Abstract
PURPOSE
To investigate the cellular effects of mitomycin C (MMC) treatment on corneal endothelial (CE) cells at clinically relevant applications and dosages.
METHODS
Radial and posterior diffusion of MMC was determined by an Escherichia coli growth inhibition bioassay. A modified version of the comet assay (single cell gel electrophoresis) was used to detect DNA cross-linking. Immunostaining detected the nuclear phosphorylated histone variant H2AX (γ-H2AX) indicating DNA double-strand breaks. Apoptosis in MMC-treated cells was detected with annexin V staining.
RESULTS
Topical application of 0.02% MMC to intact goat globes resulted in MMC in the CE at 0.37 µg/mL and produced a significant increase in CE DNA cross-linking with as little as 6 seconds of topical MMC treatment. DNA cross-linking was also demonstrated in cultured CE cells by using MMC exposures similar to those detected in CE of intact eyes. Such MMC treatment of CE produced elevated and persistent γ-H2AX-positive cells indicative of DNA double-strand breaks. Similarly, there was an increase in the proportion of apoptotic CE cells, evidenced by positive annexin V staining.
CONCLUSIONS
The results demonstrate that exposure to MMC at times and concentrations commonly used in refractive surgery produces cross-linking of corneal endothelial DNA, persistent DNA damage, and endothelial death via apoptosis. Current practices of MMC application during refractive surgeries may increase the potential for long-term and permanent deleterious effects on the health of the corneal endothelium.
Mitomycin C (MMC), an antibiotic isolated from Streptomyces caespitosus, is classified as an alkylating agent capable of covalently binding DNA and inducing inter- and intrastrand cross-links. The presence of such cross-links results in inhibition of DNA synthesis primarily during late G1 and S phases, although it is not cell-cycle specific. In addition, MMC inhibits RNA and protein synthesis and interacts with molecular oxygen, generating free radical damage to DNA and protein. 1 MMC is predominantly used as a systemic chemotherapeutic agent; however, off-label usage of MMC is common during ophthalmic procedures, such as glaucoma filtering surgery, lacrimal surgery, treatment of ocular neoplasia, ocular cicatrization surgery, and corneal refractive surgery.2
Currently, the use of adjuvant MMC as an intraoperative prophylactic for reducing haze has become increasingly widespread in photorefractive keratectomy (PRK).2–9 As a topical adjunct during refractive surgery, MMC is exclusively used to modulate corneal wound healing and, through its effects on stromal keratocytes, to prevent active fibrosis, abnormal collagen deposition, and haze formation. The induction of apoptosis of keratocytes by MMC seems to play a central role in preventing the formation of clinically significant haze.10 –12
MC applied topically has been shown to penetrate the cornea and can be detected in corneal tissue and the aqueous humor well after initial application13–15; thus, treatment with MMC during refractive surgery has the potential to impact ocular tissues other than the target keratocytes. Of particular interest is the corneal endothelium, a monolayer of nonregenerating cells essential to the clarity of the cornea. Initially, several clinical studies regarding corneal endothelial cell density and morphology indicate few short-term complications with the use of adjuvant MMC during refractive surgeries16–18; however, recent studies have suggested potential adverse effects of such treatments. The corneal endothelium appears to exhibit significant vulnerability to the toxicity of MMC. Single intraoperative MMC applications over a denuded stroma result in dose-dependent corneal edema and endothelial apoptosis.19 Similarly, significant endothelial cell loss has been reported in MMC-treated eyes within a few months after photorefractive keratectomy.20 In addition, numerous case reports have suggested that a single intraoperative application of MMC during other ocular procedures may be responsible for corneal endothelial damage and loss.21–24 Thus, as the surgical use of MMC increases, the need to define its cellular effects at relevant clinical applications and dosages on corneal endothelial cells is of high importance.
In this study, we assessed the effects of a single MMC application on corneal endothelial cells, using concentrations and times relevant to current clinical practice. We used a modified version of the highly sensitive comet assay (single cell gel electrophoresis) to detect DNA cross-linking and examined the phosphorylated histone variant H2AX (γ-H2AX) to demonstrate DNA double-strand breaks. Finally, we assessed MMC-treated cells for annexin V staining to detect apoptosis. Our results clearly demonstrate that exposure to MMC at the times and concentrations commonly used in refractive surgery produced cross-linking of corneal endothelial DNA, persistent DNA damage, and endothelial death via apoptosis.
MATERIALS AND METHODS
Animals and Cell Culture
Fresh whole goat eyes were obtained from a local butcher within a few hours of death. For indicated experiments, whole globes were used immediately as described below. To isolate corneal endothelial (CE) cells, we excised corneas with a scleral rim and placed them endothelium side up in plastic tube caps. The CE cells were enzymatically detached by direct application of 0.05% trypsin/0.5 mM EDTA (Sigma-Aldrich, St. Louis, MO) with incubation at 37°C and harvested by gentle scraping. The CE cells were separated from Descemet’s membrane and cell suspensions were broken up by gentle pipetting and sieved through a 70-µm nylon mesh. The suspended CE cells were centrifuged, and pelleted cells used for comet assay were directly resuspended in 1% low-melting-point (LMP) agarose and used as described below. CE cells for culture were resuspended in DMEM (no. 1569; Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum (Hyclone, Logan, UT), gentamicin, penicillin/streptomycin, and amphotericin B and cultured in 25 cm2 cell culture flasks at 37°C in 5% CO2 until 90% confluent. The cells were passaged on a cell dissociation enzyme (TrypLE Express; Invitrogen), centrifuged, resuspended, and plated on sterile glass coverslips for γ-H2AX detection or in six-well plates for annexin V detection. Cell culture pellets used for the comet assay were directly resuspended in 1% LMP agarose and used as described later. Confluent passage-one or -two cells were used in all cell culture experiments.
MMC Treatment
MMC (Sigma-Aldrich) was dissolved to desired concentrations in phosphate-buffered saline (PBS) for whole globe treatments and in DMEM with 10% FBS for cell culture studies. We referred to established MMC treatment protocols used in refractive surgery for the treatment of whole intact globes.3–6 First, the corneal epithelium was treated with 20% ethanol within an 8-mm trephine for 20 seconds followed by mechanical epithelium debridement of the 8-mm area with a beaver blade. Immediately after debridement, a corneal shield (Medtronic Solan, Jacksonville, FL) soaked with 200 µg/mL (0.02%) MMC in PBS was placed over the denuded area for 6, 12, 30, 60, or 120 seconds. The control globes were not treated with MMC. After the MMC sponge was removed, the eyes were copiously washed with 30 mL of PBS and then placed in a humid chamber for 30 minutes before isolation of CE cells as described earlier. Laser ablation was performed on a subset of intact whole globes before MMC treatment (Visx Star S4 Excimer Laser System; Visx Technology, Santa Clara, CA) with a fluence of 160 mJ/cm2 per pulse at 10.0 Hz. The laser was programmed for a 75-µm ablation depth with a 6.0-mm optical zone and a 0.3-mm transition zone. To allow for DNA repair in a subset of corneal buttons, we excised the corneas after MMC treatment, placed them in 35-mm2 tissue culture plates, and immersed them in culture medium at 37°C in 5% CO2 for 24 hours before CE cell isolation.
For comet assay of cultured CE cells, we treated confluent cultures with MMC concentrations ranging from 0.02 to 200 µg/mL for 10, 30, or 60 minutes and isolated cells per the protocol above.
For treatment of cultured CE cells to detect γ-H2AX and annexin V, we selected a MMC concentration of 0.4 µg/mL (0.00004%) which we determined via growth inhibition bioassay to be present in the corneal endothelium after topical treatment of intact globes with 0.02% MMC. Furthermore, this concentration was similar to those found in the aqueous humor 30 minutes to 1 hour after MMC treatment in previous studies.13,14 We treated confluent monolayers of CE cells with 0.4 µg/mL MMC in DMEM/10% FBS for 30 minutes at 37°C in 5% CO2 and then immediately removed the medium, washed the cell layer with PBS, and replaced the medium with fresh culture medium. The cells were incubated 24, 48, or 72 hours before assaying for γ-H2AX or annexin V.
Determination of Radial and Posterior Diffusion of MMC
Determination of the extent of radial and posterior diffusion of MMC in whole globes after topical exposure to 0.02% MMC was assayed by sectioning the excised cornea into radial or anterior to posterior (coronal) portions and estimating concentrations of MMC in the tissue with an Escherichia coli growth-inhibition bioassay. For radial sectioning, MMC-treated corneas were immediately cut into concentric rings with 8-, 6-, and 4-mm trephines; minced; and placed into tubes containing PBS. For coronal sections, groups of five 40-µm-thick sections of treated corneas, cut with a sliding microtome, were combined, minced, and placed into tubes containing 1 mL of PBS. Fifty microliters of each extract was pipetted onto a minimum inhibitory concentration (MIC) disc, which was then placed onto an E. coli plate, and incubated overnight. The resulting zone of inhibition of growth of E. coli was measured manually and compared to a standard curve to determine the concentration of MMC present in the extracts. Radial measurements were made from duplicate assays and coronal measurements were obtained from a single assay.
Modified Alkaline Comet Assay and Analysis
The CE cell suspensions were pelleted and resuspended in 80 µL of 1% LMP agarose. The resuspended cells were immediately transferred to microscope slides precoated with 1% normal melting-point agarose, covered with coverslips, and chilled over ice for 20 minutes. The coverslips were removed and the slides placed in lysis solution (10 mM Tris, 100 mM EDTA, 2.5 M NaCl [pH 10], 1% Triton-X) over ice for 60 minutes. Lysis solution was removed, and the slides were rinsed three times with distilled water. The slides were then irradiated with 5 Gy (Cs137 source) γ-irradiation to induce random double-strand DNA breaks. After irradiation, DNA was alkaline-denatured by immersion of slides in cold 3 M NaOH, 10 mM EDTA for 30 minutes. Electrophoresis was conducted in the same buffer at 25 V (0.8 V/cm) for 40 minutes at room temperature. The slides were incubated in neutralization buffer (0.4 mM Tris, pH 7.5) for 10 minutes and then stained with SYBR gold (Invitrogen) in TE (10 mM Tris-HCl, 1 mM EDTA [pH 8.0]). Coverslips were placed over the slides which were allowed to dry overnight protected from the light.
DNA from individual cells was visualized using an epifluorescence microscope (TE2000; Nikon, Melville, NY) with a 10× objective (Pan Fluor; Nikon). Photography and analysis of the samples were performed in a masked fashion. Images were captured with a 12-bit digital camera (SPOT; Diagnostic Instruments, Sterling Heights, MI). All comet analysis was performed using software designed for this purpose (CometScore software; TriTek Corp., Sumerduck, VA) which measures a wide range of fluorescence parameters for selected comets. The primary measurement, %DNA-in-head, reflected the relative distribution of DNA in each comet. In both the intact globe and cell culture studies, more than 1000 comets were analyzed in each of two independent experiments.
γ-H2AX Detection
Confluent CE cells grown on sterile glass coverslips were exposed to a single 30-minute treatment with 0.4 µg/mL MMC, rinsed, and continued in culture for 24, 48, or 72 hours. MMC-treated CE cells were briefly rinsed in PBS and then fixed with 3% paraformaldehyde in PBS for 20 minutes at room temperature. After the cells were rinsed with PBS, they were permeabilized with 0.1% Triton-X in PBS for 1 minute, rinsed with PBS, and stored in 50% glycerol in PBS (vol/vol) at 4°C until staining. Nonspecific binding was blocked with 10% heat-inactivated goat serum for 1 hour at room temperature. After they were washed with PBS, the CE cells were incubated overnight at 4°C with anti-γ-H2AX polyclonal rabbit antibody (AF2288; R&D Systems, Minneapolis, MN) diluted 1:800 in 1% bovine serum albumin (BSA) in PBS. The cells were washed and incubated with DAPI nuclear stain, 0.5 µg/mL and Alexa Fluor 488 – conjugated goat anti-rabbit IgG, 1 µg/mL (A-11094; Invitrogen) for 1 hour at room temperature. The coverslips were placed onto slides, and the nuclei were visualized with an inverted microscope (TE200-E Eclipse; Nikon) with a 40× oil objective (Nikon). A digital camera (CoolSnapHQ; Roper, Tucson, AZ) was used for image acquisition. Manual counting, processing, and analysis were performed (MetaMorph Software; Molecular Devices, Sunnyvale, CA). Fifteen images from each treatment group were analyzed in each of two independent experiments.
Annexin V Detection
Annexin V detection (TACS Annexin V-FITC Apoptosis Detection kit; R&D Systems) was performed on confluent CE cells grown in six-well culture plates 24 or 48 hours after a single 30-minute treatment with 0.4 µg/mL MMC, according to the manufacturer’s protocol. Nuclei were counterstained with Hoechst 33342 dye at 1 µg/mL (Invitrogen). Cells were photographed and analyzed as for γ-H2AX detection. Ten images representing different locations throughout the culture were analyzed from each treatment group.
Statistics
Statistical analysis was performed with one-way analysis of variance (ANOVA) followed by the Dunnett multiple comparison test (GraphPad Prism 4 software; GraphPad Software, San Diego, CA). The Dunnett test was used to compare values for MMC treatment groups with those of the control cultures. P < 0.05 was considered significant for all tests.
RESULTS
MMC in the Cornea after a Single Topical Treatment
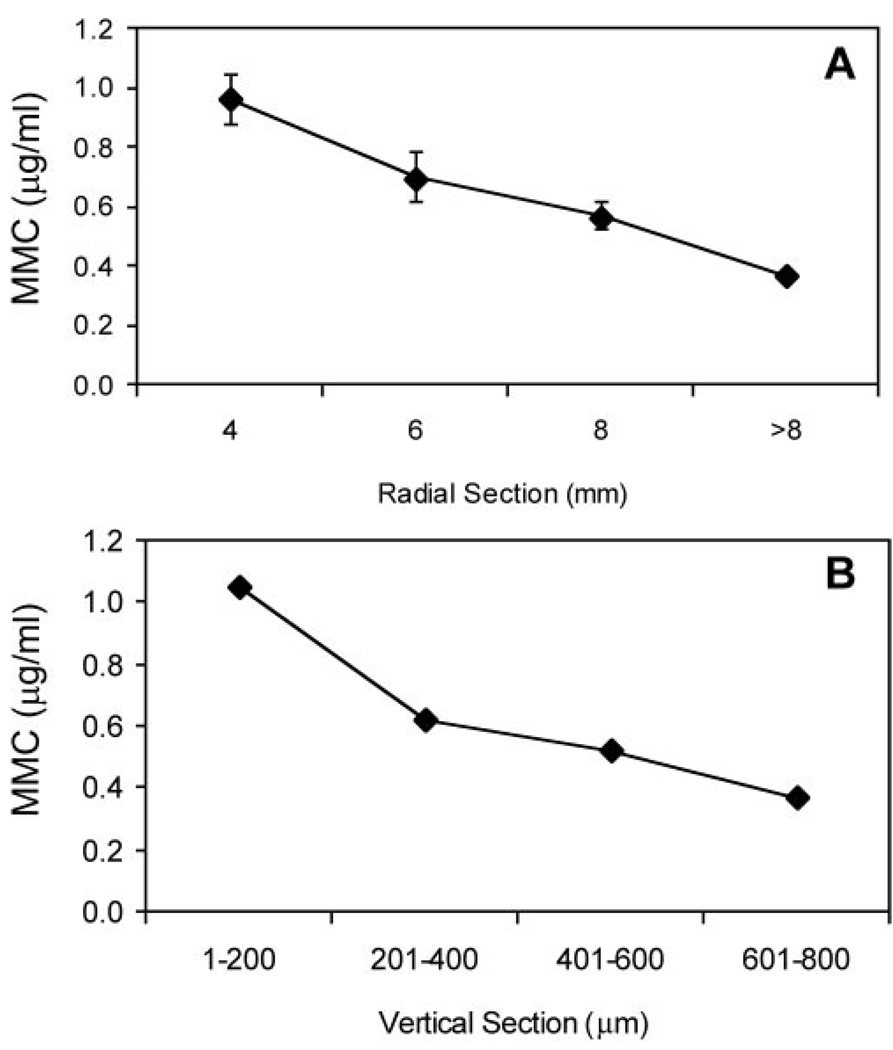
A single topical treatment of MMC (0.02%) for 2 minutes on a de-epithelialized cornea resulted in measurable MMC concentrations throughout the entire cornea (Fig. 1). Radial sections of MMC-treated corneas revealed concentrations of MMC from 1.05 µg/mL in the central area to 0.37 µg/mL in the periphery (>8 mm), demonstrating the rapid diffusion of MMC from the central cornea to the periphery (Fig. 1A). Coronal sections of MMC-treated corneas revealed the presence of MMC in all layers of the cornea after treatment, ranging from 1.05 µg/mL in the anterior stroma (1–200-µm section) to 0.37 µg/mL in the CE (601–800-µm section; Fig. 1B). This concentration was similar to those found in the aqueous humor 30 minutes to 1 hour after MMC treatment in previous studies.13,14
FIGURE 1.
Corneal concentration of MMC 2 minutes after a single topical administration of 0.02% MMC on a whole globe, as determined by growth inhibition bioassay. The zone of inhibition of E. coli growth was compared to a standard curve to determine MMC concentrations. (A) MMC concentration decreases radially, away from the site of application. (B) CE concentration is approximately 0.37 µg/mL at the depth of the endothelium (601–800 µm). Mean ± SEM.
Induction of DNA Cross-Linking in the CE
Modified Comet Assay
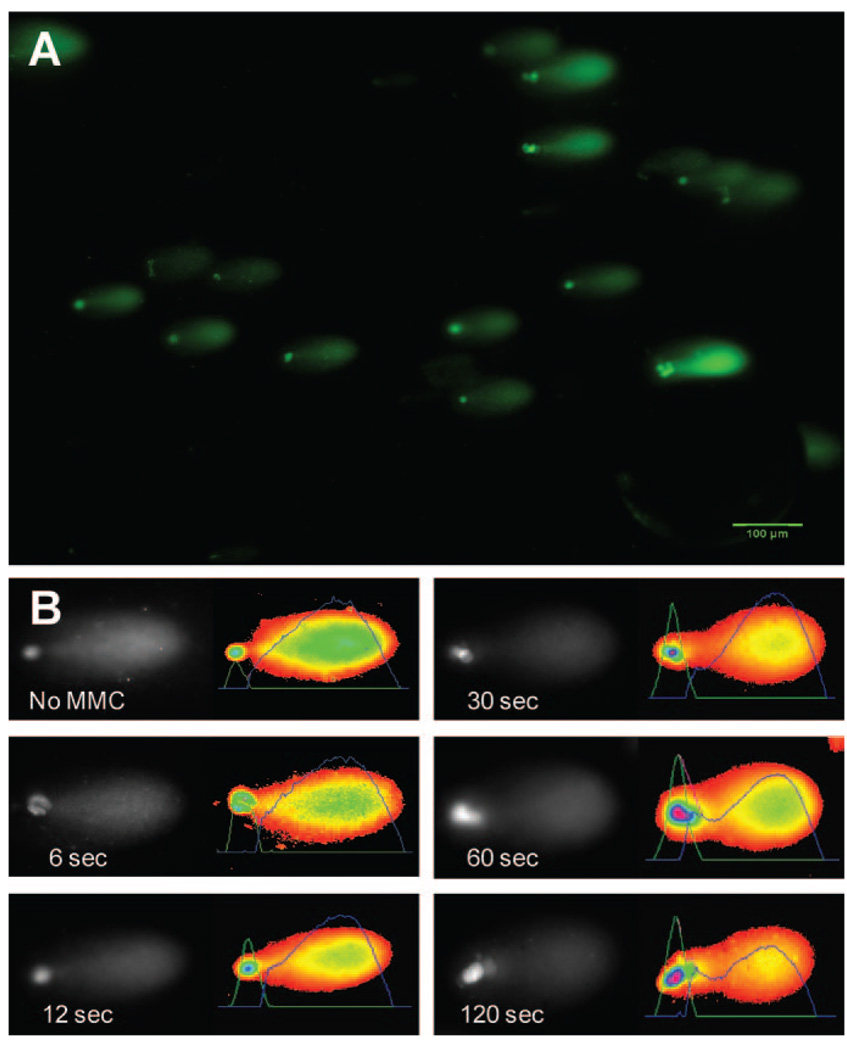
We investigated the cross-linking effects of MMC treatment on goat CE by using a modification of the comet assay previously developed by McKenna et al.25 to evaluate MMC-induced DNA cross-linking damage in RT4 cells. Figure 2A is a representative image of the results of the comet assay illustrating staining of DNA from individual CE cells after fragmentation and denaturation. Distinct head and tail regions are seen in the DNA from each cell, representing DNA fragments with differing electrophoretic mobility. DNA fragments without cross-links are smaller and migrate more rapidly (i.e., in the tail portion of the comet). In contrast, DNA fragments with MMC-induced cross-links resist full denaturation in alkaline conditions and exhibit decreased electrophoretic mobility, thereby forming the head of the comet. Figure 2B provides a representative image illustrating the increasing percentage of DNA remaining in the head (%DNA-in-head) of the comet as MMC treatment time increased.
FIGURE 2.
Single cell gel electrophoresis (modified alkaline comet assay) of CE cell DNA. (A) Representative images of individual CE cell DNA after electrophoresis show migration patterns with a comet-like appearance. (B) Individual comets are oriented with the head to the left. The image on the right of each shows a pseudocolor of the image with an overlaid graphic representation of staining intensity. This intensity is proportional to the amount of DNA at that location in the gel. Area under these curves was used calculate %DNA-in-head. With increasing length of treatment of MMC, more DNA was observed in the head of comets.
MMC-Induced DNA Cross-Links in Intact Globes
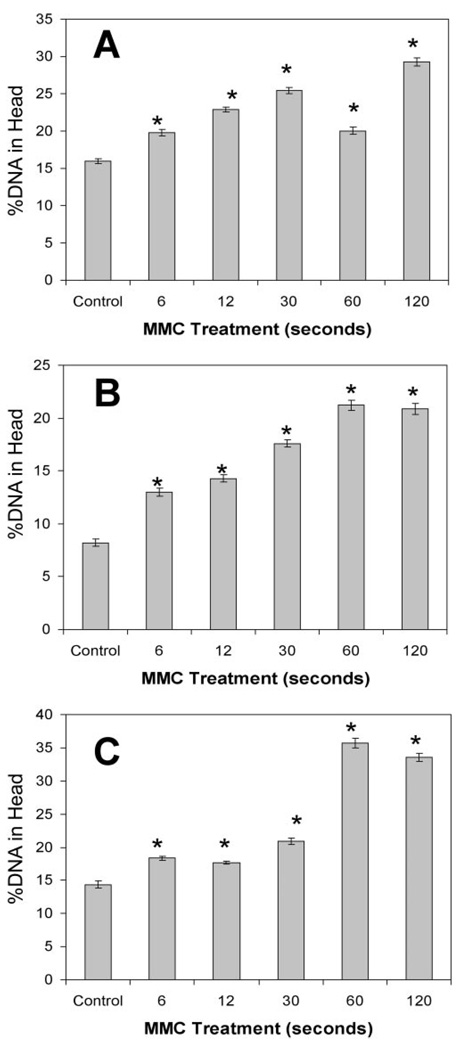
Quantitative analysis of images similar to those in Figure 2 found that a single topical application of 0.02% MMC on intact whole globes caused DNA cross-linking in CE cells at all treatment times (Fig. 3). More than 1000 individual comets were analyzed in each experiment. In experiment A, we used epithelial debridement followed by MMC treatments. The presence of the epithelial basement membrane did not prevent the cross-linking effects of MMC, as all treated groups had significantly more %DNA-in-head than did the untreated control (Fig. 3A; ANOVA P < 0.05, Dunnett P < 0.01). In experiment B, we used epithelial debridement followed by laser ablation and MMC treatments. In these conditions, which emulate PRK protocols, all MMC treatment groups had significantly more %DNA-in-head when compared with the untreated control (Fig. 3B; ANOVA P < 0.05, Dunnett P < 0.01). Experiment C was similar to experiment A, but MMC treatment was followed by a 24-hour incubation period to allow for repair. In this experiment, MMC-induced DNA cross-linking in the endothelium was not significantly reduced after 24 hours, as all MMC treatment groups had significantly more %DNA-in-head than did the control (Fig. 3C; ANOVA P < 0.05, Dunnett P < 0.01). Across all trials, there was also an apparent time response between the length of MMC treatment and amount of DNA cross-linking evident as %DNA-in-head.
FIGURE 3.
CE DNA cross-linking induced by MMC treatment of whole globes. %DNA-in-head was calculated for comets (as in Fig. 2) derived from CE cells after topical MMC treatment of intact goat eyes. (A) 0.02% MMC treatment followed epithelial debridement. (B) MMC treatment followed epithelial debridement and 75 µm stromal ablation. (C) Eyes treated with MMC after epithelial debridement (as in A) were incubated 24 hours at 37°C, to allow DNA repair. *Significant difference from control; ANOVA P < 0.0001, Dunnett P < 0.01. Mean ± SEM.
MMC-Induced DNA Cross-Links in Cultured CE Cells
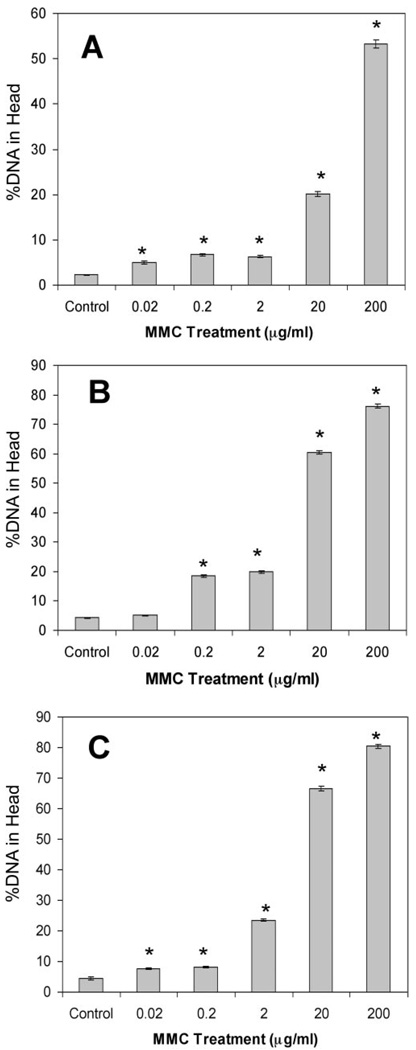
Confluent monolayers of goat CE cells also exhibited significant DNA cross-linking similar to that shown in Figure 3 in MMC-treated intact globes. MMC concentrations ranging from 0.02 to 200 µg/mL all resulted in significant DNA cross-linking when compared with the untreated control (Fig. 4; ANOVA P < 0.05, Dunnett P < 0.05). Increasing the length of exposure to MMC at all concentrations, except 0.2 µg/mL, resulted in increased DNA cross-linking, similar to the results in intact globes.
FIGURE 4.
Sensitivity of CE cells to DNA cross-linking by MMC. Goat CE cells in culture were treated with a range of MMC concentrations, and DNA cross-linking was determined using the comet assay as described in Figure 2 at three exposure times. (A) Ten-minute exposure to MMC at all concentrations induced significant DNA cross-links compared to controls. (B) Thirty-minute exposure to MMC at all concentrations (except 0.02 µg/mL, Dunnett P > 0.05) induced significant DNA cross-links compared with controls. (C) Sixty-minute exposure to MMC at all concentrations induced significant DNA cross-links compared with the control. *Significant differences from control; ANOVA P < 0.0001, Dunnett P < 0.01. Mean ± SEM.
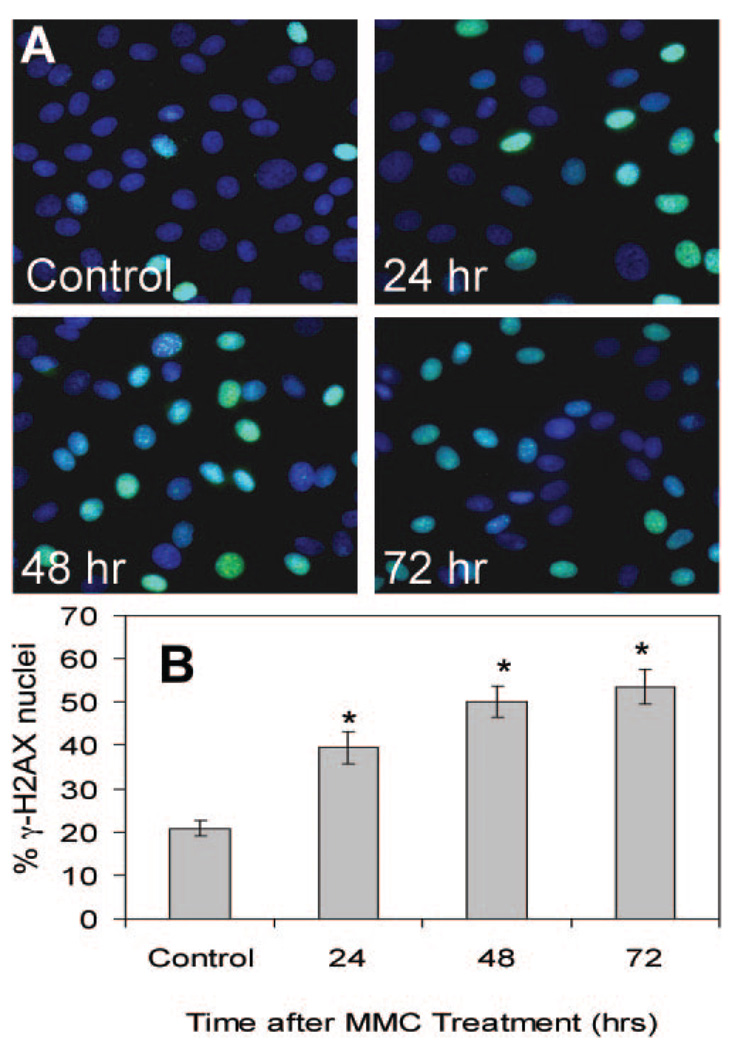
Persistent and Elevated γ-H2AX Staining after MMC Treatment
γ-H2AX nuclear foci represent phosphorylation at Ser139 of histone variant H2AX and are sensitive markers for DNA double-strand breaks.26 Dephosphorylation of histone H2AX accompanies subsequent effective repair of the DNA damage.27 As shown in Figure 5A, CE cells treated with 0.4 µg/mL MMC for 30 minutes developed γ-H2AX-positive nuclei, demonstrating the presence of DNA double-strand breaks. Recovery for 24, 48, and 72 hours after MMC treatment showed no decrease in the percentage of γ-H2AX-positive nuclei (Fig. 5B). MMC treatment therefore produces a long-term activation of γ-H2AX in CE cells, a phenomenon previously reported to be transient in cells undergoing DNA repair.28
FIGURE 5.
H2AX phosphorylation (γ-H2AX) induced by MMC treatment. (A) Confluent CE cells were treated with MMC at 0.4 µg/mL for 30 minutes and nuclei were stained with DAPI (blue) and anti-γ-H2AX anti-body (green) at 24, 48, and 72 hours after treatment. (B) The proportion of nuclei with γ-H2AX staining was similar to that in (A). *Significant differences from control; ANOVA P < 0.0001, Dunnett P < 0.01. The control cells were assayed at 72 hours. Mean ± SEM.
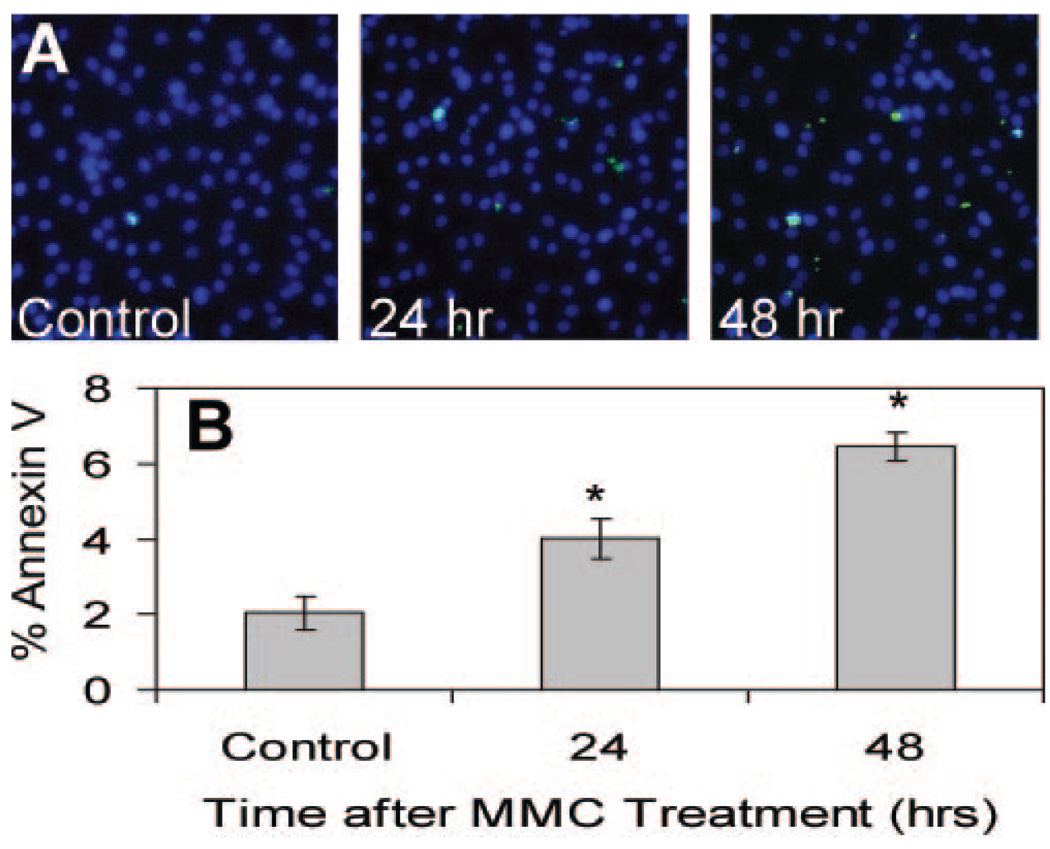
Effect of MMC on the Proportion of Apoptotic Cells
To examine the potential irreversible effects of MMC-induced DNA damage, we examined confluent CE cells for annexin V binding to phosphatidylserine flipping, an early signal of apoptosis. CE cells briefly treated with 0.4 µg/mL MMC for 30 minutes showed increased annexin V staining compared with the control (Fig. 6A). MMC treatment increased the proportion of annexin V cells, with the number labeled becoming significantly different from the control at 24 and 48 hours after treatment (Fig. 6B). Similar results were obtained after treating confluent CE cells with higher concentrations of MMC (data not shown).
FIGURE 6.
MMC exposure initiates endothelial apoptosis. (A) Confluent CE cells were treated with MMC 0.4 µg/mL for 30 minutes and stained with Hoechst 33342 (blue) and annexin V-FITC (green) 24 and 48 hours later. Cells positive for annexin V-FITC are apoptotic. (B) The proportion of apoptotic cells after MMC treatment was significantly elevated above the untreated control. *Significant differences from control; ANOVA P < 0.0001, Dunnett P < 0.01. The control cells were assayed at 48 hours. Mean ± SEM.
DISCUSSION
Recent studies have demonstrated the presence of MMC in the anterior chamber after photorefractive keratectomy,13–15 dose-dependent corneal edema after a single application of MMC on the corneal surface,19 and significant endothelial cell loss occurring within a few months after PRK with MMC.20 Our study has added new evidence of the effects of MMC on the CE, which DNA repair mechanisms did not appear to reverse. We confirmed both radial and coronal diffusion of MMC after a single topical treatment that extended to the CE. Using a sensitive, recognized measure of DNA cross-linking, the comet assay, we detected significant levels of cross-linked DNA in MMC-treated CE cells. In addition, we observed persistent DNA double-strand breaks and an increase in the proportion of apoptotic cells after a brief exposure to MMC.
Our study design included three separate trials to examine the effects of MMC treatment on intact globes across a range of conditions while emulating a standard protocol of photorefractive keratectomy with intraoperative MMC treatment. It is important to emphasize that our MMC application times ranged from 6 to 120 seconds, including the range of currently recommended MMC application times. Our results showed that the presence or absence of the epithelial basement membrane and Bowman’s layer did not affect the ability for MMC to induce cross-links in CE DNA. Ablation of 75 µm of the stroma was within the range of ablation for human PRK and has been thought to facilitate MMC access to the CE.13 In the eyes that underwent laser ablation, we observed the greatest absolute difference between the control and the 6-second treatment; however, there was no other evidence of increased risk. Finally, allowing the excised corneas 24 hours to repair the MMC-induced DNA damage produced an observable difference between treatment times less than and greater than 60 seconds. However, even with time for repair of the DNA cross-links, all treatment groups had significantly more cross-linked DNA than the untreated controls.
The effects of MMC concentration and treatment times on DNA cross-linking in CE cells were better defined in the cell culture experiments (Fig. 4). The cross-linking effects of MMC were evident across a wide range of concentrations and even 10-fold below the amounts we detected in the CE after a single topical treatment (Fig. 1). Although there is continuous turnover of aqueous humor content and significant decrease in MMC concentration over time,13–15 we found that even the brief 10-minute exposure to 0.02 µg/mL MMC was long enough to induce significant DNA cross-linking in cultured CE cells (Fig. 4A).
Despite evidence that human CE appears protected from UV irradiation damage,29 the ability of these cells to repair other DNA damage such as MMC-induced cross-linking is essentially unknown. McKenna et al.25 found in the bladder tumor cell line RT4 that almost all MMC-induced cross-links were repaired within 24 hours, reversing the characteristic changes in DNA migration in the modified comet assay. In contrast, we observed no reversal of the %DNA-in-head proportion after 24 hours in culture. In fact the proportion of cross-linked DNA in most treatment conditions actually increased 24 hours after MMC treatment (compare Fig. 3A with Fig. 3C). We also observed significant γ-H2AX positive nuclei in MMC-treated confluent cultures (Fig. 5). This histone becomes phosphorylated at the site of double-strand DNA breaks and is dephosphorylated during repair. Our observation that MMC induces γ-H2AX phosphorylation is consistent with the comet data indicating DNA damage induced by MMC. The presence of phosphorylated γ-H2AX foci is believed to represent a signal for repair and to stimulate recruitment of DNA repair enzymes. Phosphorylation of γ-H2AX in most systems is transient, however, and dephosphorylation occurs after repair has been completed.27 The persistence of the activated γ-2HAX in CE cells is an unusual observation. It suggests that little or no DNA repair of the induced breaks occurs in these quiescent cells. The nonproliferative state of the CE may limit its ability to initiate and repair MMC-induced cross-links, leaving it increasingly vulnerable to DNA damage. Current models of DNA cross-linking repair suggest that the coordinated action of several DNA repair pathways, including nucleotide excision repair and double-strand break repair, may not be as efficient in nonreplicating cells, since they are initiated during active DNA replication.30 In repair-deficient mammalian cells, only 40 DNA cross-links per cell have been estimated to be lethal.31 Additional studies are needed to evaluate the capacity to repair MMC-induced DNA cross-links in the CE.
The presence of DNA cross-linking in the CE is likely to be associated with the decreased endothelial cell density, endothelial apoptosis, and potential corneal edema observed in previous studies.19–24 Evidence of MMC-induced apoptosis was found in rabbit CE after epithelial debridement and MMC treatment for 2 minutes.19 Given the ocular penetration profile of a single topical application of MMC as well as our evidence of MMC-induced DNA cross-links and apoptosis in cultured CE cells (Fig. 6), it is probable that in vivo the CE layer is at risk to MMC-induced DNA damage and potential apoptosis. Because the human CE is nonproliferative in vivo, it is already considered fragile to insults and responds only through cell enlargement and migration as a means of repair.32 When CE cell density decreases below the level of 1000 cell/mm2, the number of pump sites per cell progressively increases; however, pump function is eventually inadequate to maintain the balanced hydration of the stroma and corneal edema ensues.33 Disruption of the normal function of CE with a genotoxic stressor such as MMC may eventually accelerate this pathologic process.
Recent acknowledgment of MMC toxicity has led to the development of methods to limit corneal exposure, mostly through reductions in concentration and exposure times. Efforts to reduce the initial recommendation of 120 seconds to shorter exposure times16,17 as well as lower concentrations34 have since been in practice. Despite these efforts, it is noteworthy that we observed significant DNA cross-linking after as few as 6 seconds of MMC exposure and at concentrations well below what has been detected in the CE and aqueous humor. Our results suggest that current practices in MMC application during refractive surgeries may be increasing the potential for long-term and permanent effects on the health of the CE.
Acknowledgments
The authors thank Kira Lathrop for help with image analysis and Nancy Sawyer for assistance with laser treatment.
Supported by National Eye Institute Grants EY016415, EY009368, and P30-EY08098; a Research to Prevent Blindness Medical Student Fellowship, and Eye and Ear Foundation of Pittsburgh. JLF is a Jules and Doris Stein Research to Prevent Blindness Professor.
Footnotes
Disclosure: D.S. Roh, None; A.L. Cook, None; S.S. Rhee, None; A. Joshi, None; R. Kowalski, None; D.K. Dhaliwal, None; J.L. Funderburgh, None
References
- 1.Chabner BA, Amrein PC, Druker BJ, et al. Antineoplastic agents. In: Brunton LL, Lazo JS, Parker KL, editors. Goodman and Gilman’s The Pharmacological Basis of Therapeutics. 11th ed. New York: McGraw Hill; 2006. [Google Scholar]
- 2.Abraham LM, Selva D, Casson R, Leibovitch I. Mitomycin: clinical applications in ophthalmic practice. Drugs. 2006;66:321–340. doi: 10.2165/00003495-200666030-00005. [DOI] [PubMed] [Google Scholar]
- 3.Majmudar PA, Forstot SL, Dennis RF, et al. Topical mitomycin-C for subepithelial fibrosis after refractive corneal surgery. Ophthalmology. 2000;107:89–94. doi: 10.1016/s0161-6420(99)00019-6. [DOI] [PubMed] [Google Scholar]
- 4.Carones F, Vigo L, Scandola E, Vacchini L. Evaluation of the prophylactic use of mitomycin-C to inhibit haze formation after photorefractive keratectomy. J Cataract Refract Surg. 2002;28:2088–2095. doi: 10.1016/s0886-3350(02)01701-7. [DOI] [PubMed] [Google Scholar]
- 5.Lane HA, Swale JA, Majmudar PA. Prophylactic use of mitomycin-C in the management of a buttonholed LASIK flap. J Cataract Refract Surg. 2003;29:390–392. doi: 10.1016/s0886-3350(02)01434-7. [DOI] [PubMed] [Google Scholar]
- 6.Gambato C, Ghirlando A, Moretto E, Busato F, Midena E. Mitomycin C modulation of corneal wound healing after photorefractive keratectomy in highly myopic eyes. Ophthalmology. 2005;112:208–218. doi: 10.1016/j.ophtha.2004.07.035. discussion 219. [DOI] [PubMed] [Google Scholar]
- 7.Lee DH, Chung HS, Jeon YC, Boo SD, Yoon YD, Kim JG. Photorefractive keratectomy with intraoperative mitomycin-C application. J Cataract Refract Surg. 2005;31:2293–2298. doi: 10.1016/j.jcrs.2005.05.027. [DOI] [PubMed] [Google Scholar]
- 8.Lacayo GO, 3rd, Majmudar PA. How and when to use mitomycin-C in refractive surgery. Curr Opin Ophthalmol. 2005;16:256–259. doi: 10.1097/01.icu.0000172830.41394.7c. [DOI] [PubMed] [Google Scholar]
- 9.Bedei A, Marabotti A, Giannecchini I, et al. Photorefractive keratectomy in high myopic defects with or without intraoperative mitomycin C: 1-year results. Eur J Ophthalmol. 2006;16:229–234. doi: 10.1177/112067210601600206. [DOI] [PubMed] [Google Scholar]
- 10.Kim TI, Pak JH, Lee SY, Tchah H. Mitomycin C-induced reduction of keratocytes and fibroblasts after photorefractive keratectomy. Invest Ophthalmol Vis Sci. 2004;45:2978–2984. doi: 10.1167/iovs.04-0070. [DOI] [PubMed] [Google Scholar]
- 11.Kim TI, Tchah H, Lee SA, Sung K, Cho BJ, Kook MS. Apoptosis in keratocytes caused by mitomycin C. Invest Ophthalmol Vis Sci. 2003;44:1912–1917. doi: 10.1167/iovs.02-0977. [DOI] [PubMed] [Google Scholar]
- 12.Chang SW. Corneal keratocyte apoptosis following topical intraoperative mitomycin C in rabbits. J Refract Surg. 2005;21:446–453. doi: 10.3928/1081-597X-20050901-05. [DOI] [PubMed] [Google Scholar]
- 13.Torres RM, Merayo-Lloves J, Daya SM, et al. Presence of mitomycin-C in the anterior chamber after photorefractive keratectomy. J Cataract Refract Surg. 2006;32:67–71. doi: 10.1016/j.jcrs.2005.06.051. [DOI] [PubMed] [Google Scholar]
- 14.Song JS, Kim JH, Yang M, Sul D, Kim HM. Mitomycin-C concentration in cornea and aqueous humor and apoptosis in the stroma after topical mitomycin-C application: effects of mitomycin-C application time and concentration. Cornea. 2007;26:461–467. doi: 10.1097/ICO.0b013e318030d217. [DOI] [PubMed] [Google Scholar]
- 15.Song JS, Kim JH, Yang M, Sul D, Kim HM. Concentrations of mitomycin C in rabbit corneal tissue and aqueous humor after topical administration. Cornea. 2006;25:S20–S23. doi: 10.1097/01.ico.0000247208.93638.92. [DOI] [PubMed] [Google Scholar]
- 16.Diakonis VF, Pallikaris A, Kymionis GD, Markomanolakis MM. Alterations in endothelial cell density after photorefractive keratectomy with adjuvant mitomycin. Am J Ophthalmol. 2007;144:99–103. doi: 10.1016/j.ajo.2007.03.039. [DOI] [PubMed] [Google Scholar]
- 17.de Benito-Llopis L, Teus MA, Ortega M. Effect of mitomycin-C on the corneal endothelium during excimer laser surface ablation. J Cataract Refract Surg. 2007;33:1009–1013. doi: 10.1016/j.jcrs.2007.02.034. [DOI] [PubMed] [Google Scholar]
- 18.Goldsberry DH, Epstein RJ, Majmudar PA, et al. Effect of mitomycin C on the corneal endothelium when used for corneal subepithelial haze prophylaxis following photorefractive keratectomy. J Refract Surg. 2007;23:724–727. doi: 10.3928/1081-597X-20070901-14. [DOI] [PubMed] [Google Scholar]
- 19.Chang SW. Early corneal edema following topical application of mitomycin-C. J Cataract Refract Surg. 2004;30:1742–1750. doi: 10.1016/j.jcrs.2003.12.044. [DOI] [PubMed] [Google Scholar]
- 20.Morales AJ, Zadok D, Mora-Retana R, Martinez-Gama E, Robledo NE, Chayet AS. Intraoperative mitomycin and corneal endothelium after photorefractive keratectomy. Am J Ophthalmol. 2006;142:400–404. doi: 10.1016/j.ajo.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 21.Sihota R, Sharma T, Agarwal HC. Intraoperative mitomycin C and the corneal endothelium. Acta Ophthalmol Scand. 1998;76:80–82. doi: 10.1034/j.1600-0420.1998.760115.x. [DOI] [PubMed] [Google Scholar]
- 22.Fukuchi T, Hayakawa Y, Hara H, Abe H. Corneal endothelial damage after trabeculectomy with mitomycin C in two patients with glaucoma with cornea guttata. Cornea. 2002;21:300–304. doi: 10.1097/00003226-200204000-00012. [DOI] [PubMed] [Google Scholar]
- 23.Mietz H, Roters S, Krieglstein GK. Bullous keratopathy as a complication of trabeculectomy with mitomycin C. Graefes Arch Clin Exp Ophthalmol. 2005;243:1284–1287. doi: 10.1007/s00417-005-1170-5. [DOI] [PubMed] [Google Scholar]
- 24.Mohammadpour M, Jabbarvand M, Javadi MA. Focal corneal decompensation after filtering surgery with mitomycin C. Cornea. 2007;26:1285–1287. doi: 10.1097/ICO.0b013e318150d371. [DOI] [PubMed] [Google Scholar]
- 25.McKenna DJ, Gallus M, McKeown SR, Downes CS, McKelvey-Martin VJ. Modification of the alkaline comet assay to allow simultaneous evaluation of mitomycin C-induced DNA cross-link damage and repair of specific DNA sequences in RT4 cells. DNA Repair. 2003;2:879–890. doi: 10.1016/s1568-7864(03)00086-7. [DOI] [PubMed] [Google Scholar]
- 26.Sedelnikova OA, Pilch DR, Redon C, Bonner WM. Histone H2AX in DNA damage and repair. Cancer Biol Ther. 2003;2:233–235. doi: 10.4161/cbt.2.3.373. [DOI] [PubMed] [Google Scholar]
- 27.Chowdhury D, Keogh MC, Ishii H, Peterson CL, Buratowski S, Lieberman J. Gamma-H2AX dephosphorylation by protein phosphatase 2A facilitates DNA double-strand break repair. Mol Cell. 2005;20:801–809. doi: 10.1016/j.molcel.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 28.Downey MDD. GammaH2AX as a checkpoint maintenance signal. Cell Cycle. 2006;5:1376–1381. doi: 10.4161/cc.5.13.2899. [DOI] [PubMed] [Google Scholar]
- 29.Inoki T, Endo H, Inoki Y, et al. Damaged DNA-binding protein 2 accelerates UV-damaged DNA repair in human corneal endothelium. Exp Eye Res. 2004;79:367–376. doi: 10.1016/j.exer.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 30.McHugh PJ, Spanswick VJ, Hartley JA. Repair of DNA interstrand crosslinks: molecular mechanisms and clinical relevance. Lancet Oncol. 2001;2:483–490. doi: 10.1016/S1470-2045(01)00454-5. [DOI] [PubMed] [Google Scholar]
- 31.Lawley PD, Phillips DH. DNA adducts from chemotherapeutic agents. Mutat Res. 1996;355:13–40. doi: 10.1016/0027-5107(96)00020-6. [DOI] [PubMed] [Google Scholar]
- 32.Joyce NC. Proliferative capacity of the corneal endothelium. Prog Retin Eye Res. 2003;22:359–389. doi: 10.1016/s1350-9462(02)00065-4. [DOI] [PubMed] [Google Scholar]
- 33.Crawford KM, Ernst SA, Meyer RF, MacCallum DK. NaK-ATPase pump sites in cultured bovine corneal endothelium of varying cell density at confluence. Invest Ophthalmol Vis Sci. 1995;36:1317–1326. [PubMed] [Google Scholar]
- 34.Thornton I, Puri A, Xu M, Krueger RR. Low-dose mitomycin C as a prophylaxis for corneal haze in myopic surface ablation. Am J Ophthalmol. 2007;144:673–681. doi: 10.1016/j.ajo.2007.07.020. [DOI] [PubMed] [Google Scholar]