Abstract
OBJECTIVE
To describe retinal microvascular geometric parameters in young patients with type 1 diabetes.
RESEARCH DESIGN AND METHODS
Patients with type 1 diabetes (aged 12–20 years) had clinical assessments and retinal photography following standardized protocol at a tertiary-care hospital in Sydney. Retinal microvascular geometry, including arteriolar and venular tortuosity, branching angles, optimality deviation, and length-to-diameter ratio (LDR), were measured from digitized photographs. Associations of these geometric characteristics with diabetes duration, A1C level, systolic blood pressure (SBP), and other risk factors were assessed.
RESULTS
Of 1,159 patients enrolled, 944 (81.4%) had gradable photographs and 170 (14.7%) had retinopathy. Older age was associated with decreased arteriolar (P = 0.024) and venular (P = 0.002) tortuosity, and female subjects had larger arteriolar branching angle than male subjects (P = 0.03). After adjusting for age and sex, longer diabetes duration was associated with larger arteriolar branching angle (P ≤ 0.001) and increased arteriolar optimality deviation (P = 0.018), higher A1C was associated with increased arteriolar tortuosity (>8.5 vs. ≤8.5%, P = 0.008), higher SBP was associated with decreased arteriolar LDR (P = 0.002), and higher total cholesterol levels were associated with increased arteriolar LDR (P = 0.044) and decreased venular optimality deviation (P = 0.044). These associations remained after controlling for A1C, retinal vessel caliber, and retinopathy status and were seen in subjects without retinopathy.
CONCLUSIONS
Key diabetes-related factors affect retinal microvascular geometry in young type 1 diabetes, even in those without evidence of retinopathy. These early retinal alterations may be markers of diabetes microvascular complications.
Children and young adults with type 1 diabetes are at risk of morbidity and disability, largely due to the development of microvascular complications such as retinopathy and nephropathy (1). Endothelial dysfunction, alteration in hemodynamic milieu, and oxygen desaturation associated with diabetes, manifesting as fluctuations in microvascular pressure and flow, are hypothesized mechanisms leading to microvascular injury (2). However, there are limited clinical data on the early changes in microcirculation prior to the appearance of diabetes complications in young people with type 1 diabetes.
Quantitative assessment of microvascular structure from retinal images provides means of noninvasive assessment of microcirculation and can inform on early preclinical pathophysiological processes involved in the evolution of microvascular complications (3). In nondiabetic populations, several geometry and morphological structures of the retinal microvasculature have been previously investigated, including vascular tortuosity (4), bifurcation/branching angle (5), junctional exponent (5), and length-to-diameter ratio (LDR) (6). Murray (7) proposed that an optimal vascular architecture achieves the most efficient blood flow transport with minimum energy spent, and the geometry of the microvascular structure follows the principle of efficiency to maximize intravascular blood flow and diffusion. However, retinal microvascular geometry parameters have not been studied previously in type 1 diabetes.
In this study, we hypothesized that in young people with type 1 diabetes, alterations in retinal microvascular geometry are associated with adverse diabetes risk profiles, including longer duration of diabetes and higher A1C and blood pressure levels. We further hypothesize that these associations are present regardless of the presence of retinopathy.
RESEARCH DESIGN AND METHODS
The Sydney Pediatric Diabetes Study is a prospective, clinic-based study of children and adolescents with type 1 diabetes, aged 12–20 years at baseline, who attended the Diabetes Complications Assessment Service at the Children's Hospital at Westmead during a 12-year period from 1990 to 2002. Details of this cohort were published previously (1). Briefly, diagnosis of type 1 diabetes was made according to criteria by the Australasian Pediatric Endocrine Group Diabetes Register and National Guidelines. Baseline assessment and retinal photography was performed at the initial visit during the recruitment period. Of 1,159 participants who had retinal photographs taken at their baseline visits, we excluded those with ungradable photographs (n = 215, 18.6%), due to either poor quality of retinal photographs or having less than four big vessels that could be traced by the computer-assisted program, leaving 944 (81.4%) participants included in analyses for this report.
Risk factors assessment
Key diabetes-related characteristics assessed included pubertal stage, duration of diabetes, and levels of A1C, cholesterol, and blood pressure, which were collected at the baseline visits via interviews, clinical examinations, and laboratory investigations following standardized protocols (1). Pubertal stage was determined using Tanner stage classification by a pediatric endocrinologist. BMI was calculated by dividing subject's weight (in kilograms) with the square of height (in meters). Systolic (SBP) and diastolic (DBP) blood pressure were measured after resting for 5 min, sitting in an upright position using a standard sphygmomanometer with an appropriately sized cuff. A1C and total plasma cholesterol levels were measured following standardized laboratory procedures (1).
Retinal photography and retinal image analysis
Retinal photography was performed according to a standardized protocol, as detailed elsewhere (1). Briefly, stereoscopic retinal photographs in seven standard fields of the Early Treatment Diabetic Retinopathy Study (ETDRS) were taken on film from both eyes after pupil dilation, using a Topcon Fundus Camera (TRC 50-VT; Tokyo Optical, Tokyo, Japan) (1). Retinopathy was assessed by an ophthalmologist and defined as present when any microaneurysm/retinal hemorrhage was found in either eye (1).
For measuring retinal microvascular geometric properties, right-eye retinal photographs of each patient were digitized and analyses were performed using a semiautomated computer-assisted image program (Singapore I Vessel Assessment or SIVA, Singapore). Retinal photographs that were centered on the optic disc were viewed on two 19-inch monitors with a resolution of 1,280 × 1,024. For each retinal photograph, a trained grader, masked to participants' identities, applied the program to measure retinal microvascular geometric parameters within a concentric zone between the optic disc margin and two optic disc diameters away from the optic disc margin. The grader allowed the software to detect the center of the optic disc and divided the region into three subzones (A, B, and C) surrounding the optic disc, each zone corresponding to 0.5, 1.0, and 2.0 optic disc diameters away from the optic disc margin, respectively. Once the optic disc and the three concentric subzones were considered appropriately located, the grader executed the program to trace all vessels. This software has an ability (70–90%) to appropriately detect arterioles and venules. However, the grader checked each graded image to see if all arterioles and venules were correctly identified, based on information of parent vessels, crossing between arterioles and venules and the color of the vessels. Corrections were made, if necessary. Graders were trained and tested for his/her ability in identifying arterioles and venules by a senior grader. During the grading process of this project, 100 randomly selected images were used to test intra- and intergrader reliabilities in classification of arterioles and venules, with κ value 0.95–0.99.
Measurements were based on the biggest six arterioles and venules. Images were considered poor quality if they were blurred or had an incomplete representation of zone C and as ungradable if there were less than four gradable large arterioles or venules. The software combined the individual measurement into summary indexes of tortuosity, branching angles, optimality deviation, and LDR for arterioles and venules separately, as described below:
Vessel tortuosity (Fig. 1) reflects the shape of the vessel and is expressed as a curvature tortuosity index (4), calculated from the integral of the total squared curvature along the path of the vessel divided by the total arc length. Normal vessels are generally straight and smooth (4). Increased tortuosity has been linked with hypertension (8) and diabetic retinopathy (9), while decreased tortuosity has been associated with ischemic heart disease–related death (10), ageing, and hypertension (C. Y. Cheung, E. Lamoureux, L. Xu, W. Hsu, M. L. Lee, Q. P. Lau, J. J. Wang, P. Mitchell, T. Y. Wong).
Branching angle represents (in degrees) the angle between two daughter vessels (5) and is thought to be related to blood flow efficiency, energy cost of bulk flow, and diffusion distance (11). Zamir et al. (5) proposed that the optimal value for the branching angle is 75 degrees (Fig. 1), and increased angles have been related to decreased blood flow (12), while decreased angles are associated with ageing and hypertension (11).
Optimality deviation is determined when the junctional exponent is calculated as d1x + d2x = d0x, where d0, d1, and d2 are diameters of the parent, larger, and smaller daughter vessels, respectively (11). The greater the value of x, the larger the daughter arterioles are relative to the parent vessel. Junctional exponent provides an index of caliber sizes of two daughter vessels relative to the parent vessel and is considered to represent an optimality state of microvascular networks (5). It has been proposed that in an optimal state, the value of junctional exponent is three (5), and optimality deviation represents the deviation from this value.
LDR is calculated as the length from the midpoint of the first branch to the midpoint of the second branch divided by the diameter of the parent vessel at the first branch (6). LDR is a measure of diameter changes that are independent of refractive magnification power of the eye (6).
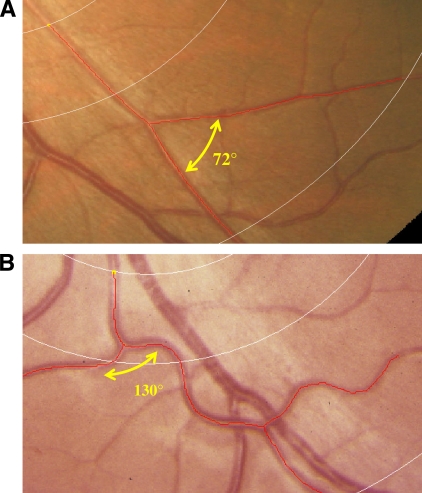
Figure 1.
Measures of retinal vessel tortuosity, branching angle, and optimality deviation. A comparison of optimal (A) and less optimal (B) arrangement of arteriolar geometry. A: Tortuosity of 0.00, branching angle of 72°, and optimality deviation of 0.16. B: Tortuosity of 47.2 × 10−3, branching angle of 130°, and optimality deviation of 0.61. A high-quality color representation of this image is available online.
Statistical analysis
All of statistical procedures were performed using Intercooled STATA 10.1 for Windows (StataCorp, College Station, TX). Retinal microvascular geometry (tortuosity, branching angle, optimality deviation, and LDR) of the right eye of each patient were used as dependent, continuous variables. Distribution of these variables was checked for normality. We used ANCOVA to compare mean retinal vessel geometric parameters by age-group (12–14 and 14–20 years), sex (female and male), pubertal stage (stage 1 to stage 5), BMI levels (≤18.0, 18.1–21.0, 21.1–25.0, and ≥25.1 kg/m2), SBP (≤120 vs. ≥120 mmHg), cholesterol (≤3.7, 3.8–4.3, 4.4–4.8, and ≥4.9 mmol/l), duration of diabetes (≤5.0, 5.1–10.0, and ≥10.1 years), and A1C (≤8.5 vs. >8.5%) (1) (see supplementary Tables in the online appendix, available at http://care.diabetesjournals.org/cgi/content/full/dc10-0055/DC1). Multiple linear regression models were subsequently used to examine the independent determinants of retinal microvascular geometric parameters as continuous variables. We constructed three models: model 1 included age and sex; model 2 additionally included BMI, SBP, cholesterol, duration of diabetes, A1C (all assessed as per SD change), presence of retinopathy, and retinal arteriolar caliber (in models for arteriolar geometric parameters) or venular caliber (in models for venular geometric parameters); and model 3 included all variables in model 2 after excluding subjects with diabetic retinopathy. We also examined interactions with these characteristics using the likelihood ratio test. Finally, sensitivity analyses were performed by including and excluding subjects with poor-quality images.
RESULTS
Of 944 patients in this study, 170 (14.7%) had retinopathy (all classified as mild nonproliferative retinopathy). The baseline characteristics of participants with and without gradable right-eye retinal photographs are shown in Table 1 and retinal microvascular measurements are shown in Table 2.
Table 1.
Baseline characteristics, according to the gradability of retinal photograph
| Characteristics | Gradable | Ungradable | P value |
|---|---|---|---|
| n | 944 | 215 | |
| Sex (% male) | 47.0 | 42.8 | 0.260 |
| Pubertal stage (%) | |||
| 1 | 5.4 | 4.6 | 0.450 |
| 2 | 11.9 | 13.8 | |
| 3 | 16.1 | 10.9 | |
| 4 | 25.4 | 28.7 | |
| 5 | 41.1 | 42.0 | |
| Age (years) | 14.1 ± 0.05 | 13.9 ± 0.10 | 0.023 |
| BMI (kg/m2) | 21.9 ± 0.11 | 21.8 ± 0.23 | 0.902 |
| SBP (mmHg) | 111.9 ± 0.38 | 113.5 ± 0.79 | 0.052 |
| DBP (mmHg) | 67.4 ± 0.26 | 67.9 ± 0.55 | 0.370 |
| Mean arterial blood pressure (mmHg) | 82.2 ± 0.26 | 83.1 ± 0.55 | 0.128 |
| Cholesterol (mmol/l) | 4.4 ± 0.03 | 4.4 ± 0.06 | 0.327 |
| Duration of diabetes (years) | 6.2 ± 0.11 | 5.6 ± 0.22 | 0.026 |
| A1C (%) | 8.5 ± 0.04 | 8.5 ± 0.09 | 0.944 |
Data are age and sex-adjusted means ± SE or %. n = number at risk.
Table 2.
Retinal microvascular parameters in 944 children and adolescents aged 12–20 years with type 1 diabetes
| Value | |
|---|---|
| Arterioles | |
| Microvascular parameters | |
| Tortuosity index (×103) | 34.2 ± 10.2 |
| Branching angle (degrees) | 83.7 ± 10.0 |
| Junctional exponent Optimality deviation (×102) | 71.9 ± 16.7 |
| LDR | 65.4 (90.2) |
| Venules | |
| Microvascular parameters | |
| Tortuosity index (×103) | 31.1 ± 10.2 |
| Branching angle (degrees) | 79.4 ± 11.1 |
| Junctional exponent Optimality deviation (×102) | 65.1 ± 19.6 |
| LDR | 72.2 (102.4) |
Data are means ± SD or median (interquartile range).
Table 3 shows that older age was associated with decreased arteriolar and venular tortuosity, and female patients on average had larger arteriolar branching angle than male patients. After adjusting for age, sex, SBP, cholesterol level, duration of diabetes, A1C level, retinopathy, and vessel caliber, the following associations were evident: 1) increasing diabetes duration was associated with increased arteriolar branching angle (P ≤ 0.002) and increased optimality deviation of arterioles (P = 0.014), 2) high A1C (>8.5 vs. ≤8.5%,) was associated with increased arteriolar tortuosity (P = 0.018), 3) increasing SBP was associated with decreasing arteriolar LDR (P = 0.001) and nonsignificantly decreasing venular LDR (0.05 < P < 0.10), and 4) increasing total cholesterol level was associated with increasing arteriolar LDR (P = 0.014) and decreasing optimality deviation of venules (P = 0.07). The observed significant associations largely remained after excluding eyes with retinopathy (n = 170, model 3) (Table 3).
Table 3.
Associations of baseline and diabetes-related factors with retinal microvascular geometry
| Arterioles |
Venules |
|||||||
|---|---|---|---|---|---|---|---|---|
| Mean difference* | P 1 | P 2 | P 3 | Mean difference* | P 1 | P 2 | P 3 | |
| Age (per SD) (1.5-year increase) | ||||||||
| Tortuosity (×103) | −0.73 (−1.37 to −0.10) | 0.024 | 0.037 | 0.035 | −0.90 (−1.54 to −0.26) | 0.006 | 0.008 | 0.002 |
| Branching angle | 0.21 (−0.41 to 0.83) | 0.502 | 0.618 | 0.957 | 0.28 (−0.41 to 0.97) | 0.840 | 0.277 | 0.748 |
| Optimality deviation (×102) | 0.09 (−0.95 to 1.13) | 0.863 | 0.754 | 0.992 | 0.40 (−1.32 to 2.12) | 0.647 | 0.842 | 0.995 |
| LDR | −1.87 (−7.90 to 4.17) | 0.543 | 0.395 | 0.071 | −2.36 (−8.26 to 3.53) | 0.432 | 0.582 | 0.483 |
| Sex (female versus male subjects) | ||||||||
| Tortuosity (×103) | 0.04 (−1.26 to 1.36) | 0.941 | 0.962 | 0.771 | −0.12 (−1.44 to 1.54) | 0.858 | 0.748 | 0.557 |
| Branching angle | 1.39 (0.11–2.66) | 0.033 | 0.019 | 0.014 | 0.60 (−0.64 to 1.83) | 0.342 | 0.472 | 0.642 |
| Optimality deviation (×102) | −0.33 (−2.46 to 1.81) | 0.762 | 0.871 | 0.820 | 0.15 (−2.29 to 3.70) | 0.932 | 0.923 | 0.877 |
| LDR | 2.77 (−9.78 to 15.3) | 0.665 | 0.883 | 0.411 | 0.19 (−12.0 to 12.4) | 0.975 | 0.971 | 0.449 |
| Duration (per SD) (3.3-year-increase) | ||||||||
| Tortuosity (×103) | 0.49 (−0.16 to 1.14) | 0.141 | 0.057 | 0.395 | −0.22 (−0.88 to 0.43) | 0.505 | 0.746 | 0.674 |
| Branching angle | 1.15 (0.52–1.78) | <0.001 | 0.002 | 0.045 | −0.26 (−0.88 to 0.36) | 0.409 | 0.983 | 0.815 |
| Optimality deviation (×102) | 1.29 (0.22–2.35) | 0.018 | 0.014 | 0.007 | 1.44 (−0.31 to 3.19) | 0.107 | 0.904 | 0.703 |
| LDR | −0.46 (−6.61 to 5.68) | 0.882 | 0.849 | 0.352 | −3.21 (−9.23 to 2.82) | 0.297 | 0.311 | 0.371 |
| A1C (≤8.5 vs. >8.5%) | ||||||||
| Tortuosity (×103) | 1.75 (0.45–3.05) | 0.008 | 0.018 | 0.014 | −0.13 (−1.44 to 1.19) | 0.848 | 0.741 | 0.465 |
| Branching angle | 0.41 (−0.86 to 1.70) | 0.520 | 0.855 | 0.868 | −0.05 (−1.29 to 1.18) | 0.936 | 0.941 | 0.961 |
| Optimality deviation (×102) | 0.97 (−1.17 to 3.10) | 0.373 | 0.355 | 0.530 | 0.84 (−2.71 to 4.38) | 0.643 | 0.657 | 0.741 |
| LDR | 0.18 (−12.4 to 12.7) | 0.977 | 0.643 | 0.362 | −0.61 (−12.8 to 11.6) | 0.922 | 0.784 | 0.546 |
| SBP (per SD) (12-mmHg increase) | ||||||||
| Tortuosity (×103) | −0.28 (−0.95 to 0.40) | 0.419 | 0.344 | 0.984 | −0.55 (−1.23 to 0.12) | 0.108 | 0.124 | 0.311 |
| Branching angle | −0.13 (−0.80 to 0.52) | 0.678 | 0.578 | 0.563 | −0.27 (−0.91 to 0.37) | 0.416 | 0.532 | 0.639 |
| Optimality deviation (×102) | 0.26 (−0.85 to 1.36) | 0.648 | 0.723 | 0.531 | 0.17 (−1.67 to 2.01) | 0.853 | 0.583 | 0.532 |
| LDR | −10.0 (−16.3 to −3.69) | 0.002 | 0.001 | 0.003 | −5.52 (−11.7 to 0.65) | 0.080 | 0.034 | 0.025 |
| Cholesterol (per SD) (0.90 mmol/l increase) | ||||||||
| Tortuosity (×103) | −0.02 (−0.70 to 0.66) | 0.948 | 0.851 | 0.415 | −0.71 (−1.40 to −0.02) | 0.043 | 0.268 | 0.084 |
| Branching angle | 0.41 (−0.26 to 1.08) | 0.231 | 0.346 | 0.874 | 0.44 (−0.20 to 1.09) | 0.180 | 0.027 | 0.107 |
| Optimality deviation (×102) | 0.36 (−0.77 to 1.49) | 0.529 | 0.773 | 0.646 | −1.90 (−3.75 to −0.06) | 0.044 | 0.007 | 0.015 |
| LDR | 6.92 (0.19–13.6) | 0.044 | 0.014 | 0.041 | 3.54 (−2.95 to 10.0) | 0.284 | 0.222 | 0.280 |
| BMI (per SD) (3.5 kg/m2 increase) | ||||||||
| Tortuosity (×103) | −0.07 (−0.77 to 0.63) | 0.841 | 0.996 | 0.595 | 0.13 (−0.57 to 0.84) | 0.713 | 0.564 | 0.289 |
| Branching angle | −0.37 (−1.06 to 0.31) | 0.285 | 0.557 | 0.848 | −0.58 (−1.24 to 0.87) | 0.088 | 0.327 | 0.738 |
| Optimality deviation (×102) | −0.18 (−1.33 to 0.96) | 0.753 | 0.871 | 0.810 | 0.44 (−1.46 to 2.36) | 0.646 | 0.493 | 0.401 |
| LDR | −2.54 (−9.58 to 4.48) | 0.477 | 0.584 | 0.506 | 3.47 (−3.40 to 10.3) | 0.322 | 0.453 | 0.580 |
*Data are mean difference (95% CI) of retinal parameters per SD increase in duration (3.3 years) or ≤8.5 vs. >8.5% A1C level and per 1-SD increase in SBP (12 mmHg), cholesterol (0.9 mmol/l), and BMI (3.5 kg/m2) using linear regression models. P1, P value of β coefficient adjusted for age and sex; P2, P value of β coefficient adjusted for age, sex, SBP, cholesterol, duration, A1C, retinopathy, and retinal vessel caliber; P3, P value of β coefficient, adjusted for age, sex, SBP, cholesterol, duration, A1C, and retinal vessel caliber, excluding subjects with retinopathy.
There were no significant interactions between age, sex, blood pressure, BMI, cholesterol, duration of diabetes, and A1C level. Analyses after excluding subjects with relatively poor image quality (n = 129, 11.1%) showed that these associations remained significant in 815 (70.3%) patients with good-quality retinal images (data not shown).
CONCLUSIONS
In young type 1 diabetes, we showed that variations in retinal microvascular geometric characteristics were associated with key diabetes-related risk factors, including longer diabetes duration and higher A1C, blood pressure, and cholesterol levels. The principal-specific findings are the following: longer duration of diabetes was associated with larger arteriolar branching angles and increasing deviation of optimality, higher A1C was associated with more tortuous arterioles, increasing SBP level was associated with smaller arteriolar and venular LDR, and increasing cholesterol level was associated with increasing arteriolar LDR and decreasing venular optimality deviation. Importantly, we showed that these associations were present even in subjects without mild retinopathy signs, suggesting that early retinal microvascular alternations are present well before the onset of clinical microvascular complications in young patients with type 1 diabetes.
There are few comparative studies. Most previous studies that have examined retinal microvascular geometric parameters have been conducted in nondiabetic populations, including healthy subjects (13), subjects with hypertension (11), or those with coronary heart disease (10). To the best of our knowledge, there has been no study reporting retinal microvascular geometric characteristics in young type 1 diabetes.
Our findings that longer duration of diabetes was associated with larger arteriolar branching angle and increasing deviation from optimality of arterioles is biologically plausible and may provide clues to early microvascular alterations in type 1 diabetes. Branching angle and junctional exponent are measures of circulatory optimality. An optimal branching angle is associated with greater efficiency in blood flow with lower energy spent (5,7). Such efficiency reduces when the branching angle is too large or when the size of daughter vessel is too large or too small relative to its parent vessel (5,7). Zamir et al. (5) have shown that departure from normal or increased deviation from optimality could result in increased workload and energy loss in maintaining the blood circulation. Branching angle and junctional exponent have been found to be impaired in atherosclerosis (14), altered blood flow (12), and endothelial dysfunction (15). Moreover, Chapman et al. (16) demonstrated that branching angle was likely to increase in response to less oxygen saturation. Thus, our finding of associations of longer diabetes duration with larger branching angle and higher optimality deviation may reflect alterations in blood flow (17), endothelial dysfunction (18), and attenuation in oxygen saturation (16). The sex difference in arteriolar branching angle, with female subjects having larger arteriolar branching angles than male subjects, could also explain our earlier observations that female patients with type 1 diabetes have greater risk for diabetic microvascular complications compared with male patients of the same age (1,3).
Diabetes is known to be associated with increased shear stress and impaired microvascular endothelium (2). Previously, increased A1C level had been shown to have a role in reduced endothelial function in people with type 1 diabetes (19). Increased vessel tortuosity has been documented to be related to increased angiogenesis (20) and endothelial dysfunction (10). We did not find a linear association between A1C levels and arteriolar tortuosity, but we observed a significant difference in tortuosity between patients with A1C ≤8.5 vs. ≥8.5%. This finding may suggest a threshold effect of the association. There have been studies showing that different cutoffs of A1C levels identify people with diabetes at high risk of microvascular injury (21,22), although there is no universal consensus on these cutoffs (22). This finding requires further confirmation.
We have previously reported that wider arteriolar caliber is associated with an increased risk of diabetic retinopathy in both type 1 and type 2 diabetes (3,23). Wider arteriolar caliber has been suggested to reflect impaired vessel autoregulation. Thus, the finding of an association of higher blood pressure levels with increasing arteriolar LDR (reflecting wider arteriolar caliber) is consistent with the influence of diabetes and blood pressure on autoregulatory processes in small blood vessels.
There have been few studies on cholesterol levels on microvascular structure, with most previous studies reporting associations between cholesterol level and retinal microvascular changes in nondiabetic populations showing inconsistent results (24,25). Therefore, our findings that higher total cholesterol levels was associated with changes in arteriolar LDR and venular optimality deviation suggests that lipids may also have an influence on microvasculature in young type 1 diabetes.
The strengths of the study are apparent. First, our study includes a large cohort of young patients with type 1 diabetes, with a participation rate of >80%. Second, our study included an objective quantitative measurement of the geometry of retinal microvasculature using computer programs. The cross-sectional nature of our study implies that longitudinal studies are needed to determine the temporal sequence of these associations. A number of images (18.6%) were ungradable and thus excluded from the analysis. However, baseline characteristics and diabetes-related risk factors were largely similar in participants with and without gradable images. Therefore, exclusion of participants with ungradeable images did not substantially influence our findings. In addition, there are several potential sources of measurement errors in assessing branching/bifurcation angles, particularly in subjects with poor retinal image quality, given that the computer software needs some manual intervention. However, as the measurement errors are random, these study findings are unlikely to be altered substantially by random errors.
In summary, our study demonstrated that in young type 1 diabetes, subtle alterations in retinal microvascular geometric parameters are associated with key diabetes-related characteristics, including duration of diabetes and A1C and blood pressure levels. These findings were present in those without any signs of retinopathy, suggesting an effect of these risk factors on the microcirculation prior to development of clinical complications. Some of the observed associations are related to known effects of diabetes on microvasculature; for other associations, the underlying mechanisms remain to be determined. Nonetheless, these data support the concept that retinal microvascular geometric changes might represent a novel marker indicative of early diabetes-related microvascular injury in patients with type 1 diabetes. Further studies are warrant to determine if these early retinal microvascular geometric alterations can predict the subsequent development of overt microvascular complications.
Supplementary Material
Acknowledgments
This study is supported by the National Health and Medical Research Council Grant 475605 (to T.Y.W., K.D., and A.J.), the Juvenile Diabetes Research Foundation Innovative Grant (to T.Y.W., K.D., A.J., and N.C.), and the Royal Victorian Eye and Ear Hospital Research Grant (to N.C.).
No potential conflicts of interest relevant to this article were reported.
We thank Dr. Amirul Islam, PhD, for the statistical guide and for advice on this article.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1. Mohsin F, Craig ME, Cusumano J, Chan AK, Hing S, Lee JW, Silink M, Howard NJ, Donaghue KC: Discordant trends in microvascular complications in adolescents with type 1 diabetes from 1990 to 2002. Diabetes Care 2005;28:1974–1980 [DOI] [PubMed] [Google Scholar]
- 2. Tooke JE: Microvascular function in human diabetes: a physiological perspective. Diabetes 1995;44:721–726 [DOI] [PubMed] [Google Scholar]
- 3. Cheung N, Rogers SL, Donaghue KC, Jenkins AJ, Tikellis G, Wong TY: Retinal arteriolar dilation predicts retinopathy in adolescents with type 1 diabetes. Diabetes Care 2008;31:1842–1846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hart WE, Goldbaum M, Cote B, Kube P, Nelson MR: Measurement and classification of retinal vascular tortuosity. Int J Med Inform 1999;53:239–252 [DOI] [PubMed] [Google Scholar]
- 5. Zamir M, Medeiros JA, Cunningham TK: Arterial bifurcations in the human retina. J Gen Physiol 1979;74:537–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. King LA, Stanton AV, Sever PS, Thom SA, Hughes AD: Arteriolar length-diameter (L:D) ratio: a geometric parameter of the retinal vasculature diagnostic of hypertension. J Hum Hypertens 1996;10:417–418 [PubMed] [Google Scholar]
- 7. Murray CD: The physiological principle of minimum work: I. the vascular system and the cost of blood volume. Proc Natl Acad Sci U S A 1926;12:207–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hughes AD, Martinez-Perez E, Jabbar AS, Hassan A, Witt NW, Mistry PD, Chapman N, Stanton AV, Beevers G, Pedrinelli R, Parker KH, Thom SA: Quantification of topological changes in retinal vascular architecture in essential and malignant hypertension. J Hypertens 2006;24:889–894 [DOI] [PubMed] [Google Scholar]
- 9. Frank RN: Diabetic retinopathy. N Engl J Med 2004;350:48–58 [DOI] [PubMed] [Google Scholar]
- 10. Witt N, Wong TY, Hughes AD, Chaturvedi N, Klein BE, Evans R, McNamara M, Thom SA, Klein R: Abnormalities of retinal microvascular structure and risk of mortality from ischemic heart disease and stroke. Hypertension 2006;47:975–981 [DOI] [PubMed] [Google Scholar]
- 11. Stanton AV, Wasan B, Cerutti A, Ford S, Marsh R, Sever PP, Thom SA, Hughes AD: Vascular network changes in the retina with age and hypertension. J Hypertens 1995;13:1724–1728 [PubMed] [Google Scholar]
- 12. Djonov V, Baum O, Burri PH: Vascular remodeling by intussusceptive angiogenesis. Cell Tissue Res 2003;314:107–117 [DOI] [PubMed] [Google Scholar]
- 13. Hughes AD, Wong TY, Witt N, Evans R, Thom SA, Klein BE, Chaturvedi N, Klein R: Determinants of retinal microvascular architecture in normal subjects. Microcirculation 2009;16:159–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chapman N, Dell'omo G, Sartini MS, Witt N, Hughes A, Thom S, Pedrinelli R: Peripheral vascular disease is associated with abnormal arteriolar diameter relationships at bifurcations in the human retina. Clin Sci (Lond) 2002;103:111–116 [DOI] [PubMed] [Google Scholar]
- 15. Griffith TM, Edwards DH, Davies RL, Harrison TJ, Evans KT: EDRF coordinates the behaviour of vascular resistance vessels. Nature 1987;329:442–445 [DOI] [PubMed] [Google Scholar]
- 16. Chapman N, Haimes G, Stanton AV, Thom SA, Hughes AD: Acute effects of oxygen and carbon dioxide on retinal vascular network geometry in hypertensive and normotensive subjects. Clin Sci (Lond) 2000;99:483–488 [PubMed] [Google Scholar]
- 17. Bursell SE, Clermont AC, Kinsley BT, Simonson DC, Aiello LM, Wolpert HA: Retinal blood flow changes in patients with insulin-dependent diabetes mellitus and no diabetic retinopathy. Invest Ophthalmol Vis Sci 1996;37:886–897 [PubMed] [Google Scholar]
- 18. Jarvisalo MJ, Raitakari M, Toikka JO, Putto-Laurila A, Rontu R, Laine S, Lehtimaki T, Ronnemaa T, Viikari J, Raitakari OT: Endothelial dysfunction and increased arterial intima-media thickness in children with type 1 diabetes. Circulation 2004;109:1750–1755 [DOI] [PubMed] [Google Scholar]
- 19. Chan NN, Vallance P, Colhoun HM: Endothelium-dependent and -independent vascular dysfunction in type 1 diabetes: role of conventional risk factors, sex, and glycemic control. Arterioscler Thromb Vasc Biol 2003;23:1048–1054 [DOI] [PubMed] [Google Scholar]
- 20. Yamakawa K, Bhutto IA, Lu Z, Watanabe Y, Amemiya T: Retinal vascular changes in rats with inherited hypercholesterolemia–corrosion cast demonstration. Curr Eye Res 2001;22:258–265 [DOI] [PubMed] [Google Scholar]
- 21. Chaturvedi N, Bandinelli S, Mangili R, Penno G, Rottiers RE, Fuller JH: Microalbuminuria in type 1 diabetes: rates, risk factors and glycemic threshold. Kidney Int 2001;60:219–227 [DOI] [PubMed] [Google Scholar]
- 22. Donaghue KC, Fairchild JM, Craig ME, Chan AK, Hing S, Cutler LR, Howard NJ, Silink M: Do all prepubertal years of diabetes duration contribute equally to diabetes complications? Diabetes Care 2003;26:1224–1229 [DOI] [PubMed] [Google Scholar]
- 23. Rogers SL, Tikellis G, Cheung N, Tapp R, Shaw J, Zimmet PZ, Mitchell P, Wang JJ, Wong TY: Retinal arteriolar caliber predicts incident retinopathy: the Australian Diabetes, Obesity and Lifestyle (AusDiab) Study. Diabetes Care 2008;31:761–763 [DOI] [PubMed] [Google Scholar]
- 24. Leung H, Wang JJ, Rochtchina E, Wong TY, Klein R, Mitchell P: Dyslipidaemia and microvascular disease in the retina. Eye (Lond) 2005;19:861–868 [DOI] [PubMed] [Google Scholar]
- 25. Wong TY, Islam FM, Klein R, Klein BE, Cotch MF, Castro C, Sharrett AR, Shahar E: Retinal vascular caliber, cardiovascular risk factors, and inflammation: the Multi-Ethnic Study of Atherosclerosis (MESA). Invest Ophthalmol Vis Sci 2006;47:2341–2350 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.