Abstract
A food supplement containing fish oils, urtica dioica, zinc, and vitamin E (Phytalgic®) for osteoarthritis (OA) has now been tested in a placebo-controlled trial for 3 months and according to the authors has a very large clinical effect, considerably larger than that of any other known product. Even experts endorsing nutraceuticals for OA symptoms would probably agree that a nutraceutical with an effect size above 0.5 is rarely seen. Despite our concerns about the fact that trial registration took place after the study was completed and the likelihood that patients would note the taste of fish, a circumstance that would lead to detection bias, we consider these data promising though with a high risk of bias.
Given the modest results of ordinary pharmacological therapy for osteoarthritis (OA), it was of great interest to see the results by Jacquet and colleagues [1] in the previous issue of Arthritis Research & Therapy. The authors tested a new nutraceutical, a food supplement marketed as Phytalgic®, in a randomized controlled trial (RCT) design. The protocol of this trial was registered in ClinicalTrials.gov (NCT00666523) [2]. However, one aspect of concern is whether the registration was pre specified. The registration claims that exactly 81 patients will be randomly assigned. How can a protocol registration foresee a random assignment of 41 patients to one group and 40 to the other group when it is a consequence of excluding 14 non-eligible patients, as presented in the CONSORT (CONsolidated Standards of Reporting Trials) Statement?
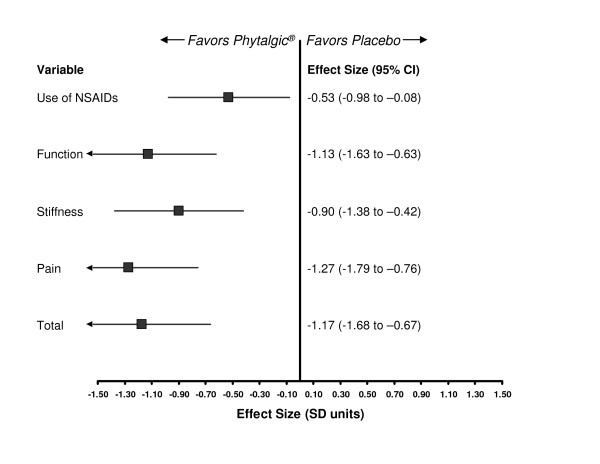
The authors present data for Phytalgic® [1] which are considerably more promising than expected and thus should be scrutinized for clinical effect and possible bias [3]. According to the authors, Phytalgic® consists of capsules containing fish oils, urtica dioica, zinc, and vitamin E. Jacquet and colleagues [1] randomly assigned some 81 OA patients to receive either Phytalgic® or a matching placebo consisting of 'non-fish oil'. Participants were an average of 57 years of age (range of 28 to 84 years) at entry, had either knee or hip OA, and were regular users of nonsteroidal anti-inflammatory drugs (NSAIDs) or analgesics. The primary outcome of this 3-month trial was use of NSAIDs or analgesics at follow-up. According to ClinicalTrials.gov [2], Jacquet and colleagues [1] considered the WOMAC (Western Ontario and McMaster Universities Osteoarthritis Index) function scale a secondary outcome measure, and none of the other WOMAC subscales is mentioned in the trial registration. In accordance with recent standards on how to evaluate the results of OA trials [3,4], Figure 1 presents a summary of findings as generic effect sizes (ESs) based on the standardized mean difference, comparing the experimental drug (Phytalgic®) with a placebo, for each of the continuous outcomes measured on different scales.
Figure 1.
Forest plot of outcomes showing effect sizes comparing Phytalgic® with placebo in osteoarthritis patients, presented as standardized mean differences. CI, confidence interval; NSAID, nonsteroidal anti-inflammatory drug; SD, standard deviation.
The results of this trial were remarkable. For example, the ES for pain reduction was -1.27, which corresponds to a very large ES and indicates that Phytalgic® is 76% more efficacious than intra-articular corticosteroid therapy for knee OA [4]. We find that very hard to believe.
During the last decade, the use of nutraceuticals has generated a great deal of interest. In our experience from trials [5] and in the results of meta-analyses on ASU (avocado-soybean unsaponifiable) (ES = -0.39) [6], rosehip powder (ES = -0.37) [7], and diacerein (ES = -0.24) [8], we have never seen anything as efficacious as Phytalgic® [3]. The same thing applies in the glucosamine area. It is now becoming evident that preparations with glucosamine hydrochloride do not ameliorate OA [9], and results of trials on different glucosamine sulfate preparations are very conflicted with lots of inconsistency [3,9]. The glucosamine sulfate product from Rottapharm Madaus (Monza, Italy) is one exception to this [10]. While trials of this particular preparation showed promise in the early days with large clinical effects on OA in smaller studies, later and presumably more strictly led RCTs with less bias have claimed results that are more moderate, with an anticipated overall ES on pain of -0.33 standard deviation units (95% confidence interval -0.49 to -0.17) [10]. Even OA experts who endorse nutra ceuticals (for example, glucosamine) would probably agree that a nutraceutical with an ES above 0.5 is rarely seen.
There is empirical evidence that OA trials may be affected by selection and detection bias [11]. Allegedly, few patients noted the taste of fish oil during 12 weeks of taking such capsules three times per day. We argue that a fishy taste in the mouth might certainly cause detection bias. Assessment of the trial reporting in terms of risk of bias, the use of random assignment, and subsequent concealment of allocation would qualify as adequate (that is, low risk of selection bias); it seems reasonable that at baseline the patients in the study groups were similar with respect to prognostic factors. The reporting of double-blinding supports a low risk of performance bias as the authors state that the manufacturer provided both the Phytalgic® and placebo capsules and that it claimed that they were identical and indistinguishable. We argue, however, that it might be difficult to hide the taste of fish oil during a 3-month trial, probably as difficult as it is to hide the taste of ginger [5]. Finally, deviations from protocol and loss to follow-up often lead to the exclusion of patients after they have been allocated to treatment groups, and this may introduce attrition bias [12]. We are concerned about the fact that the trial registration was done after study completion (April 2008). Thus, we would categorize the risk of attrition bias as being at best unclear as there is a possibility that some patients were excluded from the analyses. Although the authors performed their analyses according to the intention-to-treat principle on what they claim is the correct sample size, we worry about the fact that the attrition rate was 10% (4/40) in the placebo group, whereas only 2% (1/41) withdrew from Phytalgic®.
With that said, we are now faced with some very promising results of Phytalgic® [1], and further experience is needed to show whether this product on a larger scale will become a relevant treatment option for OA [3,7]. As previously pointed out, the largest studies and the studies that are strictly monitored by good clinical practices are usually directly sponsored by the product manufacturers [10]. A fully independent analysis of a product like Phytalgic® would require funding from official organizations (for example, the National Institutes of Health, which indeed needs reshuffling of its priorities). These initial data on Phytalgic® would seem to justify such action. If these data are confirmed, a goldmine has been struck and OA therapy is in for dramatic changes.
Abbreviations
ES: effect size; NSAID: nonsteroidal anti-inflammatory drug; OA: osteoarthritis; RCT: randomized controlled trial; WOMAC: Western Ontario and McMaster Universities Osteoarthritis Index.
Competing interests
The funding agencies (The Oak Foundation and The Danish Rheumatism Association) had no role in writing the report or in the decision to submit the manuscript for publication. Neither of the authors is affiliated with or funded by any manufacturer of drugs or nutraceuticals. RC is statistical editor for the Cochrane Musculoskeletal Group and a member of the GRADE Working Group. RC and HB have received research or institutional support, educational grants, equipment, services, or expenses from Abbott (Abbott Park, IL, USA), Amgen (Thousand Oaks, CA, USA), Astellas Pharma (Tokyo, Japan), Axellus (Oslo, Norway), Bristol-Myers Squibb Company (Princeton, NJ, USA), Cambridge Manufacturing Company Limited (Corby, UK), Dansk Droge (now part of Orkla ASA, Oslo, Norway), DSM Nutritional Products (Basel, Switzerland), Laboratoires Expanscience (Courbevoie, France), Hyben Vital ApS (Tranekær, Denmark), HypoSafe A/S (Lyngby, Denmark), Mundi pharma (Cambridge, UK), Norpharma A/S (Hørsholm, Denmark), Pharmavie (Ivry-sur-Seine, France), Pfizer Inc (New York, NY, USA), Roche (Basel, Switzerland), sanofi-aventis (Paris, France), Scandinavian Clinical Nutrition (Stockholm, Sweden), and Wyeth (Madison, NJ, USA).
See related research article by Jacquet et al., http://arthritis-research.com/content/11/6/R192
Contributor Information
Robin Christensen, Email: Robin.Christensen@frh.regionh.dk.
Henning Bliddal, Email: Henning.Bliddal@frh.regionh.dk.
Acknowledgements
This work was supported by grants from The Oak Foundation, The Danish Rheumatism Association, and Copenhagen University Hospital (Frederiksberg, Denmark).
References
- Jacquet A, Girodet PO, Pariente A, Forest K, Mallet L, Moore N. Phytalgic(R) a food supplement, vs placebo in patients with osteoarthritis of the knee or hip: a randomised double-blind placebo-controlled clinical trial. Arthritis Res Ther. 2009;11:R192. doi: 10.1186/ar2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ClinicalTrials.gov homepage. http://clinicaltrials.gov
- Bliddal H, Christensen R. The treatment and prevention of knee osteoarthritis: a tool for clinical decision-making. Expert Opin Pharmacother. 2009;10:1793–1804. doi: 10.1517/14656560903018911. [DOI] [PubMed] [Google Scholar]
- Zhang W, Moskowitz RW, Nuki G, Abramson S, Altman RD, Arden N, Bierma-Zeinstra S, Brandt KD, Croft P, Doherty M, Dougados M, Hochberg M, Hunter DJ, Kwoh K, Lohmander LS, Tugwell P. OARSI recommendations for the management of hip and knee osteoarthritis, Part I: Critical appraisal of existing treatment guidelines and systematic review of current research evidence. Osteoarthritis Cartilage. 2007;15:981–1000. doi: 10.1016/j.joca.2007.06.014. [DOI] [PubMed] [Google Scholar]
- Bliddal H, Rosetzsky A, Schlichting P, Weidner MS, Andersen LA, Ibfelt HH, Christensen K, Jensen ON, Barslev J. A randomized, placebo-controlled, cross-over study of ginger extracts and ibuprofen in osteoarthritis. Osteoarthritis Cartilage. 2000;8:9–12. doi: 10.1053/joca.1999.0264. [DOI] [PubMed] [Google Scholar]
- Christensen R, Bartels EM, Astrup A, Bliddal H. Symptomatic efficacy of avocado-soybean unsaponifiables (ASU) in osteoarthritis (OA) patients: a meta-analysis of randomized controlled trials. Osteoarthritis Cartilage. 2008;16:399–408. doi: 10.1016/j.joca.2007.10.003. [DOI] [PubMed] [Google Scholar]
- Christensen R, Bartels EM, Altman RD, Astrup A, Bliddal H. Does the hip powder of Rosa canina (rosehip) reduce pain in osteoarthritis patients? - a meta-analysis of randomized controlled trials. Osteoarthritis Cartilage. 2008;16:965–972. doi: 10.1016/j.joca.2008.03.001. [DOI] [PubMed] [Google Scholar]
- Bartels EM, Bliddal H, Schondorff PK, Altman RD, Zhang W, Christensen R. Symptomatic efficacy and safety of diacerein in the treatment of osteoarthritis: a meta-analysis of randomized placebo-controlled trials. Osteoarthritis Cartilage. 2009. in press . [DOI] [PubMed]
- Vlad SC, LaValley MP, McAlindon TE, Felson DT. Glucosamine for pain in osteoarthritis: why do trial results differ? Arthritis Rheum. 2007;56:2267–2277. doi: 10.1002/art.22728. [DOI] [PubMed] [Google Scholar]
- Reginster JY. The efficacy of glucosamine sulfate in osteoarthritis: financial and nonfinancial conflict of interest. Arthritis Rheum. 2007;56:2105–2110. doi: 10.1002/art.22852. [DOI] [PubMed] [Google Scholar]
- Nuesch E, Reichenbach S, Trelle S, Rutjes AW, Liewald K, Sterchi R, Altman DG, Juni P. The importance of allocation concealment and patient blinding in osteoarthritis trials: a meta-epidemiologic study. Arthritis Rheum. 2009;61:1633–1641. doi: 10.1002/art.24894. [DOI] [PubMed] [Google Scholar]
- Nuesch E, Trelle S, Reichenbach S, Rutjes AW, Burgi E, Scherer M, Altman DG, Juni P. The effects of excluding patients from the analysis in randomised controlled trials: meta-epidemiological study. BMJ. 2009;339:b3244. doi: 10.1136/bmj.b3244. [DOI] [PMC free article] [PubMed] [Google Scholar]