Abstract
OBJECTIVE
We previously described a cross-sectional association between serum uric acid and reduced glomerular filtration rate (GFR) in nonproteinuric patients with type 1 diabetes. Here, we prospectively investigated whether baseline uric acid impacts the risk of early progressive renal function loss (early GFR loss) in these patients.
RESEARCH DESIGN AND METHODS
Patients with elevated urinary albumin excretion (n = 355) were followed for 4–6 years for changes in urinary albumin excretion and GFR. The changes were estimated by multiple determinations of albumin-to-creatinine ratios (ACRs) and serum cystatin C (GFRcystatin).
RESULTS
At baseline, the medians (25th–75th percentiles) for uric acid, ACR, and GFRcystatin values were 4.6 mg/dl (3.8–5.4), 26.2 mg/g (15.1–56.0), and 129 ml/min per 1.73 m2 (111–145), respectively. During the 6-year follow-up, significant association (P < 0.0002) was observed between serum uric acid and development of early GFR loss, defined as GFRcystatin decline exceeding 3.3% per year. In baseline uric acid concentration categories (in mg/dl: <3.0, 3.0–3.9, 4.0–4.9, 5.0–5.9, and ≥6), the risk of early GFR loss increased linearly (9, 13, 20, 29, and 36%, respectively). This linear increase corresponds to odds ratio 1.4 (95% CI 1.1–1.8) per 1 mg/dl increase of uric acid. The progression and regression of urinary albumin excretion were not associated with uric acid.
CONCLUSIONS
We found a clear dose-response relation between serum uric acid and risk of early GFR loss in patients with type 1 diabetes. Clinical trials are warranted to determine whether uric acid–lowering drugs can halt renal function decline before it becomes clinically significant.
The paradigm of early diabetic nephropathy in type 1 diabetes has changed during the past few years. Previously, it focused on urinary leakage of small amounts of albumin, designated microalbuminuria, which was believed to predict progression to overt proteinuria and ultimately end-stage renal disease (ESRD) (1). However, recent studies (2,3) documented that rather than progression, regression to normoalbuminuria is the fate of microalbuminuria in the majority of patients. Progression to proteinuria is the fate in a minority only. At the same time, the onset of progressive renal function loss was observed in a subset of these patients well before the onset of proteinuria (4). Because this decline occurs while renal function is still normal, we label it “early” progressive renal function loss (early GFR loss). Whereas microalbuminuria may regress, remain stable, or progress during the course of diabetes, early GFR loss progresses to chronic kidney disease (CKD) and ESRD (4–6).
Because early GFR loss is detectable while renal function is normal or elevated, an opportunity may exist for effective intervention many years before the occurrence of meaningful loss of renal function. However, our knowledge of early GFR loss determinants is limited (4). Discovery of these determinants, especially modifiable ones, may enable the design of more effective programs for the prevention of CKD and ESRD.
A body of evidence suggests that elevated serum uric acid may cause kidney injury in animal models (7). Also, mounting evidence points to a role of elevated serum uric acid in the development of kidney disease in humans. In cohort studies of subjects without diabetes, hyperuricemia predicted the development of CKD stage 3 (8) and ESRD (9,10), and in community-based cohorts moderately elevated serum uric acid concentrations were associated with the development of CKD stage 3 (11,12). Recently, in a type 1 diabetic cohort in Denmark, moderately elevated uric acid concentrations were found to be associated with risk of proteinuria during an 18-year follow-up (13). However, changes in renal function were not examined.
The Second Joslin Study on the Natural History of Microalbuminuria in Type 1 Diabetes (Second Joslin Study) is a follow-up study designed to determine the natural history of early diabetic nephropathy in nonproteinuric patients with type 1 diabetes. In baseline data, high-normal serum uric acid concentration was strongly associated with reduced glomerular filtration rate (GFR), as estimated by serum cystatin C (GFRcystatin) (14). Whether elevated serum uric acid preceded or followed reduced GFR could not be determined in that cross-sectional data. Therefore, we prospectively measured GFRcystatin during 4–6 years of follow-up and investigated the association between baseline serum uric acid concentration and changes in renal function. A secondary aim was to explore the role of serum uric acid in the progression and regression of urinary albumin excretion.
RESEARCH DESIGN AND METHODS
The study protocol and informed consent procedures were approved by the Committee on Human Subjects of the Joslin Diabetes Center.
This study is a prospective follow-up study on the impact of baseline levels of serum uric acid on changes in renal function and urinary albumin excretion over the next 4–6 years of follow-up.
Participants included in this report are members of the Second Joslin Study, a prospective follow-up study described previously (14). Briefly, any patient aged 18–64 years with type 1 diabetes who attended the Joslin Clinic during 2003–2005 and had at least two albumin-to-creatinine ratios (ACRs) determined during the 2 preceding years was potentially eligible for enrollment with an entry examination. Eligibility was restricted further to New England residents with diabetes diagnosed before age 40 years and who were continuously treated with insulin. Exclusion criteria included presence of proteinuria, ESRD, kidney transplant, or comorbidity such as HIV, hepatitis C, or non–diabetes-related kidney disease. Ninety percent of patients identified themselves as white. A total of 667 patients were examined and enrolled in the Second Joslin Study.
Previously we found that risk of early GFR loss was very low in patients with type 1 diabetes and low normoalbuminuria (median ACR <10 mg/g in women and 8 mg/g in men) (4), we excluded such patients (n = 183) from the follow-up phase of the Second Joslin Study. The remaining 484 patients had baseline high normoalbuminuria (ACR median 8–24 mg/g in women and 10–16 mg/g in men, n = 180) or microalbuminuria (ACR median 25–354 mg/g in women and 17–249 mg/g in men, n = 304) and were followed until the end of 2009 with the aim of examining and obtaining serum and urine specimens annually.
For this study, we excluded 35 patients with CKD stage 3 or higher at baseline, two patients without baseline uric acid measurements, 35 patients treated with diuretics, and 1 treated with allopurinol. Of the remaining 411 patients eligible for follow-up, 355 (86%) had multiple measures (median = 5) of serum cystatin C to estimate GFR and multiple determinations of ACR (median = 5) during 4 years of follow-up.
Entry examination and measurements of exposures
At the entry examination, a trained study recruiter administered a questionnaire on medical and diabetes history, including medication usage; obtained blood and urine samples; and measured seated blood pressures twice, separated by a 5-min rest. Medical record review supplemented questionnaire information as needed.
Clinical characteristics (A1C, ACR, and serum cholesterol and HDL) measured at routine clinic visits during the preceding 2 years were retrieved from electronic laboratory records. The median of repeated determinations within the 2-year interval (including entry examination) was used as the baseline value. All other baseline characteristics were measured at entry examination.
Details of the assay for uric acid have been published previously (14). Uric acid was measured using a timed end point method on a Beckman Coulter Synchron CX9. Of the patients enrolled in this study, 262 had follow-up uric acid measurements within 2 years of baseline measurement. The correlation coefficient (Spearman) between first and second measurements was 0.78. In our lab, the interassay coefficient of variation for uric acid was <2%.
Follow-up examination
Participants were followed yearly to measure GFRcystatin and level of microalbuminuria over the next 4–6 years. The examination and collection of specimens coincided with patients' routine clinic visits. Patients who stopped coming to the clinic were examined at their homes.
Measurement of GFRcystatin
Serum cystatin C has been shown to estimate GFR and track GFR changes over time well in patients with diabetes and normal or elevated renal function (4,15,16). In 2009, serum cystatin C was measured in stored (−85 C) baseline and follow-up samples in the collaborative studies clinical laboratory at the University of Minnesota using the Dade-Behring-Siemens BN ProSpec (Siemens Healthcare Diagnostics, Deerfield, IL). The method's interassay laboratory coefficient of variation is ∼4.7%. GFRcystatin was estimated from the serum concentration of cystatin C using a recently improved conversion formula (17) (127.7 × CysC−1.17 × age−0.13 × [0.91 if female, 1.06 if black]) (13).
Measurement of urinary albumin excretion
The protocol for measuring urinary ACR in spot urines during prior clinic visits and during follow-up remained unchanged as described previously (14).
Definition of renal outcomes
While our primary outcome is early GFR loss, the study design allowed simultaneous assessment of changes in renal function and changes in urinary albumin excretion. The definition of these outcomes follows.
Early GFR loss
We calculated individual-specific slopes of GFRcystatin during follow-up with linear mixed-effects regression (PROC MIXED, SAS version 9.1; SAS Institute, Cary, NC). From these slopes, we derived each individual's percent change per year in GFRcystatin. Next, we grouped patients into 1) those with early GFR loss (if the loss of GFRcystatin exceeded 3.3% per year) and 2) those with stable renal function (if the loss of GFRcystatin was 3.3% or less per year). This criterion, a loss exceeding than 3.3% per year, was derived by us previously (4) and corresponds to the 2.5th percentile of the distribution of GFR slopes in an independent, similarly aged population without diabetes (18).
CKD stage 3 or greater
CKD stage 3 is defined as a GFRcystatin value <60 ml/min per 1.73 m2. Patients with CKD stage 3 or greater at baseline were excluded. If CKD stage 3 developed during the 4–6 years of follow-up, the outcome date was that of the first GFRcystatin <60 ml/min per 1.73 m2.
Change in ACR
To evaluate changes in urinary albumin excretion for each patient, we divided observation time into three 2-year intervals. The median ACR measurement in the 2-year interval preceding enrollment was the baseline value. Similarly, during the first two 2-year intervals after enrollment, the median ACR measurement in each was considered the first and second follow-up value, respectively. ACR progression was defined as a doubling of baseline ACR value during either of these intervals. ACR regression was defined as a halving of baseline ACR value during either interval.
Statistical methods
All statistical analyses were performed in SAS version 9.1 (SAS Institute). Comparisons between those with early GFR loss and those with stable renal function at the end of follow-up used medians and Wilcoxon rank sum tests for continuous variables and percentages and χ2 tests for categorical variables. The odds of developing early GFR loss or ACR progression or regression were estimated with logistic regression. Characteristics associated with uric acid or early GFR loss were evaluated as potential confounders in multivariate models. Characteristics were retained if they changed the relation between uric acid and early GFR loss by ≥10%. Controlling for baseline values of an exposure of interest is controversial if the study outcome is change in that same characteristic over time (19). We provide results both ways: controlling and not controlling for baseline values of GFRcystatin for early GFR loss or ACR for change in ACR.
Longitudinal measures of log-transformed GFRcystatin levels were also analyzed using a linear mixed-effects model. This model takes into account irregular time measurements and correlation between longitudinal observations. In building the model, we applied a likelihood ratio test to fixed effects of uric acid and confounders. To account for correlation between longitudinal observations, two random effects, namely random intercept and random slope for time, were also included. In addition, the linear mixed-effects framework allowed deriving and making inference about the distribution of subject-specific slopes (and subsequently percent changes per year) for any subject after adjusting for potential confounders.
RESULTS
At the end of follow-up, study subjects were divided into two groups according to the primary outcome: the presence or absence of early GFR loss. A loss in GFRcystatin that exceeded 3.3% per year was classified as early GFR loss (n = 79), all others (n = 276) were considered to have stable renal function.
The two groups did not differ with regard to sex, but patients with early GFR loss were older at both diabetes diagnosis and study entry and had a longer duration of diabetes (Table 1). The two groups did not differ with regard to A1C or clinical characteristics associated with insulin resistance, such as insulin dose, BMI, and serum cholesterol (total or HDL). Patients with early GFR loss had significantly lower GFRcystatin, higher urinary ACR, and a higher prevalence of microalbuminuria at baseline. Early GFR loss patients were also more frequently treated with renoprotective drugs, although the two groups had similar systolic and diastolic blood pressures. Both groups had similar cigarette-smoking history. Most notably, those with early GFR loss had significantly higher serum uric acid levels than patients with stable renal function (P < 0.0001).
Table 1.
Characteristics of nonproteinuric patients with type 1 diabetes according to whether renal function was stable during 4–6 years of follow-up or early GFR loss developed
| Characteristic* | Stable renal function | Early GFR loss | P value |
|---|---|---|---|
| n | 276 | 79 | |
| Baseline characteristics | |||
| % men | 55 | 56 | 0.92 |
| Age at diabetes diagnosis (years) | 17 ± 10 | 20 ± 11 | 0.02 |
| Duration of diabetes (years) | 21 ± 9 | 24 ± 10 | 0.02 |
| Age (years) | 38 ± 12 | 44 ± 11 | <0.0001 |
| A1C (%) | 8.2 (7.4–9.1) | 8.3 (7.6–9.5) | 0.17 |
| Insulin dose (units/kg/day) | 0.63 (0.53–0.83) | 0.64 (0.48–0.86) | 0.83 |
| BMI (kg/m2) | 26 (23–29) | 26 (24–32) | 0.15 |
| Serum cholesterol (mg/dl) | 184 (166–204) | 185 (167–204) | 0.96 |
| Serum HDL (mg/dl) | 54 (46–65) | 53 (46–64) | 0.74 |
| GFRcystatin (ml/min per 1.73 m2) | 131 (116–147) | 115 (93–136) | <0.0001 |
| ACR (mg/g) | 34.6 (22.2–63.8) | 51.6 (29.9–114.9) | <0.0001 |
| % with microalbuminuria | 56 | 75 | 0.003 |
| % with renoprotective treatment | 50 | 68 | 0.005 |
| Systolic blood pressure (mmHg) | 120 (111–128) | 122 (114–129) | 0.13 |
| Diastolic blood pressure (mmHg) | 70 (68–79) | 72 (68–77) | 0.60 |
| Cigarette smoking | |||
| % current | 18 | 13 | 0.18 |
| % past | 24 | 34 | |
| Serum uric acid (mg/dl) | 4.5 (3.7–5.3) | 5.10 (4.4–5.7) | <0.0001 |
| Follow-up characteristics | |||
| Duration of follow-up (years) | 4.8 (4.1–5.5) | 4.9 (3.9–5.7) | 0.56 |
| Number of cystatin determinations | 5 (4–6) | 5 (4–6) | 0.33 |
| Number of ACR determinations | 5 (4–7) | 6 (4–7) | 0.58 |
| Last GFRcystatin (ml/min per 1.73 m2) | 124 (109–139) | 86 (65–99) | By design |
| Percent change per year in GFRcystatin | −1.8 (−2.4 to −1.2) | −4.4 (−5.0 to −3.8) | By design |
| Progressed to CKD stage 3 or greater [n (%)] | 3 (1) | 19 (24) | <0.0001 |
| Last ACR (mg/g)† | 17.1 (9.8, 42.1) | 40.3 (13.8, 128.5) | <0.0001 |
| Progression of ACR [n (%)] | 48 (17) | 27 (34) | 0.001 |
| Regression of ACR [n (%)] | 103 (73) | 26 (33) | 0.47 |
Data are medians (25th–75th percentiles) and P values from Wilcoxon rank sum test for continuous variables (except for age at diabetes diagnosis, duration, and age, which are means ± SD and t tests) and percents with P values from χ2 test for categorical data.
*Values for A1C, serum cholesterol, serum HDL, and ACR were the medians of all determinations during the 2-year interval ending with the entry examination.
†Median of all ACR measurements in the second 2-year follow-up interval.
Table 1 also shows results of follow-up. The last GFRcystatin in patients with stable renal function was similar to that at baseline (124 vs. 131 ml/min per 1.73 m2), whereas it was significantly lower (86 vs. 115 ml/min per 1.73 m2) in those with early GFR loss. Median change per year in GFRcystatin was −1.8 and −4.4%, respectively. CKD stage 3 developed in 22 patients, among 19 patients with early GFR loss and among 3 those with stable renal function.
The two groups were also compared with regard to urinary albumin excretion during the 4-year follow-up. In the second follow-up interval, the median ACR value in the early GFR loss group was significantly higher than in the stable renal function group, as noted in the baseline interval, and in neither group did the median values increase by the end of follow-up. To take into account the baseline differences in ACR values, follow-up changes in ACR were expressed as fold changes from baseline in each interval. Progression was defined as a doubling of ACR in either interval, and regression was defined as a halving of ACR in either interval. ACR progressed in 34% of case subjects with early GFR loss compared with 17% of control subjects with stable renal function (P = 0.001). ACR regressed equally in the two groups (33% of case and in 37% of control subjects).
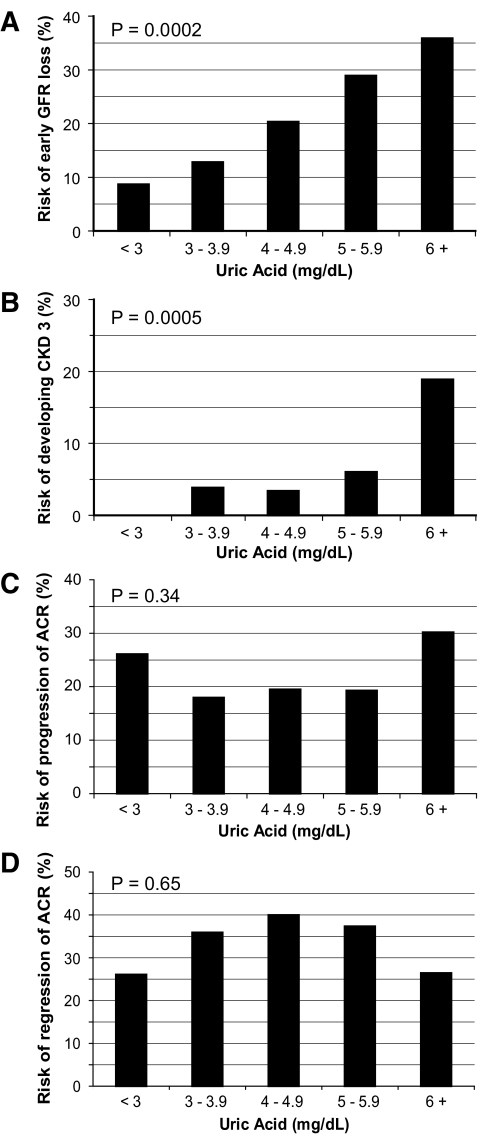
To further examine the relation between baseline uric acid and development of renal outcomes, we analyzed the occurrence of these outcomes according to five categories of baseline serum uric acid (in mg/dl: <3.0, 3–3.9, 4–4.9, 5–5.9, and ≥6). A clear dose-response relation is evident between increasing baseline serum uric acid levels and subsequent occurrence of early GFR loss (Fig. 1A). During the 4–6 years of follow-up, early GFR loss developed in 9, 13, 20, 29, and 36% of individuals in the five categories of increasing serum uric acid, respectively (P for trend = 0.0002).
Figure 1.
Risks during 4–6 years follow-up of early GFR loss (A), CKD stage 3 (B), progression of ACR (C), or regression of ACR according to baseline concentration of serum uric acid (D). Baseline serum uric acid values (mg/dl) were <3.0 for 23 patients, between 3.0 and 3.9 for 78 patients, between 4.0 and 4.9 for 118 patients, between 5.0 and 5.9 for 96 patients, and ≥6.0 for 53 patients. Median uric acid concentration in the cohort was 4.6 mg/dl. A: Test for trend in risk of early GFR loss with baseline serum uric acid category was significant, P = 0.0002. B: Test for trend in risk of CKD stage 3 with baseline serum uric acid category significant, P = 0.0005. C: Test for trend in risk of progression of ACR with baseline uric acid category was not statistically significant, P = 0.34. D: Test for trend in risk of regression of ACR with baseline uric acid category was not statistically significant, P = 0.65.
Similarly, we explored a complementary outcome to early GFR loss (i.e., development of CKD stage 3 [or greater]). Although CKD stage 3 developed in only 22 patients during follow-up, a strong dose-response relation between baseline serum uric acid categories and development of CKD stage 3 is evident in Fig. 1B. In the five categories of increasing serum uric acid, 0, 4, 3, 6, and 19% of individuals developed CKD stage 3, respectively (P for trend = 0.0005). The association between baseline serum uric acid and progression of ACR (Fig. 1C) was not significant for trend (P = 0.34). Similarly, the association between baseline serum uric acid and regression of ACR (Fig. 1D) was not significant for trend (P = 0.65).
Estimates from multivariate logistic regression of the effect of a 1.0 mg/dl increase in serum uric acid on these renal outcomes are summarized in Table 2. The unadjusted odds ratio of developing early GFR loss with baseline serum uric acid change by 1 mg/dl was 1.5 (95% CI 1.3–1.9), and this estimate was little changed by adjustment for urinary ACR, sex, and A1C or even adjustment for baseline GFRcystatin. This association can be re-expressed as an individual with a serum uric acid of 6 mg/dl having twice the risk of developing early GFR loss as an individual with a serum uric acid of 4 mg/dl (odds ratio per 2 mg/dl change = 1.9). There was no effect measure modification (interaction) by sex, A1C, baseline GFRcystatin, or baseline ACR. Due to the small number of case subjects, we could not pursue a fully adjusted model for the time-to-event analysis of CKD stage 3. The lack of association between serum uric acid and progression or regression of ACR was confirmed by logistic regression in both the univariate and multivariate analyses (Table 2).
Table 2.
Odds ratios for a 1.0 mg/dl increase in baseline serum uric acid concentration for the odds of developing selected renal outcomes during 4–6 years of follow-up
| Renal outcome | Unadjusted measure of association (95% CI) | Adjusted measure of association (95% CI)* | Adjusted measure of association including adjustment for baseline level of outcome† (95% CI) |
|---|---|---|---|
| Early GFR loss | 1.5 (1.3–1.9) | 1.5 (1.2–1.9) | 1.4 (1.1–1.8) |
| Progression of ACR | 1.1 (0.9–1.4) | 1.0 (0.8–1.3) | 1.0 (0.8–1.3) |
| Regression of ACR | 1.0 (0.8–1.1) | 1.1 (1.0–1.4) | 1.1 (1.0–1.4) |
*Adjusted for ACR, sex, and A1C in models with outcome early GFR loss; adjusted for baseline GFRcystatin, sex, and A1C in models with outcomes progression or regression of ACR.
†Adjusted for baseline GFRcystatin, ACR, sex, and A1C in models with outcome early GFR loss; adjusted for baseline ACR, GFRcystatin, sex, and A1C in models with outcomes progression or regression of ACR.
To further study the association between serum uric acid and early GFR loss, we analyzed continuous measures of changes in GFRcystatin using a linear mixed-effects model adjusting for all previous confounders. The interaction between changes in GFRcystatin over time and serum uric acid was statistically significant (P = 0.01). This interaction can be interpreted in terms of differing adjusted average percent change per year for each of the five categories of serum uric acid presented in Fig. 1. The average percent change for the five categories, from lowest to highest serum uric acid, was −0.6, −1.4, −1.8, −2.2, and −3.1% per year, respectively.
CONCLUSIONS
In this large, prospective cohort study of type 1 diabetic patients without proteinuria, we found that serum uric acid was a significant independent predictor of the development of early GFR loss after controlling for multiple confounders. For each 1 mg/dl increase in serum uric acid, there was a 40% increase in the odds of developing early GFR loss (odds ratio = 1.4 [95% CI 1.1, 1.8]). This increase in risk was linear across the entire normal range of serum uric acid levels. In contrast with these findings, we did not find any association between baseline serum uric acid and progression or regression of urinary albumin excretion. Our study is the first to provide prospective evidence of the “uncoupling” of determinants of early GFR loss from determinants of changes in urinary albumin excretion, the two cardinal manifestations of early diabetic nephropathy in type 1 diabetes.
Recently, in a type 1 diabetic cohort with long follow-up in Denmark, elevated baseline uric acid concentrations were associated with the development of proteinuria but did not impact risk of microalbuminuria (13). Since that study did not report measures of change in renal function we cannot compare results for our main outcome. Our study aimed to study renal function changes during early diabetic nephropathy when patients had only minimally elevated ACR. With longer follow-up, we may have obtained similar results as the Danish cohort. However, as we showed in our recent study (5), declining renal function begins before proteinuria develops.
Although our findings are derived from an observational study, it is intriguing to consider that uric acid might play a pathogenic role in the development of early GFR loss in type 1 diabetes. This hypothesis can be tested further in randomized, controlled trials (RCTs) since drugs to reduce serum uric acid levels are available, being commonly used in gout therapy. These drugs reduce uric acid levels either by inhibiting its synthesis (allopurinol and febuxostat) or by increasing the urinary excretion of uric acid (probenecid, benzbromarone, and sulfinpyrazole) (20,21). Several RCTs have shown that treatment with allopurinol improves brachial artery vasodilation in patients with chronic heart failure (22) and type 2 diabetes (23), but only a handful of studies, all in individuals without diabetes, have examined changes in renal function as the primary outcomes. One of these has shown that treatment with allopurinol can retard the renal function decline among patients with CKD and hyperuricemia (24). No RCT has been conducted to investigate the effect of uric acid–lowering drugs on the prevention of early renal function loss. However, in a prospective study of 48 hyperuricemic patients who were given allopurinol for 3 months, uric acid decreased from 8.0 to 5.5 mg/dl, while GFR increased from 79 to 93 ml/min per 1.73 m2 (25). Importantly, such improvement in GFR occurred in the absence of a reduction of proteinuria.
The Second Joslin Study is a well-characterized, large prospective cohort study with excellent follow-up over 4–6 years, which allowed us to determine different phenotypes/outcomes of early diabetic nephropathy. However, some perceived and real limitations of this study should be considered. First, although we showed that repeated uric acid measurements taken up to 2 years apart were highly correlated (r = 0.78), some exposure misclassification may have occurred due to the reliance on a single baseline uric acid measurement. However, such nondifferential misclassification would bias the results toward the null hypothesis, making our findings of association even more notable. Second, we estimated renal function from serum cystatin C concentrations rather than measuring GFR directly. Although indirect, this method has been shown to estimate GFR well even in normal or elevated ranges, and multiple measurements of GFRcystatin over time have been demonstrated to be a reliable tool to assess changes of renal function in longitudinal studies (4,15,16). Importantly, to minimize spurious variation in the determination of GFRcystatin changes over time, all serum cystatin C measurements (n = 1,871) were carried out in 2009, in the same laboratory and using the same reagents. Furthermore, to calculate individual GFRcystatin slopes, we utilized appropriate methods of longitudinal analysis (i.e., linear mixed-effects model), taking into account irregular time measurements and correlation between longitudinal observations. Finally, since our study is an observational one, any conclusion about a causal relationship between serum uric acid and the development of early GFR loss in type 1 diabetes should be considered as tentative. This hypothesis will have to be tested by adequately powered RCTs evaluating the effect of uric acid–lowering drugs on the decline of renal function specifically among individuals with diabetes.
Acknowledgments
This research was supported by National Institutes of Health Grant DK 041526 (to A.S.K.) and Claude Pepper Center Grants AG08808 and AG024824 (to A.T.G.).
No potential conflicts of interest relevant to this article were reported.
We thank Shu Chen for her analysis of continuous measures of change.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1. Parving H-H, Mauer M, Ritz E: Diabetic nephropathy. In The Kidney. 7th ed. Brenner BM: Ed. Philadelphia, Elsevier, 2004, pp. 1777–1818 [Google Scholar]
- 2. Caramori ML, Fioretto P, Mauer M: The need for early predictors of diabetic nephropathy risk: is albumin excretion rate sufficient? Diabetes 2000;49:1399–408 [DOI] [PubMed] [Google Scholar]
- 3. Perkins BA, Ficociello LH, Silva KH, Finkelstein DM, Warram JH, Krolewski AS: Regression of microalbuminuria in type 1 diabetes. N Engl J Med 2003;348:2285–2293 [DOI] [PubMed] [Google Scholar]
- 4. Perkins BA, Ficociello LH, Ostrander BE, Silva KH, Weinberg J, Warram JH, Krolewski AS: Microalbuminuria and the risk for early progressive renal function decline in type 1 diabetes. J Am Soc Nephrol 2007;18:1353–1361 [DOI] [PubMed] [Google Scholar]
- 5. Perkins BA, Ficociello LH, Roshan B, Warram JH, Krolewski AS: In patients with type 1 diabetes and new-onset microalbuminuria the development of advanced chronic kidney disease may not require progression to proteinuria. Kidney Int 2010;77:57–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jerums G, Panagiotopoulos S, Premaratne E, MacIsaac RJ: Integrating albuminuria and GFR in the assessment of diabetic nephropathy. Nat Rev Nephrol 2009;5:397–406 [DOI] [PubMed] [Google Scholar]
- 7. Mazzali M, Kanellis J, Han L, Feng L, Xia YY, Chen Q, Kang DH, Gordon KL, Watanabe S, Nakagawa T, Lan HY, Johnson RJ: Hyperuricemia induces a primary renal arteriolopathy in rats by a blood pressure-independent mechanism. Am J Physiol Renal Physiol 2002;6:F991–F997 [DOI] [PubMed] [Google Scholar]
- 8. Domrongkitchaiporn S, Sritara P, Kitiyakara C, Stitchantrakul W, Krittaphol V, Lolekha P, Cheepudomwit S, Yipintsoi T: Risk factors for development of decreased kidney function in a southeast Asian population: a 12-year cohort study. J Am Soc Nephrol 2005;16:791–799 [DOI] [PubMed] [Google Scholar]
- 9. Iseki K, Ikemiya Y, Inoue T, Iseki C, Kinjo K, Takishita S: Significance of hyperuricemia as a risk factor for developing ESRD in a screened cohort. Am J Kidney Dis 2004;44:642–650 [PubMed] [Google Scholar]
- 10. Ishani A, Grandits GA, Grimm RH, Svendsen KH, Collins AJ, Prineas RJ, Neaton JD: Association of single measurements of dipstick proteinuria, estimated glomerular filtration rate, and hematocrit with 25-year incidence of end-stage renal disease in the multiple risk factor intervention trial. J Am Soc Nephrol 2006;17:1444–1452 [DOI] [PubMed] [Google Scholar]
- 11. Obermayr RP, Temml C, Gutjahr G, Knechtelsdorfer M, Oberbauer R, Klauser-Braun R: Elevated uric acid increases the risk for kidney disease. J Am Soc Nephrol 2008;19:2251–2253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hsu CY, Iribarren C, McCulloch CE, Darbinian J, Go AS: Risk factors for end-stage renal disease: 25-year follow-up. Arch Intern Med 2009;169:342–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hovind P, Rossing P, Tarnow L, Johnson RJ, Parving H-H: Serum uric acid as a predictor for development of diabetic nephropathy in type 1 diabetes: an inception cohort study. Diabetes 2009;58:1668–1671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rosolowsky ET, Ficociello LH, Maselli NJ, Niewczas MA, Binns AL, Roshan B, Warram JH, Krolewski AS: High-normal serum uric acid is associated with impaired glomerular filtration rate in nonproteinuric patients with type 1 diabetes. Clin J Am Soc Nephrol 2008;3:706–713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Perkins BA, Nelson RG, Ostrander BE, Blouch KL, Krolewski AS, Myers BD, Warram JH: Detection of renal function decline in patients with diabetes and normal or elevated GFR by serial measurements of serum cystatin C concentration: results of a 4-year follow-up study. J Am Soc Nephrol 2005;16:1404–1412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Premaratne E, MacIsaac RJ, Finch S, Panagiotopoulos S, Ekinci E, Jerums G: Serial measurements of cystatin C are more accurate than creatinine-based methods in detecting declining renal function in type 1 diabetes. Diabetes Care 2008;31:971–973 [DOI] [PubMed] [Google Scholar]
- 17. Stevens LA, Coresh J, Schmid CH, Feldman HI, Froissart M, Kusek J, Rossert J, Van Lente F, Bruce RD, 3rd, Zhang YL, Greene T, Levey AS: Estimating GFR using serum cystatin C alone and in combination with serum creatinine: a pooled analysis of 3,418 individuals with CKD. Am J Kidney Dis 2008;51:395–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lindeman RD, Tobin J, Shock NW: Longitudinal studies on the rate of decline in renal function with age. J Am Geriatr Soc 1985;33:278–285 [DOI] [PubMed] [Google Scholar]
- 19. Glymour MM, Greenland S: Causal diagrams. In Modern Epidemiology. 3rd ed. Rothman KJ, Greenland S, Lash TL: Eds. Philadelphia, Lippincott Williams and Wilkins, 2008, p. 183–209 [Google Scholar]
- 20. Perez-Ruiz F, Alonso-Ruiz A, Calabozo M, Herrero-Beites A, García-Erauskin G, Ruiz-Lucea E: Efficacy of allopurinol and benzbromarone for the control of hyperuricemia: a pathogenic approach to the treatment of primary chronic gout. Ann Rheum Dis 1998;57:545–549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Becker MA, Schumacher R, Wortman RL, MacDonald PA, Eustace D, Palo WA, Streit J, Joseph-Ridge N: Febuxostat compared with allopurinol in patients with hyperuricemia and gout. N Engl J Med 2005;353:2450–2461 [DOI] [PubMed] [Google Scholar]
- 22. Farquharson CA, Butler R, Hill A, Belch JJ, Struthers AD: Allopurinol improves endothelial dysfunction in chronic heart failure. Circulation 2002;106:221–226 [DOI] [PubMed] [Google Scholar]
- 23. Butler R, Morris AD, Belch JJ, Hill A, Struther AD: Allopurinol normalizes endothelial dysfunction in type 2 diabetes with mild hypertension. Hypertension 2000;35:746–751 [DOI] [PubMed] [Google Scholar]
- 24. Siu YP, Leung KT, Tong MK, Kwan TH: Use of allopurinol in slowing the progression of renal disease through its ability to lower serum uric acid level. Am J Kidney Dis 2006;47:51–59 [DOI] [PubMed] [Google Scholar]
- 25. Kanbay M, Ozkara A, Selcoki Y, Isik B, Turgut F, Bavbek N, Uz E, Akcay A, Yigitoglu R, Covic A: Effect of treatment of hyperuricemia with allopurinol on blood pressure, creatinine clearance, and proteinuria in patients with normal renal functions. Int Urol Nephrol 2007;39:1227–1233 [DOI] [PubMed] [Google Scholar]