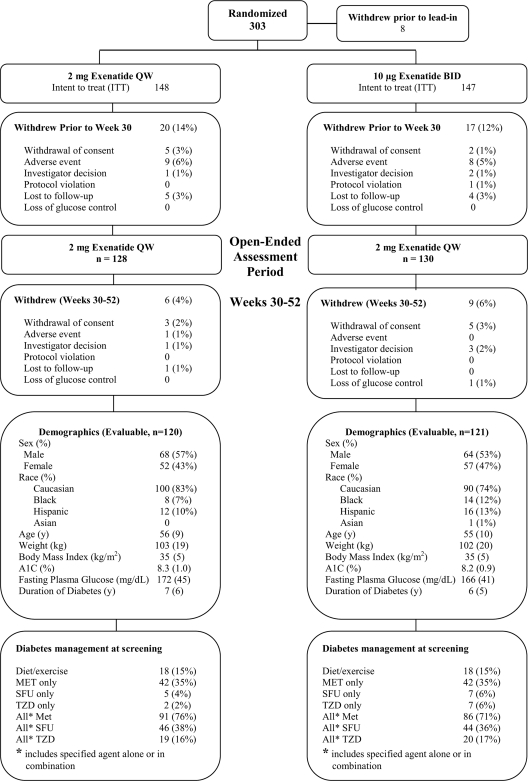
Of the 295 ITT patients, 258 patients (87%) continued into the second phase of the trial (Fig. 1). Demographics and background therapy were similar between the two 52-week evaluable cohorts. Overall, 243 patients completed 52 weeks of treatment, representing 82% of the original ITT population and 94% of the population entering the second predefined treatment period.
Figure 1.
Enrollment, patient disposition, and baseline characteristics. Of the 303 patients originally randomized to the study, 258 entered the subsequent 22-week assessment period. Fifteen patients withdrew during the 22-week assessment period, resulting in an evaluable population of n = 241 (n = 120 QW only; n = 121 BID→QW). Demographics of the ITT population are available in Drucker et al. (17) and represent the information at the original baseline. MET, metformin; SFU, sulfonylurea; TZD, thiazolidinedione.
Efficacy of exenatide QW
During this 22-week assessment period, patients who continued exenatide QW treatment maintained improvements in A1C (Fig. 2A), with a least squares mean (95% CI) change from baseline A1C of −2.1% (−2.2 to −1.9%) at week 30 and −2.0% (−2.1 to −1.8%) at week 52. The time course and durability of A1C improvements were similar for the ITT population and 52-week evaluable population (Fig. 2B). Patients who switched from exenatide BID to exenatide QW (week 30 A1C reduction −1.8% [−1.9 to −1.6%]) exhibited further improvements in glycemic control such that they achieved the same A1C reduction (−2.0%) and mean A1C (6.6%) at week 52 as patients receiving only exenatide QW. After 52 weeks of treatment, 71% of all patients achieved A1C <7.0% and 54% achieved A1C ≤6.5% (similar between cohorts) (Fig. 2D). In patients with a baseline A1C <9.0% (baseline A1C 7.8%), A1C reduction at week 52 was −1.2% (−1.4 to −1.1%) and −1.3% (−1.5 to −1.2%) in the exenatide QW-only and exenatide BID→exenatide QW groups, respectively (supplementary Fig. 1, available in an online appendix at http://care.diabetesjournals.org/cgi/content/full/dc09-1914/DC1). Larger reductions in A1C at week 52 were achieved in patients with a baseline A1C ≥9.0%: −2.8% (−3.1 to −2.5%) for the exenatide QW-only group (baseline A1C 9.7%) and −2.6% (−3.0 to −2.3%) in the exenatide BID→exenatide QW group (baseline A1C 9.6%).
Body weight decreased similarly in both treatment groups. At week 52, the least squares mean (95% CI) changes in body weight were −4.1 kg (−5.3 to −2.9 kg) and −4.5 kg (−5.7 to −3.3 kg) in the exenatide QW-only and exenatide BID→exenatide QW groups, respectively (Fig. 2E). The distribution of the change in A1C with respect to changes in body weight is shown in Fig. 2F. Ninety-seven percent of all patients achieved reductions in A1C over 52 weeks (similar between cohorts) and most patients (77 and 79% of exenatide QW-only and exenatide BID→exenatide QW, respectively) achieved reductions in both A1C and body weight. Significant A1C reductions were observed in both treatment groups regardless of weight loss (supplementary Fig. 2, available in an online appendix).
Glucose control during transition from exenatide BID to exenatide QW
In patients who received exenatide QW for 52 weeks, least squares mean (95% CI) reductions in FPG at week 30 (−46 mg/dl [−52 to −40 mg/dl]) were maintained through week 52 (−47 mg/dl [−53 to −41 mg/dl]) (Fig. 2C). Patients who switched from exenatide BID to exenatide QW achieved a similar reduction in FPG at week 52 (−43 mg/dl [−49 to −37 mg/dl]) (Fig. 2C) relative to patients who received exenatide QW for 52 weeks. Subsequent to week 30, BID-treated patients who switched to exenatide QW experienced a transient rise in mean FPG concentration followed by a rapid decrease within 2 weeks of the switch (Fig. 2C). By 3–4 weeks after initiation of QW treatment, the mean FPG in this group of subjects had returned to levels observed before they switched to exenatide QW, which was followed by further improvements such that ultimately the two groups exhibited similar reductions in FPG. Patients concomitantly taking an SFU (n = 44) reduced their SFU dose to the minimum recommended dose at week 30 according to protocol and experienced a greater rise in FPG during the transition than patients not taking an SFU (data not shown). At week 52, 45% (n = 20) of these patients received a lower SFU dose compared with their SFU dose immediately before switching to exenatide QW, whereas 43% (n = 19) received the same SFU dose and 9% (n = 4) increased the SFU dose at week 52 compared with week 30. One patient discontinued SFU treatment after switching to exenatide QW.
Effects on blood pressure and fasting lipids
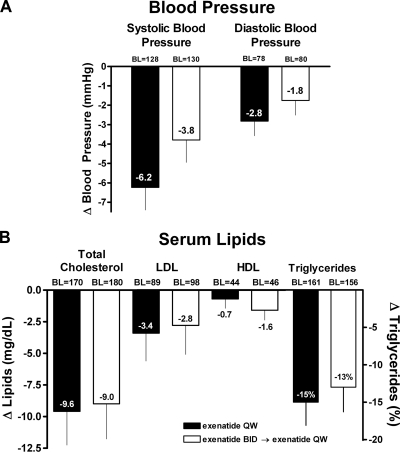
Clinically significant blood pressure improvements were observed in patients treated with exenatide QW for 52 weeks (least squares mean [95% CI] from baseline: systolic blood pressure [SBP] −6.2 mmHg [95% CI −8.5 to −3.9 mmHg], and diastolic blood pressure [DBP] −2.8 mmHg [−4.3 to −1.3 mmHg]) and in patients switching from exenatide BID to exenatide QW (SBP −3.8 mmHg [−6.1 to −1.5 mmHg] and DBP −1.8 mmHg [−3.2 to −0.3 mmHg]) (Fig. 3A). Fifty and 46% of patients (exenatide QW and exenatide BID→exenatide QW, respectively) with elevated SBP (≥130 mmHg) at baseline achieved normal SBP at week 52. Changes in concomitant antihypertensive medications were only allowed if deemed necessary by the investigator. The majority (84%) of the 154 subjects who had been using an antihypertensive medication at screening and completed 52 weeks of treatment did not change their dose. Of patients who did change their dose, 9 patients increased their dose, 13 patients decreased their dose, and 1 patient stopped the medication. In addition, 14 patients initiated antihypertensive medication after screening. Improvements in serum lipid profiles were demonstrated in both treatment groups, with clinically significant reductions in total cholesterol (−9.6 mg/dl [−14.8 to −4.3 mg/dl] and −9.0 mg/dl [−14.5 to −3.6 mg/dl]) (Fig. 3B) and triglycerides (−15% [−21 to −9%].
Figure 3.
Change from baseline in blood pressure and serum lipids. A: Patients (ITT population) in both treatment groups exhibited favorable changes (least squares mean change from baseline ± SE) in blood pressure after 52 weeks of treatment. B: Treatment with exenatide QW was associated with favorable changes in serum lipids (evaluable population). Data are presented as least squares mean ± SE for all lipids except for triglycerides (geometric least squares mean milligrams per deciliter baseline; geometric least squares mean percent change ± SE from baseline). ITT population: exenatide QW-only n = 148; exenatide BID→exenatide QW n = 147. Evaluable population: exenatide QW-only n = 120; exenatide BID→exenatide QW n = 121. BL, baseline.
Safety and tolerability
Treatment-emergent adverse events that occurred for the first time or worsened during this second phase of DURATION-1 were similar to those observed during the 30-week assessment period (Table 1) (17). Nausea was predominantly mild in intensity. No severe nausea was reported. Twenty-one patients (8%; n = 4 for exenatide QW-only and n = 17 exenatide BID→exenatide QW) reported injection-site related adverse events including bruising, erythema, hemorrhage, induration, pain, and pruritus. Mild to moderate injection site pruritus was observed after switching from exenatide BID to exenatide QW in six patients (five of these patients did not experience pruritus during the initial 30-week assessment). No injection site–related adverse events led to withdrawal. There were no episodes of major hypoglycemia; the incidence of minor hypoglycemia was low and was limited to patients using a concomitant SFU during weeks 30–52 (9% of patients receiving an SFU) (supplementary Table 1, available in an online appendix). Antibodies to exenatide titer peaked at week 6 for both treatment groups (geometric mean ± SE 33.2 ± 8.2 and 12.6 ± 3.4 for exenatide QW-only and exenatide BID→exenatide QW groups, respectively). At week 52, antibody titers to exenatide were 12.8 ± 3.4 and 8.9 ± 2.1 for exenatide QW-only and exenatide BID→exenatide QW groups, respectively (supplementary Table 2, available in an online appendix). Antibodies to exenatide were not predictive of individual A1C change or incidence of treatment-emergent adverse events. One patient withdrew because of an adverse event (worsening of type 2 diabetes) during the 22-week assessment period (exenatide QW-only group). No cases of pancreatitis were reported.
Table 1.
Treatment-emergent adverse events ≥5% incidence during open-ended assessment period (week 30 through week 52)
| Preferred term | Exenatide QW (n = 128) | Exenatide BID→Exenatide QW (n = 130) |
|---|---|---|
| Upper respiratory tract infection | 12.5 | 12.3 |
| Diarrhea | 8.6 | 6.9 |
| Nausea | 7.0 | 7.7 |
| Nasopharyngitis | 7.8 | 4.6 |
| Sinusitis | 4.7 | 6.9 |
| Vomiting | 6.3 | 4.6 |
| Urinary tract infection | 2.3 | 5.4 |
| Injection site bruising | 0 | 5.4 |
Data are % unless otherwise indicated. Adverse events that occurred for the first time or existed before week 30 and worsened after the first injection at week 30 through study termination are reported.
CONCLUSIONS
The improvements in glycemic control and body weight observed after 30 weeks of exenatide QW treatment (17) were sustained in patients continuing exenatide QW treatment for 52 weeks. Patients switching from exenatide BID to exenatide QW exhibited further improvements in glycemic control. Similar improvements in glycemic control, body weight, and cardiovascular risk factors were observed at week 52 regardless of initial randomized treatment. Although the absence of a comparator arm is a limitation of this study and the open-label trial design inherently offers the potential for bias and can affect patient expectations, the results of this study demonstrated reductions in A1C and body weight comparable to those in a previously reported double-blind, placebo-controlled study of exenatide QW at the same dose (18) and were consistent with continuous exenatide exposure. These improvements were greater than those previously observed with exenatide BID in both placebo-controlled (9–12) and open-label comparator trials (13–15). It is important to note that improvements in A1C were observed across the spectrum of baseline A1C values and weight loss categories. Treatment with exenatide QW was generally well tolerated and treatment-emergent adverse events were generally mild to moderate. As in the initial 30-week phase, the only incidences of mild to moderate hypoglycemia occurred in patients concomitantly receiving an SFU.
It is noteworthy that patients switching from exenatide BID to exenatide QW experienced a transient elevation in FPG, which generally improved within 2 weeks after initiation of QW therapy. This transient rise in FPG can be largely attributed to the time required for plasma exenatide levels to reach the therapeutic range after initiation of exenatide QW and was of a magnitude (∼15 mg/dl) that is unlikely to be associated with symptoms or harm over a short period of time. In addition, subjects who switched treatment and were using a concomitant SFU were required to reduce their SFU dose to the minimum recommended dose for initiation of exenatide QW treatment. Therefore, the transient rise in FPG concentration may also reflect SFU dose adjustments. It is important to note that the highest point in the transient FPG rise represented a mean decrease of −16 mg/dl from baseline. There was no clinically significant effect on overall glycemic control after the switch to exenatide QW (mean rise in mean A1C was ∼+0.1%). A1C values subsequently declined below those observed with 30 weeks of exenatide BID treatment and by week 44 matched those observed with continuous exenatide QW treatment. Overall, these findings suggest that switching from exenatide BID to QW, although associated with a transient increase in glycemia, ultimately leads to significantly improved glycemic control.
Achieving glycemic targets remains an elusive goal for many patients with type 2 diabetes. Furthermore, improving weight and cardiovascular risk markers and avoiding hypoglycemia are desired components of a diabetes treatment program. GLP-1R agonists were recently recommended as second-step treatment option in patients in whom avoidance of hypoglycemia or promotion of weight loss is a major concern (1). In the present study, the transition from exenatide BID to exenatide QW was made without additional safety and tolerability concerns. Furthermore, the clinical benefits of exenatide QW were maintained and resulted in a mean A1C of 6.6% after 52 weeks of treatment.
Supplementary Material
Acknowledgments
The University of North Carolina has contracts with a variety of companies for the services of J.B.B. as an investigator and/or consultant, including Amylin Pharmaceuticals, Bristol-Myers Squibb, Eli Lilly, GlaxoSmithKline, Hoffman-La Roche, Merck, Novo Nordisk, Pfizer, sanofi-aventis, and Wyeth. D.J.D. has served as an advisor or consultant within the past 12 months to Amylin Pharmaceuticals, Arena Pharmaceuticals, Arisaph Pharmaceuticals, Eli Lilly, GlaxoSmithKline, Hoffman La Roche, Isis Pharmaceuticals, Merck Research Laboratories, Metabolex, Novartis Pharmaceuticals, Novo Nordisk, and Transition Pharmaceuticals. Neither D.J.D. nor his family members hold stock directly or indirectly in any of these companies. J.B.B. and D.J.D. had full access to the primary data and led decisions on content. K.L.T., T.K., B.W., H.H., K.W., L.Z.S., and L.P. are employees and stockholders of Amylin Pharmaceuticals. M.T. is an employee and stockholder of Eli Lilly & Co. Amylin Pharmaceuticals and Eli Lilly & Company were study sponsors and thus were involved in the study design, protocol development, and the collection, review and analysis of the data. No other potential conflicts of interest relevant to this article were reported.
Parts of this study were presented in abstract form at the 69th annual meeting of the American Diabetes Association, New Orleans, Louisiana, 5–9 June 2009 and at the 45th European Association for the Study of Diabetes Annual Meeting, Vienna, Austria, 29 September–2 October 2009.
We thank the exenatide DURATION-1 clinical team for their assistance in the conduct, reporting, and quality control of the studies, the DURATION-1 study site investigators (Sherwyn Schwartz, Sam Miller, Richard Weinstein, Bethany Klopfenstein, Andrew Ahmann, Julio Rosenstock, John Pullman, Fred Whitehouse, G.M. Gollapudi, Douglas Schumacher, Mervyn Weerasinghe, Peter Weissman, Lyle Myers, Eric Klein, Thomas Littejohn, Thomas Moretto, Jon Shapiro, Daniel Lorber, Athena Philis-Tsimikas, David Kayne, Richard Bergenstal, Elizabeth Stevens, Danny Sugimoto, Bruce Berwald, Diane Krieger, Dean Kereiakes, Gary Lewis, Robert Henry, Mark Comianos, Munni Selagamsetty, Ronald Mayfield, John Buse, and Daniel Drucker) and their staffs for conducting the study, the study patients for their participation, and Alkermes for the development and manufacturing of the long-acting release formulation of exenatide.
Footnotes
Clinical trial reg. no. NCT00308139, www.clinicaltrials.gov.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1. Nathan DM, Buse JB, Davidson MB, Ferrannini E, Holman RR, Sherwin R, Zinman B: Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2009;32:193–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) group. Lancet 1998;352:837–853 [PubMed] [Google Scholar]
- 3. Brown JB, Nichols GA, Perry A: The burden of treatment failure in type 2 diabetes. Diabetes Care 2004;27:1535–1540 [DOI] [PubMed] [Google Scholar]
- 4. Matthews DR, Cull CA, Stratton IM, Holman RR, Turner RC: UKPDS 26: sulphonylurea failure in non-insulin-dependent diabetic patients over six years. UK Prospective Diabetes Study (UKPDS) Group. Diabet Med 1998;15:297–303 [DOI] [PubMed] [Google Scholar]
- 5. Pontiroli AE, Calderara A, Pozza G: Secondary failure of oral hypoglycaemic agents: frequency, possible causes, and management. Diabetes Metab Rev 1994;10:31–43 [DOI] [PubMed] [Google Scholar]
- 6. Turner RC, Cull CA, Frighi V, Holman RR: Glycemic control with diet, sulfonylurea, metformin, or insulin in patients with type 2 diabetes mellitus: progressive requirement for multiple therapies (UKPDS 49). UK Prospective Diabetes Study (UKPDS) Group. JAMA 1999;281:2005–2012 [DOI] [PubMed] [Google Scholar]
- 7. Edwards CM, Stanley SA, Davis R, Brynes AE, Frost GS, Seal LJ, Ghatei MA, Bloom SR: Exendin-4 reduces fasting and postprandial glucose and decreases energy intake in healthy volunteers. Am J Physiol Endocrinol Metab 2001;281:E155–E161 [DOI] [PubMed] [Google Scholar]
- 8. Nielsen LL, Young AA, Parkes DG: Pharmacology of exenatide (synthetic exendin-4): a potential therapeutic for improved glycemic control of type 2 diabetes. Regul Pept 2004;117:77–88 [DOI] [PubMed] [Google Scholar]
- 9. Buse JB, Henry RR, Han J, Kim DD, Fineman MS, Baron AD: Effects of exenatide (exendin-4) on glycemic control over 30 weeks in sulfonylurea-treated patients with type 2 diabetes. Diabetes Care 2004;27:2628–2635 [DOI] [PubMed] [Google Scholar]
- 10. DeFronzo RA, Ratner RE, Han J, Kim DD, Fineman MS, Baron AD: Effects of exenatide (exendin-4) on glycemic control and weight over 30 weeks in metformin-treated patients with type 2 diabetes. Diabetes Care 2005;28:1092–1100 [DOI] [PubMed] [Google Scholar]
- 11. Kendall DM, Riddle MC, Rosenstock J, Zhuang D, Kim DD, Fineman MS, Baron AD: Effects of exenatide (exendin-4) on glycemic control over 30 weeks in patients with type 2 diabetes treated with metformin and a sulfonylurea. Diabetes Care 2005;28:1083–1091 [DOI] [PubMed] [Google Scholar]
- 12. Zinman B, Hoogwerf BJ, Durán García S, Milton DR, Giaconia JM, Kim DD, Trautmann ME, Brodows RG: The effect of adding exenatide to a thiazolidinedione in suboptimally controlled type 2 diabetes: a randomized trial. Ann Intern Med 2007;146:477–485 [DOI] [PubMed] [Google Scholar]
- 13. Barnett AH, Burger J, Johns D, Brodows R, Kendall DM, Roberts A, Trautmann ME: Tolerability and efficacy of exenatide and titrated insulin glargine in adult patients with type 2 diabetes previously uncontrolled with metformin or a sulfonylurea: a multinational, randomized, open-label, two-period, crossover noninferiority trial. Clin Ther 2007;29:2333–2348 [DOI] [PubMed] [Google Scholar]
- 14. Heine RJ, Van Gaal LF, Johns D, Mihm MJ, Widel MH, Brodows RG: Exenatide versus insulin glargine in patients with suboptimally controlled type 2 diabetes: a randomized trial. Ann Intern Med 2005;143:559–569 [DOI] [PubMed] [Google Scholar]
- 15. Nauck MA, Duran S, Kim D, Johns D, Northrup J, Festa A, Brodows R, Trautmann M: A comparison of twice-daily exenatide and biphasic insulin aspart in patients with type 2 diabetes who were suboptimally controlled with sulfonylurea and metformin: a non-inferiority study. Diabetologia 2007;50:259–267 [DOI] [PubMed] [Google Scholar]
- 16. Klonoff DC, Buse JB, Nielsen LL, Guan X, Bowlus CL, Holcombe JH, Wintle ME, Maggs DG: Exenatide effects on diabetes, obesity, cardiovascular risk factors and hepatic biomarkers in patients with type 2 diabetes treated for at least 3 years. Curr Med Res Opin 2008;24:275–286 [DOI] [PubMed] [Google Scholar]
- 17. Drucker DJ, Buse JB, Taylor K, Kendall DM, Trautmann M, Zhuang D, Porter L: Exenatide once weekly versus twice daily for the treatment of type 2 diabetes: a randomised, open-label, non-inferiority study. Lancet 2008;372:1240–1250 [DOI] [PubMed] [Google Scholar]
- 18. Kim D, MacConell L, Zhuang D, Kothare PA, Trautmann M, Fineman M, Taylor K: Effects of once-weekly dosing of a long-acting release formulation of exenatide on glucose control and body weight in subjects with type 2 diabetes. Diabetes Care 2007;30:1487–1493 [DOI] [PubMed] [Google Scholar]
- 19. World Medical Association Declaration of Helsinki. Recommendations guiding physicians in biomedical research involving human subjects. JAMA 1997;277:925–926 [PubMed] [Google Scholar]
- 20. Fineman MS, Bicsak TA, Shen LZ, Taylor K, Gaines E, Varns A, Kim D, Baron AD: Effect on glycemic control of exenatide (synthetic exendin-4) additive to existing metformin and/or sulfonylurea treatment in patients with type 2 diabetes. Diabetes Care 2003;26:2370–2377 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.