Abstract
OBJECTIVE
To determine the pharmacokinetic and pharmacodynamic properties of an oral insulin (OI) formulation compared with subcutaneously injected regular human insulin (RHI).
RESEARCH DESIGN AND METHODS
Ten male patients with type 2 diabetes (means ± SD; A1C 7.0 ± 1.1%; BMI 28.3 ± 2.7 kg/m2) received either 300 units of insulin combined with 400 mg of delivery agent orally or 15 units RHI subcutaneously under isoglycemic clamp conditions.
RESULTS
Maximum insulin concentration was greater and onset of action was faster with OI (Cmax 93 ± 71 vs. 33 ± 11 μU/ml; AUCGIR(0−1h) 173 ± 86 vs. 27 ± 32 mg/kg; P < 0.05). Mean insulin concentration and glucose infusion rate returned to baseline within 3 h after OI administration. Relative bioavailability of OI was 7 ± 4% (1st 2 h).
CONCLUSIONS
This proof-of-concept study demonstrated that absorption of OI is feasible under fasting conditions. OI has a fast onset and a short duration of action but also shows a rather high between-subject variability in absorption.
Oral administration of insulin has the potential advantage of a more physiological action by its direct effect on hepatic glucose production (1,2). Thus far various oral insulin approaches however have only partially produced satisfactory results (1,3,4). Gastrointestinal absorption of insulin is hampered by factors such as enzymatic degradation and lack of permeation through epithelial cells (5). Noncovalent interaction of the novel drug-carrier molecule monosodium N-(4-chlorosalicyloyl)-4-aminobutyrate (4-CNAB) with insulin might create more favorable physico-chemical properties for gastrointestinal insulin absorption (6,7). In this study, 4-CNAB has been combined with human insulin to facilitate gastrointestinal insulin absorption.
RESEARCH DESIGN AND METHODS
A single-center, open-label, randomized, two-period cross-over, isoglycemic glucose clamp study was used to determine the pharmacokinetic and pharmacodynamic properties of an oral insulin (OI) formulation with 4-CNAB compared with subcutaneous regular human insulin (RHI). The protocol was approved by an independent ethics committee and the study was performed in accordance with the Declaration of Helsinki. A total of 14 male adult patients diagnosed with type 2 diabetes for over a year and without insulin therapy were screened after providing written informed consent (see all the inclusion and exclusion criteria in the online appendix, available at http://care.diabetesjournals.org/cgi/content/full/dc09-1807/DC1). Ten patients were enrolled. One patient withdrew consent during the first clamp visit and was replaced, resulting in ten patients (age 55 ± 9 years [means ± SD]; A1C 7.0 ± 1.1%; BMI 28.3 ± 2.8 kg/m2) completing the study. Subjects received either 300 units OI combined with 400 mg 4-CNAB in two capsules (each containing 150 units OI plus 200 mg 4-CNAB) or a subcutaneous injection of 15 units RHI (Humulin R 100 units/ml; Eli Lilly, Indianapolis, IN) on two separate dosing days separated by 1–20 days. Patients did not take oral hypoglycemic agents 24 h prior to each dosing.
Glucose clamp procedure
After an overnight fast, patients were connected to a Biostator (MTB Medizintechnik, Ulm, Germany). The clamp level, set to the subject's fasting blood glucose concentration, was established by intravenous infusions of insulin (Actrapid; Novo Nordisk, Bagsvaerd, Denmark) (0.2 mU · kg−1 · min−1 from 2 h predosing until the end of the experiment) and a variable infusion of glucose. At t = 0, one of the two study medications was administered. The pharmacokinetic (insulin levels) and pharmacodynamic (glucose infusion rates [GIRs]) responses to the study medication were measured for 6 h. The safety parameters studied included adverse events, laboratory data, vital signs, physical examinations, and electrocardiograms.
Statistical analysis methods
Area under the curve for plasma insulin concentration (AUCINS) and GIR (AUCGIR) were calculated with the trapezoidal rule. Insulin and GIR values were corrected for the baseline intravenous insulin infusion by subtracting the mean insulin concentrations or GIR in the last hour before study drug administration from all postdosing values. Individual GIR profiles were then smoothed using a polynomial function of the 6th order and maximum GIR (GIRmax), time to GIRmax (TGIRmax), time to half-maximum GIR before reaching GIRmax (TGIR-50%-early), and time to half-maximum GIR after reaching GIRmax (TGIR-50%-late) were calculated. Relative bioavailability and biopotency were calculated as the dose-corrected ratios of individual AUCs below insulin or GIR profiles after oral and subcutaneous application (8).
One subject was excluded from the bioavailability and biopotency analysis due to a very low pharmacokinetic and metabolic response to RHI administration. In addition, two subjects with an absent metabolic response to RHI administration in the 1st hour after application were excluded from the biopotency analysis in the 0-1 h period. Two-sided signed Wilcoxon rank sum tests and Kruskal-Wallis nonparametric tests were used for comparisons between treatments. P < 0.05 was considered to be statistically significant.
RESULTS
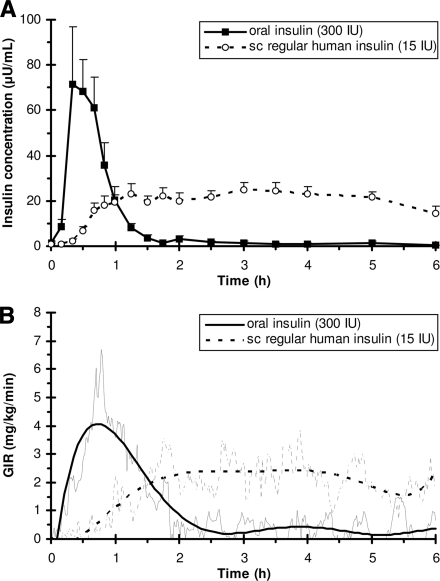
Figure 1 shows the pharmacokinetic (A) and pharmacodynamic (B) responses to oral and subcutaneous insulin administrations. All subjects showed early enhanced pharmacokinetic and pharmacodynamic responses after OI administration. Maximum plasma insulin concentration (Cmax) was significantly higher (93 ± 71 vs. 33 ± 11 μU/ml) and time to Cmax (Tmax) was significantly shorter (27 ± 9 vs. 161 ± 83 min) with OI administration. Relative bioavailability of OI for the 0-1 h, 0-2 h, and 0-6 h periods were 26 ± 28%, 7 ± 4%, and 2 ± 1%, respectively. Respective values for biopotency were 55 ± 92%, 12 ± 9%, and 3 ± 1%.
Figure 1.
A: Pharmacokinetic (plasma insulin concentration) response to administration of an oral insulin formulation (uninterrupted line) and subcutaneous regular human insulin (dotted line) at time = 0 h. Plasma insulin concentrations are presented as means ± SEM. AUCINS(0−1h): 2,559 ± 1,831 vs. 542 ± 296 μU · min−1 · ml−1, P < 0.05, AUCINS(0−6h): 3,225 ± 2,320 vs. 7,004 ± 2,440 μU · min−1 · ml−1, P < 0.05. B: Pharmacodynamic (GIR) response. GIRs are given for mean raw (thin line) and smoothed (bold line) data. AUCGIR(0−1h): 173 ± 86 vs. 27 ± 32 mg/kg, P < 0.05. AUCGIR(0−2h): 297 ± 143 vs. 137 ± 107 mg/kg, P < 0.05. AUCGIR(0−6h): (374 ± 135 vs. 651 ± 380 mg/kg). TGIRmax: 40 ± 16 vs. 255 ± 108 min, P < 0.05. TGIR-50%-early: 13 ± 6 vs. 150 ± 87 min, P < 0.05. TGIR-50%-late: 115 ± 79 vs. >360 min, P < 0.05. GIRmax: 4.4 ± 2.2 vs. 3.6 ± 1.8 mg · kg−1 · min−1. sc, subcutaneous.
Plasma C-peptide concentrations showed no significant increase in any of the experiments and were not significantly different between the two treatments.
No adverse events and no clinically relevant changes in vital signs, electrocardiograms, or standard safety laboratory parameters were observed.
CONCLUSIONS
This is the first glucose clamp study demonstrating that OI is absorbed under fasting conditions and exhibits early enhanced pharmacokinetic and pharmacodynamic responses. The duration of action of OI was much shorter than that of RHI with a return to the baseline effect within 2–3 h. No safety concerns arose from this short-term study with single dose administrations.
The fast pharmacokinetic and metabolic time-profiles of OI observed in this study may be advantageous in patients with type 2 diabetes by restoring normal first phase insulin secretion (9) and potentially leading to an improvement in glycemic control (10). OI's onset of action seems to be in the range of (or even faster than) what is published for subcutaneous fast–acting analogues (11), but a head-to-head comparison has yet to be done. OI should also have the advantage of reaching the liver in high concentration through the portal vein after gastrointestinal absorption resulting in a more physiological and stronger effect on hepatic glucose production and a weaker effect on the peripheral tissues (potentially avoiding hypoglycemia) than subcutaneous insulin preparations.
In this small proof-of-concept study, only a relatively small amount (7% for the 2-h period after drug administration) of OI is absorbed under fasting conditions with an SD of 4%. Thus, variability in absorption (coefficient of variation 60–70%) is about as high for OI as that reported for NPH insulin (11) but may increase further with prandial administration. Because of the narrow therapeutic window for insulin, this high between-subject variability might restrict clinical use of OI to patients with a high endogenous insulin secretion capacity. This first pilot study just provides a proof-of-concept for OI under fasting conditions. Additional investigations are therefore needed in particular to determine the pharmacodynamic (intraindividual) variability and the effect of food on oral insulin absorption before longer-term studies could further elucidate the clinical potential of this OI formulation.
Supplementary Material
Acknowledgments
Profil Institut für Stoffwechselforschung GmbH received financial support for conducting this study. C.K., E.Z., L.H., and T.H. received financial support for conducting this study. C.K., L.H., and T.H. are shareholders in Profil Institut für Stoffwechselforschung GmbH, which receives research grants from pharmaceutical companies. M.C.C. and G.R. are employed by and own stock in Emisphere Technologies, the developer of the oral insulin formulation studied. No other potential conflicts of interest relevant to this article were reported.
Parts of this study were presented in abstract and oral forms at the 38th annual meeting of the Deutsche Diabetes Gesellschaft, Bremen, Germany, 28–31 May 2003 and at the 63rd Scientific Sessions of the American Diabetes Association, New Orleans, Louisiana, 13–17 June 2003.
We thank Ehud Arbit, MD, and Michael Goldberg, MD, for their contributions to this study.
Footnotes
Clinical trial reg. no. NCT00982254; clinicaltrials.gov.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1. Wajcberg E, Miyazaki Y, Triplitt C, Cersosimo E, DeFronzo RA: Dose-response effect of a single administration of oral hexyl-insulin monoconjugate 2 in healthy nondiabetic subjects. Diabetes Care 2004;27:2868–2873 [DOI] [PubMed] [Google Scholar]
- 2. Blackard WG, Nelson NC: Portal and peripheral vein immunoreactive insulin concentrations before and after glucose infusion. Diabetes 1970;19:302–306 [DOI] [PubMed] [Google Scholar]
- 3. Yadav N, Morris G, Harding SE, Ang S, Adams GG: Various non-injectable delivery systems for the treatment of diabetes mellitus. Endocr Metab Immune Disord Drug Targets 2009;9:1–13 [DOI] [PubMed] [Google Scholar]
- 4. Heinemann L, Jacques Y: Oral insulin and buccal insulin: a critical reappraisal. J Diabetes Sci Technol 2009;3:568–584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lassmann-Vague V, Raccah D: Alternatives routes of insulin delivery. Diabete Metab 2006;32:513–522 [DOI] [PubMed] [Google Scholar]
- 6. Hoffman A, Qadri B: Eligen insulin–a system for the oral delivery of insulin for diabetes. IDrugs 2008;11:433–441 [PubMed] [Google Scholar]
- 7. Malkov D, Angelo R, Wang HZ, Flanders E, Tang H, Gomez-Orellana I: Oral delivery of insulin with the eligen technology: mechanistic studies. Curr Drug Deliv 2005;2:191–197 [DOI] [PubMed] [Google Scholar]
- 8. Heinemann L, Traut T, Heise T: Time-action profile of inhaled insulin. Diabet Med 1997;14:63–72 [DOI] [PubMed] [Google Scholar]
- 9. Nesher R, Cerasi E: Modeling phasic insulin release: immediate and time-dependent effects of glucose. Diabetes 2002;51(Suppl. 1):S53–S59 [DOI] [PubMed] [Google Scholar]
- 10. Bruce DG, Chisholm DJ, Storlien LH, Kraegen EW: Physiological importance of deficiency in early prandial insulin secretion in non-insulin-dependent diabetes. Diabetes 1988;37:736–744 [DOI] [PubMed] [Google Scholar]
- 11. Heise T, Nosek L, Rønn BB, Endahl L, Heinemann L, Kapitza C, Draeger E: Lower within-subject variability of insulin detemir in comparison to NPH insulin and insulin glargine in people with type 1 diabetes. Diabetes 2004;53:1614–1620 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.