Abstract
Objective
To compare modafinil to placebo for reducing methamphetamine (MA) use, improving retention, and reducing depressive symptoms and MA cravings. Rates of adverse events and cigarette smoking with modafinil versus placebo were also compared.
Methods
Following a 2-week, non-medication lead-in period, 71 treatment-seeking MA dependent participants were randomly assigned to modafinil (400 mg once daily; N= 34) or placebo (once daily; N= 37) for 12-weeks under double-blind conditions. Participants attended clinic thrice weekly to provide urine samples analyzed for MA-metabolite, to complete research assessments, and to receive contingency management and weekly cognitive behavioral therapy (CBT) sessions.
Results
There were no statistically significant effects for modafinil on MA use, retention, depressive symptoms, or MA cravings in pre-planned analyses. Outcomes for retention and MA use favored modafinil in a post hoc analysis among participants with low CBT attendance and among participants with baseline high frequency of MA use (MA use on >18 of past 30 days), but did not reach statistical significance in these small subgroups. Modafinil was safe and well tolerated and did not increase cigarette smoking.
Conclusions
Modafinil was no more effective than placebo at 400 mg daily in a general sample of MA users. A post hoc analysis showing a trend favoring modafinil among subgroups with baseline high frequency MA use and low CBT attendance suggests that further evaluation of modafinil in MA users is warranted.
Keywords: Methamphetamine, modafinil, pharmacotherapy, randomized clinical trial
1. Introduction
Each year, 24.7 million people use amphetamine or methamphetamine (MA) worldwide, which represents more consumers than that for heroin or cocaine (United Nations Office on Drugs and Crime, 2008). While the number of Americans estimated to have used MA in the past 30 days dropped 21% from 2006 to 2007 (SAMHSA Office of Applied Statistics, 2007), the number of users who report recent MA use who also meet criteria for stimulant abuse or dependence nearly doubled between 2002 and 2005 (SAMHSA Office of Applied Statistics, 2005). MA abuse continues to be a major issue in the criminal justice system, with 47% of a national sample of US sheriffs reporting that MA remains their number one drug problem (National Association of Counties, 2007) and is the most common drug reported at admission to substance abuse treatment in the Western US (Substance Abuse and Mental Health Services Administration. Office of Applied Studies, 2009) and among pregnant women nationwide (Terplan et al., 2009). In addition, chronic MA use is associated with significant health-related complications including high rates of HIV infection (Braine et al., 2005; Colfax et al., 2005).
While effective behavioral treatments for MA abuse are available (Lee and Rawson, 2008), approaches combining behavioral and pharmacotherapy are likely to be most effective. Yet currently, no medications have demonstrated proven effectiveness for treatment of MA abuse. Medications that have failed to show efficacy in well controlled clinical trials for MA abuse/dependence include the selective serotonin reuptake inhibitors fluoxetine (Batki et al., 1999) and sertraline (Shoptaw et al., 2006), amlodipine, a calcium channel blocker (Batki et al., 2001), the tricyclic antidepressant imipramine (Galloway et al., 1996), the GABA-ergic medications baclofen and gabapentin (Heinzerling et al., 2006), ondansetron, a 5HT3 receptor antagonist (Johnson et al., 2008), and mirtazapine, an antidepressant with adrenergic and serotonergic activity (Cruickshank et al., 2008). Methylphenidate, a stimulant, reduced amphetamine use, while aripiprazole, a D2 dopamine receptor partial agonist, increased amphetamine use relative to placebo in a randomized trial from Finland (Tiihonen et al., 2007), and naltrexone, an opioid antagonist, reduced amphetamine use significantly more than placebo in a preliminary trial in Sweden (Jayaram-Lindstrom et al., 2008). High-dose dexamphetamine maintenance treatment resulted in significantly improved treatment retention and reductions in methamphetamine dependence in a randomized, placebo-controlled trial (Longo et al., 2009). Recently, two separate randomized placebo-controlled trials found that bupropion, an antidepressant with mild stimulating effects and dopaminergic activity, reduces MA use in the context of cognitive behavioral therapy, but only among participants with baseline lower frequency of MA use (Elkashef et al., 2007; Shoptaw Heinzerling et al., 2008).
Modafinil is a non-amphetamine type stimulant that acts as a wakefulness-promoting drug, and is approved for managing excessive daytime sleepiness due to narcolepsy, obstructive sleep apnea, and shift work disorder. While modafinil’s mechanism of action has not been definitively determined, recent evidence suggests a role for dopaminergic and noradrenergic systems. For example, modafinil inhibits human dopamine- (DAT) and norepinephrine transporters in vitro (Madras et al., 2006) and produced DAT blockade and increased extracellular dopamine in the caudate, putamen, and nucleus accumbens in vivo in a human PET study (Volkow et al., 2009). Modafinil’s effect on wakefulness was abolished in knock-out mice lacking DAT (Wisor et al., 2001) or D2 dopamine receptors (Qu et al., 2008) while in humans, modafinil’s effect on wakefulness was influenced by genotype for the functional Val158Met polymorphism in the catechol-O-methyltransferase gene (Bodenmann et al., 2009). Furthermore, in human lab studies, prazosin, an alpha1-adrenoreceptor antagonist, blocked the cognitive-enhancing effect of modafinil (Winder-Rhodes et al., 2009) while effects of modafinil on tonic noradrenergic activity in the locus coeruleus on functional MRI were associated with performance on a cognitive control task (Minzenberg et al., 2008). Clinically, modafinil has stimulating effects that may ameliorate MA withdrawal symptoms (McGregor et al., 2008) but appears to be less euphorigenic with a lower abuse liability than traditional stimulants (O'Brien et al., 2006; Vosburg et al., 2009). Modafinil improves cognition in a variety of domains that are impaired in chronic MA users, including memory, attention, executive function, and cognitive control in healthy adults as well as patients with ADHD, depression, and schizophrenia (Minzenberg and Carter, 2008). Two clinical trials of modafinil for cocaine dependence suggest that modafinil is effective among cocaine dependent participants without co-morbid alcohol dependence (Anderson et al., 2009; Dackis et al., 2005).
Clinical studies of modafinil for methamphetamine dependence have primarily investigated modafinil at the 200 mg daily dose recommended for its approved indication, excessive daytime sleepiness. One human laboratory study found reductions in MA-related subjective effects with modafinil 200 mg daily, although results were not statistically significant (De La Garza et al., 2009). In a single-blind study of modafinil 200 mg daily plus cognitive behavioral therapy among 13 HIV positive gay men with methamphetamine abuse/dependence, modafinil was well tolerated and retention was high with 77% of participants completing the trial (McElhiney et al., 2009). In the first randomized, double-blind, placebo-controlled trial of modafinil for methamphetamine dependence, modafinil 200 mg daily was no more effective than placebo in pre-planned analyses of retention and MA use in the full sample (Shearer et al., 2009). There was a trend towards greater reductions in stimulant use with modafinil among medication compliant participants (p=0.07) and self-reported stimulant use at end of treatment was significantly lower for modafinil relative to placebo in a post hoc analysis limited to methamphetamine dependent participants without co-morbid opioid dependence. Furthermore, counseling attendance was significantly associated with treatment outcomes regardless of treatment group assignment.
MA dependent individuals typically use high doses of MA, up to 4 g/day in some reports (Cruickshank and Dyer, 2009), and therefore doses of modafinil greater than 200 mg daily may be required to treat MA dependence. Modafinil 400 mg daily was safe and well tolerated among 8 methamphetamine dependent participants in an open-label study (McGaugh et al., 2009), but this dose has not been evaluated in randomized, placebo-controlled trials for MA dependence. Therefore, we performed a randomized, double-blind, placebo-controlled trial of modafinil 400 mg daily for the treatment of MA dependence. We hypothesized that modafinil would result in greater reductions in MA use, improved retention, and reduced somatic and depressive symptoms relative to placebo.
2. Methods
2.1 Participants
Study participants were 71 MA dependent outpatients seeking treatment in the Los Angeles area. All participants met the following inclusion criteria: (1) 18 years of age or older; (2) current MA dependence verified by the Structured Clinical Interview for the DSM-IV-TR (SCID; (Spitzer et al., 1995)); (3) willing and able to comply with study procedures; (4) willing and able to provide written informed consent; and (5) if female and of childbearing potential, not pregnant or lactating, and willing to use an acceptable method of birth control.
Participants met none of the following exclusion criteria: (1) a medical condition that would interfere with safe study participation, such as active tuberculosis, unstable cardiac, renal, or liver disease, unstable diabetes, or elevated liver enzymes (SGOT or SGPT) greater than four times the upper limit of normal; (2) a current neurological disorder (e.g., organic brain disease, dementia) or major psychiatric disorder not due to substance abuse (e.g., schizophrenia or bipolar illness) as assessed by the SCID and a medical history which would make study agent compliance difficult or which would compromise informed consent, or recent (past 30 days) history of suicide attempts and/or current serious suicidal intention or plan; (3) currently taking prescription medication that is contraindicated for use with modafinil; (4) current dependence on cocaine, opiates, alcohol, or benzodiazepines, as defined by DSM-IV-TR criteria; (5) a history of alcohol dependence within the past three years; (6) a history of mitral valve prolapse, left ventricular hypertrophy, cardiac arrhythmias, angina, myocardial infarction, acute coronary syndrome (unstable angina), cardiac syncope or presyncope, or any EKG abnormalities that suggests the presence of one of these conditions; (7) a systolic blood pressure greater than 160, or a diastolic blood pressure greater than 100 (i.e. cutoffs for stage 2 hypertension), or a heart rate greater than 70% of the maximum heart rate expected for their age (0.70(220-age)) at any of the screening visits; (8) a history of narcolepsy; (9) a history of sensitivity to modafinil; or (10) any other circumstances that, in the opinion of the investigators, would compromise participant safety.
2.2 Procedures
Study activities occurred at two UCLA clinical research sites in the Los Angeles area (Hollywood and Rancho Cucamonga). All study protocols were approved by the UCLA IRB. The study was registered with ClinicalTrials.gov (identifier: NCT00469508).
2.2.1. Recruitment
Potential study participants were recruited from the community using advertisements for a study of experimental medications for MA dependence. All recruitment materials referred interested individuals to a toll free phone number for further information. Trained recruitment specialists scheduled an in-person admission interview for interested participants at the study site to describe the study in further detail and complete the informed consent procedure.
2.2.2. Design
The study used a randomized, double-blind, placebo-controlled clinical trial design with an active medication condition (modafinil 400mg) and a matching placebo. A psychosocial/behavioral platform of cognitive behavioral therapy (CBT) and contingency management (CM) was also provided. After completing a 2-week baseline/screening period for eligibility, participants were randomly assigned by personnel not associated with the study to receive either modafinil or placebo, in conjunction with CBT and CM, for 12 weeks. An urn randomization procedure (Stout et al., 1994) was used to provide multivariate balance on three factors known to influence outcomes in outpatient stimulant treatment trials: (1) baseline level of MA use (< 2 MA- positive versus 2 or more MA-positive urine samples during the two week baseline period), (2) level of cognitive dysfunction (above or below one standard deviation below the published means for the MicroCog computerized battery (Powell et al., 2004)), (3) and gender.
Participants visited the study clinic three times per week (Monday, Wednesday and Friday) to provide urine samples, to conduct medication exchanges and monitor participant safety, to complete study assessments and to receive psychosocial/behavioral treatments. At termination, participants underwent a repeat physical examination, including blood work and an EKG, with the study physician, followed by four weeks of weekly visits to assess adverse events and potential withdrawal symptoms and a brief health check at the final visit. All study activities were provided free of charge. Other than non-cash vouchers earned as part of the CM intervention, participants were not compensated for participation.
2.2.3 Assessments
A battery of measures determined study eligibility, assessed participant safety and documented treatment efficacy. The SCID was used to identify past and current substance use diagnoses and to verify inclusion and exclusion criteria. The ASI-Lite (McLellan et al., 1992) was used to estimate the severity of participants’ reported addiction-related problems in seven areas of functioning: medical, employment, drug use, alcohol use, legal, family/social, and psychiatric. The BDI-II (Steer et al., 1999) was used to monitor participants’ depressive symptoms at baseline and weekly during the treatment period. MA cravings were assessed at baseline and weekly during the treatment period using a visual analog scale that ranges from 0 (no cravings) to 100 (most intense cravings possible).
Medication adherence was measured using weekly pill counts justified against participant reports of medication taking to calculate the proportion of dispensed medication doses that were taken. Participants met with the study physician bi-monthly to receive a 2-week supply of medication in a blister package in exchange for the previous two weeks’ blister package with any unused medications by which to complete the pill count. Urine samples were collected thrice weekly throughout the study period. All samples between 93–100° F at the time of collection were considered valid. Urine samples were analyzed immediately onsite using radioimmunoassay (Branan Medical Corp., Irvine, CA) for qualitative tests of MA-metabolite.
2.2.4. Psychosocial counseling
All participants received a standard counseling program consisting of weekly individual CBT sessions during the medication phase of the study. Counseling was delivered by a masters- or doctoral-level trained therapist who received training in the use of the 12-week CBT program and familiarity with its manualized format (Carroll, 1998). To maintain fidelity of the counseling program, counselors met once weekly with the principal investigator (S.S.) to receive corrective feedback and individual clinical supervision.
2.2.5. Contingency management
A CM intervention was provided during the 2 week baseline period and 12 week medication treatment period. Non-cash vouchers for goods and services promoting a healthy drug-free lifestyle were earned for MA metabolite-free urine samples, on an escalating schedule for the first 4 weeks, then remaining at this for the remaining 10 weeks prior to discontinuation. The voucher for the initial MA-free sample was worth $3.00. Vouchers increased in value by $1.00 for each consecutive MA-free sample, through the end of the 4th week. All MA-free samples from weeks 5–14 were worth $15.00 for participants who remained abstinent. Participants who produced a sample positive for MA metabolite, or who failed to submit urine samples, did not receive a voucher for that particular visit and their subsequent voucher value was reduced to the initial $3.00, with a reset to $15 after three consecutive MA-free urine specimens. The maximum that could be earned for providing MA-metabolite free urine samples at all visits throughout the entire study was $537 in vouchers.
2.2.6. Medication procedures
Experimental drug and matching placebo were obtained through the manufacturing company (Cephalon Inc., Frazer, PA). Doses of the study medication were as follows: modafinil 200mg per day (two 100mg tablets per day taken in the morning) for the first 3 days of the study followed by an increase to 400 mg per day (four 100 mg tablets per day taken at one time in the morning) until the last three days of the trial, when the dose was titrated down to 200mg per day for the final 3 days. Participants ingested the first dose of study medication under the supervision of the study physician, were monitored for one hour, and then were dispensed a two-week supply of study medication in blister packages and instructed on how to self-administer the medication at home. Participants were required to bring the experimental drug packages to the site at each visit for pills counts to monitor drug adherence. The study physician was allowed to order a permanent dose reduction (200mg per day) for participants who experienced adverse events deemed to be medication related.
2.2.7. Safety procedures
Participants underwent a medical history and physical examination, EKG and routine laboratory studies during screening and at study termination. Participants’ vital signs were collected at each study visit. Any vital sign abnormalities during study visits prompted immediate notification of the study physician who assessed the patient and took appropriate clinical action. Participant suicidal intention was closely monitored using the BDI-II, participant self-report and counseling sessions. Participants who showed any signs of suicidal behavior were evaluated by study staff trained to respond with the appropriate steps needed. Weekly rounds were held with the full study team and the status of each participant was reviewed to assess medical and psychiatric safety, experimental drug compliance, and data completion.
2.3. Data Analysis
All analyses used an “intention-to-treat” approach. The primary study outcome was MA use as assessed via urine drug screens. Secondary outcomes were treatment retention, MA cravings, depressive symptoms, adverse events, and cigarette smoking. The following aggregate measures of urine drug screen results were calculated: the Treatment Effectiveness Score (TES; the sum of the number of MA-free urine samples submitted per participant) and the Joint Probability Index (JPI) at the end of 6 and 12 weeks of treatment (the number of MA-free urine specimens submitted by participants in each treatment group at the time point divided by the number of participants randomized to the treatment group; (Ling et al., 1997)), the longest period of uninterrupted MA abstinence, and the proportion of participants abstinent during the final two weeks of treatment (weeks 11 and 12). Differences in aggregate urine measures between modafinil and placebo groups were assessed using a t-test for the TES and longest MA abstinence, Z statistic for the JPI, and Chi square test for the proportion abstinent during weeks 11 and 12. Study retention was measured as the number of days between the first dose of study medication and the participants’ last clinic visit. Survival analysis was performed using the Cox proportional hazards model. Longitudinal models using GEE were used to assess changes in the following outcomes among participants receiving modafinil and placebo during the 12 week treatment period: MA use as assessed via urine drug screen results, depressive symptoms, MA cravings, and cigarette smoking. Post hoc analyses compared treatment outcomes for modafinil and placebo separately among participants categorized by baseline frequency of MA use, with high frequency defined as self-reported MA use on more than 18 of the past 30 days and low frequency defined as MA use on 18 or fewer of the past 30 days, a cut-point that has previously been shown to correlate with differential medication response among MA users (Elkashef et al., 2008), and cognitive behavioral therapy (CBT attendance; 0–2 sessions versus 3 or more of the 12 possible sessions).
3. Results
3.1 Baseline demographic, drug use, and psychiatric characteristics
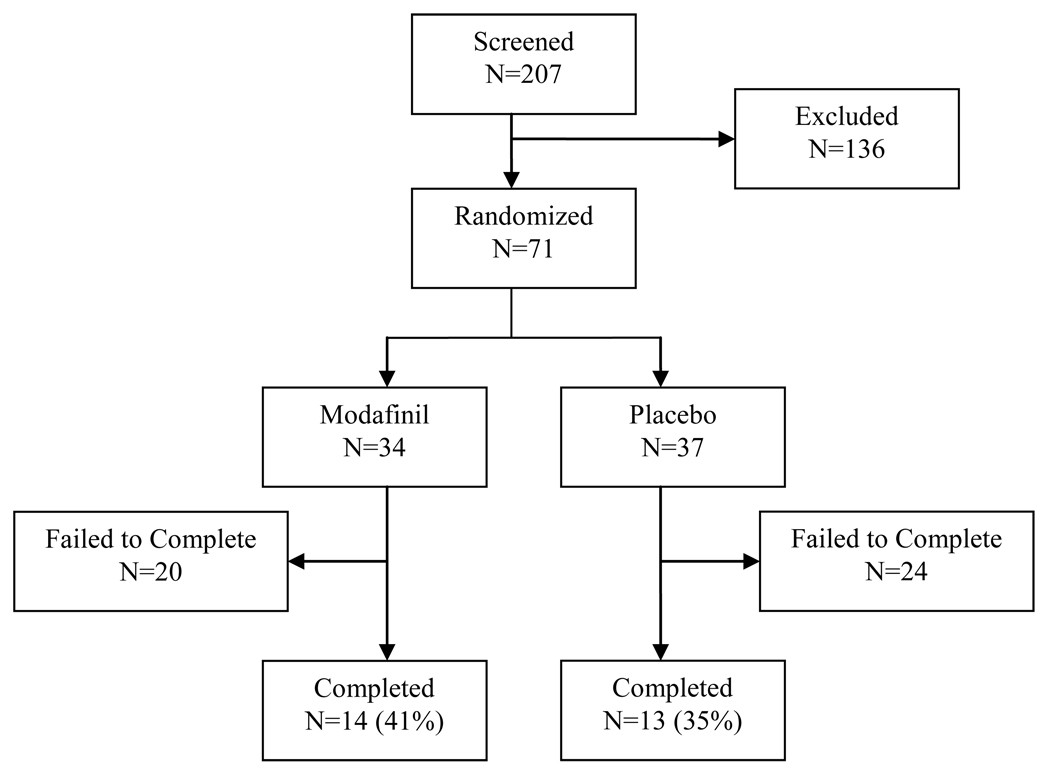
A total of 207 treatment-seeking individuals provided informed consent and entered the 2-week baseline/screening period. Of these participants, 71 met all inclusion criteria and no exclusion criteria and were randomized into the study (Figure 1). Table 1 shows the baseline characteristics of these 71 participants by randomly assigned treatment group assignment (modafinil versus placebo). The majority of participants in both the modafinil and placebo groups were either White or Hispanic ethnicity, while all four black participants were in the modafinil group and both Asian participants were in the placebo group (χ2 = 6.3, d.f. = 3, p = 0.10). Participants in the modafinil group had higher baseline ASI composite scores in the drug (t = 2.0, d.f. = 69, p = 0.05) and family/social (t = 2.0, d.f. = 69, p = 0.04) domains. There were no other significant differences between participants assigned to the modafinil and placebo groups.
Figure 1.
Study participant flow chart
Table 1.
Participant demographic, drug use, and psychiatric characteristics by treatment condition (modafinil or placebo).
| Mean (SD) or % (N) | ||
|---|---|---|
| Modafinil (N=34) |
Placebo (N=37) |
|
| Age (in years) | 39.1 (11.1) | 37.8 (10.1) |
| Ethnicity | ||
| White | 50% (17) | 51.4% (19) |
| Hispanic | 38.2% (13) | 43.2% (16) |
| Black | 11.8% (4) | 0% (0) |
| Asian | 0% (0) | 5.4% (2) |
| Gender | ||
| Male | 73.5% (25) | 67.6% (25) |
| Female | 26.5% (9) | 32.4% (12) |
| Marital Status | ||
| Married | 32.3% (11) | 18.9% (7) |
| Never married | 47.1% (16) | 59.5% (22) |
| Divorced/separated | 20.6% (7) | 21.6% (9) |
| Education (in years) | 13.6 (2.4) | 13.5 (1.9) |
| Employment | ||
| Full-time | 58.8% (20) | 45.9% (17) |
| Part-time | 2.9% (1) | 24.3% (9) |
| Unemployed1 | 23.5% (8) | 18.9% (7) |
| Student/retired/military | 14.7% (5) | 10.8% (4) |
| Income in past 30 days (US$) | 2,202 (3,847) | 1,939 (2,493) |
| Years MA use, lifetime | 15.6 (11.1) | 13.4 (10.6) |
| Days MA use (past 30d) | 9.4 (7.1) | 9.2 (7.0) |
| Route of MA administration | ||
| Smoking | 73.5% (25) | 64.9% (24) |
| Nasal | 20.6% (7) | 27.0% (10) |
| Injection | 5.9% (2) | 5.4% (2) |
| Oral | 0% (0) | 2.7% (1) |
| Days cocaine use (past 30 d) | 0.09 (0.38) | 0.24 (0.86) |
| Days cannabis use (past 30 d) | 5.4 (9.2) | 3.9 (8.1) |
| Days alcohol use (past 30 d) | 5.5 (8.1) | 3.0 (5.3) |
| Current cigarette smoker | 70.6% (24) | 75.7% (28) |
| ASI composite scores | ||
| Medical | 0.23 (0.30) | 0.15 (0.28) |
| Employment | 0.41 (0.33) | 0.47 (0.29) |
| Alcohol | 0.11 (0.16) | 0.07 (0.10) |
| Drug | 0.25 (0.09) a | 0.21 (0.10) |
| Legal | 0.09 (0.15) | 0.05 (0.12) |
| Family/social | 0.27 (0.21) a | 0.16 (0.22) |
| Psychiatric | 0.21 (0.21) | 0.20 (0.21) |
| Beck Depression Inventory score2 | 16 (11.1) | 14 (9.9) |
Includes one person in controlled environment in the modafinil group
Mean BDI at baseline (excludes six cases where BDI was not collected at baseline)
Significant at the .05-level for a two-tailed test.
3.2 Urine Drug Screen Results
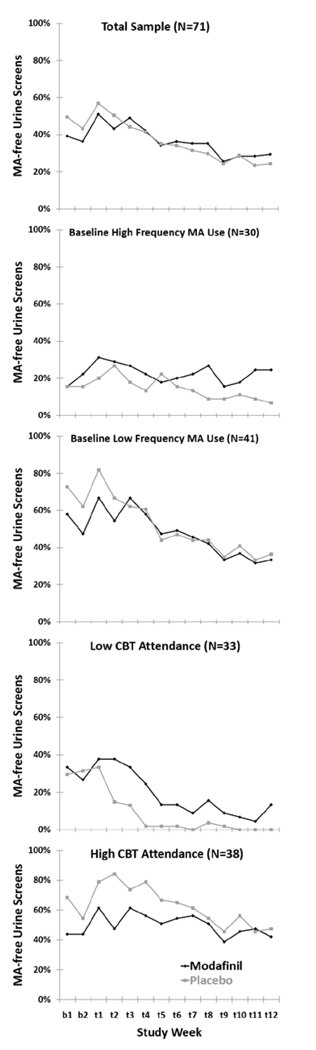
Participants in the modafinil group were missing on average 7 of the 36 (19%, range 0 – 21) possible thrice-weekly urine drug screens during the 12 week medication treatment period while participants in the placebo group were missing on average 5 of the 36 urine drug screens (13%, range 0 – 17) which was not statistically significant (χ2 = 2.60, d.f. = 1, p = 0.11). There were no statistically significant differences in aggregate measures of urine drug screen results in pre-planned analyses of the total sample or post hoc analyses among participants with baseline higher and lower frequency of MA use, although there was a trend (p<0.10) favoring modafinil over placebo in urine outcomes among participants with low CBT attendance (Table 2). The proportion of participants with MA metabolite- free urine drug screens during the 12 week medication treatment period was similar among participants in the modafinil and placebo groups in the total sample and among participants with baseline higher and lower frequency of MA use (Figure 2). Among participants with low CBT attendance, the proportion of participants with MA-free urine screens was higher for modafinil relative to placebo (Figure 2). In a GEE model, baseline frequency of MA use (OR = 0.15, 95% confidence interval 0.08 – 0.31, p < 0.0001 for participants with higher relative to lower frequency of baseline MA use), CBT attendance (OR = 1.20, 95% confidence interval 1.08 – 1.34, p < 0.001 for each additional CBT session attended), but not treatment group assignment (OR = 0.78, 95% confidence interval 0.39 – 1.56, p = 0.49 for modafinil relative to placebo), were significantly associated with the probability of providing MA-free urine drug screens across the 12 week medication treatment period.
Table 2.
Treatment outcome measures for participants in each treatment condition (modafinil versus placebo) in the total sample, and separately among participants with baseline higher frequency (more than 18 of the past 30 days) versus lower frequency (18 or fewer of the past 30 days) methamphetamine (MA) use and low cognitive behavioral therapy (CBT) attendance (0–2 sessions) versus high CBT attendance (3 or more sessions).
| Total Sample | High Freq. MA Use | Low Freq. MA Use | Low CBT Attendance | High CBT Attendance | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Modafinil (N=34) |
Placebo (N=37) |
Modafinil (N=15) |
Placebo (N=15) |
Modafinil (N=19) |
Placebo (N=22) |
Modafinil (N=15) |
Placebo (N=18) |
Modafinil (N=19) |
Placebo (N=19) |
|
| Retention | ||||||||||
| Completed treatment period, % (N) | 41% (14) | 35% (13) | 40% (6) | 20% (3) | 42% (8) | 45% (10) | 20% (3) | 6% (1) | 58% (11) | 63% (12) |
| Days retained, mean | 57 | 47 | 54 | 37 | 59 | 53 | 36 b | 21 b | 73 | 70 |
| Urine drug screen aggregates | ||||||||||
| JPI, week 6 1 | 0.53 | 0.43 | 0.33 | 0.27 | 0.68 | 0.54 | 0.27 | 0.06 | 0.74 | 0.79 |
| JPI, week 12 1 | 0.35 | 0.30 | 0.33 | 0.13 | 0.37 | 0.41 | 0.13 b | 0 b | 0.53 | 0.58 |
| Treatment effectiveness score 2 | 13.1 | 12.7 | 8.3 | 5.2 | 16.9 | 17.9 | 6.5 b | 2.2 b | 18.4 | 22.7 |
| Longest MA Abstinence, mean (days) | 18 | 17 | 12.3 | 7 | 23 | 24 | 9 b | 5 b | 26 | 30 |
| Abstinent weeks 11 and 12, % (N) | 27% (9) | 27% (10) | 13% (2) | 8% (1) | 37% (7) | 41% (9) | 6% (1) | 0% (0) | 42% (8) | 53% (10) |
| Depressive symptoms 3 | ||||||||||
| BDI score, baseline, mean | 16 | 14 | 13 | 15 | 19 | 13 | 15 | 11 | 17 | 17 |
| BDI score, week 12, mean | 7 | 10 | 7 | 12 | 7 | 8 | 8 | 11 | 6 | 8 |
| Methamphetamine craving 3 | ||||||||||
| Visual analog scale, baseline, mean | 59 | 56 | 64 a | 85 a | 56 | 39 | 66 | 64 | 54 | 49 |
| Visual analog scale, week 12, mean | 41 | 29 | 47 | 37 | 37 | 24 | 51 | 42 | 34 b | 16 b |
The number of MA-free urine specimens submitted at the final visit in the week divided by the number of participants randomized to the treatment condition.
The average of MA-free urine specimens provided during the treatment period by participants in each treatment condition.
Last observation carried forward when week 12 missing
Significantly different at the .05-level in a two-tailed test.
Significantly different at the .10-level in a two-tailed test.
Figure 2.
Proportion of participants with methamphetamine (MA) metabolite- free urine drug screens during the two week baseline period (b1, b2) and 12 week medication treatment period (t1–t12) in the total sample, and separately for participants with baseline lower frequency (18 or fewer of the past 30 days) versus higher frequency (more than 18 of the past 30 days) MA use and low CBT attendance (0–2 sessions) versus high CBT attendance (3 or more sessions).
3.3 Retention
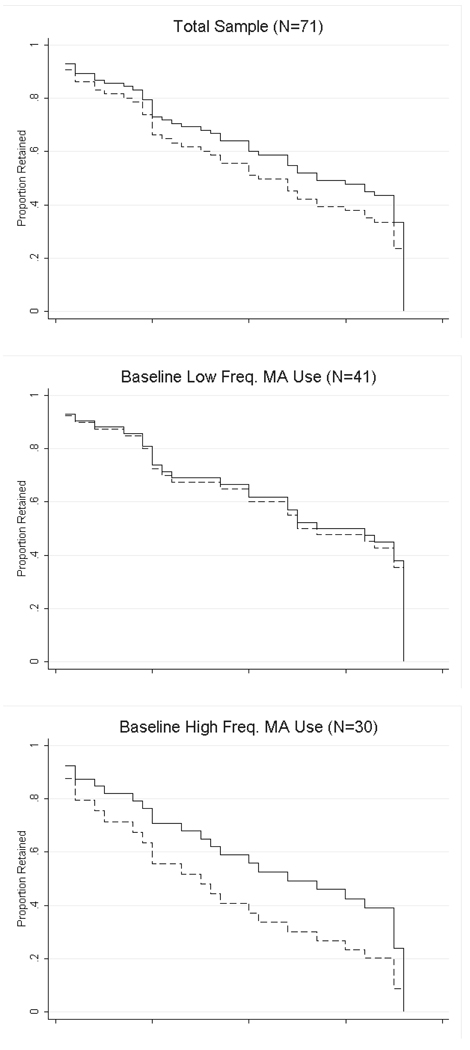
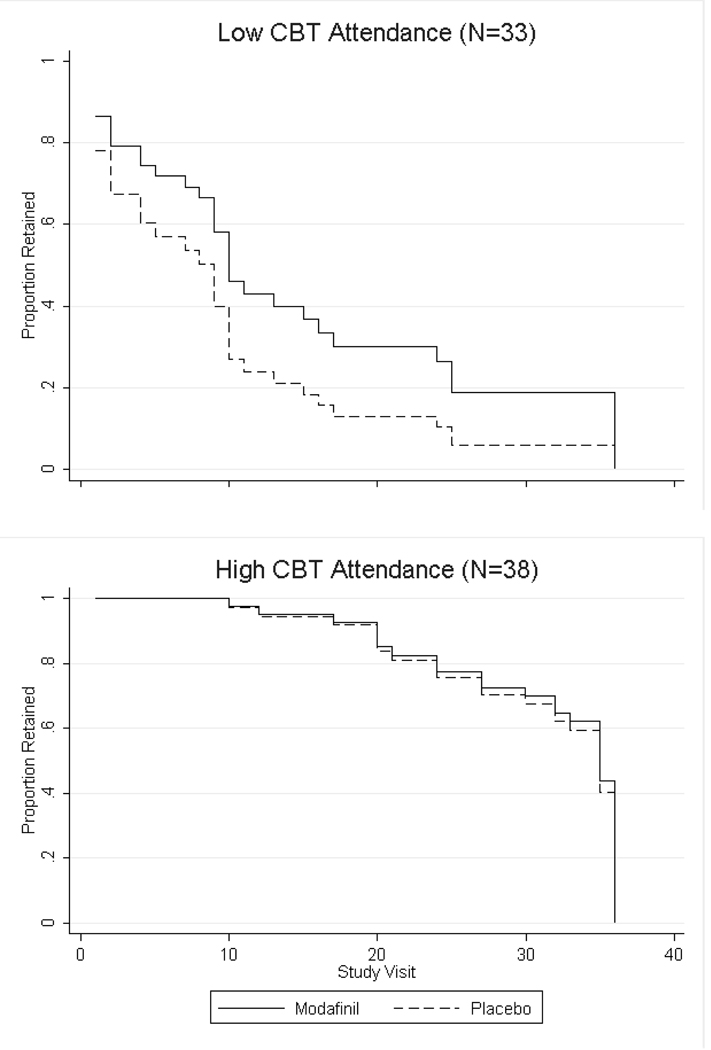
Forty one percent of participants in the modafinil group completed the 12 week medication treatment period (attended at least one visit during week 12) compared to 35% of participants in the placebo group (χ2 = 0.27, d.f. = 1, p = 0.60; Table 2). Participants in the modafinil group were retained on average 57 days compared to 47 days for participants in the placebo group (t = 1.41, d.f. = 69, p = 0.16). In post hoc analyses among participants with baseline higher frequency of MA use, 40% of participants in the modafinil group completed the medication period compared to 20% for the placebo group (χ2 = 1.43, d.f. = 1, p = 0.23) and the mean number of days retained was 54 days for participants in the modafinil group compared to 37 days for placebo (t = 1.46, d.f. = 28, p = 0.15). Among participants with baseline lower frequency of MA use, 42% of participants in the modafinil group completed the treatment period compared with 45% for placebo (χ2 = 0.05, d.f. = 1, p = 0.83) and the mean number of days retained was 59 days for modafinil and 53 days for placebo (t = 0.67, d.f. = 39, p = 0.51). Among participants with low CBT attendance, 20% of participants receiving modafinil completed the treatment period compared to 6% of participants in the placebo group (χ2 = 1.60, d.f. = 1, p = 0.21) while participants in the modafinil group were retained for 36 days on average compared to 21 days in the placebo group (t = 1.66, d.f. = 31, p = 0.10). Fifty eight percent of participants receiving modafinil with high CBT attendance completed the treatment period compared to 63% of participants with high CBT attendance receiving placebo (χ2 = 0.11, d.f. = 1, p = 0.74) with mean retention in the modafinil group 73 days versus 70 days in the placebo group (t = 0.47, d.f. = 36, p = 0.64). Survival analysis showed there was no significant difference in retention between participants in the modafinil versus placebo groups for the total sample (χ2 = 0.80, d.f. = 1, p = 0.37). In post hoc analyses, participants were retained for longer with modafinil than placebo among participants with higher frequency of baseline MA use and low CBT attendance (Figure 3), but neither of these were statistically significant in Cox regression analyses (χ2 = 1.99, d.f. = 1, p = 0.16 for Cox regression among higher frequency of baseline use group and χ2 = 2.17, d.f. = 1, p = 0.34 for low CBT attendance group).
Figure 3.
Survival analysis depicting the proportion of participants retained in each treatment condition (modafinil versus placebo) throughout the 36 study visits (12 weeks) in the total sample, and separately for participants with baseline lower frequency (18 or fewer of the past 30 days) versus higher frequency (more than 18 of the past 30 days) methamphetamine (MA) use and low CBT attendance (0–2 sessions) versus high CBT attendance (3 or more sessions).
3.4 Depressive Symptoms
Depressive symptoms decreased during the medication treatment period, but there were no statistically significant differences in depressive symptoms at end of treatment between participants receiving modafinil or placebo in the total sample or separately among participants with higher versus lower frequency of baseline MA use or high versus low CBT attendance (Table 2). In a GEE model predicting weekly Beck Depressive Index scores, there was a trend towards a significant effect for time (β = −0.17, 95% confidence interval −0.35 – 0.01, p = 0.06) but no significant effect for treatment group assignment (β = −0.68, 95% confidence interval −4.02 – 2.67, p = 0.69).
3.5 Methamphetamine Cravings
MA cravings decreased during the medication treatment period, but there were no statistically significant differences in MA cravings at end of treatment between participants receiving modafinil or placebo in the total sample or separately among participants with higher or lower frequency of baseline MA use (Table 2). There was a trend (p < 0.10) towards higher end of treatment cravings with modafinil among participants with higher CBT attendance. In a GEE model predicting weekly MA craving visual analog scale scores, there was a significant effect for time (β = −0.68, 95% confidence interval −1.28 to −0.07, p = 0.03) but no significant effect for treatment group assignment (β = 5.04, 95% confidence interval −8.57 – 18.65, p = 0.47).
3.6 Cigarette Smoking
Among the 52 participants who reported smoking cigarettes during the 12 week medication treatment period, there was no significant effect of treatment group assignment on weekly assessments of the self-reported average number of cigarettes smoked per day (β = 0.75, 95% confidence interval −3.29 – 4.80, p = 0.71 in GEE model).
3.7 Medication Adherence and Tolerability
Participants in the modafinil group returned on average 73% of the two-week medication blister packages they were dispensed compared to 63% in the placebo group (t = 1.24, d.f. = 69, p = 0.22). There were three participants in the modafinil group and eight participants in the placebo group who returned none of the dispensed medication blister packages and therefore had no pill count data available. Among participants with pill count data available, participants in the modafinil group took on average 91% of dispensed study medication compared to 83% in the placebo group (t = 1.60, d.f. = 58, p = 0.12). Four of the 34 participants in the modafinil group required study medication dose reductions from 400 mg daily to 200 mg daily as compared to 3 of the 37 participants in the placebo group, which was not a statistically significant difference (χ2 = 0.27, d.f. = 1, p = 0.61). Reasons for the dose reductions included complaints of anxiety (2 in each condition) or sleep problems (2 in modafinil, 1 in placebo). Study medication was discontinued in 3 participants in the modafinil group versus 8 participants in the placebo group (χ2 = 2.22, d.f. = 1, p = 0.14). Reasons for study medication discontinuation for both conditions included elevated blood pressure (1 each) and anxiety (1 each). One modafinil participant was discontinued due to elevated heart rate. The remaining discontinuations were in the placebo condition and involved acute psychosis (n=2), participant request (n=2), sleep problem (n=1) and hives (n=1).
3.8 Results of Psychosocial and Behavioral Treatment
Participants in the modafinil group attended on average 4.4 of the 12 possible weekly cognitive behavioral therapy sessions, as compared to 3.9 sessions on average for participants in the placebo group (t = 0.57, d.f. = 69, p = 0.57). Sixty percent of participants with high frequency MA use at baseline had low CBT attendance (0–2 sessions) compared to 37% of participants with baseline low frequency MA use (χ2 = 3.8, d.f. = 69, p = 0.05) although number of days with MA use in the past 30 days at baseline and subsequent CBT sessions attended in the total sample were not significantly correlated (r2 = −0.25, p=0.23).
Participants in the modafinil group received on average $113 of the $537 possible from the contingency management intervention reinforcing MA-free urine drug screens, while participants in the placebo group received $139 (t = −0.70, d.f. = 69, p = 0.49).
3.9 Adverse Events
There were four serious adverse events, none of which were deemed to be study medication-related. An HIV infected participant in the modafinil group developed a skin abscess due to injection drug use, a second HIV infected participant in the modafinil group was admitted to the hospital for pneumonia and otitis media, and a third HIV infected participant in the modafinil group was admitted to the hospital for acute epididymitis. One participant in the placebo group was admitted to a psychiatric hospital for acute MA-induced psychosis. There were a greater number of adverse events reported by participants in the modafinil group (151) than in the placebo group (82), the majority of which were mild for both groups. The most frequently reported adverse events reported by participants in the modafinil group were headache (32% (N = 11) of participants receiving modafinil, versus 19% (N = 7) for placebo), insomnia (29% (N = 10) for modafinil versus 19% (N = 7) for placebo), nausea (21% (N = 7) for modafinil versus 11% (N = 4) for placebo), upper respiratory infection symptoms (21% (N = 7) for modafinil versus 8% (N = 3) for placebo) and cough (15% (N = 5) for modafinil versus no participants for placebo).
4. Discussion
In this randomized, double-blind trial of modafinil 400 mg daily for the treatment of MA dependence, there was no significant effect for modafinil relative to placebo on MA use, retention, depressive symptoms or cravings. In post-hoc analyses among participants with higher frequency of MA use at baseline (MA use on more than 18 of the past 30 days) and participants with low CBT attendance (0 – 2 of a possible 12 sessions), outcomes for retention, MA use, depressive symptoms, and cravings were all in the direction favoring modafinil, although none of these differences reached statistical significance. Modafinil was safe and well tolerated and did not increase cigarette smoking in our sample of MA dependent participants. In summary, this study does not show efficacy for 400 mg daily of modafinil for the treatment for MA dependence in generalized populations of MA users, although additional studies among subgroups of MA users with high frequency of MA use and/or low CBT attendance are warranted.
The 400 mg daily dose of modafinil evaluated in this study is higher than the 200 mg daily dose recommended for modafinil’s approved indication for the treatment of excessive daytime sleepiness disorders (Cephalon, 2008), but was safe and well tolerated, with similar rates of medication adherence, dose reductions, and medication discontinuation in the modafinil and placebo groups. Previous clinical studies in cocaine dependence found similar outcomes for modafinil at 400 mg and 200 mg doses (Anderson et al., 2009; Dackis et al., 2005; Hart et al., 2008) while modafinil’s dopaminergic effects in a human imaging study were equivalent for 400 mg and 200 mg doses (Volkow et al., 2009). The only previous clinical trial of modafinil for MA dependence examined modafinil 200 mg daily and failed to find an effect for modafinil relative to placebo in pre-planned analyses of retention and MA use, although there was a trend favoring modafinil among highly compliant participants and those without co-morbid opioid dependence (Shearer et al., 2009). Results of the Shearer et al study and the current study together suggest that modafinil across a range of clinically relevant doses (200 mg and 400 mg) is no more effective than placebo in general samples of MA users, although there may be an effect for modafinil in certain subgroups, particularly those with more severe MA use disorders at treatment entry.
Outcomes of post hoc analyses among participants with higher baseline frequency of MA use and with low CBT attendance were in the direction favoring modafinil for retention and MA use, although these findings did not achieve statistical significance. The study was not powered to detect differences within these subgroups (N ≈ 15), and therefore further evaluation of these possible effects in appropriately powered samples may be warranted. Of note, participants with baseline high frequency of MA use were more likely to have low CBT attendance and therefore it is possible that baseline MA use is confounding the relationship observed between modafinil response and low CBT attendance. Previous studies have found differential medication effects related to baseline MA use frequency (Elkashef et al., 2008; Shoptaw et al., 2008) and although the reason for a possible differential effect for modafinil in high- but not low-frequency MA users is not clear, it could be related to modafinil’s effects on cognitive function. Studies have found greater impairment of cognitive function and control among high-frequency/heavy MA users (Monterosso et al., 2005; Simon et al., 2000) while effects of modafinil on cognition appear to be greatest among groups with greater baseline cognitive dysfunction (Hunter et al., 2006; Randall et al., 2004; Randall et al., 2005). MA users with higher frequency of MA use may benefit more from modafinil’s cognitive enhancing effects due to greater baseline cognitive dysfunction. Alternately, modafinil’s stimulant effects may counteract greater MA withdrawal symptoms in high- relative to low-frequency MA users. Finally, response to the cognitive behavioral therapy platform in the trial may have obscured a potential modafinil effect among lower frequency MA users and/or those with high CBT attendance. If this were confirmed in future trials, then modafinil in conjunction with lower intensity behavioral treatment, such as the “medication management” platform used for alcohol dependence pharmacotherapy (Anton et al., 2006) could provide an alternative for patients unable to receive CBT for MA dependence.
A recent trial of modafinil 200 mg daily for smoking cessation was terminated early due to an increase in smoking and nicotine withdrawal symptoms with modafinil relative to placebo (Schnoll et al., 2008). Studies estimate that between 87% – 92% of MA users seeking treatment smoke cigarettes (Weinberger and Sofuoglu, 2009), with 73% of the sample in this study reporting cigarette smoking. As a result, an increase in cigarette smoking among MA users treated with modafinil would have significant negative consequences for both individual and public health. In contrast to the smoking cessation trial in non-MA users, there was no increase in cigarette smoking with modafinil relative to placebo among cigarette smoking MA users in this study. The reason for these different results is not clear, but may be due to the different modafinil dosage, the lack of a smoking cessation intervention in this study, differences between MA using and non-MA using cigarette smokers, and/or interactions between MA and nicotine.
A limitation of the study is that the study is powered to detect at least a moderate effect for modafinil (d = 0.50) in the overall sample assuming that a medication lacking at least a moderate sized effect in the overall sample would lack clinical significance. Meaningful and differential medication effects reliably observed for high- versus low-frequency MA users in this and other trials suggest that baseline MA-use severity is an important behavioral phenotype that has strong ramifications on outcomes and may guide future development of medications for subtypes of MA dependence. The investigation of differential medication response among subgroups of MA users is a priority for future studies.
In conclusion, there was no effect for modafinil relative to placebo in pre-planned analyses of MA use, retention, depressive symptoms, or cravings. Results among participants with baseline higher frequency of MA use and those with low CBT attendance were in the direction favoring modafinil, but did not reach statistical significance in these small subgroups.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson AL, Reid MS, Li SH, Holmes T, Shemanski L, Slee A, Smith EV, Kahn R, Chiang N, Vocci F, Ciraulo D, Dackis C, Roache JD, Salloum IM, Somoza E, Urschel HC, 3rd, Elkashef AM. Modafinil for the treatment of cocaine dependence. Drug Alcohol Depend. 2009;104:133–139. doi: 10.1016/j.drugalcdep.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anton RF, O'Malley SS, Ciraulo DA, Cisler RA, Couper D, Donovan DM, Gastfriend DR, Hosking JD, Johnson BA, LoCastro JS, Longabaugh R, Mason BJ, Mattson ME, Miller WR, Pettinati HM, Randall CL, Swift R, Weiss RD, Williams LD, Zweben A. Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial. JAMA. 2006;295:2003–2017. doi: 10.1001/jama.295.17.2003. [DOI] [PubMed] [Google Scholar]
- Batki S, Moon J, Delucchi K, Hersh D, Bradley M, Aguillon-Doms C, Mendelson J, Jones R, Panganiban T, Everhart T, Mengis M, Smolar S, Helmke H, Jacob P., III Amlodipine treatment of methamphetamine dependence, a controlled outpatient trial: preliminary analysis. The 63rd Annual Scientific Meeting of the College on Problems of Drug Dependence; Scottsdale, AZ. 2001. [Google Scholar]
- Batki SL, Moon J, Bradley M, Hersh D, Smolar S, Mengis M, Delucchi K, Sexe D, Bennett S, Lefkowitz E, Chu W, Morello L, Jacob P, III, Jones RT. Fluoxetine in methamphetamine dependence-a controlled trial: preliminary analysis. The 61st Annual Scientific Meeting of the College on Problems of Drug Dependence, Acapulco; Mexico. 1999. [Google Scholar]
- Bodenmann S, Xu S, Luhmann UFO, Arand M, Berger W, Jung HH, Landolt HP. Pharmacogenetics of Modafinil After Sleep Loss: Catechol-O-Methyltransferase Genotype Modulates Waking Functions But Not Recovery Sleep. Clin Pharmacol Ther. 2009;85:296–304. doi: 10.1038/clpt.2008.222. [DOI] [PubMed] [Google Scholar]
- Braine N, Des Jarlais DC, Goldblatt C, Zadoretzky C, Turner C. HIV risk behavior among amphetamine injectors at U.S. syringe exchange programs. AIDS Educ Prev. 2005;17:515–524. doi: 10.1521/aeap.2005.17.6.515. [DOI] [PubMed] [Google Scholar]
- Carroll KM. A cognitive-behavioral approach: treating cocaine addiction. Bethesda, MD: NIDA Therapy Manuals for Drug Addiction; 1998. [Google Scholar]
- Cephalon, Inc. Provigil (modafinil) Tablets [C-IV], FDA Approved Package Insert. Frazier, PA: Cephalon, Inc; 2008. [Google Scholar]
- Colfax G, Coates TJ, Husnik MJ, Huang Y, Buchbinder S, Koblin B, Chesney M, Vittinghoff E. Longitudinal patterns of methamphetamine, popper (amyl nitrite), and cocaine use and high-risk sexual behavior among a cohort of san francisco men who have sex with men. J Urban Health. 2005;82:i62–i70. doi: 10.1093/jurban/jti025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruickshank CC, Dyer KR. A review of the clinical pharmacology of methamphetamine. Addiction (Abingdon, England) 2009;104:1085–1099. doi: 10.1111/j.1360-0443.2009.02564.x. [DOI] [PubMed] [Google Scholar]
- Cruickshank CC, Montebello ME, Dyer KR, Quigley A, Blaszczyk J, Tomkins S, Shand D. A placebo-controlled trial of mirtazapine for the management of methamphetamine withdrawal. Drug Alcohol Rev. 2008;27:326–333. doi: 10.1080/09595230801935672. [DOI] [PubMed] [Google Scholar]
- Dackis CA, Kampman KM, Lynch KG, Pettinati HM, O'Brien CP. A double-blind, placebo-controlled trial of modafinil for cocaine dependence. Neuropsychopharmacology. 2005;30:205–211. doi: 10.1038/sj.npp.1300600. [DOI] [PubMed] [Google Scholar]
- De La Garza R, 2nd, Zorick T, London ED, Newton TF. Evaluation of modafinil effects on cardiovascular, subjective, and reinforcing effects of methamphetamine in methamphetamine-dependent volunteers. Drug Alcohol Depend. 2009 doi: 10.1016/j.drugalcdep.2009.08.013. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkashef AM, Rawson RA, Anderson AL, Li SH, Holmes T, Smith EV, Chiang N, Kahn R, Vocci F, Ling W, Pearce VJ, McCann M, Campbell J, Gorodetzky C, Haning W, Carlton B, Mawhinney J, Weis D. Bupropion for the treatment of methamphetamine dependence. Neuropsychopharmacology. 2008;33:1162–1170. doi: 10.1038/sj.npp.1301481. [DOI] [PubMed] [Google Scholar]
- Galloway GP, Newmeyer J, Knapp T, Stalcup SA, Smith D. A controlled trial of imipramine for the treatment of methamphetamine dependence. J Subst Abuse Treat. 1996;13:493–497. doi: 10.1016/s0740-5472(96)00154-7. [DOI] [PubMed] [Google Scholar]
- Hart CL, Haney M, Vosburg SK, Rubin E, Foltin RW. Smoked cocaine self-administration is decreased by modafinil. Neuropsychopharmacology. 2008;33:761–768. doi: 10.1038/sj.npp.1301472. [DOI] [PubMed] [Google Scholar]
- Heinzerling KG, Shoptaw S, Peck JA, Yang X, Liu J, Roll J, Ling W. Randomized, placebo-controlled trial of baclofen and gabapentin for the treatment of methamphetamine dependence. Drug Alcohol Depend. 2006;85:177–184. doi: 10.1016/j.drugalcdep.2006.03.019. [DOI] [PubMed] [Google Scholar]
- Hunter MD, Ganesan V, Wilkinson ID, Spence SA. Impact of modafinil on prefrontal executive function in schizophrenia. Am J Psychiatry. 2006;163:2184–2186. doi: 10.1176/appi.ajp.163.12.2184. [DOI] [PubMed] [Google Scholar]
- Jayaram-Lindstrom N, Hammarberg A, Beck O, Franck J. Naltrexone for the treatment of amphetamine dependence: a randomized, placebo-controlled trial. Am J Psychiatry. 2008;165:1442–1448. doi: 10.1176/appi.ajp.2008.08020304. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Ait-Daoud N, Elkashef AM, Smith EV, Kahn R, Vocci F, Li SH, Bloch DA. A preliminary randomized, double-blind, placebo-controlled study of the safety and efficacy of ondansetron in the treatment of methamphetamine dependence. Int J Neuropsychopharmacol. 2008;11:1–14. doi: 10.1017/S1461145707007778. [DOI] [PubMed] [Google Scholar]
- Lee NK, Rawson RA. A systematic review of cognitive and behavioural therapies for methamphetamine dependence. Drug Alcohol Rev. 2008;27:309–317. doi: 10.1080/09595230801919494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling W, Shoptaw S, Wesson D, Rawson RA, Compton M, Klett CJ. Treatment effectiveness score as an outcome measure in clinical trials. NIDA Res Monogr. 1997;175:208–220. [PubMed] [Google Scholar]
- Longo M, Wickes W, Smout M, Harrison S, Cahill S, White JM. Randomized controlled trial of dexamphetamine maintenance for the treatment of methamphetamine dependence. Addiction (Abingdon, England) 2009 Oct 21; doi: 10.1111/j.1360-0443.2009.02717.x. Electronic publication 2009. [DOI] [PubMed] [Google Scholar]
- Madras BK, Xie ZH, Lin ZC, Jassen A, Panas H, Lynch L, Johnson R, Livni E, Spencer TJ, Bonab AA, Miller GM, Fischman AJ. Modafinil occupies dopamine and norepinephrine transporters in vivo and modulates the transporters and trace amine activity in vitro. J Pharmacol Exp Ther. 2006;319:561–569. doi: 10.1124/jpet.106.106583. [DOI] [PubMed] [Google Scholar]
- McElhiney MC, Rabkin JG, Rabkin R, Nunes EV. Provigil (modafinil) plus cognitive behavioral therapy for methamphetamine use in HIV+ gay men: a pilot study. Am J Drug Alcohol Abuse. 2009;35:34–37. doi: 10.1080/00952990802342907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGaugh J, Mancino MJ, Feldman Z, Chopra MP, Gentry WB, Cargile C, Oliveto A. Open-label pilot study of modafinil for methamphetamine dependence. J Clin Psychopharmacol. 2009;29:488–491. doi: 10.1097/JCP.0b013e3181b591e0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGregor C, Srisurapanont M, Mitchell A, Wickes W, White JM. Symptoms and sleep patterns during inpatient treatment of methamphetamine withdrawal: a comparison of mirtazapine and modafinil with treatment as usual. J Subst Abuse Treat. 2008;35:334–342. doi: 10.1016/j.jsat.2007.12.003. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Kushner H, Metzger D, Peters R, Smith I, Grissom G, Pettinati H, Argeriou M. The Fifth Edition of the Addiction Severity Index. J Subst Abuse Treat. 1992;9:199–213. doi: 10.1016/0740-5472(92)90062-s. [DOI] [PubMed] [Google Scholar]
- Minzenberg MJ, Carter CS. Modafinil: A review of neurochemical actions and effects on cognition. Neuropsychopharmacology. 2008;33:1477–1502. doi: 10.1038/sj.npp.1301534. [DOI] [PubMed] [Google Scholar]
- Minzenberg MJ, Watrous AJ, Yoon JH, Ursu S, Carter CS. Modanfinil Shifts Human Locus Coeruleus to Low-Tonic, High-Phasic Activity During Functional MRI. Science. 2008;322:1700–1702. doi: 10.1126/science.1164908. [DOI] [PubMed] [Google Scholar]
- Monterosso JR, Aron AR, Cordova X, Xu JS, London ED. Deficits in response inhibition associated with chronic methamphetamine abuse. Drug Alcohol Depend. 2005;79:273–277. doi: 10.1016/j.drugalcdep.2005.02.002. [DOI] [PubMed] [Google Scholar]
- National Association of Counties. The Meth Epidemic: The changing demographics of methamphetamine. [accessed on September 7 2008];2007 http://www.naco.org/Template.cfm?Section=Meth_Action_Clearinghouse.
- O'Brien CP, Dackis CA, Kampman K. Does modafinil produce euphoria? Am J Psychiatry. 2006;163:1109. doi: 10.1176/ajp.2006.163.6.1109. [DOI] [PubMed] [Google Scholar]
- Powell D, Kaplan E, Whitla D, Weintraub S, Catlin R, Funkenstein H. MicroCog™: Assessment of Cognitive Functioning. San Antonio, TX: Windows® Edition Pearson Education, Inc; 2004. [Google Scholar]
- Qu WM, Huang ZL, Xu XH, Matsumoto N, Urade Y. Dopaminergic D-1 and D-2 receptors are essential for the arousal effect of modafinil. J Neurosci. 2008;28:8462–8469. doi: 10.1523/JNEUROSCI.1819-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall DC, Fleck NL, Shneerson JM, File SE. The cognitive-enhancing properties of modafinil are limited in non-sleep-deprived middle-aged volunteers. Pharmacol Biochem Behav. 2004;77:547–555. doi: 10.1016/j.pbb.2003.12.016. [DOI] [PubMed] [Google Scholar]
- Randall DC, Shneerson JM, File SE. Cognitive effects of modafinil in student volunteers may depend on IQ. Pharmacol Biochem Behav. 2005;82:133–139. doi: 10.1016/j.pbb.2005.07.019. [DOI] [PubMed] [Google Scholar]
- SAMHSA Office of Applied Statistics. NSDUH Report. [accessed on September 7 2008];Methamphetamine Use, Abuse, and Dependence: 2002, 2003, and 2004. 2005 http://www.drugabusestatistics.samhsa.gov/2k5/meth/meth.htm.
- SAMHSA Office of Applied Statistics. National Survey on Drug use and Health. [accessed on September 7 2008];2007 http://www.oas.samhsa.gov/nsduhLatest.htm.
- Schnoll RA, Wileyto EP, Pinto A, Leone F, Gariti P, Siegel S, Perkins KA, Dackis C, Heitjan DF, Berrettini W, Lerman C. A placebo-controlled trial of modafinil for nicotine dependence. Drug Alcohol Depend. 2008;98:86–93. doi: 10.1016/j.drugalcdep.2008.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shearer J, Darke S, Rodgers C, Slade T, van Beek I, Lewis J, Brady D, McKetin R, Mattick RP, Wodak A. A double-blind, placebo-controlled trial of modafinil (200 mg/day) for methamphetamine dependence. Addiction (Abingdon, England) 2009;104:224–233. doi: 10.1111/j.1360-0443.2008.02437.x. [DOI] [PubMed] [Google Scholar]
- Shoptaw S, Heinzerling KG, Rotheram-Fuller E, Steward T, Wang J, Swanson AN, De La Garza R, Newton T, Ling W. Randomized, placebo-controlled trial of bupropion for the treatment of methamphetamine dependence. Drug Alcohol Depend. 2008;96:222–232. doi: 10.1016/j.drugalcdep.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoptaw S, Huber A, Peck J, Yang X, Liu J, Jeff D, Roll J, Shapiro B, Rotheram-Fuller E, Ling W. Randomized, placebo-controlled trial of sertraline and contingency management for the treatment of methamphetamine dependence. Drug Alcohol Depend. 2006;85:12–18. doi: 10.1016/j.drugalcdep.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Simon SL, Domier C, Carnell J, Brethen P, Rawson R, Ling W. Cognitive impairment in individuals currently using methamphetamine. Am J Addict. 2000;9:222–231. doi: 10.1080/10550490050148053. [DOI] [PubMed] [Google Scholar]
- Spitzer R, Williams J, Gibbbon M, First M. The Structured Clinical Interview for DSM-IV. Washington, D.C: American Psychiatric Press; 1995. [Google Scholar]
- Steer RA, Clark DA, Beck AT, Ranieri WF. Common and specific dimensions of self-reported anxiety and depression: the BDI-II versus the BDI-IA. Behav Res Ther. 1999;37:183–190. doi: 10.1016/s0005-7967(98)00087-4. [DOI] [PubMed] [Google Scholar]
- Stout RL, Wirtz PW, Carbonari JP, Del Boca FK. Ensuring balanced distribution of prognostic factors in treatment outcome research. J Stud Alcohol Suppl. 1994;12:70–75. doi: 10.15288/jsas.1994.s12.70. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Office of Applied Studies. Highlights - 2007. Rockville, MD: National Admissions to Substance Abuse Treatment Services; 2009. Treatment Episode Data Set (TEDS) [Google Scholar]
- Terplan M, Smith EJ, Kozloski MJ, Pollack HA. Methamphetamine use among pregnant women. Obstet Gynecol. 2009;113:1285–1291. doi: 10.1097/AOG.0b013e3181a5ec6f. [DOI] [PubMed] [Google Scholar]
- Tiihonen J, Kuoppasalmi K, Fohr J, Tuomola P, Kuikanmaki O, Vorma H, Sokero P, Haukka J, Meririnne E. A comparison of aripiprazole, methylphenidate, and placebo for amphetamine dependence. Am J Psychiatry. 2007;164:160–162. doi: 10.1176/ajp.2007.164.1.160. [DOI] [PubMed] [Google Scholar]
- United Nations Office on Drugs and Crime. World Drug Report, 2008. Vienna, Austria: 2008
- Volkow ND, Fowler JS, Logan J, Alexoff D, Zhu W, Telang F, Wang GJ, Jayne M, Hooker JM, Wong C, Hubbard B, Carter P, Warner D, King P, Shea C, Xu YW, Muench L, Apelskog-Torres K. Effects of Modafinil on Dopamine and Dopamine Transporters in the Male Human Brain Clinical Implications. JAMA. 2009;301:1148–1154. doi: 10.1001/jama.2009.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vosburg SK, Hart CL, Haney M, Rubin E, Foltin RW. Modafinil does not serve as a reinforcer in cocaine abusers. Drug Alcohol Depend. 2009 September 22; doi: 10.1016/j.drugalcdep.2009.09.002. Electronic publication 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger AH, Sofuoglu M. The impact of cigarette smoking on stimulant addiction. Am J Drug Alcohol Abuse. 2009;35:12–17. doi: 10.1080/00952990802326280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winder-Rhodes S, Chamberlain S, Idris M, Robbins T, Sahakian B, Muller U. Effects of modafinil and prazosin on cognitive and physiological functions in healthy volunteers. J Psychopharmacol. 2009 June 3; doi: 10.1177/0269881109105899. Electronic publication, 2009. [DOI] [PubMed] [Google Scholar]
- Wisor JP, Nishino S, Sora I, Uhl GH, Mignot E, Edgar DM. Dopaminergic role in stimulant-induced wakefulness. J Neurosci. 2001;21:1787–1794. doi: 10.1523/JNEUROSCI.21-05-01787.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]