Abstract
Fresh frozen plasma (FFP) is indicated for the management of massive bleedings. Recent audits suggest physician knowledge of FFP is inadequate and half of the FFP transfused in critical care is inappropriate. Trauma is among the largest consumers of FFP. Current trauma resuscitation guidelines recommend FFP to correct coagulopathy only after diagnosed by laboratory tests, often when overt dilutional coagulopathy already exists. The evidence supporting these guidelines is limited and bleeding remains a major cause of trauma-related death. Recent studies demonstrated that coagulopathy occurs early in trauma. A novel early formula-driven haemostatic resuscitation proposes addressing coagulopathy early in massive bleedings with FFP at a near 1:1 ratio with red blood cells. Recent retrospective reports suggest such strategy significantly reduces mortality, and its use is gradually expanding to nontraumatic bleedings in critical care. The supporting studies, however, have bias limiting the interpretation of the results. Furthermore, logistical considerations including need for immediately available universal donor AB plasma, short life after thawing, potential waste and transfusion-associated complications have challenged its implementation. The present review focuses on FFP transfusion in massive bleeding and critically appraises the evidence on formula-driven resuscitation, providing resources to allow clinicians to develop informed opinion, given the current deficient and conflicting evidence.
Introduction
Fresh frozen plasma (FFP) is a blood product that has been available since 1941 [1]. Initially used as a volume expander, it is currently indicated for the management and prevention of bleeding in coagulopathic patients [1-3]. The evidence on FFP transfusion is scant and of limited quality [4].
Estimates state that 25 to 30% of all critical care patients receive FFP transfusions [5,6]. Despite its commonality, only 37% of the physicians in a recent study correctly responded to basic questions about FFP, including the volume of one unit [7]. An audit on transfusion practices suggested that one-half of all FFP transfused to critical care patients is inappropriate [5].
Massive haemorrhage is among the most challenging issues in critical care, affecting trauma patients, surgical patients, obstetric patients and gastrointestinal patients [3,8,9]. In trauma, a recent series of retrospective clinical studies suggests that early and aggressive use of FFP at a 1:1 ratio with red blood cells (RBC) improves survival in cases of massive haemorrhage [10-19]. Because bleeding is directly responsible for 40% of all trauma-related deaths, this strategy - also known as haemostatic damage control or formula-driven resuscitation - has received substantial attention worldwide. This early formula-driven haemostatic resuscitation proposes transfusion of FFP at a near 1:1 ratio with RBC, thus addressing coagulopathy from the beginning of the resuscitation and potentially reducing mortality. Nevertheless, this strategy requires immediate access to large volumes of thawed universal donor FFP, which is challenging to implement.
Despite conflict with existing guidelines, early formula-driven haemostatic resuscitation use is expanding and is gradually being used in nontraumatic bleedings in critical care [20]. Both the existing guidelines and early formula-driven haemostatic resuscitation are supported by limited evidence, generating controversies and challeng ing clinical decisions in critical care (Table 1). The objective of the present article is to review the evidence on FFP in the management of massive traumatic haemorrhage and to critically appraise early formula-driven haemostatic resuscitation, providing the reader with resources to develop an informed opinion on the current controversy.
Table 1.
Arguments for and against the adoption of early formula-driven haemostatic resuscitation in trauma
| Pros | Cons | |
|---|---|---|
| Mortality | Retrospective studies suggesting a reduction in mortality from exsanguinations | Data limited by survivorship bias |
| Increase in FFP and platelet use might increase the risk of acute lung injury, multiple organ failure, thrombosis, sepsis and death | ||
| Coagulopathy | Prevention and treatment of coagulopathy due to transfusion of clotting factors | Difficult to identify patients early on who will develop coagulopathy and in fact need transfusion of FFP and platelets |
| Minimize crystalloid use (decrease the risk of dilution) | Uncertainty about the ideal dose of FFP in the trauma situation | |
| Laboratory tests | No need for coagulation tests | Unnecessary exposure to AB plasma (in some countries, a higher risk of transfusion-related acute lung injury due to higher proportion of female donors) |
| Avoid the delay of waiting for blood test results | ||
| Blood bank systems | More timely issuing of blood components | The waste of FFP will increase (shortage of AB plasma) |
| No time needed to thaw FFP (AB plasma available at all times) | May increase the complications associated with FFP and platelet transfusion | |
| Decrease the need for communication between blood bank and the medical team |
FFP, fresh frozen plasma.
Plasma basics
'Fresh frozen plasma' is a confusing term as plasma cannot be fresh and frozen at the same time. Fresh refers to timing from collection to freezing, and frozen refers to the long-term storage condition. FFP transfusion must be ABO compatible, with AB being the universal type, lacking anti-A and anti-B antibodies. Only 4% of the population is AB, resulting in chronic shortage of this blood type [21].
Preparation and composition
FFP is prepared from either single units of whole blood (a whole blood-derived unit is approximately 250 ml) or plasma collected by apheresis (usually 500 ml) [1,2,22]. FFP is collected in citrate-containing anticoagulation solution, frozen within 8 hours and stored at -30°C for up to 1 year. FFP contains all of the clotting factors, fibrinogen (400 to 900 mg/unit), plasma proteins (particularly albumin), electrolytes, physiological anticoagulants (protein C, protein S, antithrombin, tissue factor pathway inhibitor) and added anticoagulants [1,2].
Plasma frozen within 24 hours of collection is termed frozen plasma (PF24), containing 15 to 20% lower factor VIII levels than FFP [23,24]. PF24 is common in countries using the buffy-coat method, in which RBC and plasma are extracted after hard spin from whole blood and platelets recovered after a second soft spin within 24 hours of collection. PF24 has similar clinical indications as FFP [2,23,24].
FFP is commonly thawed in a water bath over 20 to 30 minutes, but US Food and Drug Administration-approved microwaves can thaw 2 units of plasma in 2 to 3 minutes [1]. After thawing, the activity of labile clotting factors such as factor V and factor VIII decline gradually, and most countries recommend FFP use within 24 hours [25,26]. In some countries, FFP is used up to 5 days after thawing. The consequences of transfusing stored, thawed 5-day-old plasma is not completely understood, but the activity of factor VIII is expected to drop by >50%, and the activity of factor V and factor VII drops to about 20% 5 days after thawing [27].
Photochemically treated FFP and solvent detergent FFP are approved methods of inactivating pathogens in some jurisdictions. Both methods cause loss of clotting factors, particularly factor VIII. Some solvent detergent FFP preparations have reduced activity of protein S and α2-antiplasmin, and have been associated with thrombo-embolic complications [28,29]. These solvent detergent preparations are extensively used in some European countries, while solvent detergent FFP was withdrawn in North America due to concerns of Parvovirus transmission [1].
Risks
FFP can transmit infectious diseases, albeit rarely. Screen ing and pathogen inactivation reduced transmission rates of HIV to 1:7.8 million, of hepatitis C virus to 1:2.3 million and of hepatitis B virus to 1:153,000 units transfused [30]. In the UK, concerns over Creutzfeldt-Jakob disease - a rare but rapidly progressive spongiform encephalopathy - led to leukocyte depletion in all blood products and recommendations to use FFP from areas of low epidemicity [31,32].
Other important complications relate to blood immunogenicity, increasingly recognized over the past two decades, particularly transfusion-related acute lung injury (TRALI) and transfusion-associated circulatory overload [33,34]. TRALI is the commonest cause of transfusion-related death [33,34]. Two mechanisms have mostly been implicated in TRALI. Donor plasma antibodies react with human leukocyte antigens, causing complement activation, endothelial damage, neutrophil activation and lung capillary leak. Anti-human leukocyte antigens and anti-neutrophil antibodies are commonly found in plasma from multiparous female donors, and the TRALI frequency is higher in recipients from female donors [35-37]. To minimize the risk of TRALI, a male-only plasma policy has been adopted in many countries - with marked reductions in TRALI [35]. Another potential mechanism involves interactions of biologically active mediators in stored plasma and lung endothelial cells. Other important transfusion-related complications include acute haemolytic reaction from anti-A and anti-B antibodies, and anaphylaxis [22].
Massive bleeding
Massive bleeding is defined as the loss of one blood volume within 24 hours, or as 50% blood loss within 3 hours or a bleeding rate of 150 ml/minute [38]. The physiological derangements and complications are proportional to the blood loss and to the time to correct shock. Loss of one blood volume and replacement with RBC only results in clotting factor levels dropping to approximately 30%, the minimal level thought to be required for adequate haemostasis [3,39]. Lower levels significantly prolong the prothrombin time and the activated partial thromboplastin time above 1.5× normal [1]. FFP transfusion to replace clotting factors is often recommended for these patients but no studies exist supporting this practice [4]. Replacing one blood volume or more without FFP results in dilutional coagulopathy, diffuse microvascular bleeding and increased mortality [40,41].
Current guidelines for FFP in massive bleeding
The principles of managing massive haemorrhage include rapid control of bleeding; replenishing the intravascular volume with crystalloid followed by RBC and, once coagulopathy is present or suspected, then adding FFP, platelets and cryoprecipitate; along with correction of acidosis and hypothermia. Most current guidelines [1,39,42-44], including the European and US guidelines, recommend transfusing FFP, platelets and cryoprecipitate only when laboratory assays detect a deficit. The goal is to correct the assays as follows: FFP to correct the prothrombin time/activated partial thrombo plastin time to <1.5× normal, platelets to raise the count to ≥50 × 109/l and cryoprecipitate to raise fibrinogen to ≥1.0 g/l [1,42-44]. Where a laboratory is not available, these products are recommended after large infusions of crystalloid and RBC. The usual FFP dose in massive bleeding is 15 to 20 ml/kg or 3 to 6 units, which aims to raise clotting factors levels above 30% [3,38,39].
Current crystalloid-based resuscitation guidelines initiate FFP transfusion late, often after more than one blood volume is lost and the patients have clinically overt coagulopathy [40,41]. Most recommendations are based on observations and expert opinion, often lacking high-level evidence. Many recommendations originated in studies conducted in nontrauma settings and when RBC units had 150 to 300 ml plasma [1]. Currently, RBC preparations contain only minimal residual plasma (≤30 ml). Despite worldwide acceptance of similar resuscitation principles, bleeding remains the second overall cause of death in trauma - becoming the first cause of death following hospital admission [45-47].
Trauma-associated coagulopathy
Haemorrhage is directly responsible for 40% of all trauma-related deaths [45,46], and many deaths are potentially preventable. Current resuscitation strategies invariably fail to prevent coagulopathy in massive bleedings. Multiple causes have traditionally been implicated in trauma coagulopathy, including clotting factor consumption and dilution, hypothermia and acidosis - all linked to large-volume crystalloid infusion and late replacement of clotting elements [40,41]. The management of massive trauma bleeding started changing when Brohi and MacLeod and colleagues separately described that trauma coagulopathy occurs early, and is present on hospital admission in 25% of all severely traumatized patients [48,49]. Further studies suggest that early coagulopathy is initiated by shock and the amount of tissue destruction, independent of clotting factor consumption or dilution (Figure 1), and is associated with a threefold mortality increase [48,49].
Figure 1.
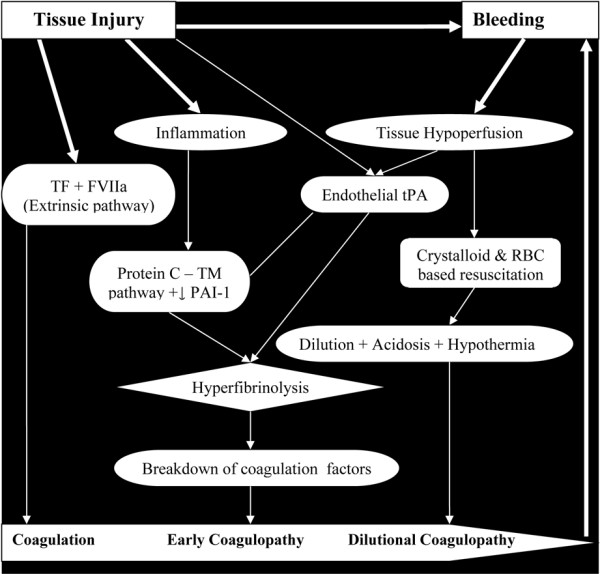
Recently proposed mechanism for coagulopathy in trauma. Tissue trauma activates the coagulation process via tissue factor (TF) and activated factor VII (FVIIa), formerly named the extrinsic pathway, to stop bleeding. Concomitantly, endothelial damage/ischaemia leads to release of physiologic anticoagulants and antifibrinolytics (that is, thrombomodulin (TM), protein C and tissue plasminogen activator (tPA)) due to inflammation and tissue hypoperfusion, to prevent thrombosis. Early coagulopathy develops when there is an imbalance in this process, with excessive anticoagulation, hyperfibrinolysis and consumption of clotting factors. Resuscitation with crystalloid and red blood cells (RBC) can cause/worsen dilution, acidosis and hypothermia. PAI-1, plasminogen activator inhibitor 1.
A unique coagulopathy in traumatic brain injury has long been suspected, where the release of brain tissue factor causes systemic activation of coagulation (dissemi-nated intravascular coagulation), exhaustion of clotting elements and hyperfibrinolysis [50,51]. While coagulo-pathy is common and critically important in traumatic brain injury, the controversial existing evidence suggests it may not differ from trauma coagulopathy in general [51].
The early trauma coagulopathy concept has challenged the current crystalloid-based resuscitation that ignores coagulopathy until it becomes overt. Over the past 2 years, a haemostatic blood-based resuscitation - commonly termed damage control resuscitation - proposes a series of early and aggressive strategies to treat or prevent early trauma-associated coagulopathy [52,53]. This resuscitation entails the use of thawed plasma as the primary resuscitation fluid, limited use of crystalloid, targeted systolic blood pressure at approximately 90 mmHg to prevent renewed bleeding, early activation of a massive transfusion protocol with fixed ratios of FFP:platelets:cryoprecipitate:RBC (approximately 1:1:1:1), liberal use of recombinant activated factor VII (rFVIIa) and the use of fresh whole blood for the most severely injured combat casualties [52,53].
Critical appraisal of the evidence on early formula-driven haemostatic resuscitation
The first reports suggesting aggressive FFP transfusions were computer simulation models. In 2003, Hirshberg and colleagues published a haemodilution model of exsanguination, calculated the changes in coagulation and predicted an optimal FFP:RBC ratio of 2:3 to adequately replenish clotting factors [54]. Ho and colleagues predicted 1 to 1.5 units FFP to each RBC to prevent dilutional coagulopathy in mathematical models [55].
Since 2007, growing numbers of retrospective military and civilian papers have studied early formula-driven haemostatic resuscitation with different FFP:RBC ratios (mostly near 1:1) and mortality [11-20,56,57]. Overall, these studies demonstrate a significant association between higher ratios and lower mortality in massive traumatic bleedings, with absolute mortality reductions ranging between 15 and 62% [11-20]. These figures surpass any predictions of potentially preventable deaths in trauma [47]. While the survival advantage of early and aggressive FFP transfusion in early formula-driven resuscitation cannot be ignored, the evidence behind it has limitations that are discussed next.
Survival advantage
Borgman and colleagues reviewed 246 massively transfused (≥10 units RBC/24 hours) combatants and analysed mortality at three different FFP:RBC ratios (1:8, 1:2.5 and 1:1.4) [11]. A 55% absolute reduction in mortality occurred between the highest and lowest ratios. While mortality reduction was impressive, patients with a higher FFP:RBC ratio (1:1.4) had a longer median time to death (38 hours) than those with a lower ratio (2 hours). These data suggest that lower ratio patients may not have lived long enough to receive FFP. Another study by the same group on civilian trauma patients reported a similarly impressive survival advantage for higher ratios than lower ratios, but also a markedly dissimilar time to death (35 hours versus 4 hours) [58]. Both studies disclose survivorship bias, where arguably patients had to survive long enough to receive FFP, thus questioning their conclusions.
Addressing survivorship bias
Two studies specifically addressed the survivorship bias in high-FFP:RBC studies. Scalea and colleagues used stepwise logistic regression analysis on 806 patients, demonstrating no survival benefit for higher ratios when early deaths were excluded [56]. This study has its own limitations, however, including a failure to report the time to intensive care unit admission, an inability to include major factors (acidosis and coagulation) in the statistical model and a surprisingly low mortality (6%) for massive transfusions. Snyder and colleagues also attemp-ted to correct for survivorship bias in another study where mortality in high (>1:2) and low (<1:2) ratios was compared in regression models [57]. Using the FFP:PRBC ratio as a fixed value at 24 hours, as in many studies on this topic, the high ratio resulted in better survival. This survival advantage was lost, however, when the ratio was treated as a time-dependent variable (relative risk = 0.84, 95% confidence interval = 0.47 to 1.5). These two studies dispute the survival advantage suggested by the previous studies with such bias.
Time to intervention
The delay to thaw and initiate FFP transfusion leads to another important limitation: timing to initiate and reach the high FFP:RBC ratio. Early formula-driven resusci-tation proposes that FFP should be initiated early, ideally with the first RBC unit at the start of resuscitation [52,53]. Considering that even laboratory-guided resuscitation eventually results in a high FFP:RBC ratio, a critical difference in formula-driven resuscitation is the early implementation of a high ratio. No studies to date have reported on transfusing pre-thawed FFP along with the first RBC units or on the time to reach the 1:1 ratio. Snyder and colleagues stated that the median time to the first RBC was 18 minutes from arrival, while the first FFP was transfused more than 1 hour later [57].
The commonly used definition of massive bleeding as transfusions over 24 hours ignores the fact that 80% of all massive transfusions occur within the first 6 hours of hospitalization, at which point either bleeding reduces substantially or the patient dies [59]. A multicentre study involving 16 trauma centres, 452 massively bleeding trauma patients and transfusion rates within 6 hours of hospitalization (rate <1:4, rate of 1:4 to 1:1 and rate ≥1:1) concluded that early high FFP:RBC and platelet:RBC ratios improved survival [19]. Despite limitations, including significant differences in the baseline Glasgow coma scale and therefore the severity of head injuries between groups, the study provides better evidence that reaching high FFP:platelet:RBC ratios within the first hours of admission is associated with mortality reduction.
Missing data, co-interventions and heterogeneity
Data on timing to initiate FFP transfusions, on timing to reach the 1:1 ratio and on transfusions during the first 6 hours are equally missing in the studies supporting early formula-driven haemostatic resuscitation and in existing guidelines, limiting comparisons between the different strategies.
Spinella and colleagues reported in 708 military patients transfused with ≥1 units RBC that FFP transfusion was associated with increased survival (odds ratio = 1.17, 95% confidence interval = 1.06 to 1.29; P = 0.002) [12]. Missing data on the International Normalized Ratio, not measured in one-half of the patients, and heterogeneity with nonsurviving patients being significantly more coagulopathic than that for surviving patients, International Normalized Ratio 2.06 versus 1.4 (P < 0.001) on admission, however, challenge their conclusion.
Aggressive and early FFP transfusion is part of damage control resuscitation, which also proposes crystalloid restriction, rFVIIa and other interventions. A small study on 40 combat casualties resuscitated with a package containing whole blood, rFVIIa, crystalloid restriction and a high FFP:RBC ratio illustrates the complexity of analysing multiple co-interventions [52]. Combatants receiving the package had better survival compared with historical controls managed with similar FFP:RBC ratios but not rFVIIa, whole blood and significantly less blood transfusion [60]. In this study, multiple co-interventions make it impossible to establish the contribution of any of them.
Two other studies analysed survival before and after implementation of massive transfusion protocols [13,17]. Both studies demonstrated better survival with the protocol despite no difference in 24-hour FFP transfusion before and after protocol implementation and despite FFP:RBC ratios other than 1:1. The results could be interpreted as the protocol, and not the high FFP:RBC ratios, leading to better survival.
Potential harm
In a study demonstrating the survival advantage of aggressive FFP transfusion in the intensive care unit, Gonzalez and colleagues reported an unusual high incidence of early and lethal acute respiratory distress syndrome [10]. The aggressive FFP transfusion was aimed at correcting the International Normalized Ratio to ≤1.3, probably an unattainable goal given that the International Normalized Ratio of FFP is near 1.3 [61-63]. Considering that the deaths might represent transfusion-associated circulatory overload or TRALI, the study raises concerns on the aggressive FFP transfusion strategy. In a separate study of 415 trauma patients [64], early acute respiratory distress syndrome (before day 4) occurred significantly more among those patients transfused more FFP. Some studies, however, suggest that the adoption of early and aggressive FFP transfusion in fact reduces the overall exposure to blood and blood products [19]. Here also, the evidence is conflicting and precludes definitive conclusions.
Ethical and logistical considerations
In many countries, blood transfusion requires written informed consent, which is deferred only in life-threatening situations, including massive bleeding. The proposal to transfuse FFP early and aggressively raises important ethical considerations. First, traumatic massive bleeding carries upm to 40% mortality even when current resuscitation guidelines are strictly followed, and early coagulopathy increases mortality threefold. The marked reduction in mortality recently reported with early and high FFP:RBC resuscitation has prompted many trauma centres to adopt this strategy. The evidence behind early formula-driven haemostatic resuscitation is concordant with recent advances in the understanding of early trauma coagulopathy, but they also have methodological flaws and bias that seriously question the survival benefit.
Many trauma centres keep thawed AB plasma (uni-versal donor) available at all times for resuscitation. In countries that have implemented policies favouring male-only plasma to minimize the risks of TRALI, supplying AB plasma becomes an even greater challenge. Other ethical, logistical and financial considerations include the potential waste of unused thawed FFP, a so far untouched issue, plus the financial costs of haemostatic protocols, and the use of AB plasma on non-AB patients (potential increased risk from exposure to female FFP).
The answer to these challenges is not readily available or intuitive, particularly contrasting with the high mortality and lack of evidence supporting the existing guidelines. For now, the clinical decision continues to be based on observations, judgement and evidence transplanted from other fields. Definitive answers will only come from better understanding the pathophysiology of coagulation and prospective clinical trials, which may be years away. The challenges to such clinical trials are summarized in Table 2.
Table 2.
Challenges and proposed solutions to future clinical trials on haemostatic resuscitation
| Most important challenges | Proposed solutions |
|---|---|
| Avoid survivorship bias | Exclude patients not expected to live long enough to receive plasma |
| Precise documentation of the time of transfusions and death | |
| Perform analysis of transfusion as a time-dependent variable | |
| Avoid contamination of the control arm and avoid delay in initiating 1:1 transfusions in the intervention arm | Transfusion guidelines for both arms clear and easy to follow |
| Close cooperation between blood bank, trauma, anaesthesia and critical care | |
| Thawed AB plasma 24/7 or rapid thawing (microwave) | |
| Minimize time for results of laboratory tests - consider point-of-care testing | |
| Multiple interventions concomitantly tested | Standardize all aspects of resuscitation (that is, amount and type of intravenous fluid; procoagulant drugs) in control and intervention groups |
| Measure clotting factor levels | |
| Discriminate coagulopathic from mechanical bleeding | Measure indicators of coagulopathy: |
| • Thromboelastography | |
| • Clotting factor assays | |
| • Markers of hyperfibrinolysis | |
| • Tissue hypoperfusion (lactate, base deficit) | |
| • Progression of bleeding by computerized tomography scan (that is, progression brain | |
| contusion, retroperitoneal haematomas) | |
| • Ask the physician's opinion (that is, surgeon, anaesthetist, intensivist) | |
| Immediate cessation of component therapy | Evidence that bleeding has stopped |
| Consider ending by 6 hours | |
| Outcome | Consider restoration of haemostasis competence |
| Need for large samples | Consider a feasibility trial prior to a large multicentre trial to identify major challenges |
| Consent | Need for delayed consent |
Conclusion
The current knowledge regarding coagulopathy and FFP precludes the development of evidence-based guidelines. Existing guidelines for the management of massive bleeding recommend late FFP transfusion, based on conventional coagulation assays, which correlate poorly with clinically bleeding.
Early formula-driven haemostatic resuscitation has challenged this approach and has proposed early and aggressive FFP transfusion at a FFP:RBC ratio near 1:1, thus treating or preventing early trauma coagulopathy. Initial studies have reported significant reductions in mortality, but are uncontrolled and methodologically flawed, particularly by survivorship bias. Presently, clinical decisions should be based in assessing the pros and cons of both strategies while considering local resources and individual clinical context.
Prospective clinical trials are urgently needed to determine whether early formula-driven haemostatic resuscitation should be adopted or forgotten, to better understand trauma-associated coagulopathy and to develop evidence-based massive transfusion guidelines. Other areas for future research include improving the diagnosis of coagulopathy and evaluating novel products such as thawing microwaves for faster release of blood products.
Abbreviations
FFP: fresh frozen plasma; RBC: red blood cells; rFVIIa: recombinant factor VII activated; TRALI: transfusion-related acute lung injury.
Competing interests
SR has received speaker's fees and honorarium (as a member of the Scientific Advisory Board) from NovoNordisk A/S, manufacturer of NovoSeven (recombinant factor VIIa). The other authors declare that they have no competing interests.
See related letter by Daban et al., http://ccforum.com/content/14/2/412
Contributor Information
Bartolomeu Nascimento, Email: Bartolomeu.nascimento@sunnybrook.ca.
Jeannie Callum, Email: Jeannie.callum@sunnybrook.ca.
Gordon Rubenfeld, Email: Gordon.rubenfeld@sunnybrook.ca.
Joao Baptista Rezende Neto, Email: ljrezende@yahoo.com.br.
Yulia Lin, Email: yulia.lin@sunnybrook.ca.
Sandro Rizoli, Email: sandro.rizoli@sunnybrook.ca.
Acknowledgements
The authors acknowledge Dr Alina Toma for the excellent contributions to references and editing of the manuscript.
References
- O'Shaughnessy DF, Atterbury C, Bolton Maggs P, Murphy M, Thomas D, Yates S, Williamson LM. British Committee for Standards in Haematology, Blood Transfusion Task Force. Guidelines for the use of fresh-frozen plasma, cryoprecipitate and cryosupernatant. Br J Haematol. 2004;126:11–28. doi: 10.1111/j.1365-2141.2004.04972.x. [DOI] [PubMed] [Google Scholar]
- Spence RK. Clinical use of plasma and plasma fractions. Best Pract Res Clin Haematol. 2006;19:83–96. doi: 10.1016/j.beha.2005.01.035. [DOI] [PubMed] [Google Scholar]
- Erber WN, Perry DJ. Plasma and plasma products in the treatment of massive hemorrhage. Best Pract Res Clin Haematol. 2006;19:97–112. doi: 10.1016/j.beha.2005.01.026. [DOI] [PubMed] [Google Scholar]
- Stanworth SJ, Brunskill SJ, Hyde CJ, McClelland DB, Murphy MF. Is fresh frozen plasma clinically effective? A systematic review of randomized controlled trials. Br J Haematol. 2004;126:139–152. doi: 10.1111/j.1365-2141.2004.04973.x. [DOI] [PubMed] [Google Scholar]
- Lauzier F, Cook D, Griffith L, Upton J, Crowther M. Fresh frozen plasma transfusion in critically ill patients. Crit Care Med. 2007;35:1655–1659. doi: 10.1097/01.CCM.0000269370.59214.97. [DOI] [PubMed] [Google Scholar]
- Rao MP, Boralessa H, Morgan C, Soni N, Goldhill DR, Brett SJ, Boralessa H, Contreras M. North Thames Blood Interest Group. Blood component use in critically ill patients. Anaesthesia. 2002;57:530–534. doi: 10.1046/j.1365-2044.2002.02514.x. [DOI] [PubMed] [Google Scholar]
- Rock G, Berger R, Pinkerton P, Fernandes B. A pilot study to assess physician knowledge in transfusion medicine. Transfus Med. 2002;12:125–128. doi: 10.1046/j.1365-3148.2002.00363.x. [DOI] [PubMed] [Google Scholar]
- Dutton RP. Goals of therapy in common bleeding emergencies. Pharmacotherapy. 2007;27(9 Pt 2):85S–92S. doi: 10.1592/phco.27.9part2.85S. [DOI] [PubMed] [Google Scholar]
- Zimmerman LH. Causes and consequences of critical bleeding and mechanisms of blood coagulation. Pharmacotherapy. 2007;27(9 Pt 2):45S–56S. doi: 10.1592/phco.27.9part2.45S. [DOI] [PubMed] [Google Scholar]
- Gonzalez EA, Moore FA, Holcomb JB, Miller CC, Kozar RA, Todd SR, Cocanour CS, Balldin BC, McKinley BA. Fresh frozen plasma should be given earlier to patients requiring massive transfusion. J Trauma. 2007;62:112–119. doi: 10.1097/01.ta.0000250497.08101.8b. [DOI] [PubMed] [Google Scholar]
- Borgman MA, Spinella PC, Perkins JG, Grathwohl KW, Repine T, Beekley AC, Sebesta J, Jenkins D, Wade CE, Holcomb JB. The ratio of blood products transfused affects mortality in patients receiving massive transfusions at a combat support hospital. J Trauma. 2007;63:805–813. doi: 10.1097/TA.0b013e3181271ba3. [DOI] [PubMed] [Google Scholar]
- Spinella PC, Perkins JG, Grathwohl KW, Beekley AC, Niles SE, McLaughlin DF, Wade CE, Holcomb JB. Effect of plasma and red blood cell transfusions on survival in patients with combat related traumatic injuries. J Trauma. 2008;64(2 Suppl):S69–S77. doi: 10.1097/TA.0b013e318160ba2f. discussion S77-S78. [DOI] [PubMed] [Google Scholar]
- Cotton BA, Gunter OL, Isbell J, Au BK, Robertson AM, Morris JA Jr, St Jacques P, Young PP. Damage control hematology: the impact of a trauma exsanguination protocol on survival and blood product utilization. J Trauma. 2008;64:1177–1182. doi: 10.1097/TA.0b013e31816c5c80. [DOI] [PubMed] [Google Scholar]
- Duchesne JC, Hunt JP, Wahl G, Marr AB, Wang YZ, Weintraub SE, Wright MJ, McSwain NE Jr. Review of current blood transfusions strategies in a mature level I trauma center: were we wrong for the last 60 years? J Trauma. 2008;65:272–276. doi: 10.1097/TA.0b013e31817e5166. discussion 276-278. [DOI] [PubMed] [Google Scholar]
- Gunter OL Jr, Au BK, Isbell JM, Mowery NT, Young PP, Cotton BA. Optimizing outcomes in damage control resuscitation: identifying blood product ratios associated with improved survival. J Trauma. 2008;65:527–534. doi: 10.1097/TA.0b013e3181826ddf. [DOI] [PubMed] [Google Scholar]
- Maegele M, Lefering R, Paffrath T, Tjardes T, Simanski C, Bouillon B. Working Group on Polytrauma of the German Society of Trauma Surgery (DGU) Redblood-cell to plasma ratios transfused during massive transfusion are associated with mortality in severe multiple injury: a retrospective analysis from the Trauma Registry of the Deutsche Gesellschaft für Unfallchirurgie. Vox Sang. 2008;95:112–119. doi: 10.1111/j.1423-0410.2008.01074.x. [DOI] [PubMed] [Google Scholar]
- Johansson PI, Stensballe J. Effect of haemostatic control resuscitation on mortality in massively bleeding patients: a before and after study. Vox Sang. 2009;96:111–118. doi: 10.1111/j.1423-0410.2008.01130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira PG, Inaba K, Shulman I, Salim A, Demetriades D, Brown C, Browder T, Green D, Rhee P. Impact of plasma transfusion in massively transfused trauma patients. J Trauma. 2009;66:693–697. doi: 10.1097/TA.0b013e31817e5c77. [DOI] [PubMed] [Google Scholar]
- Zink KA, Sambasivan CN, Holcomb JB, Chisholm G, Schreiber MA. A high ratio of plasma and platelets to packed red blood cells in the first 6 hours of massive transfusion improves outcomes in a large multicenter study. Am J Surg. 2009;197:565–570. doi: 10.1016/j.amjsurg.2008.12.014. discussion 570. [DOI] [PubMed] [Google Scholar]
- Forcione DG, Alam HB, Kalva SP, Misdraji J. Case records of the Massachusetts General Hospital. Case 9-2009. An 81-year-old man with massive rectal bleeding. N Engl J Med. 2009;360:1239–1248. doi: 10.1056/NEJMcpc0810836. [DOI] [PubMed] [Google Scholar]
- Petrides M, Stack G, Cooling L, Maes LY. Carbohydrate Blood Group Antigens and Collections. Practical Guide to Transfusion Medicine. 2. 4, Chapter 2. Bethesda, MD: AABB Press; 2007. pp. 59–91. [Google Scholar]
- Callum JL, Pinkerton PH. Blood Easy 2. A Guide to Transfusion Medicine. 2. Toronto: Helen Stevenson Savattuq Inc; 2005. [Google Scholar]
- O'Neill EM, Rowley J, Hansson-Wicher M, McCarter S, Ragno G, Valeri CR. Effect of 24-hour whole-blood storage on plasma clotting factors. Transfusion. 1999;39:488–491. doi: 10.1046/j.1537-2995.1999.39050488.x. [DOI] [PubMed] [Google Scholar]
- Smith JF, Ness PM, Moroff G, Luban NL. Retention of coagulation factors in plasma frozen after extended holding at 1-6°C. Vox Sang. 2000;78:28–30. doi: 10.1046/j.1423-0410.2000.7810028.x. [DOI] [PubMed] [Google Scholar]
- Triulzi DJ, editor. American Association of Blood Banks. Blood Transfusion Therapy: A Physician's Handbook. 7. Bethesda, MD: AABB; 2002. [Google Scholar]
- United Kingdom Blood Transfusion Services/National Institute for Biological Standards and Control. Guidelines for the Blood Transfusion Services in the UK. 6. http://www.transfusionguidelines.org.uk [Google Scholar]
- Shehata N, Blajchman M, Heddle N. Coagulation factors in FFP and cryosupernatant. Transfus Med. 2001;11:391–401. doi: 10.1046/j.1365-3148.2001.00115.x. [DOI] [Google Scholar]
- Jain N, Kirschbaum N, Gaines A, Coignard B, Jarvis W, Silverman T. Pulmonary embolism in liver transplant setting associated with the use of solvent detergent plasma [abstract] J Thromb Haemost. 2003;1(Suppl 1):OC159. [Google Scholar]
- Yarranton H, Cohen H, Pavord SR, Benjamin S, Hagger D, Machin SJ. Venous thromboembolism associated with the management of acute thrombotic thrombocytopenic purpura. Br J Haematol. 2003;121:778–785. doi: 10.1046/j.1365-2141.2003.04360.x. [DOI] [PubMed] [Google Scholar]
- O'Brien SF, Yi QL, Fan W, Scalia V, Kleinman SH, Vamvakas EC. Current incidence and estimated residual risk of transfusion-transmitted infections in donations made to Canadian Blood Services. Transfusion. 2007;47:316–325. doi: 10.1111/j.1537-2995.2007.01108.x. [DOI] [PubMed] [Google Scholar]
- Det Norske Veritas. Assessment of the Risk of Exposure to vCJD Infectivity in Blood and Blood Products. A Report to Spongiform Encephalopathy Advisory Committee. London: Det Norske Veritas; 1999. [Google Scholar]
- Murphy ME. In: Practical Transfusion Medicine. Murphy MF, Pamphilon DH, editor. Oxford: Blackwell Science; 2001. Febrile reactions and TRALI; pp. 157–163. [Google Scholar]
- Popovsky MA. Pulmonary consequences of transfusion: TRALI and TACO. Transfus Apher Sci. 2006;34:243–244. doi: 10.1016/j.transci.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Renaudier P, Rebibo D, Waller C, Schlanger S, Vo Mai MP, Ounnoughene N, Breton P, Cheze S, Girard A, Hauser L, Legras JF, Saillol A, Willaert B, Caldani C. Pulmonary complications of transfusion: TACO and TRALI, a review. Transfus Clin Biol. 2009. in press . [DOI] [PubMed]
- Wright SE, Snowden CP, Athey SC, Leaver AA, Clarkson JM, Chapman CE, Roberts DR, Wallis JP. Acute lung injury after ruptured abdominal aortic aneurysm repair: the effect of excluding donations from females from the production of fresh frozen plasma. Crit Care Med. 2008;36:1796–1802. doi: 10.1097/CCM.0b013e3181743c6e. [DOI] [PubMed] [Google Scholar]
- Marik PE, Corwin HL. Acute lung injury following blood transfusion: expanding the definition. Crit Care Med. 2008;36:3080–3084. doi: 10.1097/CCM.0b013e31818c3801. [DOI] [PubMed] [Google Scholar]
- Jawa RS, Anillo S, Kulaylat MN. Transfusion-related acute lung injury. J Intensive Care Med. 2008;23:109–121. doi: 10.1177/0885066607312994. [DOI] [PubMed] [Google Scholar]
- Stainsby D, MacLennan S, Thomas D, Isaac J, Hamilton PJ. British Committee for Standards in Haematology. Guidelines on the management of massive blood loss. Br J Haematol. 2006;135:634–641. doi: 10.1111/j.1365-2141.2006.06355.x. [DOI] [PubMed] [Google Scholar]
- Dizk WH. Component Therapy Before Bedside Procedures. Transfusion Therapy. 2. Vol. 1. Bethesda, MD: AABB Press; 2005. pp. 2–23. Vol 7:203-241. [Google Scholar]
- Cotton BA, Guy JS, Morris JA Jr, Abumrad NN. The cellular, metabolic, and systemic consequences of aggressive fluid resuscitation strategies. Shock. 2006;26:115–121. doi: 10.1097/01.shk.0000209564.84822.f2. [DOI] [PubMed] [Google Scholar]
- Tieu BH, Holcomb JB, Schreiber MA. Coagulopathy: its pathophysiology and treatment in the injured patient. World J Surg. 2007;31:1055–1064. doi: 10.1007/s00268-006-0653-9. [DOI] [PubMed] [Google Scholar]
- American Society of Anesthesiologists Task Force on Perioperative Blood Transfusion and Adjuvant Therapies. Practice guidelines for perioperative blood transfusion and adjuvant therapies: an updated report by the American Society of Anesthesiologists Task Force on Perioperative Blood Transfusion and Adjuvant Therapies. Anesthesiology. 2006;105:198–208. doi: 10.1097/00000542-200607000-00030. [DOI] [PubMed] [Google Scholar]
- Iorio A, Basileo M, Marchesini E, Materazzi M, Marchesi M, Esposito A, Palazzesi GP, Pellegrini L, Pasqua BL, Rocchetti L, Silvani CM. The good use of plasma. A critical analysis of five international guidelines. Blood Transfus. 2008;6:18–24. doi: 10.2450/2008.0041-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spahn DR, Cerny V, Coats TJ, Duranteau J, Fernández-Mondéjar E, Gordini G, Stahel PF, Hunt BJ, Komadina R, Neugebauer E, Ozier Y, Riddez L, Schultz A, Vincent JL, Rossaint R. Task Force for Advanced Bleeding Care in Trauma. Management of bleeding following major trauma: a European guideline. Crit Care. 2007;11:R17. doi: 10.1186/cc5686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauaia A, Moore FA, Moore EE, Moser KS, Brennan R, Read RA, Pons PT. Epidemiology of trauma deaths: a reassessment. J Trauma. 1995;38:185–193. doi: 10.1097/00005373-199502000-00006. [DOI] [PubMed] [Google Scholar]
- Kauvar DS, Lefering R, Wade CE. Impact of hemorrhage on trauma outcome: an overview of epidemiology, clinical presentations, and therapeutic considerations. J Trauma. 2006;60(6 Suppl):S3–S11. doi: 10.1097/01.ta.0000199961.02677.19. [DOI] [PubMed] [Google Scholar]
- Acosta JA, Yang JC, Winchell RJ, Simons RK, Fortlage DA, Hollingsworth-Fridlund P, Hoyt DB. Lethal injuries and time to death in a level I trauma center. J Am Coll Surg. 1998;186:528–533. doi: 10.1016/S1072-7515(98)00082-9. [DOI] [PubMed] [Google Scholar]
- Brohi K, Singh J, Heron M, Coats T. Acute traumatic coagulopathy. J Trauma. 2003;54:1127–1130. doi: 10.1097/01.TA.0000069184.82147.06. [DOI] [PubMed] [Google Scholar]
- MacLeod JB, Lynn M, McKenney MG, Cohn SM, Murtha M. Early coagulopathy predicts mortality in trauma. J Trauma. 2003;55:39–44. doi: 10.1097/01.TA.0000075338.21177.EF. [DOI] [PubMed] [Google Scholar]
- Gando S. Tissue factor in trauma and organ dysfunction. Semin Thromb Hemost. 2006;32:48–53. doi: 10.1055/s-2006-933340. [DOI] [PubMed] [Google Scholar]
- Gando S, Nanzaki S, Kemmotsu O. Coagulofibrinolytic changes after isolated head injury are not different from those in trauma patients without head injury. J Trauma. 1999;46:1070–1076. doi: 10.1097/00005373-199906000-00018. discussion 1076-1077. [DOI] [PubMed] [Google Scholar]
- Holcomb JB, Jenkins D, Rhee P, Johannigman J, Mahoney P, Mehta S, Cox ED, Gehrke MJ, Beilman GJ, Schreiber M, Flaherty SF, Grathwohl KW, Spinella PC, Perkins JG, Beekley AC, McMullin NR, Park MS, Gonzalez EA, Wade CE, Dubick MA, Schwab CW, Moore FA, Champion HR, Hoyt DB, Hess JR. Damage control resuscitation: directly addressing the early coagulopathy of trauma. J Trauma. 2007;62:307–310. doi: 10.1097/TA.0b013e3180324124. [DOI] [PubMed] [Google Scholar]
- Beekley AC. Damage control resuscitation: a sensible approach to the exsanguinating surgical patient. Crit Care Med. 2008;36(7 Suppl):S267–S274. doi: 10.1097/CCM.0b013e31817da7dc. [DOI] [PubMed] [Google Scholar]
- Hirshberg A, Dugas M, Banez EI, Scott BG, Wall MJ Jr, Mattox KL. Minimizing dilutional coagulopathy in exsanguinating hemorrhage: a computer simulation. J Trauma. 2003;54:454–463. doi: 10.1097/01.TA.0000053245.08642.1F. [DOI] [PubMed] [Google Scholar]
- Ho AM, Dion PW, Cheng CA, Karmakar MK, Cheng G, Peng Z, Ng YW. A mathematical model for fresh frozen plasma transfusion strategies during major trauma resuscitation with ongoing hemorrhage. Can J Surg. 2005;48:470–478. [PMC free article] [PubMed] [Google Scholar]
- Scalea TM, Bochicchio KM, Lumpkins K, Hess JR, Dutton R, Pyle A, Bochicchio GV. Early aggressive use of fresh frozen plasma does not improve outcome in critically injured trauma patients. Ann Surg. 2008;248:578–584. doi: 10.1097/SLA.0b013e31818990ed. [DOI] [PubMed] [Google Scholar]
- Snyder CW, Weinberg JA, McGwin G Jr, Melton SM, George RL, Reiff DA, Cross JM, Hubbard-Brown J, Rue LW, Kerby JD. The relationship of blood product ratio to mortality: survival benefit or survival bias? J Trauma. 2009;66:358–362. doi: 10.1097/TA.0b013e318196c3ac. discussion 362-364. [DOI] [PubMed] [Google Scholar]
- Holcomb JB, Wade CE, Michalek JE, Chisholm GB, Zarzabal LA, Schreiber MA, Gonzalez EA, Pomper GJ, Perkins JG, Spinella PC, Williams KL, Park MS. Increased plasma and platelet to red blood cell ratios improves outcome in 466 massively transfused civilian trauma patients. Ann Surg. 2008;248:447–458. doi: 10.1097/SLA.0b013e318185a9ad. [DOI] [PubMed] [Google Scholar]
- Kashuk JL, Moore EE, Johnson JL, Haenel J, Wilson M, Moore JB, Cothren CC, Biffl WL, Banerjee A, Sauaia A. Postinjury life threatening coagulopathy: is 1:1 fresh frozen plasma:packed red blood cells the answer? J Trauma. 2008;65:261–270. doi: 10.1097/TA.0b013e31817de3e1. discussion 270-271. [DOI] [PubMed] [Google Scholar]
- Fox CJ, Gillespie DL, Cox ED, Mehta SG, Kragh JF Jr, Salinas J, Holcomb JB. The effectiveness of a damage control resuscitation strategy for vascular injury in a combat support hospital: results of a case control study. J Trauma. 2008;64(2 Suppl):S99–S106. doi: 10.1097/TA.0b013e3181608c4a. discussion S106-S107. [DOI] [PubMed] [Google Scholar]
- Holland LL, Foster TM, Marlar RA, Brooks JP. Fresh frozen plasma is ineffective for correcting minimally international normalized ratios. Transfusion. 2005;45:1234–1235. doi: 10.1111/j.1537-2995.2005.00184.x. [DOI] [PubMed] [Google Scholar]
- Doyle S, O'Brien P, Murphy K, Fleming C, O'Donnell J. Coagulation factor content of solvent/detergent plasma compared with fresh frozen plasma. Blood Coagul Fibrinolysis. 2003;14:283–287. doi: 10.1097/00001721-200304000-00010. [DOI] [PubMed] [Google Scholar]
- Nifong TP, Light J, Wenk RE. Coagulation stability and sterility of thawed S/D-treated plasma. Transfusion. 2002;42:1581–1584. doi: 10.1046/j.1537-2995.2002.00246.x. [DOI] [PubMed] [Google Scholar]
- Sperry JL, Ochoa JB, Gunn SR, Alarcon LH, Minei JP, Cuschieri J, Rosengart MR, Maier RV, Billiar TR, Peitzman AB, Moore EE. Inflammation the Host Response to Injury Investigators. An FFP:PRBC transfusion ratio ≥1:1.5 is associated with a lower risk of mortality after massive transfusion. J Trauma. 2008;65:986–993. doi: 10.1097/TA.0b013e3181878028. [DOI] [PubMed] [Google Scholar]


