Abstract
OBJECTIVE
Age at onset of type 1 diabetes influences the risk of microvascular complications. However, the long-term risk of proliferative retinopathy within the wide spectrum of age at onset of type 1 diabetes is less well known.
RESEARCH DESIGN AND METHODS
A sample of 1,117 consecutively recruited patients was drawn from the FinnDiane Study population (4,800 patients). Type 1 diabetes was defined as age at onset ≤40 years, insulin treatment initiated within 1 year, and C-peptide ≤0.3 nmol/l. Retinopathy status was graded based on ophthalmic records and/or fundus photographs. The risk of proliferative retinopathy was studied in age-at-onset groups 0–4, 5–14, and 15–40 years.
RESULTS
The mean durations to proliferative retinopathy were 24.3 (22.7–25.9) years in the 0–4 years group, 20.1 (19.2–21.1) years in the 5–14 years group, and 21.6 (19.8–23.3) years in the 15–40 years group (P < 0.001). In a Cox regression model, with A1C, blood pressure, sex, and BMI as covariates, the highest risk of proliferative retinopathy was observed in the 5–14 years group (hazard ratio 1.90 [95% CI 1.45–2.48], P < 0.001). Diabetes onset 0–4 vs. 5–14 years made no difference in the long-term risk of proliferative retinopathy (P = 0.2). When split into two groups, age at onset <15 years was associated with a higher long-term risk than age at onset ≥15 years (1.82 [1.40–2.36], P < 0.001).
CONCLUSIONS
Age at onset significantly modifies the long-term risk of proliferative retinopathy. The highest risk is in age-at-onset group 5–14 years, whereas the lowest risk is in age-at-onset group 15–40 years.
Proliferative retinopathy is a severe microvascular complication in patients with type 1 diabetes. After 20 years of diabetes, almost all patients with type 1 diabetes and 58% of patients with type 2 diabetes show signs of retinopathy. When retinopathy worsens, severe visual loss eventually threatens 5–10% of the patients (1). The most severe form of retinopathy is proliferative retinopathy, and most of the patients with this complication will become blind after 5–10 years without treatment (2). The prevalence of proliferative retinopathy varies between 13 and 50% after 15–25 years of diabetes in patients who need insulin (3,4).
Several risk factors for diabetic retinopathy have already been identified. The prevalence of any retinopathy is strongly related to duration of diabetes and glycemic control (1,4). Poor glycemic control increases both the incidence and the progression of retinopathy (5). Male sex and high blood pressure further increase the risk of retinopathy (3). Genetic factors are also likely to play a major role (6). It is of note that the age at onset of diabetes may modify the metabolic phenotype of the patients and thus predispose certain patients to diabetic retinopathy. In particular, diabetes onset at age <5 years may have a protective effect on the development of retinopathy (7,8). Thus, it can be hypothesized that because the incidence of type 1 diabetes is on the rise and the increase has been greatest in children aged 0–4 years (9), there may possibly be a decrease in the overall risk of retinopathy. The studies so far have not focused on the onset of type 1 diabetes in adulthood, and therefore the number of patients with late-onset diabetes has been rather small and not large enough to study the effect of late age at onset on the risk of proliferative retinopathy. Furthermore, it is not yet known whether young age at onset is a protective factor in the long term or whether it only delays the onset of proliferative retinopathy. Therefore, the aim of this study was to elucidate how the age at onset of type 1 diabetes influences the long-term risk of proliferative retinopathy in patients with type 1 diabetes.
RESEARCH DESIGN AND METHODS
The present study is a retrospective cohort study, undertaken as part of the ongoing FinnDiane Study (Finnish Diabetic Nephropathy Study), which has since 1997 collected comprehensive data from patients with type 1 diabetes at 92 centers throughout Finland, including primary care as well as secondary and tertiary care hospitals with the aim of identifying genetic and environmental risk factors for diabetes complications. All adult patients with type 1 diabetes at these centers have been invited to participate in the FinnDiane Study, and 78% have responded positively (10). The recruitment strategy is random because the only inclusion criterion is type 1 diabetes, and every patient at the recruiting centers has been invited. The FinnDiane Study has to date recruited 4,800 patients with type 1 diabetes, and although it is not by strict definition a population-based study, the distribution of the patients closely follows the distribution of the general population in Finland. Thus, there is no bias in the sampling procedure regarding the geographic location or selection of patients in the recruiting centers. The protocol is in accordance with the Declaration of Helsinki and was approved by the ethics committee of the Helsinki University Central Hospital.
Inclusion of patients in the present study was based on the ascending order of the FinnDiane patient identification numbers. Thus, the consecutively recruited patients from the very beginning of the FinnDiane Study were the first to be included in this study. This approach has two advantages. First the sampling frame should be as random as possible with regard to ophthalmic status and the treatment of diabetes and its complications. Second, these patients had the longest duration of type 1 diabetes because they were the first ones to participate in the FinnDiane study.
We obtained fundus photographs and/or records of fundus examinations performed by a specialist in ophthalmology for 1,117 consecutively recruited patients. These patients were required to have onset of diabetes at the age of ≤40 years, C-peptide ≤0.3 nmol/l (11), and insulin treatment initiated within 1 year of diagnosis. For 972 of 1,117 (87%) patients, C-peptide concentrations were <0.033 nmol/l, which represents the detection limit of the assay (Human C-Peptide RIA Kit, Linco Research., St. Charles, MO). Records of fundus examinations by a specialist in ophthalmology were available for 917 of 1,117 (82%) patients, and fundus photographs were available for 851 of 1,117 (76%) patients. The patients who had fundus photographs available (n = 851) had been photographed a total of 2,792 times, on a median of 3 (interquartile range [IQR] 1–5) times per patient. Both records and photographs were available for 651 of 1,117 (58%) patients. All available data were used to score the severity and progression of retinopathy, a procedure handled by an ophthalmologist (K.H.) unaware of the demographic data and the presence or absence of other complications. The Early Treatment of Diabetic Retinopathy Study (ETDRS)-grading scale was used, for which 10 represents no retinopathy, ≥61 represents proliferative retinopathy, and 81–85 represents advanced retinopathy (12). The eye with the more severe retinopathy was used to represent the overall retinopathy severity for a particular patient. In this study laser treatment alone was not taken as evidence of proliferative retinopathy because severe nonproliferative retinopathy is also an indication for scatter laser photocoagulation.
Data on medication, cardiovascular status, diabetes complications, hypertension, and cardiovascular disease were obtained using a standardized questionnaire, which was completed by the patient's attending physician. Blood pressure was measured twice in the sitting position using a mercury sphygmomanometer after a rest of at least 10 min. Mean arterial blood pressure was calculated according to the formula: mean arterial pressure = diastolic blood pressure + ⅓ (systolic blood pressure − diastolic blood pressure). Anthropometric data, such as height and weight were recorded, and blood was drawn for the laboratory measurements, including A1C. Data on all-cause mortality were obtained from a national registry maintained by the Population Register Centre of Finland.
Statistical analysis
Data are presented as means (95% CI) for continuous, normally distributed variables and medians (IQR) for nonnormally distributed variables. SEs are given for mean differences. Kaplan-Meier survival analysis was used to estimate time without proliferative retinopathy and a Mantel-Cox log-rank test was used to compare survival distributions among different age-at-onset groups. The risk of proliferative retinopathy within the age-at-onset groups was estimated with a Cox proportional hazards model, controlling for clinically significant covariates. One-way ANOVA, adjusted for multiple comparisons (Sidak), was used to compare the mean differences of these groups. Proportions were compared with a Kruskal-Wallis test. A previously published macro for SAS statistical software (SAS, Cary, NC) was used to compute the cumulative incidence of proliferative retinopathy and cumulative mortality before the development of proliferative retinopathy accounting for competing events (13). All other statistical calculations were performed with SPSS 15.0 (SPSS, Chicago, IL, USA).
RESULTS
Tables 1 and 2 show the clinical characteristics of the patients studied. The male-to-female ratio was 596:521. Mean age at onset of type 1 diabetes was 13.7 (95% CI 13.1–14.1) years and the mean duration of diabetes was 25.0 (24.4–25.7) years (Table 1). Proliferative retinopathy was found in 367 of 1,117 (33%) patients. The highest proportion of patients with proliferative retinopathy was found in age-at-onset group 0–4 years (47.0% [95% CI 39.5–54.6]), the second highest in age-at-onset group 5–14 years (38.8% [34.7.5–42.9]), and the lowest in age-at-onset group 15–40 years (18.9% [15.0–22.7]) (Table 2). Ophthalmic follow-up data were available for a median of 10.8 (IQR 5.9–17.4) years after the diagnosis of proliferative retinopathy.
Table 1.
Clinical characteristics of patients without any retinopathy compared with patients with nonproliferative and proliferative retinopathy
| No retinopathy | Nonproliferative retinopathy | Proliferative retinopathy | All patients | |
|---|---|---|---|---|
| n | 258 | 492 | 367 | 1,117 |
| Duration of diabetes (years) | 13.7 (12.6–14.7)* | 24.9 (24.1–25.8)* | 33.1 (32.2–34.0)* | 25.0 (24.4–25.7) |
| Age at onset (years) | 17.2 (16.2–18.3)* | 14.0 (13.2–14.8)* | 10.6 (9.9–11.3)* | 13.7 (13.1–14.1) |
| A1C (%) | 8.1 (7.9–8.3)† | 8.5 (8.3–8.6)* | 8.7 (8.5–8.8)* | 8.4 (8.4–8.5) |
| Mean arterial pressure (mmHg) | 93.7 (92.4–94.9)* | 96.6 (95.7–97.5)* | 102.3 (101.1–103.5)* | 97.8 (97.2–98.5) |
| BMI (kg/m2) | 24.3 (23.9–24.7) | 25.2 (24.9–25.5)* | 25.5 (25.1–25.9)* | 25.1 (24.8–25.3) |
| Mortality | 5 (1.9)* | 19 (3.9)* | 75 (20.4)* | 99 (8.9) |
Data are means (95% CI) or n (%). One-way ANOVA, adjusted for multiple comparisons (Sidak). Differences in proportions were compared with the Kruskal-Wallis test.
*Significant difference between all three groups (P < 0.05).
†Significant difference between two groups.
Table 2.
Clinical characteristics of patients in different age-at-onset groups
| n | Duration of diabetes (years) | PDR (%) | Duration to PDR (years) | A1C (%) | MAP (mmHg) | C-peptide <0.033 nmol/l (%) | BMI (kg/m2) | Mortality (%) | |
|---|---|---|---|---|---|---|---|---|---|
| Age-at-onset group | |||||||||
| 0–4 years | 168 | 31.12 (29.5–32.7)* | 47.0 (39.5–54.6)¶ | 24.3 (22.7–25.9)¶ | 8.8 (8.5–9.0)¶ | 96.6 (94.9–98.3) | 96.4 (92.4–98.7)¶ | 24.8 (24.3–25.4) | 8.9 |
| 5–14 years | 546 | 26.1 (25.1–27.0)* | 38.8 (34.7–42.9)¶ | 20.1 (19.2–21.1)* | 8.5 (8.4–8.6) | 97.3 (96.4–98.2) | 89.9 (87.4–92.5) | 24.9 (24.6–25.2) | 9.3 |
| >15 years | 403 | 21.0 (19.9–22.1)* | 18.9 (15.0–22.7)* | 21.6 (19.8–23.3) | 8.3 (8.1–8.4)* | 99.0 (97.9–100.1) | 79.2 (75.2–83.1)* | 25.4 (25.0–25.8) | 8.2 |
| All patients | 1,117 | 25.0 (24.4–25.7) | 32.9 (30.0–35.6) | 21.3 (20.6–22.1) | 8.4 (8.4–8.5) | 97.8 (97.2–98.5) | 87.0 (85.0–89.0) | 25.0 (24.8–25.3) | 8.9 |
Data are means (95% CI) unless indicated otherwise. One-way ANOVA, adjusted for multiple comparisons (Sidak). Differences in proportions were compared with the Kruskal-Wallis test.
*,¶Significant difference (P < 0.05) between two groups. *Significant difference between all three groups (P < 0.05). MAP, mean arterial pressure; PDR, proliferative diabetic retinopathy.
Mean duration from onset of diabetes to proliferative retinopathy was 21.3 (95% CI 20.6–22.1) years in all patients. The longest duration to proliferative retinopathy was 24.3 (22.7–25.9) years in the 0–4 years group (n = 79), whereas the shortest was 20.1 (19.2–21.1) years in the 5–14 years group (n = 212). In the 15–40 years group the duration to proliferative retinopathy was 21.6 (19.8–23.3) years (n = 76) (Table 2). The mean ± SE difference in the duration of diabetes to proliferative retinopathy between age-at-onset groups 0–4 and 5–14 years was statistically significant (4.2 ± 0.9 years, P < 0.001). However, the mean difference between the other two groups did not reach statistical significance. The youngest age-at-onset group had the longest duration of diabetes (31.12 [95% CI 29.5–32.7] years), highest A1C (8.8 [8.5–9.0]%), and the highest proportion of patients with C-peptide below the detection limit (96.4 [92.4–98.7]%) (Table 2). Patients with proliferative retinopathy had the highest mortality (20.4%), the highest BMI (25.5 [25.1–25.9] kg/m2), and the longest duration of diabetes (33.1 [32.2–34.0] years) (Table 1).
In the Cox proportional hazards model, with potentially significant risk factors (A1C, blood pressure, sex, and BMI) as covariates, onset of diabetes between 5 and 14 years of age increased the risk of proliferative retinopathy the most (hazard ratio [HR] 1.90 [95% CI 1.45–2.48], P < 0.001) as contrasted with age-at-onset group 15–40 years (Table 3). Similarly, patients with age at onset 0–4 years had a higher risk of proliferative retinopathy (1.61 [1.16–2.23], P = 0.002). As for the other covariates, the risk of proliferative retinopathy increased 15% with every unit increase in A1C percentage (1.15 [1.07–1.23], P < 0.001) and 3% with every 1 mmHg increase in mean arterial blood pressure (1.03 [1.02–1.04], P < 0.001). Male sex and BMI did not influence the risk of proliferative retinopathy. When age-at-onset group 0–4 years was compared with the age-at-onset group with the highest risk (5–14 years), the long-term risk of proliferative retinopathy, adjusted for the above-mentioned covariates, showed no difference (0.85 [0.65–1.10], P = 0.2). The worst prognosis seems to be in age-at-onset group 5–9 years. However, if compared with age at onset 10–14 years, this difference did not reach statistical significance (1.29 [0.98–1.70], P = 0.07). Because the patients in groups 5–9 and 10–14 years did not have a significantly different risk, they are presented as a combined age-at-onset group 5–14 years in this study.
Table 3.
Risk of proliferative retinopathy in age-at-onset groups 0–4 years and 5–14 years compared with age-at-onset group 15–40 years: Cox proportional hazards model
| Variable | HR (95% CI) | P value |
|---|---|---|
| Age at onset 15–40 years | 1 | |
| Age at onset 0–4 years | 1.61 (1.16–2.23) | 0.004 |
| Age at onset 5–14 years | 1.90 (1.45–2.48) | < 0.001 |
| Male sex | 1.07 (0.86–1.33) | 0.6 |
| A1C (%) | 1.15 (1.07–1.23) | < 0.001 |
| Mean arterial pressure (mmHg) | 1.03 (1.02–1.04) | < 0.001 |
| BMI | 1.02 (0.99–1.05) | 0.3 |
Age-at-onset group 0–4 years, n = 164; age-at-onset group 5–14 years, n = 537; age-at-onset group 15–40 years, n = 400.
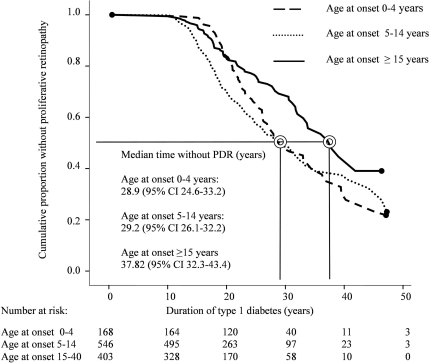
The Kaplan-Meier survival analysis stratified by various age-at-onset groups illustrates the progression to proliferative retinopathy (Fig. 1). Median times without proliferative retinopathy were 28.9 (24.6–33.2), 29.2 (26.1–32.2), and 37.8 (32.3–43.4) years in age-at-onset groups 0–4 years, 5–14 years, and 15–40 years, respectively (P < 0.001, Mantel-Cox log-rank test) (Fig. 1). Despite the initial delay in the progression to proliferative retinopathy in the youngest age-at-onset group, the long-term risk between age-at-onset groups 0–4 and 5–14 years was no different (P = 0.224, Mantel-Cox log-rank test) (Fig. 1). However, there was a significant difference when the patients were split into two groups according to age at onset before or after 15 years of age (P < 0.001, Mantel-Cox log-rank test). Median times without proliferative retinopathy were 28.9 (95% CI 26.4–31.4) years for the patients with age at onset before 15 years and 37.8 (95% CI 32.3–43.4) years for the patients with age at onset after 15 years. The risk of proliferative retinopathy was significantly higher in those patients with age at onset before 15 years versus after 15 years, when adjusted for the above-mentioned covariates (HR 1.82 [95% CI 1.40–2.36], P < 0.001).
Figure 1.
Kaplan-Meier survival analysis of the cumulative proportion of patients without proliferative retinopathy (PDR) in 1,117 patients stratified into three groups according to age at onset (P < 0.001, Mantel-Cox log-rank test).
A total of 99 of the 1,117 patients (8.9%) had died during the follow-up. Of these 75 of 99 (75.8%) had proliferative retinopathy (Table 1). The remaining 24 of 99 (24.2%) patients did not have proliferative retinopathy.
CONCLUSIONS
This study shows that the patients with the youngest age at onset (0–4 years) have the longest mean duration of type 1 diabetes without proliferative retinopathy (24.3 [95% CI 22.7–25.9] years). This observation is in line with earlier findings regarding diabetic retinopathy and nephropathy (7,8,14). However, the long-term risk of proliferative retinopathy is no different between age-at-onset groups 0–4 and 5–14 years despite the initial advantage for those with earlier onset of diabetes. Ultimately, the survival curves for these two groups cross each other when the duration of diabetes approaches 30 years. The survival curve for those patients with age at onset of type 1 diabetes at 15–40 years appears to be consistently better than that for patients with younger age of onset.
Good self-care of diabetes correlates with good metabolic control (15). It can be hypothesized that it may be easier to learn good self-care skills at a very young age compared with the prepubertal period (14). Learning good self-care skills may take a longer time in prepubertal children compared with young children, which would explain the delayed onset of proliferative retinopathy in the youngest patients. In addition to behavioral factors, hormonal changes in puberty may contribute to worse metabolic control (16). Furthermore, the initial advantage for the younger patients may be due to more stringent management of type 1 diabetes, because this has been shown to reduce the decline in insulin production (17).
Diabetes onset at puberty has been linked to a less aggressive form of type 1 diabetes (18), and it has also been observed that β-cells are better preserved when type 1 diabetes begins in the adulthood (19). We observed a longer time without proliferative retinopathy as well as a lower risk of proliferative retinopathy for age-at-onset group 15–40 years. This finding could be explained by preservation of β-cells as these patients also had the highest C-peptide concentrations. The Diabetes Control and Complications Trial data indicated that patients with any residual C-peptide secretion, but especially those with the highest stimulated concentrations, had a reduced incidence of retinopathy and nephropathy (20). The role of C-peptide has been somewhat controversial, because it has not been linked to retinopathy in other studies (3). In our study those patients with a higher C-peptide concentration had less proliferative retinopathy, later age at onset, and lower A1C (Tables 1 and 2). The association of age at onset with the risk of diabetic retinopathy may not be limited to type 1 diabetes. It has recently been noted that a higher age at onset of diabetes reduces the risk of retinopathy in type 2 diabetic patients as well (21).
An advantage of our study is that the timing of onset of proliferative retinopathy is robust, because it was based on several examinations and/or serial fundus photographs. Only in 19 of 367 (5.2%) patients was proliferative retinopathy discovered at their first fundus examination by an ophthalmologist. Thus, there were no available reference points for these patients before they had developed proliferative retinopathy. Because these 19 patients comprise only 5.2% of all the patients with proliferative retinopathy, the possible inaccuracy introduced by these patients was judged to be negligible, and they were kept in the study. Importantly, this decision also kept the original random sampling frame intact. All of the other patients (n = 348) had had at least one ophthalmic examination on a median of 0.7 (0.3–1.8) years before the diagnosis. In addition, records of treatment and follow-up were available for nearly all (364 of 367) patients with proliferative retinopathy. Retinal photography has been reported to be the most sensitive screening method for diabetic retinopathy. The sensitivity is in excess of 80% in detecting proliferative retinopathy (22). Ophthalmoscopy has less sensitivity but conversely a higher specificity. It provides good results in the hands of trained professionals such as ophthalmologists and diabetologists, especially when used in repeated examinations (22). In Finland, the national guidelines for the screening of diabetic retinopathy were published already in 1992 and updated in 2006, along the European guidelines, which emphasize fundus photography as the preferable screening method (23). As a result, fundus photographs were available for as many as 851 of 1,117 (76%) patients.
The generalizability of the present results may be limited by the fact that the FinnDiane Study is not by strict definition a population-based study. In any case, a possible selection bias is unlikely because the geographic distribution of the FinnDiane patients closely follows the distribution of the general population, and the patient recruitment at the participating centers can be considered random. Furthermore, the diagnosis and treatment of diabetes and its complications are fairly uniform across Finland. There has been only one population-based study on the prevalence of proliferative retinopathy among patients with type 1 diabetes in Finland in a sample of 1,067 patients, and the results are comparable to ours (24). Another potentially important issue is the competing risk of death. Accounting for the cumulative mortality by using a previously published SAS macro (13) did not change the outcome of either Kaplan-Meier or Cox regression analysis. Interestingly, 75 of 99 patients who had died during the follow-up had developed proliferative retinopathy. The ultimate long-term risk of proliferative retinopathy appears therefore to be roughly 75% for the FinnDiane study population. Finally, there may also be a confounding cohort effect in the present study, because the oldest patients may have a worse prognosis than the younger ones attributable to improvements in medical care of diabetes (25). However, we tested the data for such a cohort effect in two ways. The first way was to use the year of onset of diabetes as a continuous variable in the Cox regression model. The other was to use the decade of onset of diabetes as a categorical variable. Both of these variables were statistically nonsignificant.
In summary, our study shows a longer time free of proliferative retinopathy in the youngest patients. It appears that this initial advantage gradually wears off in the long term. In contrast, those patients with age at onset of type 1 diabetes between 15 and 40 years may have a consistently better prognosis than any patient group with age at onset <15 years.
Supplementary Material
Acknowledgments
This study was supported by grants from Folkhälsan Research Foundation, Wilhelm and Else Stockmann Foundation, Finnish Eye Foundation, and Eye and Tissue Bank Foundation and by a special governmental grant for health sciences research (TYH 3263).
No potential conflicts of interest relevant to this article were reported.
We acknowledge all of the physicians and nurses at each center participating in the collection of patients.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1. Klein R: The epidemiology of diabetic retinopathy: findings from the Wisconsin Epidemiologic Study of Diabetic Retinopathy. Int Ophthalmol Clin 1987;27:230–238 [DOI] [PubMed] [Google Scholar]
- 2. Deckert T, Simonsen SE, Poulsen JE: Prognosis of proliferative retinopathy in juvenile diabetics. Diabetes 1967;16:728–733 [DOI] [PubMed] [Google Scholar]
- 3. Klein R, Knudtson MD, Lee KE, Gangnon R, Klein BE: The Wisconsin Epidemiologic Study of Diabetic Retinopathy: XXII. The twenty-five-year progression of retinopathy in persons with type 1 diabetes. Ophthalmology 2008;115:1859–1868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rossing K, Jacobsen P, Rossing P, Lauritzen E, Lund-Andersen H, Parving HH: Improved visual function in IDDM patients with unchanged cumulative incidence of sight-threatening diabetic retinopathy. Diabetes Care 1998;21:2007–2015 [DOI] [PubMed] [Google Scholar]
- 5. Retinopathy and nephropathy in patients with type 1 diabetes four years after a trial of intensive therapy. The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group. N Engl J Med 2000;342:381–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hietala K, Forsblom C, Summanen P, Groop PH: FinnDiane Study Group. Heritability of proliferative diabetic retinopathy. Diabetes 2008;57:2176–2180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kostraba JN, Dorman JS, Orchard TJ, Becker DJ, Ohki Y, Ellis D, Doft BH, Lobes LA, LaPorte RE, Drash AL: Contribution of diabetes duration before puberty to development of microvascular complications in IDDM subjects. Diabetes Care 1989;12:686–693 [DOI] [PubMed] [Google Scholar]
- 8. Donaghue KC, Fairchild JM, Craig ME, Chan AK, Hing S, Cutler LR, Howard NJ, Silink M: Do all prepubertal years of diabetes duration contribute equally to diabetes complications? Diabetes Care 2003;26:1224–1229 [DOI] [PubMed] [Google Scholar]
- 9. Harjutsalo V, Sjöberg L, Tuomilehto J: Time trends in the incidence of type 1 diabetes in Finnish children: a cohort study. Lancet 2008;371:1777–1782 [DOI] [PubMed] [Google Scholar]
- 10. Fagerudd J, Forsblom C, Pettersson-Fernholm K, Groop PH: FinnDiane Study Group. Implementation of guidelines for the prevention of diabetic nephropathy. Diabetes Care 2004;27:803–804 [DOI] [PubMed] [Google Scholar]
- 11. Madsbad S, Alberti KG, Binder C, Burrin JM, Faber OK, Krarup T, Regeur L: Role of residual insulin secretion in protecting against ketoacidosis in insulin-dependent diabetes. Br Med J 1979;2:1257–1259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Davis MD, Fisher MR, Gangnon RE, Barton F, Aiello LM, Chew EY, Ferris FL, 3rd, Knatterud GL: Risk factors for high-risk proliferative diabetic retinopathy and severe visual loss: Early Treatment Diabetic Retinopathy Study Report #18. Invest Ophthalmol Vis Sci 1998;39:233–252 [PubMed] [Google Scholar]
- 13. Bergstralh E: SAS macro: comprisk. Department of Biostatistics, Mayo Clinic College of Medicine, Rochester, MN, USA: 2004. [Google Scholar]
- 14. Finne P, Reunanen A, Stenman S, Groop PH, Grönhagen-Riska C: Incidence of end-stage renal disease in patients with type 1 diabetes. JAMA 2005;294:1782–1787 [DOI] [PubMed] [Google Scholar]
- 15. Wysocki T, Harris MA, Wilkinson K, Sadler M, Mauras N, White NH: Self-management competence as a predictor of outcomes of intensive therapy or usual care in youth with type 1 diabetes. Diabetes Care 2003;26:2043–2047 [DOI] [PubMed] [Google Scholar]
- 16. Acerini CL, Williams RM, Dunger DB: Metabolic impact of puberty on the course of type 1 diabetes. Diabetes Metab 2001;27:S19–S25 [PubMed] [Google Scholar]
- 17. Sherry NA, Tsai EB, Herold KC: Natural history of β-cell function in type 1 diabetes. Diabetes 2005;54(Suppl. 2):S32–S39 [DOI] [PubMed] [Google Scholar]
- 18. Kordonouri O, Danne T, Enders I, Weber B: Does the long-term clinical course of type I diabetes mellitus differ in patients with prepubertal and pubertal onset? Results of the Berlin Retinopathy Study. Eur J Pediatr 1998;157:202–207 [DOI] [PubMed] [Google Scholar]
- 19. Karjalainen J, Salmela P, Ilonen J, Surcel HM, Knip M: A comparison of childhood and adult type I diabetes mellitus. N Engl J Med 1989;320:881–886 [DOI] [PubMed] [Google Scholar]
- 20. Steffes MW, Sibley S, Jackson M, Thomas W: β-Cell function and the development of diabetes-related complications in the diabetes control and complications trial. Diabetes Care 2003;26:832–836 [DOI] [PubMed] [Google Scholar]
- 21. Wong J, Molyneaux L, Constantino M, Twigg SM, Yue DK: Timing is everything: age of onset influences long-term retinopathy risk in type 2 diabetes, independent of traditional risk factors. Diabetes Care 2008;31:1985–1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hutchinson A, McIntosh A, Peters J, O'Keeffe C, Khunti K, Baker R, Booth A: Effectiveness of screening and monitoring tests for diabetic retinopathy—a systematic review. Diabet Med 2000;17:495–506 [DOI] [PubMed] [Google Scholar]
- 23. Retinopathy Working Party. A protocol for screening for diabetic retinopathy in Europe. Diabet Med 1991;8:263–267 [PubMed] [Google Scholar]
- 24. Virtamo T, Summanen P, Tuomilehto J, Laatikainen L: Diabetic retinopathy and visual impairment in juvenile onset diabetics in Finland (Abstract). Diabetes Metab 2001;27(Suppl. 2):2S14 [Google Scholar]
- 25. Wong TY, Mwamburi M, Klein R, Larsen M, Flynn H, Hernandez-Medina M, Ranganathan G, Wirostko B, Pleil A, Mitchell P: Rates of progression in diabetic retinopathy during different time periods: a systematic review and meta-analysis. Diabetes Care 2009;32:2307–2313 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.