Abstract
Introduction
Bisphosphonates are the most commonly used drugs for the prevention and treatment of osteoporosis. Although a recent FDA review of the results of clinical trials reported no clear link between bisphosphonates and serious or non-serious atrial fibrillation (AF), some epidemiologic studies have suggested an association between AF and bisphosphonates.
Methods
We conducted a meta-analysis of non-experimental studies to evaluate the risk of AF associated with bisphosphonates. Studies were identified by searching MEDLINE and EMBASE using a combination of the Medical Subject Headings and keywords. Our search was limited to English language articles. The pooled estimates of odds ratios (OR) as a measure of effect size were calculated using a random effects model.
Results
Seven eligible studies with 266,761 patients were identified: three cohort, three case-control, and one self-controlled case series. Bisphosphonate exposure was not associated with an increased risk of AF [pooled multivariate OR 1.04, 95% confidence interval (CI) 0.92-1.16] after adjusting for known risk factors. Moderate heterogeneity was noted (I-squared score = 62.8%). Stratified analyses by study design, cohort versus case-control studies, yielded similar results. Egger's and Begg's tests did not suggest an evidence of publication bias (P = 0.90, 1.00 respectively). No clear asymmetry was observed in the funnel plot analysis. Few studies compared risk between bisphosphonates or by dosing.
Conclusions
Our study did not find an association between bisphosphonate exposure and AF. This finding is consistent with the FDA's statement.
Introduction
Osteoporosis is a major public health threat for an estimated 44 million Americans or 55% of the population 50 years of age and older [1]. There were approximately two million new fractures in the US in 2005 with a direct medical cost of US$17 billion [2]. Bisphosphonates are the main therapy for postmenopausal and glucocorticoid-induced osteoporosis. Currently, four oral or intravenous forms of bisphosphonates (alendronate, risedronate, ibandronate, and zoledronic acid) are approved for the treatment of osteoporosis in the US. Known side effects of bisphosphonates include gastrointestinal mucosal irritation, such as esophageal ulcer, bone pain, muscle cramps or pain, hyperphosphatemia, hypocalcemia, and rarely osteonecrosis of the jaw [3,4].
An increased risk of atrial fibrillation (AF) has been noted with use of bisphosphonates, especially with intravenous zoledronic acid [5-7]. AF is the most common arrhythmia among older adults. Common risk factors include older age, male gender, dilated cardiomyopathy, diabetes, hypertension, coronary artery disease, obstructive sleep apnea, cardiac surgery, high-dose corticosteroid therapy, alcohol excess, and alcohol withdrawal [8-10]. AF contributes to approximately 35% of strokes in an octogenarian population, increases the overall risk of stroke by five-fold, and is associated with particularly severe strokes [11]. With the aging population, AF and its consequences will become an increasingly common medical and economic problem.
In October 2007, the US Food and Drug Administration (FDA) first announced that the agency was assessing the potential risk of AF associated with bisphosphonate use [12]. After reviewing data from spontaneous post-marketing reports and clinical trials of bisphosphonates, the FDA stated that healthcare providers and patients should not change either their prescribing practices or their use of bisphosphonates [12]. A year later, the FDA released updated information based on further information from four placebo-controlled clinical trials and concluded that there was no clear association between bisphosphonate exposure and the rate of serious or non-serious AF events [13]. At that time, the FDA stated that that they would explore the feasibility of conducting additional epidemiologic studies to further examine the issue. Subsequently, a number of large population-based studies examining the risk of AF with bisphosphonates have been published [7,14-19]. The results from two meta-analytic studies [20,21] are also available but information one could draw from these studies are somewhat limited owing to a small study size and significant heterogeneity among the included studies. Population-based epidemiologic studies offer several advantageous over clinical trial data, particularly to study unexpected and rare adverse reactions such as AF in bisphosphonate users (incidence rate of AF is less than 2 per 100 person-year [14,17]). Clinical trials are often underpowered to detect rare adverse events and lack of generalizability owing to specific inclusion and exclusion criteria [22]. Given such potential limitations of clinical trials and meta-analyses of clinical trials, we therefore conducted a systematic review and meta-analysis of non-experimental studies to determine the association between the use of bisphosphonates and the risk of AF or flutter.
Materials and methods
Data sources
We searched two major electronic databases -- MEDLINE (1981 to September 2009), EMBASE (1981 to September 2009) -- for studies of the association between AF and the use of bisphosphonates in patients with osteoporosis. We also searched abstracts from scientific meetings (American College of Rheumatology and International Conference on Pharmacoepidemiology and Therapeutic Risk Management) and bibliographies of identified reports and review articles for additional references. We imposed no geographic restrictions. Our search strategy is described in Figure 1. Only studies published in English were included.
Figure 1.
Search strategy.
Study eligibility and selection
To be eligible for inclusion, we only considered population-based non-experimental studies of adult patients, studies reporting the risk of AF or flutter associated with bisphosphonate use, and studies examining the effects of bisphosphonates for the prevention or treatment of osteoporosis or fractures. Studies of patients with malignancy such as breast or prostate cancer were excluded. Two authors (SYK and MJK) independently screened each of the potential titles, abstracts, and/or full-texts to determine if they were eligible for inclusion. Areas of disagreement or uncertainty were resolved by consensus.
Data abstraction and quality assessment
All data were independently abstracted in duplicate by two authors (SYK and MJK) using a data abstraction form. Data on the study characteristics, such as author name, year of publication, country, sample size, mean age of patients, type of bisphosphonate used, and number of outcome, unadjusted and multivariate, or adjusted, and risk of AF, were collected. The Newcastle-Ottawa Scale was used to assess the quality of studies [23] (Tables 1 and 2). A quality score was calculated on the basis of three major components: selection of the study groups (0 to 4 points), quality of the adjustment for confounding (0 to 2 points) and ascertainment of the outcome of interest in the cohorts (0 to 3 points). A higher score represents better methodological quality. Adjustment for known AF risk factors, duration of follow up of at least one year for cohort studies, and adequate outcome ascertainment were criteria of higher quality.
Table 1.
Summary of included cohort studies and characteristics
| Study, year, country | Subjects | Mean age (years) |
Mean follow-up (years) | Type of bisphosphonate | AF definition | Total AF events (n) | Variables matched and/or controlled | Quality assessment score§ |
|---|---|---|---|---|---|---|---|---|
| Bunch and colleagues [15], 2009, USA | 9,623 (98 BIS users and 9,525 non-users) in angiographic research database | 60 | 4.1 | Alendronate Etidronate Ibandronate Risedronate Zoledronic acid |
Hospital discharge ICD-9 codes and EKG data | 974 | Age, HTN, hyperlipidemia, DM, HF, CAD, etc | 4/2/3 |
| 37,485 (7,489 BIS users and 29,996 non-users) in a commercial health care system database | 51 | 4.6 | 1012 | |||||
| Abrahamsen and colleagues [14], 2009, Denmark | 43,033 patients (14,302 BIS users and 28,731 non-users) with fractures in the National Hospital Discharge register | 74 | 2.7 | Alendronate Clodronate Etidronate Ibandronate Risedronate |
Prescription for a cardiac glycoside +/- ICD-10 codes | 797 * | Age, gender, fracture sites, medications use for DM, HTN, hypercholesterolemia, and thrombosis, and Charlson index | 4/2/3 |
| Huang and colleagues [17], 2009, Taiwan | 27,257 women (21,037 BIS users and 6,220 raloxifene users) with osteoporosis in the National Health Insurance database | 74 | 0.6 | Alendronate | Inpatient or outpatient ICD-9 codes | 821 | DM, CAD, COPD, history of AF, HTN, and total defined daily dose of drugs | 3/2/2 |
* only in BIS users, § Methodologic quality assessment score based on Newcastle-Ottawa Scale [23]; selection (0 to 4), comparability (0 to 2), and outcome score (0 to 3) points with a higher score representing a better quality.
AF, atrial fibrillation; BIS, bisphosphonates; CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; DM, diabetes mellitus; EKG, electrocardiogram; HF, heart failure; HTN, hypertension; ICD, International Classification of Diseases.
Table 2.
Summary of included case-control studies and characteristics
| Study, Year, Country | Subjects | Mean age (year) |
Mean duration (year) | Type of bisphosphonate | AF definition | Total AF events (n) | Variables matched and/or controlled | Quality assessment score§ |
|---|---|---|---|---|---|---|---|---|
| Heckbert and colleagues [7], 2008, USA | 1,685 women in a commercial health care system database | 73 | 20 | Alendronate | ICD-9 codes verified by medical records +/- EKG | 719 | Age, HTN, calendar year, osteoporosis, and any cardiovascular disease | 4/2/3 |
| Sorensen and colleagues [19], 2008, Denmark | 81,640 women in the National Registry of Patients | >75 | Not reported | Alendronate Etidronate |
ICD-8 and 10 codes | 13586 | Age, county, cardiovascular disease, renal failure, DM, HRT, fracture, cancer, alcoholism, pulmonary disease, anti-thyroid drugs, osteoporosis, steroid use, etc | 3/2/3 |
| Grosso and colleagues* [16], 2009, UK | 2,195 women with AF in the UK General Practice Research Database | 82 | 13 | Alendronate Risedronate |
Diagnosis codes and Read codes | 2195 | Age+ | 3/2/3 |
| Levesque and colleagues [18], 2009, Canada | 63,843 adults (BIS users and raloxifene users) in the Quebec health database | 75 | 4 | Alendronate Etidronatea Risedronatea |
Not reported | 2149 | Age, cohort entry year, follow-up, CAD, HTN, and use of HRT | N/A |
AF, atrial fibrillation; BIS, bisphosphonates; CAD, coronary artery disease; DM, diabetes mellitus; EKG, electrocardiogram; HRT, hormone replacement therapy; HTN, hypertension; ICD, International Classification of Diseases.
* self-controlled case-series. + no other adjustment not needed in self-controlled study, N/A, not applicable (only the abstract was available at the time of this study), a risks specific to etidronate or risedronate not used in meta-analysis, § Methodologic quality assessment score based on Newcastle-Ottawa Scale [23]; selection (0 to 4), comparability (0 to 2), and outcome score (0 to 3) points with a higher score representing a better quality.
Statistical analysis
We chose to use odds ratio (OR) as a measure of effect size because it is one of the most commonly used effect measures for observational studies [24]. We assumed that either relative risk or hazard ratio was equivalent to OR as the probability of the outcome, AF, was expected to be low [25]. Pooled estimates of multivariate ORs were calculated using the DerSimonian and Laird random effects model [26,27] for AF. This statistical technique weighs individual studies by sample size and variance (both within- and between-study variance) and yields a pooled point estimate of OR and a 95% confidence interval (CI). The DerSimonian and Laird technique was considered an appropriate pooling technique because of the relative heterogeneity of the source population in each study. Subgroup analysis by study design was performed. We also evaluated the presence of heterogeneity across trials by using the I2 statistic, which quantifies the percentage of variability that can be attributed to between-study differences [28]. Sensitivity analysis was performed to examine the effect of studies lacking information on methodologic quality on the pooled estimates. To assess the potential for publication bias, we performed the Begg's and the Egger's tests and constructed funnel plots to visualize possible asymmetry [29]. Due to the limited number of studies, no further subgroup analysis or meta-regression was considered. All statistical analyses were performed in Stata 10 (Stata Corp, College Station, TX, USA). We followed the Meta-analyses of Observational Studies in Epidemiology guidelines [30] in the report of this meta-analysis.
Results
Study characteristics and quality
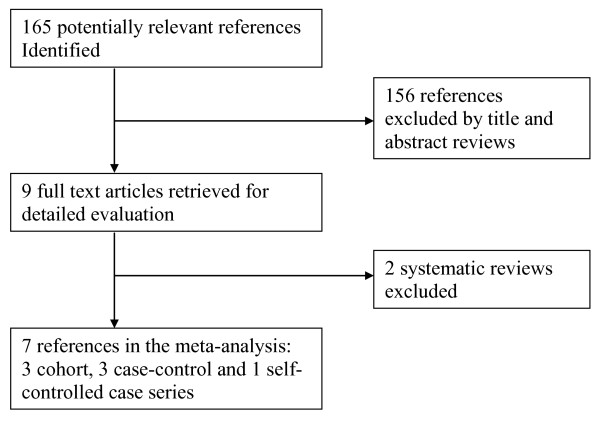
The electronic search retrieved 160 potentially relevant references (Figure 2). A manual search of bibliographies of relevant review papers and the published abstracts of the annual meetings of the American College of Rheumatology and the International Conference on Pharmacoepidemiology (2008 to 2009) identified five additional references. On initial screening, 156 of 165 were excluded based on titles and abstracts. Nine full-text articles were subsequently retrieved for detailed evaluation. Of those, seven studies - three cohort, three case-control, and one self-controlled case series [7,14-19] - representing data from 266,761 patients were finally included in the meta-analysis and two systematic reviews [20,21] were excluded. The characteristics of the included studies and of their participants are summarized in Tables 1 and 2. The self-controlled case series study by Grosso and colleagues [16] was considered as a case-control study for analysis.
Figure 2.
Selection of studies included in the analysis.
One study was completed in Taiwan [17], and all others used population-based data from either North America [7,15,18] or Europe [14,16,19]. The number of subjects in each study ranged from 1,685 to 81,640. The majority of study subjects were aged over 70 years. Two studies compared bisphosphonate users with raloxifene users [17,18] and the rest compared bisphosphonate users with non-users [7,14-16,19]. One study [15] included zoledronic acid and the others only considered oral bisphosphonates. In the study by Levesque and colleagues [18], the risk of AF specific to alendronate, risedronate, and etidronate was reported; however, only the risk of AF among alendronate users was included in our meta-analysis because the majority of the included studies focused on either alendronate or a combination of different bisphosphonates as a drug class. Results from two different patient cohorts were available in the study by Bunch and colleagues [15].
Most studies were of moderate to high quality although only three studies defined the outcome of AF based on a combination of diagnosis codes and clinical records [7,14,15]. Quality of the study by Levesque and colleagues [18] was not assessed because it was only available as an abstract.
Bisphosphonates and atrial fibrillation
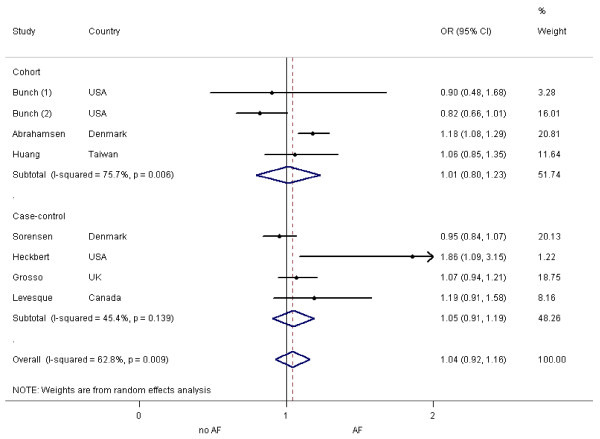
The overall pooled estimate of multivariate OR for AF based on eight patient cohorts from seven studies adjusting for known AF risk factors was 1.04 (95% CI = 0.92 to 1.16; Figure 3). Moderate heterogeneity was noted (I2 = 62.8%, P = 0.009). Our subgroup analysis stratified by study design yielded similar results. The pooled estimate of multivariate OR based on three cohort studies [14,15,17] was 1.01 (95% CI = 0.80 to 1.23) with moderate-to-severe heterogeneity (I2 = 75.7%, P = 0.01). The pooled estimate of multivariate OR based on four case-control studies [7,16,18,19] was 1.05 (95% CI = 0.91 to 1.19) with moderate heterogeneity (I2 = 45.4%, P = 0.14).
Figure 3.
Random effects analysis of the studies for the association between AF and bisphosphonate exposure, adjusted for known risk factors. Points (dot) and overall (diamond) estimates are given as odds ratios (OR) with 95% confidence interval (CI) (horizontal bar), AF, atrial fibrillation.
Sensitivity analysis was carried out by excluding the study by Levesque and colleagues [18], of which quality was not known. The pooled estimate of multivariate OR remained almost the same (1.03; 95% CI = 0.90 to 1.15).
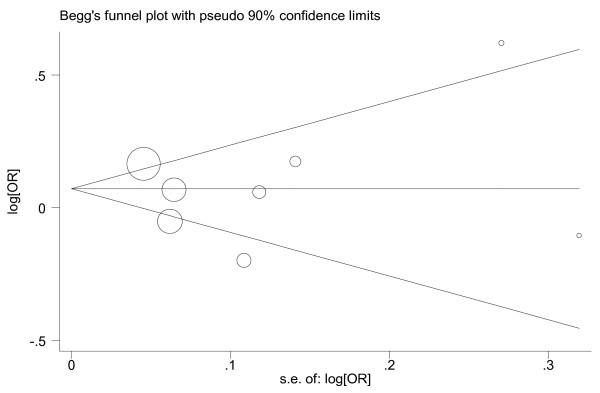
Publication bias assessment
The funnel plot was visually examined and did not reveal any obvious asymmetry (Figure 4). There was no statistical evidence of publication bias among the included studies by using Egger's (P = 0.90) and Begg's (P = 1.00) tests.
Figure 4.
Begg's funnel plot for publication bias assessment. The size of circle represents the weight of each study in our analysis. OR, odds ratio; se, standard error.
Discussion
Although a few plausible mechanisms exist for the association between the use of bisphosphonates and AF -- electrolyte imbalance such as hypocalcemia or bisphosphonate-induced inflammation affecting atrial remodeling and fibrosis [31-34] - our meta-analysis of non-experimental studies found no significant association between bisphosphonate exposure and AF.
It is possible that our result was negative because mild or asymptomatic AF cases were missed in large non-experimental studies, because the majority of the included studies defined outcomes mainly with diagnosis codes. Also, as there is only a small fraction of patients exposed to intravenous forms of bisphosphonates such as ibandronate or zoledronic acid in our meta-analysis, it still remains uncertain whether the risk of AF increases with intravenous bisphosphonates. Our study is consistent with the FDA's safety review and supported by two previously published meta-analyses [20,21]. In the previous meta-analysis of four randomized clinical trials based on total 519 (230 serious, 289 non-serious) AF events among 26,352 patients [20], a significant association was observed between the risk of serious AF events and use of bisphosphonates (OR = 1.47; 95% CI = 1.01 to 2.14), but no significant association was noted for any AF events (OR = 1.14, 95% CI = 0.96 to 1.36). The elevated risk of serious AF might have been overestimated as two of the four clinical trials [5,6,35,36] were performed to study the effect of monthly zoledronic acid infusion on osteoporosis [5,36]. Another meta-analysis by Mak and colleagues [21] showed no evidence of an increased risk of AF related to bisphosphonate use in their meta-analysis of four randomized clinical trials [5,6,36,37] and three non-experimental studies (one cohort and two case-control studies) [7,14,19]. The pooled estimate of crude ORs based on three non-experimental studies [7,14,19] was 1.25 (95% CI = 0.98 to 1.73). Our study included four additional studies [15-18] that were not analyzed in any of previous meta-analyses and yielded similar results.
Several other limitations of our study should be noted. Due to a relatively small number of studies included in our analysis, we were unable to examine the risk of AF relative to the type or dose of bisphosphonates. Non-experimental studies are subject to measured and unmeasured confounding and other biases. Thus, our meta-analysis may suffer these limitations as well. Not every study included in our meta-analysis was adjusted for all the potential risk factors of AF, as observed in Tables 1 and 2. As raw data of individual studies were not available to us, we pooled the multivariate, or adjusted, ORs from the final model of each study. In addition, significant heterogeneity between studies was noted, as often expected in meta-analyses of observational studies [38,39]. In order to account for both within- and between-study variance, we used a random effects model for our analysis. Potential sources of heterogeneity between the studies include variations in the study size, patient characteristics (age, gender, geographic location, underlying comorbidities and use of other drugs), type of bisphosphonates, exposure and outcome ascertainment methods, and the study quality. Due to the limited number of the included studies in our analysis, we could not further identify causes of heterogeneity using the meta-regression technique. Although all meta-analyses are inherently vulnerable to publication bias, we attempted to minimize this bias by searching two major electronic databases with no geographic restriction. Three different statistical tests examined the issue of publication bias and revealed no statistical evidence for significant publication bias.
Our study also has several important strengths. This meta-analysis is up to date and comprehensive including seven large epidemiologic studies conducted in various countries [7,14-19]. We assessed the quality of individual studies using the Newcastle-Ottawa Scale [23]. The majority of the included studies had adequate sample sizes, follow-up lengths (applicable for cohort studies only), and adjustments for known AF risk factors. Our pooled estimates are based on multivariate ORs of individual studies adjusting for a number of known AF risk factors. We furthermore provided a subgroup analysis of the studies by study design and yielded a similar result.
Conclusions
Our meta-analysis of published non-experimental studies suggests that there is no increased risk of AF associated with the use of bisphosphonates for osteoporosis. Physicians should be vigilant for all potential drug-associated toxicities, although the use of oral bisphosphonates probably does not increase a risk of AF, independent of known risk factors for AF. Future research studying a risk associated with intravenous bisphosphonates or a dose-response relation might provide further information.
Abbreviations
AF: atrial fibrillation; CI: confidence interval; FDA: Food and Drug Administration; OR: odds ratio.
Competing interests
SYK, MJK, and SMC declare that they have no competing interests. DHS received research grants from Merck & Co., Inc. Novartis, and Amgen, Inc.
Authors' contributions
All authors participated in the study conception. SYK, SMC, and DHS participated in the study design and data interpretation. SYK and MJK participated in data acquisition and analysis. All authors participated in manuscript preparation and revision. All authors read and approved the final manuscript.
Contributor Information
Seo Young Kim, Email: skim62@partners.org.
Min Jung Kim, Email: mkim16@partners.org.
Suzanne M Cadarette, Email: s.cadarette@utoronto.ca.
Daniel H Solomon, Email: dsolomon@partners.org.
Acknowledgements
SYK received support from the National Institutes of Health (T32 AR 055885). SMC received the New Investigator Award in the Area of Aging and Osteoporosis from the Canadian Institutes of Health Research. DHS was supported by several National Institutes of Health programs (K24 AR055989, P60 AR047782, R21 DE018750, and R01 AR056215). DHS also received research grants from Abbott Immunology, Amgen and Novartis, as well as support from BMS for an educational course on clinical research in Rheumatology. The funders of this study had no role in any aspect of the study design, implementation or evaluation and did not contribute to the decision to submit the manuscript for publication.
References
- National Osteoporosis Foundation: America's Bone Health: The state of osteoporosis and low bone mass in our nation. http://www.nof.org/advocacy/prevalence/
- Burge R, Dawson-Hughes B, Solomon D, Wong J, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005-2025. J Bone Miner Res. 2007;22:465–475. doi: 10.1359/jbmr.061113. [DOI] [PubMed] [Google Scholar]
- National Osteoporosis Foundation: The clinician's guide to prevention and treatment of osteoporosis 2008. http://www.nof.org/professionals/NOF_Clinicians_Guide.pdf
- Solomon DH, Rekedal L, Cadarette SM. Osteoporosis treatments and adverse events. Curr Opin Rheumatol. 2009;21:363–368. doi: 10.1097/BOR.0b013e32832ca433. [DOI] [PubMed] [Google Scholar]
- Black D, Delmas P, Eastell R, Reid I, Boonen S, Cauley J, Cosman F, Lakatos P, Leung P, Zulema Man MD, Carlos Mautalen C, Mesenbrink P, Hu H, Caminis J, Tong K, Rosario-Jansen T, Krasnow J, Hue T, Sellmeyer D, Eriksen E, Cummings S. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med. 2007;356:1809–1822. doi: 10.1056/NEJMoa067312. [DOI] [PubMed] [Google Scholar]
- Cummings S, Schwartz A, Black D. Alendronate and atrial fibrillation. N Engl J Med. 2007;356:1895–1896. doi: 10.1056/NEJMc076132. [DOI] [PubMed] [Google Scholar]
- Heckbert S, Li G, Cummings S, Smith N, Psaty B. Use of alendronate and risk of incident atrial fibrillation in women. Arch Intern Med. 2008;168:826–831. doi: 10.1001/archinte.168.8.826. [DOI] [PubMed] [Google Scholar]
- Chatap G, Giraud K, Vincent J. Atrial fibrillation in the elderly: facts and management. Drugs Aging. 2002;19:819–846. doi: 10.2165/00002512-200219110-00002. [DOI] [PubMed] [Google Scholar]
- Feinberg W, Blackshear J, Laupacis A, Kronmal R, Hart R. Prevalence, age distribution, and gender of patients with atrial fibrillation. Arch Intern Med. 1995;155:469–473. doi: 10.1001/archinte.155.5.469. [DOI] [PubMed] [Google Scholar]
- Hooft C van der, Heeringa J, Brusselle G, Hofman A, Witteman J, Kingma J, Sturkenboom M, Stricker B. Corticosteroids and the risk of atrial fibrillation. Arch Intern Med. 2006;166:1016–1020. doi: 10.1001/archinte.166.9.1016. [DOI] [PubMed] [Google Scholar]
- Nattel S, Opie L. Controversies in atrial fibrillation. Lancet. 2006;367:262–272. doi: 10.1016/S0140-6736(06)68037-9. [DOI] [PubMed] [Google Scholar]
- FDA. Early communication of an ongoing safety review on bisphosphonates: alendronate (Fosamax, Fosamax Plus D), etidronate (Didronel), ibandronate (Boniva), pamidronate (Aredia), risedronate (Actonel, Actonel W/Calcium), tiludronate (Skelid), and zoledronic acid (Reclast, Zometa) http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/DrugSafetyInformationforHeathcareProfessionals/ucm070303.htm
- FDA. Update of safety review follow-up to the October 1, 2007 'Early Communication about the Ongoing Safety Review of Bisphosphonates'. http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/DrugSafetyInformationforHeathcareProfessionals/ucm136201.htm
- Abrahamsen B, Eiken P, Brixen K. Atrial fibrillation in fracture patients treated with oral bisphosphonates. J Intern Med. 2009;265:581–592. doi: 10.1111/j.1365-2796.2008.02065.x. [DOI] [PubMed] [Google Scholar]
- Bunch TJ, Anderson JL, May HT, Muhlestein JB, Horne BD, Crandall BG, Weiss JP, Lappe DL, Osborn JS, Day JD. Relation of bisphosphonate therapies and risk of developing atrial fibrillation. Am J Cardiol. 2009;103:824–828. doi: 10.1016/j.amjcard.2008.11.037. [DOI] [PubMed] [Google Scholar]
- Grosso A, Douglas I, Hingorani A, MacAllister R, Smeeth L. Oral bisphosphonates and risk of atrial fibrillation and flutter in women: a self-controlled case-series safety analysis. PLoS ONE. 2009;4:e4720. doi: 10.1371/journal.pone.0004720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W, Tsai Y, Wen Y, Hsiao F, Kuo K, Tsai C. Osteoporosis treatment and atrial fibrillation: alendronate versus raloxifene. Menopause. 2010;17:57–63. doi: 10.1097/gme.0b013e3181b34749. [DOI] [PubMed] [Google Scholar]
- Levesque L, Blagojevic A, Etminan M, Brophy J. Oral bisphosphonates and the risk of atrial fibrillation in a cohort of older adults. Pharmacoepidemiol Drug Saf. 2009;18:S114. [Google Scholar]
- Sorensen H, Christensen S, Mehnert F, Pedersen L, Chapurlat R, Cummings SR, Baron JA. Use of bisphosphonates among women and risk of atrial fibrillation and flutter: population based case-control study. BMJ. 2008;336:813–816. doi: 10.1136/bmj.39507.551644.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loke Y, Jeevanantham V, Singh S. Bisphosphonates and atrial fibrillation: Systematic review and meta-analysis. Drug Saf. 2009;32:219–228. doi: 10.2165/00002018-200932030-00004. [DOI] [PubMed] [Google Scholar]
- Mak A, Cheung M, Ho R, Cheak A, Lau C. Bisphosphonates and atrial fibrillation: Bayesian meta-analyses of randomized controlled trials and observational studies. BMC Musculoskelet Disord. 2009;10:113. doi: 10.1186/1471-2474-10-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesko S, Mitchell A. In: Textbook of Pharmacoepidemiology. Strom B, Kimmel S, editor. West Sussex: John Wiley & Sons, Ltd; 2007. The use of randomized controlled trials for pharmacoepidemiology studies; pp. 311–320. [Google Scholar]
- The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm
- Walter S. Choice of effect measure for epidemiological data. J Clin Epidemiol. 2000;53:931–939. doi: 10.1016/S0895-4356(00)00210-9. [DOI] [PubMed] [Google Scholar]
- Zhang J, Yu K. What's the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. JAMA. 1998;280:1690–1691. doi: 10.1001/jama.280.19.1690. [DOI] [PubMed] [Google Scholar]
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- Egger M, Smith G, Altman D. Systematic reviews in health care. Second. London: BMJ Publishing Group; 2001. [Google Scholar]
- Higgins J, Thompson S. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- Sterne J, Egger M. Funnel plots for detecting bias in meta-analysis: Guidelines on choice of axis. J Clin Epidemiol. 2001;54:1046–1055. doi: 10.1016/S0895-4356(01)00377-8. [DOI] [PubMed] [Google Scholar]
- Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson G, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SBMDM. for the Meta-analysis Of Observational Studies in Epidemiology G. Meta-analysis of Observational Studies in Epidemiology: A Proposal for Reporting. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- Aviles RJ, Martin DO, Apperson-Hansen C, Houghtaling PL, Rautaharju P, Kronmal RA, Tracy RP, Van Wagoner DR, Psaty BM, Lauer MS, Chung MK. Inflammation as a risk factor for atrial fibrillation. Circulation. 2003;108:3006–3010. doi: 10.1161/01.CIR.0000103131.70301.4F. [DOI] [PubMed] [Google Scholar]
- Boos C, Anderson R, Lip G. Is atrial fibrillation an inflammatory disorder? Eur Heart J. 2006;27:136–149. doi: 10.1093/eurheartj/ehi645. [DOI] [PubMed] [Google Scholar]
- Dicuonzo G, Vincenzi B, Santini D, Avvisati G, Rocci L, Battistoni F, Gavasci M, Borzomati D, Coppola R, Tonini G. Fever after zoledronic acid administration is due to increase in TNF-alpha and IL-6. J Interferon Cytokine Res. 2003;23:649–654. doi: 10.1089/107999003322558782. [DOI] [PubMed] [Google Scholar]
- Vasikaran S. Bisphosphonates: an overview with special reference to alendronate. Ann Clin Biochem. 2001;38:608–623. doi: 10.1258/0004563011901037. [DOI] [PubMed] [Google Scholar]
- Karam R, Camm J, McClung M. Yearly zoledronic acid in postmenopausal osteoporosis. N Engl J Med. 2007;357:712–713. [PubMed] [Google Scholar]
- Lyles KW, Colon-Emeric CS, Magaziner JS, Adachi JD, Pieper CF, Mautalen C, Hyldstrup L, Recknor C, Nordsletten L, Moore KA, Lavecchia C, Zhang J, Mesenbrink P, Hodgson PK, Abrams K, Orloff JJ, Horowitz Z, Eriksen EF, Boonen S. Zoledronic acid and clinical fractures and mortality after hip fracture. N Engl J Med. 2007;357:1799–1809. doi: 10.1056/NEJMoa074941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black DM, Cummings SR, Karpf DB, Cauley JA, Thompson DE, Nevitt MC, Bauer DC, Genant HK, Haskell WL, Marcus R, Ott SM, Torner JC, Quandt SA, Reiss TF, Ensrud KE. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research Group. Lancet. 1996;348:1535–1541. doi: 10.1016/s0140-6736(96)07088-2. [DOI] [PubMed] [Google Scholar]
- Coory M. Comment on: heterogeneity in meta-analysis should be expected and appropriately quantified. Int J Epidemiol. 2009. in press . [DOI] [PubMed]
- Higgins J. Commentary: Heterogeneity in meta-analysis should be expected and appropriately quantified. Int J Epidemiol. 2008. pp. 1158–1160. [DOI] [PubMed]