Abstract
Transforming growth factor beta (TGFβ) is a growth factor with many faces. In our osteoarthritis (OA) research we have found that TGFβ can be protective as well as deleterious for articular cartilage. We postulate that the dual effects of TGFβ on chondrocytes can be explained by the fact that TGFβ can signal via different receptors and related Smad signaling routes. On chondrocytes, TGFβ not only signals via the canonical type I receptor ALK5 but also via the ALK1 receptor. Notably, signaling via ALK5 (Smad2/3 route) results in markedly different chondrocyte responses than ALK1 signaling (Smad1/5/8), and we postulate that the balance between ALK5 and ALK1 expression on chondrocytes will determine the overall effect of TGFβ on these cells. Importantly, signaling via ALK1, but not ALK5, stimulates MMP-13 expression by chondrocytes. In cartilage of ageing mice and in experimental OA models we have found that the ALK1/ALK5 ratio is significantly increased, favoring TGFβ signaling via the Smad1/5/8 route, changes in chondrocyte differentiation and MMP-13 expression. Moreover, human OA cartilage showed a significant correlation between ALK1 and MMP-13 expression. In this paper we summarize concepts in OA, its link with ageing and disturbed growth factor responses, and a potential role of TGFβ signaling in OA development.
Introduction
Osteoarthritis (OA) is the joint disease with the highest incidence. The disease is in general divided into primary OA and secondary OA. Primary OA has no obvious trigger, while secondary OA is the result of an evident underlying affliction. The main features of this disease are cartilage erosion, synovial fibrosis, osteophyte formation at the joint margins and sclerosis of the subchondral bone. Patients with OA suffer from joint pain and tenderness, occasional effusions and, in the long run, loss of joint function.
The etiology of primary OA is not known but several risk factors have been detected. Systemic risk factors include genetic background, ethnicity, gender and obesity, but the main risk factor for the initiation and progression of primary OA is ageing. Functional articular cartilage is maintained by the cartilage cells, chondrocytes. Changes in chondrocytes, leading to the inability of these cells to maintain the homeostasis of articular cartilage, can be expected to be at the root of OA development. In view of the fact that the principal risk factor of OA is ageing, age-related changes in chondrocytes are likely to be involved in OA development.
Changes in osteoarthritic chondrocytes
Cartilage is, on a weight basis, mainly composed of collagens and proteoglycans. Collagens - for the most part type II, type IX and type XI - provide tensile strength, while the proteoglycan aggrecan retains water in the matrix. In humans, cartilage is composed of three zones: superficial zone, middle zone and deep zone. The superficial zone contains disc-shaped chondrocytes, the cells in the middle zone cells are more spherical and the deep zone contains spherical chondrocytes arranged in columns.
Cartilage damage in OA has several characteristics. At the initial stages of OA the cartilage surface is intact but focal edema and minor fibrillations can be observed. Subsequently the superficial zone becomes fibrillated and chondrocytes are lost from this zone. Finally, fibrillations progress into fissures - a process that is followed by cartilage erosion, denudation of bone and joint deformation.
At the initial stages of OA, chondrocytes start to multiply and form multicellular clusters. In addition, chondrocytes expressing markers of hypertrophic chondrocytes are found in OA cartilage. A subpopulation of OA chondrocytes synthesizes molecules that, under normal conditions, are only expressed by terminally differentiated (hypertrophic) chondrocytes, normally found in growth plates. Expression of osteocalcin, alkaline phosphatase, c-maf, Runx2 and type X collagen has been demonstrated in OA chondrocytes [1-5]. Moreover, chondrocytes in OA cartilage express high levels of matrix metalloproteinase 13 (MMP-13), the enzyme most potently degrading type II collagen [6]. This underscores the hypertrophy-like character of OA chondrocytes since MMP-13 is highly upregulated during chondrocyte terminal differentiation, and deficiency of MMP-13 even results in impaired endochondral ossification [7,8].
During OA, cartilage matrix degradation exceeds matrix deposition resulting in net matrix loss. In contrast to what is observed in inflammatory arthritis, mRNA expression and synthesis of a number of matrix molecules is increased instead of decreased compared with normal cartilage [9,10]. Only in the very late stages of OA does synthesis of matrix molecules drop below control levels. The synthesis of the main structural component of cartilage, type II collagen, is clearly enhanced in OA cartilage [11,12]. In OA cartilage, both catabolism (for example, MMP-13 synthesis) and anabolism (type II collagen synthesis) are high. It is unclear whether elevated catabolism and enhanced anabolism is achieved by the same cells or by different chondrocyte subpopulations.
Catabolic cytokines
Catabolic cytokines have been suggested to play a dominant role in OA. Chondrocytes can be stimulated by catabolic cytokines to release cartilage degradation products, ultimately leading to damage. A cytokine that is suggested to be a principle mediator of joint damage in OA is IL-1. Chondrocytes from OA cartilage display high levels of IL-1α and IL-1β and have elevated expression of the plasma membrane-bound IL-1 receptor I, while the decoy IL-1 receptor II is downregulated in OA chondrocytes [13]. Not only do fibrillated areas show these expression patterns, but also cartilage proximal to macroscopic OA lesions demonstrates a higher binding of TNFα and IL-1β com pared with chondrocytes from morphologically normal cartilage in the same joint [14]. This indicates not only that the levels of IL-1 are increased in OA joints, but also that OA chondrocytes are more sensitive to IL-1.
IL-1 is considered a principle mediator of joint damage in OA. IL-1 has the ability to stimulate chondrocytes to degrade both aggrecan and collagen [15]. This cytokine causes destruction of cartilage by increasing enzyme activity while decreasing the synthesis of enzyme inhibitors [16]. IL-1 can stimulate chondrocytes to produce nitric oxide [17], matrix metalloproteinases [18] and aggrecanases (ADAMTS) [19], and suppresses the synthesis of aggrecan and collagen type II [20-22].
The latter is remarkable if IL-1 plays a dominant role in OA pathophysiology. IL-1 is a potent inhibitor of chondrocyte type II collagen synthesis, but type II collagen synthesis is increased during OA as discussed above. This discrepancy points to alternative players that are involved in OA. IL-1 might play a role in the induction of enzyme expression but is unlikely to be the only factor that determines development and progression of OA.
Osteoarthritic chondrocytes also express, besides catabolic factors, anabolic factors such as transforming growth factor beta (TGFβ) [23,24]. Increased synthetic activity in early OA has been found to be accompanied with an upregulation of TGFβ expression [25,26]. We propose a role for TGFβ not only as a cartilage protective agent but also as a mediator of cartilage degeneration during ageing and OA development.
Transforming growth factor beta
The TGFβ superfamily is composed of over 35 members. The family members play fundamental roles in development and homeostasis. In mammals, three isotypes of TGFβ are found: β1, β2 and β3. Expression of these three isoforms is differently regulated at the transcriptional level due to dissimilar promoter sequences [27].
TGFβ is secreted as an inactive complex and requires activation before it is able to bind to its receptor [28]. Activated TGFβ binds to the TGFβ type II receptor and forms a complex that recruits the TGFβ type I receptor, ALK5. TGFβ has also recently been shown, however, to have the ability to signal via the alternative TGFβ type I receptor ALK1 in chondrocytes. In endothelial cells, but also in chondrocytes, activation of the ALK5 route is followed by Smad2 or Smad3 phosphorylation while ALK1 has been found to result in phosphorylation of Smad1, Smad5, or Smad8 [29-31]. The activated receptor Smads form a complex with the co-Smad, Smad4 - this complex translocates to the nucleus and modifies gene expression. Interestingly, signaling via either ALK5 or ALK1 can determine the response of cells to TGFβ stimulation, which can be totally contrary [32,33]. For example, in endothelial cells ALK5 inhibits migration whereas ALK1 stimulates migration and proliferation [34].
Signaling via the Smad pathway appears to be the most important signaling pathway for TGFβ, but this is not the only pathway. Mitogen-activated protein kinase, Rho-like GTPase and phopshatidylinositol-3-kinase pathways are involved in TGFβ signaling (reviewed in [35]). Activation of TGFβ activated kinase 1 occurs independent of ALK5 kinase activity and results in P38 and JNK signaling [36]. That TGFβ activates different pathways calls attention to the fact that one has to take into account the differences in management of the TGFβ signal in different cell types and the subsequent variation in TGFβ effects.
Transforming growth factor beta and osteoarthritis
Family studies have indicated a relation between TGFβ and a disease related to OA. In Japanese women a polymorphism of TGFβ1 on position 29 (T to C, amino acid 10) positioned in the signal sequence region of TGFβ1 is related to an elevated prevalence of spinal osteophytosis and ossification of the posterior longitudinal ligament [37,38].
Asporin inhibits TGFβ-mediated expression of cartilage matrix genes such as collagen type II and aggrecan, and inhibits accumulation of proteoglycan [39]. Kizawa and colleagues found an asporin polymorphism that showed a significantly higher frequency in OA [39]. The D-14 polymorphism had a stronger inhibitory effect on TGFβ than the common D-13 repeat. This indicates that expression of D-14 results in strong TGFβ inhibition and that this is associated with OA development. When this study was repeated in a Spanish Caucasian population by Rodriguez-Lopez and colleagues, however, the higher susceptibility to OA of people with the D-14 polymorphism was not found [40]. In a subset of UK Caucasians, a trend was seen towards a higher degree of D-14 polymorphism in OA. In a different ethnic group of Asian origin, Han Chinese, the OA susceptibility was again found [41]. These studies indicate that reduced TGFβ signaling can result in OA development.
Mice deficient for Smad3 developed degenerative joint disease resembling human OA. Chondrocytes present in the articular cartilage of Smad3-deficient mice showed enhanced chondrocyte hypertrophy indicated by increased expression of type X collagen. These data indicate that Smad3 signaling is essential for repressing chondrocyte terminal differentiation. This observation is supported by studies in mice that overexpress a dominant-negative TGFβ type II receptor in skeletal tissues [42]. These mice developed progressive skeletal degeneration that strongly resembles human OA. In addition, mice that lack latent TGFβ binding protein 3 also show altered chondrocyte differentiation and early OA development [43,44]. Interference with normal TGFβ signaling apparently results in aberrations in chondrocyte differentiation and enhanced OA development.
The effects of TGFβ on chondrocytes seem to be context related. Serum factors can modulate the effect of TGFβ on chondrocyte proliferation. Growth of cultured rabbit chondrocytes decreased after TGFβ stimulation in the presence of a low serum concentration, while the cell number increased in the presence of high serum levels [45,46]. The rabbit chondrocytes demonstrated differences in TGFβ receptor expression as a function of cell cycle progression [47-49]. Moreover, expression of TGFβ receptors appeared to be changed by nitric oxide and ROS levels and OA chondrocytes became insensitive to TGFβ, which was concomitant with loss of the expression of TGFβ type II receptor on these chondrocytes [50,51]. A loss of the TGFβ type II receptor has also been observed by our own group during ageing and OA in murine models [52,53]. Moreover, proteoglycan synthesis is also differentially regulated by TGFβ in rabbit and bovine chondrocytes depending on the differentiation stage of the chondrocytes [54,55]. In calf cartilage explants, proteoglycan synthesis is stimulated by TGFβ in a dose-dependent manner [56,57]. From these observations it can be concluded that, in general, TGFβ maintains chondrocyte and cartilage homeostasis but that changes in differentiation stage and associated alterations in receptor expression modify the effect of TGFβ on chondrocyte function.
We have shown in young mice that TGFβ has favorable effects on cartilage, such as stimulation of proteoglycan synthesis in cartilage [58]. In old mice, however, stimulation of aggrecan synthesis by TGFβ is reduced - and this is associated with a loss in ALK5 expression, and TGFβ type II receptor expression, on articular chondrocytes [59]. Livne and colleagues showed in mandibular chondrocytes a strong age-related decrease in stimulation of proteoglycan synthesis by TGFβ in young mice (1 month old, +120%) and old mice (18 months old, +7%) [59,60]. Nonchondrocytic cells have also been shown to display a diminished response to TGFβ during ageing. Smooth muscle cells derived from old rats produce normal levels of TGFβ but fail to respond to the inhibitory effects of this growth factor in contrast to young cells [61]. The response to TGFβ appears to be age related and a change in TGFβ signaling can play a role in age-related diseases such as OA.
Control of chondrocyte differentiation by SMADs
Activation of the Smad1/5/8 route in chondrocytes is strongly associated with chondrocyte terminal differentiation and hypertrophy [62]. Bone morphogenetic protein itself or activation of the bone morphogenetic protein pathway (Smad1/5/8) leads, both in the growth plate and in articular chondrocytes, to expression of terminal differentiation markers [63-65]. Signaling via Smad1 cooperates with the transcription factor Runx2 (CBFA1) to induce chondrocyte terminal differentiation. This cross-talk between the bone morphogenetic proteinassociated Smads and Runx2 is essential to stimulate the expression of hypertrophy markers in differentiating chondrocytes [66]. Blocking the Smad1/5/8 route by overexpression of Smad6 reduced the expression of both type X collagen and alkaline phosphatase activity in chondrocytes, while using Smad6 antisense had an opposite effect [67]. Moreover, in vivo inhibition of Smad1/5/8 phosphorylation, as observed in Smad6 transgenic mice, was associated with delayed chondrocyte hypertrophy [68]. In articular chondrocytes treated with azacytidine, reduced Smad2 and Smad3 expression and signaling and increased Smad1/5 expression correlated with elevated synthesis of type X collagen and alkaline phosphatase. These observations clearly demonstrate that terminal differentiation of articular chondrocytes is associated with dominant signaling via the Smad1/5/8 pathway [69].
The latter observation shows not only that activation of the Smad1/5/8 route leads to terminal differentiation but also that loss of Smad2/3 can lead to induction of chondrocyte terminal differentiation. The inhibitory effects of TGFβ on chondrocyte maturation is mediated by the Smad2/3 pathway, as has been shown by overexpression of dominant negative Smad2 and Smad3 in chondrocytes. Mutant mice deficient for functional Smad3 show abnormally increased numbers of type X collagen-expressing chondrocytes in articular cartilage. Overexpression of both Smad2 and Smad3 blocked spontaneous maturation in Smad3-deficient chondrocytes [70,71]. Smad2 and Smad3 are key mediators of the inhibitory effect of TGFβ on chondrocyte terminal differentiation [72]. Without Smad2/3 signaling, chondrocytes break their quiescent state and undergo anomalous terminal differentiation. Apparently the balance between Smad1/5/8 signaling and Smad2/3 signaling controls chondrocyte differentiation.
The wnt signaling pathways are involved in chondrocyte differentiation and OA development [73]. Enhanced and decreased wnt signaling both result in cartilage loss [74,75]. Furthermore, the wnt inhibitor dickkopf1 stimulated chondrocyte apoptosis in OA joints [76]. Increased wnt signaling can have a direct effect on chondrocyte differentiation but it can also alter differentiation by variable modulation of the Smad2/3 and Smad1/5/8 pathways. wnt signaling leads to inhibition of the activity of the GSK3 kinase, which resulted in Xenopus embryos in prolonged duration of the Smad1 signal [77]. If a similar mechanism takes place in chondrocytes, enhanced wnt signaling will result in augmented terminal differentiation.
Chondrocyte differentiation is regulated by Sox9, and additional Sox molecules, but chondrocyte terminal differentiation is rigorously controlled by the transcription factor Runx2 [78,79]. Mice lacking Runx2 do not show chondrocyte terminal differentiation, and bone formation via this pathway is totally blocked [80]. Smad pathways are integrated via Runx2 to control chondrocyte terminal differentiation. Interaction of Runx2 with Smad1 facilitates the function of Runx2 in stimulating terminal differentiation, while Smad3 blocks Runx2 function [81-83]. The Smad2/3 and Smad1/5/8 balance controls the Runx2 function and terminal differentiation.
Change in transforming growth factor beta signaling in ageing chondrocytes and osteoarthritis
We have demonstrated an age-related loss of TGFβ type I receptor ALK5 and phosphorylation of Smad2/3 in murine articular cartilage [84]. Expression of non phosphorylated Smad2 was not altered during ageing. Moreover, in two experimental models of OA - the DMM (meniscus destabilization) model and STR/ORT mice (spontaneous OA) - development of the disease was correlated with a loss of ALK5 expression. Expression of the alternative TGFβ receptor, ALK1, did not decrease to a similar extent as ALK5 [85]. As a result, the ratio of ALK1/ALK5 expressing cells strongly increased in OA articular chondrocytes. During ageing of C57Bl mice, the ratio ALK1/ALK5 increased up to sixfold. In the DMM model, OA develops on the medial tibial side while the lateral side is relatively protected. A more than threefold increase in the ALK1/ALK5 ratio was observed on the medial side while the ratio on the lateral side was unaffected. STR/ORT mice develop OA starting at the medial tibia from an age of 2 to 3 months. The ALK1/ALK5 ratio was 5 on the medial tibia at an age of 3 months and was 18 in 1-year-old animals. The lateral tibia showed a ratio increase from 1 to 5 in the same period. Clearly an increased ALK1/ALK5 ratio in chondrocytes is associated with ageing and OA development [85].
We postulate that the loss of ALK5 expression and the concomitant elevated ratio of ALK1/ALK5 will have profound effects on chondrocyte behavior. The effect of TGFβ on chondrocytes will be governed by the ALK1/ALK5 ratio. A prevailing expression of ALK5 will result in a dominance of the Smad2/3 signaling route, while ALK1 dominance will result in a stronger Smad1/5/8 pathway. The balance of these routes has been shown to control chondrocyte differentiation (see above).
We and others have shown that TGFβ signals in chondrocytes not only via ALK5 but also via ALK1 [86]. Exposure of chondrocytes to TGFβ results in both Smad2/3 and Smad1/5/8 phosphorylation within 15 to 30 minutes [85]. In addition, overexpression of constitutive active ALK5 (Smad2/3) results in increased expression of aggrecan while constitutive ALK1 (Smad1/5/8) expression leads to elevated expression of MMP-13. Blocking ALK5 expression using siRNA resulted in elevated expression of MMP-13 [85]. The ALK1 (Smad1/5/8) and ALK5 (Smad3) signaling balance in chondrocytes apparently determines MMP-13 expression. In addition, a clear trend towards elevated type II collagen and aggrecan expression was observed in cells with constitutive active ALK1. Noticeably, human osteoarthritic cartilage demonstrated a significant correlation between ALK1 and MMP-13 mRNA expression and a trend (P = 0.05 to 0.1) with type II collagen and aggrecan expression. These observations indicate that ALK1 signaling can induce a chondrocyte phenotype similar to that found in OA cartilage, a phenotype with simultaneous enhanced expression of matrix molecules and MMP-13.
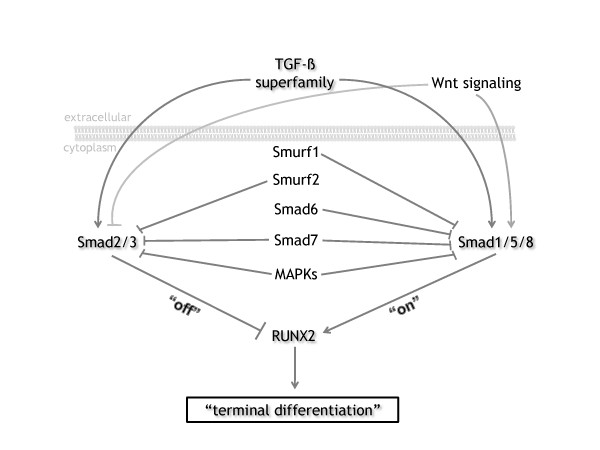
We hypothesize that articular chondrocytes reside in a quiescent state in young healthy cartilage due to the inhibitory effect of TGFβ, via Smad2/3, on the progression of chondrocyte differentiation. During ageing of chondrocytes and OA development, signaling through ALK1 and Smad1/5/8 is increased in favor of signaling via ALK5 and Smad2/3. The dominant Smad1/5/8 signaling triggers the articular chondrocytes to leave their quiescent state (Figure 1). This leads to a chondrocyte phenotype with characteristics analogous to terminal differentiated growth plate chondrocytes - a chondrocyte with an autolytic phenotype typified by degradation of its surrounding cartilage matrix, as can be found in OA cartilage.
Figure 1.
Alterations in transforming growth factor beta signaling cause changes in chondrocyte differentiation and osteoarthritis development. Transforming growth factor beta (TGFβ) can either signal by the Smad2/3 route (canonical) or the Smad1/5/8 route. Smad2/3 and Smad1/5/8 form a complex with Smad4 that enters the nucleus and modulates gene expression and Runx2 function. The signaling by Smad2/3 and Smad1/5/8 is differentially modified by a number of intracellular molecules. Both Smad routes are blocked by Smad7, while Smad6 blocks preferentially the Smad1/5/8 pathway [100,101]. wnt signaling modifies these pathways by stabilization of Smad1/5/8 [102]. Smurf1 and Smurf2 are E3 ubiquitin ligases that inhibit Smad signaling. Smurf1 triggers the degradation of Smad1/5/8 while Smurf2 stimulates mainly the degradation of Smad2/3 [103]. Mitogen-activated protein kinases (MAPKs) modulate the stability and degradation of the Smads by phosphorylation of these molecules [102].
This hypothesis can explain the often enigmatic effects of TGFβ on articular cartilage. The effect of TGFβ on chondrocytes will be determined by the relative expression of ALK5 and ALK1. In general, we have observed in young animals that TGFβ is protective for articular cartilage [84,87-90]. Prolonged exposure of cartilage to high TGFβ levels, however, induces osteoarthritic lesions in murine knee joints, starting in the deep zones [91]. We have observed that the chondrocytes in the deep zone, just above the tidemark, show high ALK1 expression (personal observation). In old animals, showing a decrease in the ALK5/ALK1 ratio, the protective effect of TGFβ is lost and TGFβ can act as an OA-inducing factor [85,92,93] (Table 1).
Table 1.
Arguments implying a role for alterations in TGFβ signaling in osteoarthritis development
| Genetic studies point to a role for TGFβ in osteoarthritis |
| Mice that express a dominant negative TGFβ type II receptor in skeletal tissues showed enhanced chondrocyte hypertrophy and osteoarthritis |
| Mice deficient for Smad3 or latent TGFβ binding protein 3 demonstrated enhanced chondrocyte hypertrophy and osteoarthritis |
| Cartilage protective effects of TGFβ are lost in ageing mice |
| ALK1/ALK5 expression ratio is increased in cartilage in ageing mice and experimental osteoarthritis |
| ALK1 overexpression results in MMP-13 upregulation in chondrocytes |
| Blocking ALK5 expression, using siRNA, leads to elevated expression of MMP-13 |
| In human osteoarthritis cartilage, ALK1 expression and MMP-13 expression significantly correlate |
| Smad2/3 signaling inhibits, while Smad1/5/8 signaling stimulates, progression of chondrocyte differentiation |
| In osteoarthritis, synthesis of matrix molecules (type II collagen) is increased - indicating no dominant role for catabolic cytokines |
| Alterations in TGFβ signaling in osteoarthritis can provide an explanation for the enigmatic observation of concomitant increased synthesis of matrix molecules (type II collagen) and increased MMP-13 production |
MMP-13, matrix metalloproteinase 13; TGFβ, transforming growth factor beta.
In conclusion, loss of the Smad2/3 signaling and relatively enhanced Smad1/5/8 signaling can explain the enigmatic observation in OA cartilage of elevated expression of both matrix molecules and proteolytic enzymes, like MMP-13. Moreover, the age-related loss of ALK5 signaling in chondrocytes can give a clue to the high correlation between ageing and OA development. Interestingly, a remarkable relationship has been reported between reduced TGFβ signaling and another, highly age-related affliction, Alzheimer's disease [94,95]. Alzheimer's disease is characterized by progressive neurodegeneration and cerebral accumulation of the β-amyloid peptide. Reduced TGFβ type II receptor expression and signaling has been demonstrated in Alzheimer's disease. Overexpression of dominant negative Smad3 causes neurodegeneration in cell cultures, indicating that loss of Smad2/3 signaling is involved. Reducing neuronal TGFβ signaling via the Smad2/3 pathway in mice resulted in age-dependent neurodegeneration. These findings show that reduced TGFβ Smad3-dependent signaling in neuronal cells increases age-dependent neurodegeneration and Alzheimer's disease-like symptoms. This observation points to a striking similarity between authentic Alzheimer's disease and Alzheimer's disease of the joint - OA.
Targets for therapy
We postulate that the OA process is driven by the loss of the Smad2/3 block on differentiation in articular chondrocytes, leading to progression of chondrocyte differentiation and an autolytic phenotype. In the early stages of OA - bearing in mind that OA is initially a focal process - not all chondrocytes will be at the same stage of differentiation. A mixture of cell populations will be present in OA cartilage. Some chondrocytes will have progressed in their differentiation to an OA chondrocyte phenotype, triggered by a loss of the Smad2/3 block. Other cells will still be in a quiescent, healthy state of differentiation. The latter cells can be targets for therapy to block further progression of the OA process. Blocking the progression of chondrocyte differentiation will block further expansion of the OA process in remaining healthy cartilage.
Loss of Smad2/3 signaling is at the root of the OA process in our view. To inhibit articular chondrocytes in their deviant differentiation, this pathway has to be stimulated at the same time as circumventing the role of the ALK1 receptor. Compounds specifically stimulating the Smad2/3 route should be developed. A similar strategy, using TGFβ mimetics, has been proposed to treat Alzheimer's disease [96]. TGFβ mimetics have already been developed that can mimic TGFβ effects on cells [97].
An alternative therapy could be stimulation of one of the other Smad2/3 routes in chondrocytes. Signaling via the activin ALK4 and ALK7 receptors leads to activation of the Smad2/3 pathway [98]. Little is known about the expression of these receptors in old chondrocytes, but potentially these receptors could be targets to enhance Smad2/3 signaling in chondrocytes in OA.
An alternative strategy would be blocking ALK1 or the Smad1/5/8 pathway in chondrocytes to block the trigger that stimulates progression of chondrocyte differentiation. Since ALK1 is involved in vessel formation, blocking ALK1 can interfere with this process [99]. As blockers of ALK1 to treat OA will be mainly applied in middle-aged and older people, additional effects of this treatment are expected to be limited. General blocking of the Smad1/5/8 pathway using kinase blockers that inhibit the activity of ALK1, ALK2, ALK3 and ALK6 is an alternative option to stop chondrocyte aberrant differentiation.
The potential side effects of the above therapies are unclear. Effects on growth plate chondrocytes will be absent since the growth plates are not present in elderly humans. The effects of stimulating the Smad2/3 pathway using TGFβ mimetics or the ALK4/7 pathway could result in side effects, such as induction of fibrosis. Blocking ALK1 will have few side effects due to the restricted effect of ALK1 in vessel formation, which is anticipated to be relatively unimportant in elderly people. The effects of general inhibition of the Smad1/5/8 pathway in elderly people are hard to predict but this might interfere with bone metabolism. Bone morphogenetic protein signaling is known to be involved in both bone formation and bone degradation, the latter by stimulation of osteoclast maturation.
Conclusion
Until now no effective therapy has been developed for OA that interferes with disease progression. Painkilling and joint replacement are the only options at this moment. The proposed treatment attacks the OA process at its core, blocking the generation of chondrocytes with an autolytic phenotype. The proposed OA mechanism and potential therapies open the venue to new strategies to treat this common crippling joint disease.
Abbreviations
IL: interleukin; MMP-13: matrix metalloproteinase 13; OA: osteoarthritis; TGFβ: transforming growth factor beta; TNF: tumor necrosis factor.
Competing interests
The authors declare that they have no competing interests.
Contributor Information
Peter M van der Kraan, Email: p.vanderkraan@reuma.umcn.nl.
Esmeralda N Blaney Davidson, Email: e.blaneydavidson@reuma.umcn.nl.
Wim B van den Berg, Email: w.vandenberg@reuma.umcn.nl.
References
- Pullig O, Weseloh G, Ronneberger D, Kakonen S, Swoboda B. Chondrocyte differentiation in human osteoarthritis: expression of osteocalcin in normal and osteoarthritic cartilage and bone. Calcif Tissue Int. 2000;67:230–240. doi: 10.1007/s002230001108. [DOI] [PubMed] [Google Scholar]
- Kirsch T, Swoboda B, Nah H. Activation of annexin II and V expression, terminal differentiation, mineralization and apoptosis in human osteoarthritic cartilage. Osteoarthritis Cartilage. 2000;8:294–302. doi: 10.1053/joca.1999.0304. [DOI] [PubMed] [Google Scholar]
- Aigner T, Reichenberger E, Bertling W, Kirsch T, Stoss H, von der MK. Type X collagen expression in osteoarthritic and rheumatoid articular cartilage. Virchows Arch B Cell Pathol Incl Mol Pathol. 1993;63:205–211. doi: 10.1007/BF02899263. [DOI] [PubMed] [Google Scholar]
- Li T, Xiao J, Wu Z, Qiu G. Over-expression of c-maf by chondrocytes in osteoarthritis. J Int Med Res. 2009;37:129–135. doi: 10.1177/147323000903700115. [DOI] [PubMed] [Google Scholar]
- Wang X, Manner PA, Horner A, Shum L, Tuan RS, Nuckolls GH. Regulation of MMP-13 expression by RUNX2 and FGF2 in osteoarthritic cartilage. Osteoarthritis Cartilage. 2004;12:963–973. doi: 10.1016/j.joca.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Reboul P, Pelletier JP, Tardif G, Cloutier JM, Martel-Pelletier J. The new collagenase, collagenase-3, is expressed and synthesized by human chondrocytes but not by synoviocytes. A role in osteoarthritis. J Clin Invest. 1996;97:2011–2019. doi: 10.1172/JCI118636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inada M, Wang Y, Byrne MH, Rahman MU, Miyaura C, Lopez-Otin C, Krane SM. Critical roles for collagenase-3 (Mmp13) in development of growth plate cartilage and in endochondral ossification. Proc Natl Acad Sci USA. 2004;101:17192–17197. doi: 10.1073/pnas.0407788101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stickens D, Behonick DJ, Ortega N, Heyer B, Hartenstein B, Yu Y, Fosang AJ, Schorpp-Kistner M, Angel P, Werb Z. Altered endochondral bone development in matrix metalloproteinase 13-deficient mice. Development. 2004;131:5883–5895. doi: 10.1242/dev.01461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser A, Fearon U, Billinghurst RC, Ionescu M, Reece R, Barwick T, Emery P, Poole AR, Veale DJ. Turnover of type II collagen and aggrecan in cartilage matrix at the onset of inflammatory arthritis in humans: relationship to mediators of systemic and local inflammation. Arthritis Rheum. 2003;48:3085–3095. doi: 10.1002/art.11331. [DOI] [PubMed] [Google Scholar]
- Poole AR, Rizkalla G, Ionescu M, Reiner A, Brooks E, Rorabeck C, Bourne R, Bogoch E. Osteoarthritis in the human knee: a dynamic process of cartilage matrix degradation, synthesis and reorganization. Agents Actions Suppl. 1993;39:3–13. doi: 10.1007/BF01972702. [DOI] [PubMed] [Google Scholar]
- Hermansson M, Sawaji Y, Bolton M, Alexander S, Wallace A, Begum S, Wait R, Saklatvala J. Proteomic analysis of articular cartilage shows increased type II collagen synthesis in osteoarthritis and expression of inhibin betaA (activin A), a regulatory molecule for chondrocytes. J Biol Chem. 2004;279:43514–43521. doi: 10.1074/jbc.M407041200. [DOI] [PubMed] [Google Scholar]
- Aigner T, Zien A, Gehrsitz A, Gebhard PM, McKenna L. Anabolic and catabolic gene expression pattern analysis in normal versus osteoarthritic cartilage using complementary DNA-array technology. Arthritis Rheum. 2001;44:2777–2789. doi: 10.1002/1529-0131(200112)44:12<2777::AID-ART465>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Martel-Pelletier J, McCollum R, DiBattista J, Faure MP, Chin JA, Fournier S, Sarfati M, Pelletier JP. The interleukin-1 receptor in normal and osteoarthritic human articular chondrocytes. Identification as the type I receptor and analysis of binding kinetics and biologic function. Arthritis Rheum. 1992;35:530–540. doi: 10.1002/art.1780350507. [DOI] [PubMed] [Google Scholar]
- Shlopov BV, Gumanovskaya ML, Hasty KA. Autocrine regulation of collagenase 3 (matrix metalloproteinase 13) during osteoarthritis. Arthritis Rheum. 2000;43:195–205. doi: 10.1002/1529-0131(200001)43:1<195::AID-ANR24>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Dingle JT. Catabolin - a cartilage catabolic factor from synovium. Clin Orthop Relat Res. 1981;156:219–231. [PubMed] [Google Scholar]
- Martel-Pelletier J. Pathophysiology of osteoarthritis. Osteoarthritis Cartilage. 2004;12(Suppl A):S31–S33. doi: 10.1016/j.joca.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Vuolteenaho K, Moilanen T, Jalonen U, Lahti A, Nieminen R, van Beuningen HM, Kraan PM van der, Moilanen E. TGFβ inhibits IL-1-induced iNOS expression and NO production in immortalized chondrocytes. Inflamm Res. 2005;54:420–427. doi: 10.1007/s00011-005-1373-6. [DOI] [PubMed] [Google Scholar]
- Pasternak RD, Hubbs SJ, Caccese RG, Marks RL, Conaty JM, DiPasquale G. Interleukin-1 stimulates the secretion of proteoglycan- and collagendegrading proteases by rabbit articular chondrocytes. Clin Immunol Immunopathol. 1986;41:351–367. doi: 10.1016/0090-1229(86)90006-1. [DOI] [PubMed] [Google Scholar]
- Takahashi N, Rieneck K, Kraan PM van der, van Beuningen HM, Vitters EL, Bendtzen K, Berg WB van den. Elucidation of IL-1/TGF-β interactions in mouse chondrocyte cell line by genome-wide gene expression. Osteoarthritis Cartilage. 2005;13:426–438. doi: 10.1016/j.joca.2004.12.010. [DOI] [PubMed] [Google Scholar]
- Bocquet J, Daireaux M, Langris M, Jouis V, Pujol JP, Beliard R, Loyau G. Effect of a interleukin-1 like factor (mononuclear cell factor) on proteoglycan synthesis in cultured human articular chondrocytes. Biochem Biophys Res Commun. 1986;134:539–549. doi: 10.1016/S0006-291X(86)80454-5. [DOI] [PubMed] [Google Scholar]
- Goldring MB, Birkhead JR, Suen LF, Yamin R, Mizuno S, Glowacki J, Arbiser JL, Apperley JF. Interleukin-1 beta-modulated gene expression in immortalized human chondrocytes. J Clin Invest. 1994;94:2307–2316. doi: 10.1172/JCI117595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatnagar R, Penfornis H, Mauviel A, Loyau G, Saklatvala J, Pujol JP. Interleukin-1 inhibits the synthesis of collagen by fibroblasts. Biochem Int. 1986;13:709–720. [PubMed] [Google Scholar]
- Redini F, Galera P, Mauviel A, Loyau G, Pujol JP. Transforming growth factor beta stimulates collagen and glycosaminoglycan biosynthesis in cultured rabbit articular chondrocytes. FEBS Lett. 1988;234:172–176. doi: 10.1016/0014-5793(88)81327-9. [DOI] [PubMed] [Google Scholar]
- Redini F, Daireaux M, Mauviel A, Galera P, Loyau G, Pujol JP. Characterization of proteoglycans synthesized by rabbit articular chondrocytes in response to transforming growth factor-beta (TGF-β) Biochim Biophys Acta. 1991;1093:196–206. doi: 10.1016/0167-4889(91)90123-F. [DOI] [PubMed] [Google Scholar]
- Goldring MB. The role of cytokines as inflammatory mediators in osteoarthritis: lessons from animal models. Connect Tissue Res. 1999;40:1–11. doi: 10.3109/03008209909005273. [DOI] [PubMed] [Google Scholar]
- Kraan PM van der, Glansbeek HL, Vitters EL, Berg WB van den. Early elevation of transforming growth factor-beta, decorin, and biglycan mRNA levels during cartilage matrix restoration after mild proteoglycan depletion. J Rheumatol. 1997;24:543–549. [PubMed] [Google Scholar]
- Govinden R, Bhoola KD. Genealogy, expression, and cellular function of transforming growth factor-beta. Pharmacol Ther. 2003;98:257–265. doi: 10.1016/S0163-7258(03)00035-4. [DOI] [PubMed] [Google Scholar]
- Lawrence DA. Latent-TGF-β: an overview. Mol Cell Biochem. 2001;219:163–170. doi: 10.1023/A:1010819716023. [DOI] [PubMed] [Google Scholar]
- Konig HG, Kogel D, Rami A, Prehn JH. TGFβ1 activates two distinct type I receptors in neurons: implications for neuronal NF-κB signaling. J Cell Biol. 2005;168:1077–1086. doi: 10.1083/jcb.200407027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnson KW, Parker WL, ten DP, Thorikay M, Philip A. ALK1 opposes ALK5/Smad3 signaling and expression of extracellular matrix components in human chondrocytes. J Bone Miner Res. 2008;23:896–906. doi: 10.1359/jbmr.080209. [DOI] [PubMed] [Google Scholar]
- Goumans MJ, Valdimarsdottir G, Itoh S, Lebrin F, Larsson J, Mummery C, Karlsson S, ten Dijke P. Activin receptor-like kinase (ALK)1 is an antagonistic mediator of lateral TGFbeta/ALK5 signaling. Mol Cell. 2003;12:817–828. doi: 10.1016/S1097-2765(03)00386-1. [DOI] [PubMed] [Google Scholar]
- Wu X, Ma J, Han JD, Wang N, Chen YG. Distinct regulation of gene expression in human endothelial cells by TGF-β and its receptors. Microvasc Res. 2006;71:12–19. doi: 10.1016/j.mvr.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Seki T, Hong KH, Oh SP. Nonoverlapping expression patterns of ALK1 and ALK5 reveal distinct roles of each receptor in vascular development. Lab Invest. 2006;86:116–129. doi: 10.1038/labinvest.3700376. [DOI] [PubMed] [Google Scholar]
- Goumans MJ, Valdimarsdottir G, Itoh S, Rosendahl A, Sideras P, ten Dijke P. Balancing the activation state of the endothelium via two distinct TGF-beta type I receptors. EMBO J. 2002;21:1743–1753. doi: 10.1093/emboj/21.7.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YE. Non-Smad pathways in TGF-β signaling. Cell Res. 2009;19:128–139. doi: 10.1038/cr.2008.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorrentino A, Thakur N, Grimsby S, Marcusson A, von BV, Schuster N, Zhang S, Heldin CH, Landstrom M. The type I TGF-β receptor engages TRAF6 to activate TAK1 in a receptor kinase-independent manner. Nat Cell Biol. 2008;10:1199–1207. doi: 10.1038/ncb1780. [DOI] [PubMed] [Google Scholar]
- Kamiya M, Harada A, Mizuno M, Iwata H, Yamada Y. Association between a polymorphism of the transforming growth factor-β1 gene and genetic susceptibility to ossification of the posterior longitudinal ligament in Japanese patients. Spine. 2001;26:1264–1266. doi: 10.1097/00007632-200106010-00017. [DOI] [PubMed] [Google Scholar]
- Yamada Y, Okuizumi H, Miyauchi A, Takagi Y, Ikeda K, Harada A. Association of transforming growth factor beta 1 genotype with spinal osteophytosis in Japanese women. Arthritis Rheum. 2000;43:452–460. doi: 10.1002/1529-0131(200002)43:2<452::AID-ANR28>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Kizawa H, Kou I, Iida A, Sudo A, Miyamoto Y, Fukuda A, Mabuchi A, Kotani A, Kawakami A, Yamamoto S, Uchida A, Nakamura K, Notoya K, Nakamura Y, Ikegawa S. An aspartic acid repeat polymorphism in asporin inhibits chondrogenesis and increases susceptibility to osteoarthritis. Nat Genet. 2005;37:138–144. doi: 10.1038/ng1496. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Lopez J, Pombo-Suarez M, Liz M, Gomez-Reino JJ, Gonzalez A. Lack of association of a variable number of aspartic acid residues in the asporin gene with osteoarthritis susceptibility: case-control studies in Spanish Caucasians. Arthritis Res Ther. 2006;8:R55. doi: 10.1186/ar1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Q, Shi D, Yi L, Ikegawa S, Wang Y, Nakamura T, Qiao D, Liu C, Dai J. Replication of the association of the aspartic acid repeat polymorphism in the asporin gene with knee-osteoarthritis susceptibility in Han Chinese. J Hum Genet. 2006;51:1068–1072. doi: 10.1007/s10038-006-0065-6. [DOI] [PubMed] [Google Scholar]
- Serra R, Johnson M, Filvaroff EH, Laborde J, Sheehan DM, Derynck R, Moses HL. Expression of a truncated, kinase defective TGF-beta type II receptor in mouse skeletal tissue promotes terminal chondrocyte differentiation and osteoarthritis. J Cell Biol. 1997;139:541–552. doi: 10.1083/jcb.139.2.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Chen L, Xu XL, Li CL, Huang CF, Deng CX. TGF-β/Smad3 signals repress chondrocyte hypertrophic differentiation and are required for maintaining articular cartilage. J Cell Biol. 2001;153:35–46. doi: 10.1083/jcb.153.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabovic B, Chen Y, Colarossi C, Zambuto L, Obata H, Rifkin DB. Bone defects in latent TGF-β binding protein (Ltbp)-3 null mice, a role for Ltbp in TGF- β presentation. J Endocrinol. 2002;175:129–141. doi: 10.1677/joe.0.1750129. [DOI] [PubMed] [Google Scholar]
- Vivien D, Galera P, Loyau G, Pujol JP. Differential response of cultured rabbit articular chondrocytes (RAC) to transforming growth factor beta (TGF-β) - evidence for a role of serum factors. Eur J Cell Biol. 1991;54:217–223. [PubMed] [Google Scholar]
- Vivien D, Galera P, Lebrun E, Daireaux M, Loyau G, Pujol JP. TGF-β-induced G2/M delay in proliferating rabbit articular chondrocytes is associated with an enhancement of replication rate and a cAMP decrease: possible involvement of pertussis toxin-sensitive pathway. J Cell Physiol. 1992;150:291–298. doi: 10.1002/jcp.1041500211. [DOI] [PubMed] [Google Scholar]
- Vivien D, Redini F, Galera P, Lebrun E, Loyau G, Pujol JP. Rabbit articular chondrocytes (RAC) express distinct transforming growth factor-beta receptor phenotypes as a function of cell cycle phases. Exp Cell Res. 1993;205:165–170. doi: 10.1006/excr.1993.1071. [DOI] [PubMed] [Google Scholar]
- Pujol JP, Galera P, Pronost S, Boumediene K, Vivien D, Macro M, Min W, Redini F, Penfornis H, Daireaux M. Transforming growth factor-beta (TGF-β) and articular chondrocytes. Ann Endocrinol (Paris) 1994;55:109–120. [PubMed] [Google Scholar]
- Boumediene K, Felisaz N, Pujol JP. Cell-cycle-dependent expression of transforming growth factor beta type I receptor correlates with differential proliferative effects of TGFβ1 in articular chondrocytes. Exp Cell Res. 1998;243:173–184. doi: 10.1006/excr.1998.4129. [DOI] [PubMed] [Google Scholar]
- Pujol JP, Chadjichristos C, Legendre F, Bauge C, Beauchef G, Andriamanalijaona R, Galera P, Boumediene K. Interleukin-1 and transforming growth factor-beta 1 as crucial factors in osteoarthritic cartilage metabolism. Connect Tissue Res. 2008;49:293–297. doi: 10.1080/03008200802148355. [DOI] [PubMed] [Google Scholar]
- Legendre F, Dudhia J, Pujol JP, Bogdanowicz P. JAK/STAT but not ERK1/ERK2 pathway mediates interleukin (IL)-6/soluble IL-6R down-regulation of type II collagen, aggrecan core, and link protein transcription in articular chondrocytes. Association with a down-regulation of SOX9 expression. J Biol Chem. 2003;278:2903–2912. doi: 10.1074/jbc.M110773200. [DOI] [PubMed] [Google Scholar]
- Blaney Davidson EN, Scharstuhl A, Vitters EL, Kraan PM van der, Berg WB van den. Reduced transforming growth factor-beta signaling in cartilage of old mice: role in impaired repair capacity. Arthritis Res Ther. 2005;7:R1338–R1347. doi: 10.1186/ar1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharstuhl A, van Beuningen HM, Vitters EL, Kraan PM van der, Berg WB van den. Loss of transforming growth factor counteraction on interleukin 1 mediated effects in cartilage of old mice. Ann Rheum Dis. 2002;61:1095–1098. doi: 10.1136/ard.61.12.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galera P, Redini F, Vivien D, Bonaventure J, Penfornis H, Loyau G, Pujol JP. Effect of transforming growth factor-beta 1 (TGF-β1) on matrix synthesis by monolayer cultures of rabbit articular chondrocytes during the dedifferentiation process. Exp Cell Res. 1992;200:379–392. doi: 10.1016/0014-4827(92)90186-C. [DOI] [PubMed] [Google Scholar]
- Kraan PM van der, Vitters E, van den BW. Differential effect of transforming growth factor beta on freshly isolated and cultured articular chondrocytes. J Rheumatol. 1992;19:140–145. [PubMed] [Google Scholar]
- Morales TI. Transforming growth factor-beta 1 stimulates synthesis of proteoglycan aggregates in calf articular cartilage organ cultures. Arch Biochem Biophys. 1991;286:99–106. doi: 10.1016/0003-9861(91)90013-9. [DOI] [PubMed] [Google Scholar]
- Morales TI, Roberts AB. Transforming growth factor beta regulates the metabolism of proteoglycans in bovine cartilage organ cultures. J Biol Chem. 1988;263:12828–12831. [PubMed] [Google Scholar]
- van Beuningen HM, Kraan PM van der, Arntz OJ, Berg WB van den. Transforming growth factor-beta 1 stimulates articular chondrocyte proteoglycan synthesis and induces osteophyte formation in the murine knee joint. Lab Invest. 1994;71:279–290. [PubMed] [Google Scholar]
- Blaney Davidson EN, Scharstuhl A, Vitters EL, Kraan PM van der, Berg WB van den. Reduced transforming growth factor-beta signaling in cartilage of old mice: role in impaired repair capacity. Arthritis Res Ther. 2005;7:R1338–R1347. doi: 10.1186/ar1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livne E, Laufer D, Blumenfeld I. Differential response of articular cartilage from young growing and mature old mice to IL-1 and TGF-β. Arch Gerontol Geriatr. 1997;24:211–221. doi: 10.1016/S0167-4943(96)00753-4. [DOI] [PubMed] [Google Scholar]
- McCaffrey TA, Falcone DJ. Evidence for an age-related dysfunction in the antiproliferative response to transforming growth factor-beta in vascular smooth muscle cells. Mol Biol Cell. 1993;4:315–322. doi: 10.1091/mbc.4.3.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mailhot G, Yang M, Mason-Savas A, Mackay CA, Leav I, Odgren PR. BMP-5 expression increases during chondrocyte differentiation in vivo and in vitro and promotes proliferation and cartilage matrix synthesis in primary chondrocyte cultures. J Cell Physiol. 2008;214:56–64. doi: 10.1002/jcp.21164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Schwarz EM, Zuscik MJ, Rosier RN, Ionescu AM, Puzas JE, Drissi H, Sheu TJ, O'keefe RJ. Retinoic acid stimulates chondrocyte differentiation and enhances bone morphogenetic protein effects through induction of Smad1 and Smad5. Endocrinology. 2003;144:2514–2523. doi: 10.1210/en.2002-220969. [DOI] [PubMed] [Google Scholar]
- Nishihara A, Fujii M, Sampath TK, Miyazono K, Reddi AH. Bone morphogenetic protein signaling in articular chondrocyte differentiation. Biochem Biophys Res Commun. 2003;301:617–622. doi: 10.1016/S0006-291X(02)03068-1. [DOI] [PubMed] [Google Scholar]
- Bau B, Haag J, Schmid E, Kaiser M, Gebhard PM, Aigner T. Bone morphogenetic protein-mediating receptor-associated Smads as well as common Smad are expressed in human articular chondrocytes but not up-regulated or down-regulated in osteoarthritic cartilage. J Bone Miner Res. 2002;17:2141–2150. doi: 10.1359/jbmr.2002.17.12.2141. [DOI] [PubMed] [Google Scholar]
- Drissi MH, Li X, Sheu TJ, Zuscik MJ, Schwarz EM, Puzas JE, Rosier RN, O'keefe RJ. Runx2/Cbfa1 stimulation by retinoic acid is potentiated by BMP2 signaling through interaction with Smad1 on the collagen X promoter in chondrocytes. J Cell Biochem. 2003;90:1287–1298. doi: 10.1002/jcb.10677. [DOI] [PubMed] [Google Scholar]
- Li X, Ionescu AM, Schwarz EM, Zhang X, Drissi H, Puzas JE, Rosier RN, Zuscik MJ, O'keefe RJ. Smad6 is induced by BMP-2 and modulates chondrocyte differentiation. J Orthop Res. 2003;21:908–913. doi: 10.1016/S0736-0266(03)00008-1. [DOI] [PubMed] [Google Scholar]
- Horiki M, Imamura T, Okamoto M, Hayashi M, Murai J, Myoui A, Ochi T, Miyazono K, Yoshikawa H, Tsumaki N. Smad6/Smurf1 overexpression in cartilage delays chondrocyte hypertrophy and causes dwarfism with osteopenia. J Cell Biol. 2004;165:433–445. doi: 10.1083/jcb.200311015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuscik MJ, Baden JF, Wu Q, Sheu TJ, Schwarz EM, Drissi H, O'keefe RJ, Puzas JE, Rosier RN. 5-Azacytidine alters TGF-β and BMP signaling and induces maturation in articular chondrocytes. J Cell Biochem. 2004;92:316–331. doi: 10.1002/jcb.20050. [DOI] [PubMed] [Google Scholar]
- Li TF, Darowish M, Zuscik MJ, Chen D, Schwarz EM, Rosier RN, Drissi H, O'Keefe RJ. Smad3-deficient chondrocytes have enhanced BMP signaling and accelerated differentiation. J Bone Miner Res. 2006;21:4–16. doi: 10.1359/JBMR.050911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Chen L, Xu X, Li C, Huang C, Deng CX. TGF-β/Smad3 signals repress chondrocyte hypertrophic differentiation and are required for maintaining articular cartilage. J Cell Biol. 2001;153:35–46. doi: 10.1083/jcb.153.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson CM, Schwarz EM, Reynolds PR, Puzas JE, Rosier RN, O'keefe RJ. Smad2 and 3 mediate transforming growth factor-beta1-induced inhibition of chondrocyte maturation. Endocrinology. 2000;141:4728–4735. doi: 10.1210/en.141.12.4728. [DOI] [PubMed] [Google Scholar]
- Zhou S, Eid K, Glowacki J. Cooperation between TGF-β and Wnt pathways during chondrocyte and adipocyte differentiation of human marrow stromal cells. J Bone Miner Res. 2004;19:463–470. doi: 10.1359/JBMR.0301239. [DOI] [PubMed] [Google Scholar]
- Zhu M, Tang D, Wu Q, Hao S, Chen M, Xie C, Rosier RN, O'Keefe RJ, Zuscik M, Chen D. Activation of β-catenin signaling in articular chondrocytes leads to osteoarthritis-like phenotype in adult beta-catenin conditional activation mice. J Bone Miner Res. 2009;24:12–21. doi: 10.1359/jbmr.080901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu M, Chen M, Zuscik M, Wu Q, Wang YJ, Rosier RN, O'Keefe RJ, Chen D. Inhibition of β-catenin signaling in articular chondrocytes results in articular cartilage destruction. Arthritis Rheum. 2008;58:2053–2064. doi: 10.1002/art.23614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng LH, Wang CJ, Ko JY, Sun YC, Su YS, Wang FS. Inflammation induction of Dickkopf-1 mediates chondrocyte apoptosis in osteoarthritic joint. Osteoarthritis Cartilage. 2009;17:933–943. doi: 10.1016/j.joca.2008.12.008. [DOI] [PubMed] [Google Scholar]
- Fuentealba LC, Eivers E, Ikeda A, Hurtado C, Kuroda H, Pera EM, De Robertis EM. Integrating patterning signals: Wnt/GSK3 regulates the duration of the BMP/Smad1 signal. Cell. 2007;131:980–993. doi: 10.1016/j.cell.2007.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furumatsu T, Ozaki T, Asahara H. Smad3 activates the Sox9-dependent transcription on chromatin. Int J Biochem Cell Biol. 2009;41:1198–1204. doi: 10.1016/j.biocel.2008.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay AM, Beck SC, Murphy JM, Barry FP, Chichester CO, Pittenger MF. Chondrogenic differentiation of cultured human mesenchymal stem cells from marrow. Tissue Eng. 1998;4:415–428. doi: 10.1089/ten.1998.4.415. [DOI] [PubMed] [Google Scholar]
- Hecht J, Seitz V, Urban M, Wagner F, Robinson PN, Stiege A, Dieterich C, Kornak U, Wilkening U, Brieske N, Zwingman C, Kidess A, Stricker S, Mundlos S. Detection of novel skeletogenesis target genes by comprehensive analysis of a Runx2-/- mouse model. Gene Expr Patterns. 2007;7:102–112. doi: 10.1016/j.modgep.2006.05.014. [DOI] [PubMed] [Google Scholar]
- Javed A, Bae JS, Afzal F, Gutierrez S, Pratap J, Zaidi SK, Lou Y, van Wijnen AJ, Stein JL, Stein GS, Lian JB. Structural coupling of Smad and Runx2 for execution of the BMP2 osteogenic signal. J Biol Chem. 2008;283:8412–8422. doi: 10.1074/jbc.M705578200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leboy P, Grasso-Knight G, D'Angelo M, Volk SW, Lian JV, Drissi H, Stein GS, Adams SL. Smad-Runx interactions during chondrocyte maturation. J Bone Joint Surg Am. 2001;83A(Suppl 1):S15–S22. [PubMed] [Google Scholar]
- Hjelmeland AB, Schilling SH, Guo X, Quarles D, Wang XF. Loss of Smad3- mediated negative regulation of Runx2 activity leads to an alteration in cell fate determination. Mol Cell Biol. 2005;25:9460–9468. doi: 10.1128/MCB.25.21.9460-9468.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaney Davidson EN, Vitters EL, Kraan PM van der, Berg WB van den. Expression of transforming growth factor-beta (TGFβ) and the TGFβ signalling molecule SMAD-2P in spontaneous and instability-induced osteoarthritis: role in cartilage degradation, chondrogenesis and osteophyte formation. Ann Rheum Dis. 2006;65:1414–1421. doi: 10.1136/ard.2005.045971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaney Davidson EN, Remst DF, Vitters EL, van Beuningen HM, Blom AB, Goumans MJ, Berg WB van den, Kraan PM van der. Increase in ALK1/ALK5 ratio as a cause for elevated MMP-13 expression in osteoarthritis in humans and mice. J Immunol. 2009;182:7937–7945. doi: 10.4049/jimmunol.0803991. [DOI] [PubMed] [Google Scholar]
- Finnson KW, Parker WL, ten DP, Thorikay M, Philip A. ALK1 opposes ALK5/Smad3 signaling and expression of extracellular matrix components in human chondrocytes. J Bone Miner Res. 2008;23:896–906. doi: 10.1359/jbmr.080209. [DOI] [PubMed] [Google Scholar]
- van Beuningen HM, Glansbeek HL, Kraan PM van der, Berg WB van den. Differential effects of local application of BMP-2 or TGF-β1 on both articular cartilage composition and osteophyte formation. Osteoarthritis Cartilage. 1998;6:306–317. doi: 10.1053/joca.1998.0129. [DOI] [PubMed] [Google Scholar]
- Glansbeek HL, van Beuningen HM, Vitters EL, Kraan PM van der, Berg WB van den. Stimulation of articular cartilage repair in established arthritis by local administration of transforming growth factor-beta into murine knee joints. Lab Invest. 1998;78:133–142. [PubMed] [Google Scholar]
- van Beuningen HM, Kraan PM van der, Arntz OJ, Berg WB van den. In vivo protection against interleukin-1-induced articular cartilage damage by transforming growth factor-beta 1: age-related differences. Ann Rheum Dis. 1994;53:593–600. doi: 10.1136/ard.53.9.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Beuningen HM, Kraan PM van der, Arntz OJ, Berg WB van den. Protection from interleukin 1 induced destruction of articular cartilage by transforming growth factor beta: studies in anatomically intact cartilage in vitro and in vivo. Ann Rheum Dis. 1993;52:185–191. doi: 10.1136/ard.52.3.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Beuningen HM, Glansbeek HL, Kraan PM van der, Berg WB van den. Osteoarthritis-like changes in the murine knee joint resulting from intra-articular transforming growth factor-beta injections. Osteoarthritis Cartilage. 2000;8:25–33. doi: 10.1053/joca.1999.0267. [DOI] [PubMed] [Google Scholar]
- Scharstuhl A, van Beuningen HM, Vitters EL, Kraan PM van der, Berg WB van den. Loss of transforming growth factor counteraction on interleukin 1 mediated effects in cartilage of old mice. Ann Rheum Dis. 2002;61:1095–1098. doi: 10.1136/ard.61.12.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaney Davidson EN, Scharstuhl A, Vitters EL, Kraan PM van der, Berg WB van den. Reduced transforming growth factor-beta signaling in cartilage of old mice: role in impaired repair capacity. Arthritis Res Ther. 2005;7:R1338–R1347. doi: 10.1186/ar1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesseur I, Zou K, Esposito L, Bard F, Berber E, Can JV, Lin AH, Crews L, Tremblay P, Mathews P, Mucke L, Masliah E, Wyss-Coray T. Deficiency in neuronal TGF-β signaling promotes neurodegeneration and Alzheimer's pathology. J Clin Invest. 2006;116:3060–3069. doi: 10.1172/JCI27341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das P, Golde T. Dysfunction of TGF-β signaling in Alzheimer's disease. J Clin Invest. 2006;116:2855–2857. doi: 10.1172/JCI30284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Zou K, Tesseur I, Wyss-Coray T. Small molecule TGF-β mimetics as potential neuroprotective factors. Curr Alzheimer Res. 2005;2:183–186. doi: 10.2174/1567205053585756. [DOI] [PubMed] [Google Scholar]
- Glaser KB, Li J, Aakre ME, Morgan DW, Sheppard G, Stewart KD, Pollock J, Lee P, O'Connor CZ, Anderson SN, Mussatto DJ, Wegner CW, Moses HL. Transforming growth factor beta mimetics: discovery of 7-[4-(4-cyanophenyl)phenoxy]-heptanohydroxamic acid, a biaryl hydroxamate inhibitor of histone deacetylase. Mol Cancer Ther. 2002;1:759–768. [PubMed] [Google Scholar]
- Bernard DJ, Lee KB, Santos MM. Activin B can signal through both ALK4 and ALK7 in gonadotrope cells. Reprod Biol Endocrinol. 2006;13:52. doi: 10.1186/1477-7827-4-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez L, Sanz-Rodriguez F, Blanco FJ, Bernabeu C, Botella LM. Hereditary hemorrhagic telangiectasia, a vascular dysplasia affecting the TGF-β signaling pathway. Clin Med Res. 2006;4:66–78. doi: 10.3121/cmr.4.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Ionescu AM, Schwarz EM, Zhang X, Drissi H, Puzas JE, Rosier RN, Zuscik MJ, O'Keefe RJ. Smad6 is induced by BMP-2 and modulates chondrocyte differentiation. J Orthop Res. 2003;21:908–913. doi: 10.1016/S0736-0266(03)00008-1. [DOI] [PubMed] [Google Scholar]
- Iwai T, Murai J, Yoshikawa H, Tsumaki N. Smad7 inhibits chondrocyte differentiation at multiple steps during endochondral bone formation and down-regulates p38 MAPK pathways. J Biol Chem. 2008;283:27154–27164. doi: 10.1074/jbc.M801175200. [DOI] [PubMed] [Google Scholar]
- Fuentealba LC, Eivers E, Ikeda A, Hurtado C, Kuroda H, Pera EM, De Robertis EM. Integrating patterning signals: Wnt/GSK3 regulates the duration of the BMP/Smad1 signal. Cell. 2007;131:980–993. doi: 10.1016/j.cell.2007.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue Y, Imamura T. Regulation of TGF-β family signaling by E3 ubiquitin ligases. Cancer Sci. 2008;99:2107–2112. doi: 10.1111/j.1349-7006.2008.00925.x. [DOI] [PMC free article] [PubMed] [Google Scholar]