Abstract
Background
An abnormal ECG during maximal exercise testing has been shown to be a powerful predictor of future coronary heart disease (CHD) mortality in asymptomatic men. However, little is known about the relationship between exercise ECG responses and CHD risk in men with diabetes mellitus.
Methods and Results
We examined the association between exercise ECG responses and mortality in 2854 men with documented diabetes mellitus (mean age 49.5 years) who completed a maximal treadmill exercise test during the period from 1974 to 2001 and who were without a previous cardiovascular disease (CVD) event at baseline. Mortality due to all causes, CHD, and CVD were the main outcome measures across categories of exercise ECG responses, with stratification by cardiorespiratory fitness, quantified as treadmill test duration. During an average follow-up of 16 years, 441 deaths (210 CVD and 133 CHD) were identified. Across normal, equivocal, and abnormal exercise ECG groups, age- and examination year-adjusted CHD mortality rates per 10 000 person-years were 23.0, 48.6, and 69.0, respectively (Ptrend<0.001). After further adjustment for fasting plasma glucose level, smoking, body mass index, hypercholesterolemia, hypertension, family history of CVD or diabetes mellitus, abnormal resting ECG responses, and cardiorespiratory fitness, hazard ratios (95% confidence intervals) were 1.00 (referent), 1.68 (1.01 to 2.77), and 2.21 (1.41 to 3.46; Ptrend<0.001). Similar patterns of associations were noted between exercise ECG testing and both CVD and all-cause mortality risk.
Conclusions
Among men with diabetes mellitus, equivocal and abnormal exercise ECG responses were associated with higher risk of all-cause, CVD, and CHD mortality.
Keywords: exercise, electrocardiography, coronary disease, diabetes mellitus
The prevalence of diabetes mellitus in 2005 was 10.8 million, affecting 7% of the total US population. In 2005 alone, 1.5 million new cases of diabetes mellitus were reported and an estimated 1.1 million people died of diabetes mellitus.1 Individuals with diabetes are known to havea2to 4 times higher risk of premature cardiovascular disease (CVD) and death than individuals of the same age who do not have diabetes mellitus.2,3 The risk of a CVD event in those with diabetes is similar to that of persons with established CVD. Therefore, a noninvasive diagnosis of subclinical CVD in patients with diabetes mellitus is potentially important and may optimize secondary preventative interventions in this high-risk population.
Although exercise testing is proven to have prognostic value, studies examining the relationship between exercise ECG responses and coronary heart disease (CHD) mortality risk in asymptomatic men with existing CVD risk factors are inconsistent. Most of the studies have shown a positive association in high-risk subgroups,4–7 although others have not.8 However, none of these studies focused on individuals with diabetes mellitus, which is considered to be a CHD risk equivalent. The American Heart Association examined this issue and concluded that no outcome data were available to show a benefit of early identification of asymptomatic CVD in diabetic patients from exercise ECG testing.9 Indeed, data on the relationship between exercise ECG tests and CVD/ CHD risk in diabetic men are sparse, and most studies used small samples. Callaham et al10 studied 1747 US veterans with diabetes mellitus and showed that exercise-induced ST-segment depression was associated with more CVD events during a mean 2 years of follow-up. In a study of 45 patients, Weiner et al11 reported that patients with diabetes with exercise-induced silent ischemia had worse outcomes in terms of CVD events than persons without diabetes with silent ischemia. Therefore, the primary aim of the present study was to evaluate the relationship between exercise ECG testing and CHD mortality in a large population of asymptomatic men with diabetes mellitus. We showed previously that a maximal exercise test performed in asymptomatic men free of CVD can predict future risk of CHD death and that an abnormal test was a more powerful predictor of risk in those with than those without conventional risk factors.12 The present study will expand this prior report and focus on men with diabetes mellitus.
Methods
Population
All participants completed a baseline clinical examination during 1974 to 2001 at the Cooper Clinic (Dallas, Tex) and were enrolled in the Aerobics Center Longitudinal Study. The participants had no history or evidence of myocardial infarction or stroke, had a body mass index ≥18.5 kg/m2, and had at least 1 year of follow-up data. Other inclusion criteria for the present analyses were that participants had completed a maximal treadmill test at baseline and had diabetes mellitus, which was defined as fasting plasma glucose concentration ≥7.0 mmol/L (126 mg/dL), a history of physician diagnosis of the disease, or insulin use.13 We identified 2854 men (21 to 84 years old) with diabetes mellitus. We excluded women from the present analysis because the small number of women with diabetes mellitus in our clinic population precluded meaningful analyses. The majority of men were white men from middle or upper socioeconomic strata. All participants gave informed consent to participate in the clinical examination and follow-up study. The Cooper Institute Institutional Review Board reviewed and approved the study protocol annually.
Clinical Examination
The baseline examination was performed after a fast of at least 12 hours and included a physician examination and a thorough preventive medical evaluation and clinical measurements. Body mass index was computed from measured height and weight. Systolic and diastolic blood pressures were recorded at rest, as the first and fifth Korotkoff sounds, by standard auscultation methods.14 Hypertension was defined as systolic blood pressure ≥140 mm Hg, diastolic blood pressure ≥90 mm Hg, or a history of physician diagnosis. Concentrations of total and high-density lipoprotein cholesterol, triglyceride, and glucose were measured with automated bioassays in accordance with the Centers for Disease Control and Prevention Lipid Standardization Program. Family history of CVD or diabetes and smoking habits were obtained from a standardized questionnaire. Resting ECG was performed according to a standard manual of operations. Conventional risk factors were defined according to American Heart Association guidelines15: hypercholesterolemia (total cholesterol ≥6.20 mmol/L [240 mg/dL]), low high-density lipoprotein (<1.03 mmol/L [40 mg/dL]), hypertriglyceridemia (≥2.26 mmol/L [200 mg/dL]), hypertension, current smoking, abnormal resting ECG response, obesity (body mass index ≥30.0 kg/m2), and a family history of premature CVD.
Exercise Test
A maximal symptom-limited treadmill exercise test was performed with a modified Balke protocol.16,17 The treadmill test began with the patient walking 88 m/min at 0% grade. At the end of the first minute, elevation was increased to 2% and thereafter was increased 1% per minute until the 25th minute. Beyond 25 minutes, elevation remained constant, whereas speed was increased each minute by 5.4 m/min until exhaustion. The test end point was volitional exhaustion or termination by the physician for medical reasons. The 12-lead ECG was monitored continuously during exercise testing. Exercise duration on this protocol is highly correlated with measured maximal oxygen uptake(r=0.92).18 Maximal metabolic equivalents (METs, 1 MET=3.5 mL O2 uptake · kg−1min−1) were estimated from the final treadmill speed and grade.19 We used our previously published age-specific distribution of treadmill duration from the overall Aerobics Center Longitudinal Study population to define fitness as low fit (lowest 20%), moderately fit (middle 40%), or high fit (upper 40%) to maintain consistency with our previous reports and because we have found that low fitness, defined in this way, is an independent predictor of mortality20,21 and morbidity.22 In brief, treadmill-time quintiles were determined for each age group (20 to 39, 40 to 49, 50 to 59, and ≥60 years). Men with a treadmill time in the first quintile were assigned to the low-fit group. Those with scores in the second through the third quintiles and scores in the fourth through the fifth quintiles constituted moderate- and high-fit groups, respectively. Thus, assignment to a fitness category was based on age norms of treadmill performance rather than an absolute fitness standard. The respective cut points for total treadmill time and METs in the low-, moderate-, and high-fitness groups were described in detail in a previous report.22 Heart rate recovery was defined as the heart rate decline during the first 5 minutes after completion of the maximal exercise test (maximal heart rate minus heart rate at 5 minutes of recovery). Heart rate reserve was calculated as 220 minus age minus resting heart rate.
Equivocal exercise ECG responses were those in which ST-segment depression was present between 0.5 and 1.0 mm in amplitude that lasted at least 0.08 seconds.17 Abnormal exercise ECG responses included rhythm and conduction disturbances and ischemic ST-T-wave abnormalities, as described in detail elsewhere.12,17 Previously, to establish the percentage of abnormal tests due to the major criteria of ST depression or elevation ≥1 mm that lasted ≥0.08 second from the J point, 357 abnormal tests among 1436 abnormal tests identified in the database as abnormal were randomly selected and reanalyzed by a group of 3 physicians. We found that 90% of these randomly selected abnormal tests were abnormal owing to ST depression or elevation with or without chest pain, and the specificity of the testing was ≈90%.12
Mortality Surveillance
All participants were followed up from the date of their baseline examination until their date of death or December 31, 2003. The National Death Index was the primary data source for mortality surveillance. The underlying cause of death was determined from the National Death Index report or by a nosologist’s review of official death certificates obtained from the department of vital records in the decedent’s state of residence. Cardiovascular disease mortality was defined by International Classification of Diseases, Ninth Revision (ICD-9) codes 390 to 449.9 before 1999 and ICD-Tenth Revision (ICD-10) codes I00 to I78 during 1999 to 2003. CHD mortality was defined by ICD-9 codes 410 to 414 before 1999 and ICD-10 codes I20 to I25 during 1999 to 2003. The average follow-up time was 15.8 years (SD=9.6, range 1.3 to 30.0 years). We computed person-years of follow-up as the sum of follow-up time among decedents and survivors. In 45 093 person-years of follow-up, 441 deaths were identified, of which 210 were due to CVD and 133 to CHD.
Statistical Analysis
Baseline characteristics of the population were examined by exercise ECG category (differences in covariates were tested by Student’s t tests for continuous variables and χ2 tests for categorical variables in Table 1) and by vital status (differences in covariates were tested with F tests for all variables in Table 1). The associations of exercise ECG testing with the risk of mortality were analyzed with risk factor-adjusted Cox proportional hazards models. The dependent variable was time to development of death of all causes, CVD, or CHD. The independent variable was exercise ECG group. Risk factors included baseline measures: age (in years), examination year, fasting glucose level (mg/dL), smoking status (current smoker or not), body mass index (kg/m2), hypercholesterolemia (yes or no), hypertension (present or not), abnormal resting ECG responses (present or not), family history of CVD or diabetes (present or not for each), heart rate reserve, and treadmill time (minutes). Tests of linear trends across risk factor categories were computed by integer scoring (values 1, 2, and 3) for the 3 categories.
Table 1.
Baseline Characteristics of 2854 Men With Diabetes Mellitus According to Vital Status and Exercise ECG Testing Outcomes, Aerobics Center Longitudinal Study, 1974–2003
| Characteristic | Vital Status | P | Exercise ECG Testing |
P for Trend |
|||
|---|---|---|---|---|---|---|---|
| Survivors | Decedents | Normal | Equivocal | Abnormal | |||
| (n=2413) | (n=441) | (n=2247) | (n=304) | (n=303) | |||
| Age, y | 48.6±9.6 | 54.2±9.3 | <0.001 | 47.9±9.3 | 53.6±9.4 | 56.9±8.5 | <0.001 |
| Follow-up, y | 15.8±9.9 | 15.6±7.8 | 0.72 | 16.3±9.8 | 13.9±8.7 | 13.4±8.6 | <0.001 |
| Body mass index, kg/m2 | 28.0±4.7 | 27.8±5.6 | 0.56 | 27.8±4.7 | 28.9±4.9 | 28.2±5.9 | 0.001 |
| Workload achieved, METs | 10.5±2.3 | 9.0±2.4 | <0.001 | 10.6±2.4 | 9.2±2.1 | 8.7±2.1 | <0.001 |
| Lipids, mmol/L | |||||||
| Total cholesterol | 5.7±2.2 | 6.0±1.2 | <0.001 | 5.7±2.3 | 5.7±1.2 | 5.8±1.3 | 0.79 |
| HDL cholesterol | 1.1±0.5 | 1.0±0.3 | 0.001 | 1.1±0.5 | 1.0±0.3 | 1.1±0.3 | 0.007 |
| Triglycerides | 2.1±2.7 | 2.5±3.1 | 0.03 | 2.1±2.1 | 2.5±2.4 | 2.5±1.8 | 0.008 |
| Fasting blood glucose, mmol/L | 7.3±2.7 | 8.0±3.3 | <0.001 | 7.2±2.7 | 8.2±3.2 | 8.1±3.0 | <0.001 |
| Resting blood pressure, mm Hg | |||||||
| Systolic | 126±15 | 131±17 | <0.001 | 125±14 | 132±18 | 134±18 | <0.001 |
| Diastolic | 84±10 | 84±11 | 0.25 | 84±10 | 86±11 | 85±10 | <0.001 |
| Maximal blood pressure, mm Hg | |||||||
| Systolic | 193±26 | 194±28 | 0.49 | 191±25 | 200±31 | 199±30 | <0.001 |
| Diastolic | 80±14 | 84±13 | <0.001 | 80±13 | 85±15 | 84±14 | <0.001 |
| Resting heart rate, bpm | 64±11 | 66±13 | 0.001 | 64±11 | 65±11 | 65±12 | 0.14 |
| Maximal heart rate, bpm | 172±17 | 164±20 | <0.001 | 174±16 | 164±19 | 159±21 | <0.001 |
| Heart rate at 5 min of recovery, bpm | 109±15 | 102±15 | <0.001 | 109±14 | 106±16 | 102±18 | <0.001 |
| Heart rate reserve, bpm | 108±20 | 98±22 | <0.001 | 110±19 | 99±20 | 94±23 | <0.001 |
| Current smoker, % | 17.9 | 22.7 | 0.02 | 19.8 | 13.5 | 14.9 | 0.006 |
| Hypertension, % | 40.9 | 57.8 | <0.001 | 39.6 | 57.9 | 58.4 | <0.001 |
| Family history of diabetes mellitus, % | 11.9 | 3.0 | <0.001 | 9.9 | 11.2 | 13.9 | 0.10 |
| Parental history of premature CVD, % | 32.0 | 42.0 | <0.001 | 32.4 | 35.5 | 39.9 | 0.02 |
| Abnormal resting ECG, % | 5.5 | 10.0 | <0.001 | 3.9 | 11.2 | 18.2 | <0.001 |
Data shown as mean±SD unless specified otherwise.
Cumulative hazard plots grouped by exposures suggested no appreciable violations of the proportional hazards assumption. We excluded events that occurred during the first year of follow-up to reduce potential confounding caused by procedure-related deaths and the potential influence of undetected subclinical disease at baseline. For survival analyses for the fitness and exercise ECG testing combinations, we combined moderately and highly fit men into a fit group; low-fit men were designated as the unfit group (20 to 39 years old, ≤10.4 METs; 40 to 49 years, ≤9.9 METs; 50 to 59 years, ≤8.5 METs; ≤60 years, ≤7.2 METs). This allowed us to create the following 6 categories: normal ECG-fit; equivocal ECG-fit; abnormal ECG-fit; normal ECG-unfit; equivocal ECG-unfit; and abnormal ECG-unfit. Kaplan-Meier survival curves were generated by the above combinations for CVD and CHD mortality. Crude and multivariate-adjusted log-rank tests were used to determine significance. All tests for statistical significance were 2-sided. A probability value <0.05 was considered significant. All statistical analyses were performed with SAS statistical software, version 9.1 (SAS Inc, Cary, NC).
The authors had full access to and take full responsibility for the integrity of the data. All authors have read and agree to the manuscript as written.
Results
At baseline, the mean age (±SD) of the study participants was 49.5±9.7 years, mean treadmill test duration was 14.9± 5.1 minutes, and mean maximal METs was 10.2± 2.4. The prevalence of unfit subjects was 28.9%. Table 1 shows baseline characteristics based on vital status and exercise ECG results. Workload achieved (METs) was higher in survivors and normal exercise ECG groups, whereas age, systolic blood pressure, heart rate recovery, and heart rate reserve were greater and current smoking, hypertension, family history of CVD, and abnormal resting ECGs occurred more frequently in decedents and abnormal exercise ECG groups.
We examined age- and examination year–adjusted relative risk for mortality due to all causes, CVD, and CHD (Table 2). Exercise ECG test results were a strong predictor of CHD mortality risk (hazard ratio [HR] 2.11, 95% confidence interval [CI] 1.29 to 3.46 for equivocal ECG and HR 3.00, 95% CI 1.95 to 4.61 for abnormal ECG, Ptrend<0.001). The other predictors of CHD death were abnormal resting ECG (HR 2.13, 95% CI 1.28 to 3.54), obesity (HR 2.81, 95% CI 1.76 to 4.51), hypertension (HR 2.15, 95% CI 1.50 to 3.08), and hypercholesterolemia (HR 1.46, 95% CI 1.04 to 2.07). The HR (95% CI) for CHD mortality associated with each 1-MET increment was 0.79 (0.73 to 0.86), and it was 0.43 (0.30 to 0.64) and 0.30 (0.19 to 0.48) in moderately and highly fit men, respectively, compared with the low-fit group. Patterns and magnitude of the association between exposure variables and all-cause and CVD mortality risk were similar to those for CHD (Table 2).
Table 2.
Age- and Examination Year-Adjusted HRs of All-Cause, CVD, and CHD Death According to Exercise ECG Testing Results and Other Potential CVD Risk Factors in 2854 Men With Diabetes Mellitus
| Variable | All-Cause HR (95% CI) | CVD HR (95% CI) | CHD HR (95% CI) |
|---|---|---|---|
| Exercise ECG testing | |||
| Normal | 1.00 (Referent) | 1.00 (Referent) | 1.00 (Referent) |
| Equivocal | 1.70 (1.30–2.22) | 2.01 (1.37–2.96) | 2.11 (1.29–3.46) |
| Abnormal | 1.77 (1.37–2.29) | 2.59 (1.84–3.66) | 3.00 (1.95–4.61) |
| P linear trend | <0.001 | <0.001 | <0.001 |
| Workload achieved, per 1-MET increment | 0.81 (0.78–0.85) | 0.81 (0.76–0.86) | 0.79 (0.73–0.86) |
| CRF | |||
| Low fit | 1.00 (Referent) | 1.00 (Referent) | 1.00 (Referent) |
| Moderate fit | 0.46 (0.37–0.57) | 0.47 (0.34–0.63) | 0.43 (0.30–0.64) |
| High fit | 0.34 (0.26–0.43) | 0.32 (0.22–0.47) | 0.30 (0.19–0.48) |
| P linear trend | <0.001 | <0.001 | <0.001 |
| Resting heart rate, per 10 bpm | 1.24 (1.15–1.34) | 1.26 (1.12–1.41) | 1.23 (1.06–1.42) |
| Maximal heart rate, per 10 bpm | 0.82 (0.78–0.87) | 0.79 (0.74–0.86) | 0.77 (0.70–0.84) |
| Heart rate at 5 min of recovery, per 10 bpm | 0.96 (0.90–1.03) | 0.93 (0.84–1.03) | 0.92 (0.81–1.04) |
| Heart rate reserve, per 10 bpm | 0.81 (0.77–0.84) | 0.78 (0.73–0.84) | 0.77 (0.71–0.84) |
| Maximal systolic blood pressure, per 10 mm Hg | 1.06 (1.02–1.10) | 1.06 (1.00–1.12) | 1.08 (1.01–1.15) |
| Maximal diastolic blood pressure, per 10 mm Hg | 1.13 (1.06–1.21) | 1.18 (1.07–1.30) | 1.14 (1.01–1.30) |
| Body mass index, kg/m2 | |||
| 18.5–24.9 | 1.00 (Referent) | 1.00 (Referent) | 1.00 (Referent) |
| ≥25.0 | 1.31 (1.05–1.63) | 1.39 (1.00–1.93) | 1.40 (0.92–2.13) |
| ≥30.0 | 2.26 (1.74–2.93) | 2.43 (1.66–3.56) | 2.81 (1.76–4.51) |
| P linear trend | <0.001 | <0.001 | <0.001 |
| Current smoker | 1.45 (1.16–1.82) | 1.72 (1.25–2.35) | 1.43 (0.95–2.15) |
| Hypertension | 1.56 (1.29–1.89) | 1.99 (1.50–2.64) | 2.15 (1.50–3.08) |
| Hypercholesterolemia* | 1.34 (1.11–1.63) | 1.41 (1.07–1.86) | 1.46 (1.04–2.07) |
| Low HDL cholesterol† | 1.01 (0.83–1.48) | 1.33 (0.87–2.04) | 1.39 (0.79–2.44) |
| Hypertriglyceridemia‡ | 1.45 (1.18–1.77) | 1.31 (0.98–1.76) | 1.40 (0.97–2.02) |
| Abnormal resting ECG | 1.25 (0.91–1.73) | 1.90 (1.26–2.87) | 2.13 (1.28–3.54) |
Hypercholesterolemia is defined as total cholesterol ≥6.20 mmol/L (240 mg/dL).
Low HDL cholesterol is defined as <1.03 mmol/L (40 mg/dL).
Hypertriglyceridemia is defined as triglyceride ≥2.26 mmol/L (200 mg/dL).
Table 3 shows a positive gradient (Ptrend<0.001) of CHD, CVD, and all-cause mortality rates across exercise ECG groups. After adjustment for risk factors, men with equivocal and abnormal exercise ECG tests had a 68% and 121% higher CHD death risk (Ptrend<0.001), a 63% and 103% higher risk of CVD death (Ptrend<0.001), and a 41% and 44% higher risk of all-cause death (Ptrend=0.003) than men with a normal ECG test, respectively.
Table 3.
Rates and HRs for All-Cause, CVD, and CHD Mortality by Exercise ECG Results in 2854 Men With Diabetes Mellitus
| No. of Deaths |
Rate* | Model 1† | Model 2‡ | |||
|---|---|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | |||
| All causes | ||||||
| Normal | 288 | 86.4 | 1.00 | Referent | 1.00 | Referent |
| Equivocal | 68 | 146.5 | 1.70 | 1.30–2.22 | 1.43 | 1.08–1.88 |
| Abnormal | 85 | 153.1 | 1.77 | 1.37–2.29 | 1.42 | 1.09–1.85 |
| P linear trend | <0.001 | 0.004 | ||||
| CVD | ||||||
| Normal | 125 | 37.7 | 1.00 | Referent | 1.00 | Referent |
| Equivocal | 34 | 75.8 | 2.01 | 1.37–2.96 | 1.65 | 1.11–2.46 |
| Abnormal | 51 | 97.7 | 2.59 | 1.84–3.66 | 1.97 | 1.37–2.83 |
| P linear trend | <0.001 | <0.001 | ||||
| CHD | ||||||
| Normal | 78 | 23.0 | 1.00 | Referent | 1.00 | Referent |
| Equivocal | 21 | 48.6 | 2.11 | 1.29–3.46 | 1.69 | 1.03–2.80 |
| Abnormal | 34 | 69.0 | 3.00 | 1.95–4.61 | 2.11 | 1.34–3.32 |
| P linear trend | <0.001 | <0.001 | ||||
Rate per 10 000 person-years adjusted for age and examination year.
Adjusted for age and examination year.
Adjusted for the above plus fasting glucose level, current smoking (yes or no), body mass index, hypercholesterolemia (yes or no), hypertension (present or not), family history of CVD or diabetes mellitus (present or not for each), abnormal resting ECG responses (present or not), heart rate reserve, and treadmill time duration.
We then examined whether other risk predictors modified the association between exercise ECG responses and CHD mortality (Table 4). The positive associations between exercise ECG testing and CHD risk were evident in categories of other risk factors, except that there appeared to be a stronger risk among men with abnormal resting ECG responses, although the test for interaction was not significant (P for interaction=0.92). This may be due to the small number of CHD deaths in this group (only 3, 7, and 9 CHD deaths occurred across normal, equivocal, and abnormal ECG exercise groups, respectively). The consistency in the direction and magnitude of association between exercise ECG testing and CHD suggested that little effect modification was present across most of the risk factor categories. We also performed stratified analyses on CVD mortality risk, with the pattern being similar (data not shown).
Table 4.
HRs for CHD Mortality According to Potential Risk Factor Categories by Exercise ECG Results in 2854 Men With Diabetes Mellitus
| Exercise ECG Testing | P for Trend | |||||
|---|---|---|---|---|---|---|
| Normal | Equivocal | Abnormal | ||||
| HR | 95% CI | HR | 95% CI | |||
| Age, y* | ||||||
| <55 | 1.00 | 2.10 | 1.01–4.37 | 2.57 | 1.17–5.64 | 0.005 |
| ≥55 | 1.00 | 1.60 | 0.80–3.20 | 2.68 | 1.49–4.81 | 0.001 |
| CRF† | ||||||
| Low | 1.00 | 1.87 | 0.97–3.61 | 1.84 | 0.97–3.50 | 0.04 |
| Moderate | 1.00 | 1.20 | 0.44–3.29 | 2.61 | 1.22–5.56 | 0.02 |
| High | 1.00 | 1.96 | 0.54–7.12 | 3.41 | 1.10–10.59 | 0.03 |
| Current smoker† | ||||||
| No | 1.00 | 1.81 | 1.03–3.18 | 2.43 | 1.49–3.95 | <0.001 |
| Yes | 1.00 | 1.35 | 0.44–4.17 | 1.55 | 0.50–4.84 | 0.40 |
| Family history of CVD† | ||||||
| No | 1.00 | 1.74 | 0.92–3.28 | 2.35 | 1.30–4.25 | 0.003 |
| Yes | 1.00 | 2.26 | 1.01–5.06 | 2.44 | 1.23–4.85 | 0.006 |
| Resting ECG responses† | ||||||
| Normal | 1.00 | 1.36 | 0.76–2.44 | 2.32 | 1.42–3.78 | <0.001 |
| Abnormal | 1.00 | 12.63 | 2.10–75.94 | 4.99 | 1.21–20.62 | 0.05 |
| Body mass index, kg/m2† | ||||||
| 18.5–24.9 | 1.00 | 1.59 | 0.56–4.57 | 2.31 | 0.92–5.80 | 0.07 |
| ≥25.0 | 1.00 | 1.79 | 1.01–3.17 | 2.30 | 1.39–3.82 | <0.001 |
| Hypertension† | ||||||
| No | 1.00 | 1.26 | 0.47–3.35 | 2.63 | 1.18–5.84 | 0.02 |
| Yes | 1.00 | 2.01 | 1.11–3.61 | 2.34 | 1.38–3.98 | <0.001 |
| Total cholesterol† | ||||||
| <6.20 mmol/L (240 mg/dl) | 1.00 | 2.42 | 1.27–4.61 | 2.95 | 1.61–5.40 | <0.001 |
| ≥6.20 mmol/L (240 mg/dl) | 1.00 | 1.12 | 0.50–2.53 | 1.83 | 0.94–3.58 | 0.09 |
| Triglycerides† | ||||||
| <2.26 mmol/L (200 mg/dL) | 1.00 | 1.52 | 0.78–2.96 | 2.32 | 1.37–3.95 | 0.002 |
| ≥2.26 mmol/L (200 mg/dL) | 1.00 | 2.13 | 0.97–4.69 | 2.18 | 0.97–4.91 | 0.03 |
Adjusted for examination year, glucose level, and each of the other risk factors (except age) in the Table.
Adjusted for examination year, glucose level, and each of the other risk factors in the Table.
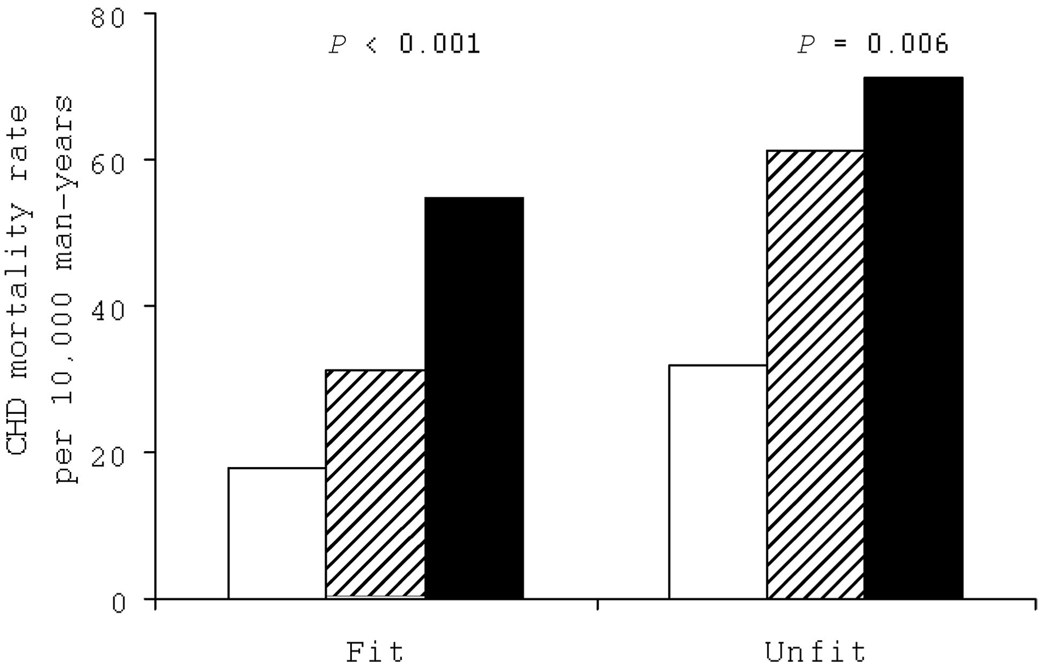
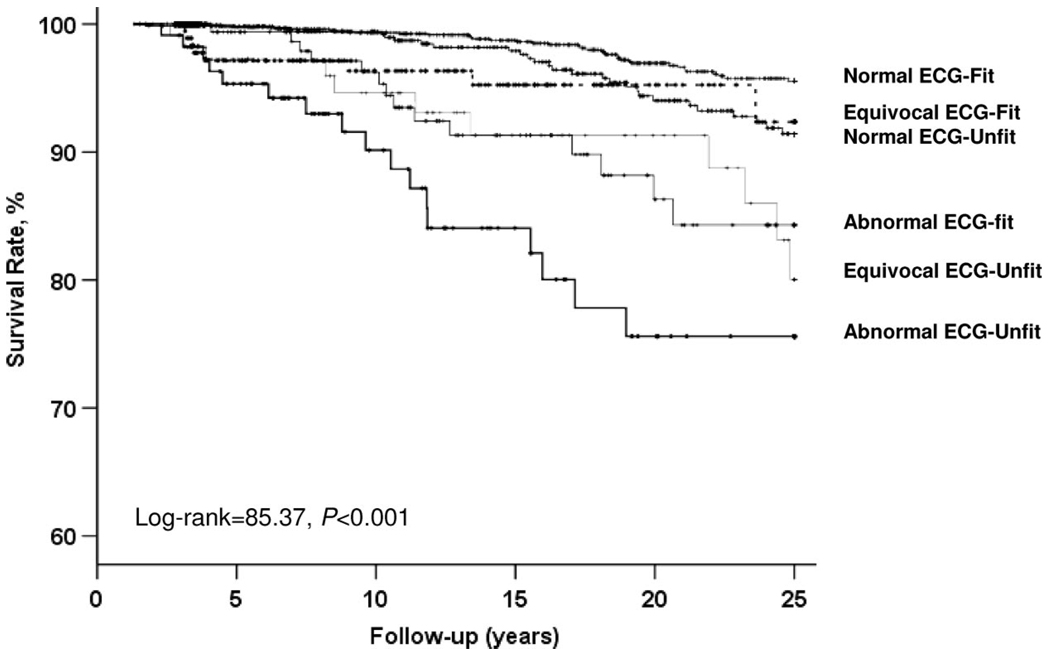
Statistical evidence existed of an interaction between exercise ECG responses and cardiorespiratory fitness (CRF) in predicting CHD death (χ2 = 17.18, P<0.001). Therefore, we examined the joint association of exercise ECG testing and CRF level on CHD mortality (Figure 1). The prevalence of abnormal ECG testing was higher in unfit men (13.9%) than in fit men (9.3%). We observed positive gradients of CHD death rates across exercise ECG testing results within fit or unfit groups (P< 0.01 each). The differences in CHD survival over time by the 6 exercise ECG testing-fitness groups are shown in Figure 2. After adjustment for all risk factors, the resulting log-rank test did not change materially (χ2=80.56, P<0.001). Individuals who had a normal exercise ECG and were fit had the best survival. Those with an abnormal exercise ECG, especially the unfit men, had the lowest survival rates during follow-up. Survival rates were higher among fit men than unfit men in each of the exercise ECG result groups. The Kaplan-Meier curves were similar when we analyzed CVD mortality rates across these 6 groups (data not shown).
Figure 1.
Age- and examination year-adjusted CHD mortality rates by fitness levels and exercise ECG results in 2854 men with diabetes mellitus. Open bars represent normal ECG; striped bars, equivocal ECG; and solid bars, abnormal ECG. P values are for a test of linear trend across ECG results.
Figure 2.
Survival free of CHD across exercise ECG results and fitness status in a mean 16-year follow-up study of 2854 men with diabetes mellitus.
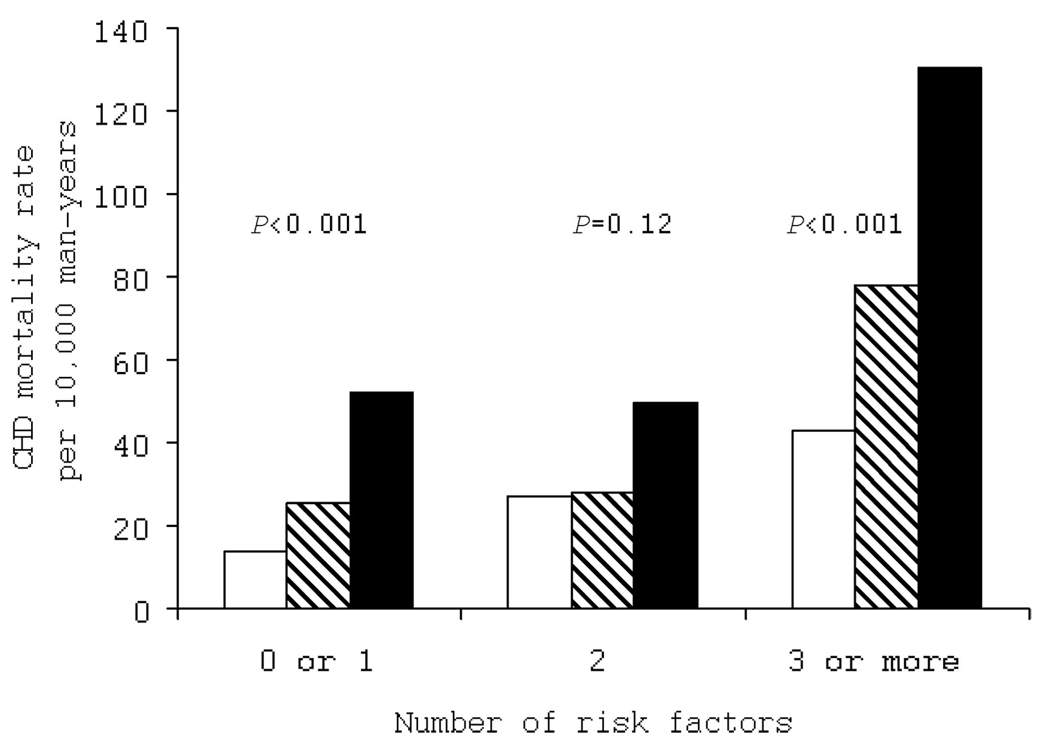
To further examine whether exercise ECG testing had prognostic value beyond an individual’s pretest probability of having a CHD event, we computed CHD mortality rates by exercise ECG test results grouped on the number of major conventional CVD risk factors at baseline (Figure 3). As expected, those with more risk factors and an abnormal exercise ECG had higher CHD mortality. We observed positive gradients for CHD mortality rates across exercise ECG categories within the 0 to 1 and 3 or more risk factor groups, but the trend in men with 2 risk factors was not significant. CVD mortality followed a similar pattern (data not shown).
Figure 3.
Age- and examination year-adjusted CHD mortality rates (per 10 000 person-years) by exercise ECG results and number of major conventional CVD risk factors (current smoking, hypertension, hypercholesterolemia, family history of CVD, abnormal resting ECG response, and obesity) in 2854 men with diabetes mellitus. Open bars represent normal ECG; striped bars, equivocal ECG; and solid bars, abnormal ECG. P values are for a test of linear trend across ECG groups. The number of men (and CHD deaths) in the normal, equivocal, and abnormal ECG groups were 1212 (24), 123 (5), and 111 (10) in those with 0 or 1 risk factors; 624 (29), 86 (5), and 107 (8) in those with 2 risk factors; and 411 (25), 95 (11), and 85 (16) in those with ≥3 risk factors, respectively.
Discussion
Individuals with diabetes mellitus have been reported to have a 2 to 4 times higher risk of CVD and death than individuals of the same age who do not have diabetes mellitus.2,3 We previously reported that an abnormal exercise test is associated with higher risk of CHD mortality in asymptomatic men and that this relation is evident within different risk factor groups.12 Exercise testing has not been recommended for patients with diabetes mellitus9 because diabetes mellitus is considered a CHD “risk equivalent,” and it has been assumed that little additional information would be obtained from exercise testing. Therefore, the primary aim of the present report was to expand on our previous report12 and to determine whether exercise testing improves risk stratification in men with diabetes mellitus. Our hypothesis was that an equivocal or abnormal exercise ECG in men with diabetes mellitus would be associated with a higher risk of all-cause, CVD, and CHD mortality than among those who had a normal exercise ECG. We observed a direct gradient of mortality risk across normal, equivocal, and abnormal exercise ECG groups, and this remained significant after adjustment for age, smoking, family history of CVD or diabetes, abnormal resting ECG responses, fasting glucose level, CRF, and factors that may be intermediate in the causal pathway between exercise ECG and CHD (body mass index, hypertension, and hypercholesterolemia). A second major finding was that the association between exercise ECG result and CHD generally was consistent within strata of other CHD predictors. The prognostic value of an abnormal exercise ECG was particularly notable in men with other coexisting risk factors at baseline. A third noteworthy issue was that fit men had a higher survival rate than unfit men within each category of exercise ECG outcome.
Because of their propensity for silent ischemia, patients with diabetes mellitus may require special consideration. Callaham et al10 studied 1747 US veterans with diabetes and showed that exercise-induced ST-segment depression was associated with more CVD events during a mean 2 years of follow-up. Weiner et al11 reported that 45 patients with diabetes mellitus who had exercise-induced silent ischemia had worse outcomes in terms of CVD events than persons without diabetes mellitus but with silent ischemia. May and colleagues23 estimated the prevalence of maximal symptom-limited bicycle ergometer exercise-induced silent ischemia in those with diabetes mellitus was 13.5%. In the present study, ≈20% of men with diabetes had abnormal or equivocal exercise ECG responses. Thus, those with diabetes mellitus and an abnormal ECG may potentially benefit from further attention.
Our finding on the positive relation between abnormal exercise ECG results and higher risk for CHD mortality are in concert with other population-based studies of asymptomatic men with other CVD risk factors.4–7 Laukkanen and colleagues4 examined 1769 middle-aged men using a maximal symptom-limited exercise stress test and found that exercise-induced silent ischemia was associated with adverse outcome largely in those who were at high risk of developing CHD because of the presence of smoking, hypercholesterolemia, or hypertension. The Framingham Heart Study Offspring Study evaluated 3043 asymptomatic men and women, all of whom underwent a symptom-limited exercise test and were followed up for 18.2 years.5 ST-segment depression provided additional prognostic information in age- and Framingham risk score-adjusted models in men, particularly among those in the highest-risk group (10-year predicted CHD risk ≥20%).5 Bruce et al6 found a 3-fold higher risk of CHD among patients with 1 or more risk factors and 2 or more abnormal features on exercise testing. Rywik et al7 reported that abnormal exercise ECG responses (≥1 mm horizontal or downsloping ST-segment depression) were associated with a doubling of risk for CHD events. However, some studies have failed to confirm a positive relation between abnormal exercise ECG testing and CHD outcomes.8 For example, Bodegard et al8 found that impaired breathing, not the exercise ECG result, predicted a high long-term risk of CHD mortality.
A few previous studies have reported the usefulness of exercise testing for predicting CHD in patients with diabetes mellitus.24,25 Elhendy et al24 evaluated the importance of exercise echocardiography for risk stratification of such patients with known or suspected ischemic heart disease. A total of 563 patients underwent symptom-limited treadmill testing that involved 1 of the 3 following protocols: Bruce, Naughton, or modified Bruce. Their data indicated that patients with diabetes mellitus who had an abnormal stress echocardiogram were at greater risk of death or nonfatal myocardial infarction than those with a normal stress echo-cardiogram.24 Lee and colleagues25 sought to determine the characteristics of exercise treadmill testing in patients with diabetes who had angina. They conducted a retrospective analysis of exercise test results in 1282 men who had undergone coronary angioplasty and were without prior myocardial infarction. They concluded that a standard exercise test has similar diagnostic characteristics in patients with diabetes mellitus and in those free of the disease.
Exercise testing is also an important predictor of survival. Prakash et al26 tested 6213 men referred for a standard maximal exercise ECG treadmill test because of complaints of angina or because they had risk factors or signs or symptoms of CHD. They found that abnormal exercise ECGs were significantly more common in those who died during follow-up, and exercise capacity (< 5 METS) was independently and significantly associated with all-cause mortality. The present findings are consistent with this report: Men with higher CRF levels have a higher survival rate than unfit men, and an abnormal exercise ECG is associated with an increased risk of all-cause mortality. These findings further demonstrate the prognostic power of exercise testing, including both the ECG response and the CRF assessment.
Results from the Aerobics Center Longitudinal Study have shown that low levels of CRF are independently associated with increased mortality in men with diabetes mellitus.2,27,28 In the present investigation, we found that men with diabetes who were unfit but had normal ECG testing had lower CHD mortality risk than men with diabetes who were fit but had equivocal or abnormal ECG testing. This finding further demonstrates the prognostic power of the exercise ECG response even in fit men with diabetes mellitus. Strengthening the public health importance of our findings, we observed that 26% of unfit men with diabetes had normal exercise ECG tests, which provides further evidence for the importance of the exercise ECG. To the best of our knowledge, no studies have examined the joint association of exercise ECG results and exercise capacity on CHD risks in men with diabetes mellitus. The present results provide evidence of an intrinsic value in encouraging healthcare professionals to perform a treadmill test to determine diabetic patients’ CRF level and exercise ECG response, which may guide both physical activity counseling and CHD prevention efforts.
Strengths of the present study include the extensive baseline examination to detect subclinical disease, the use of measured risk factors, the variety of mortality end points, the relatively long follow-up (average 15 years), the broad age range of the study population (from 21 to 82 years), and the fact that we studied the prognostic impact of an exercise ECG stress test. In the present study, all stress tests were maximal tests, and ECGs were monitored continually with 12 leads for 10 minutes after the conclusion of each test.12,17 Therefore, our methods ensured that we would capture most, if not all, abnormalities that may have occurred.
One limitation of the present study was the absence of data documenting the duration of diabetes in the study subjects. However, the literature shows that half of the prevalent cases of diabetes mellitus are undiagnosed and exist in patients without typical clinical symptoms.29 Similarly, in the present report, it is difficult to know exactly when these men were diagnosed with diabetes mellitus. Although it is impossible for us to directly assess the impact of the duration of diabetes on the results, we did adjust the examination year when they were identified as having diabetes mellitus. Although we were unable to separate individuals with type 1 versus type 2 diabetes mellitus, previous reports suggest that >90% of adults with diabetes have type 2 diabetes.30 Because the present study consisted of middle-aged men, we suspect most of the cases were type 2 diabetes. Another limitation was the predominantly white, well-educated, middle- to upper-class, and male subject group, which limits the ability to generalize the results of the present study. However, this should not affect the internal validity, and no strong reason exists to assume that the benefits of exercise testing would be any less in women or other ethnic groups. Our previous studies have shown, in analyses in which enough deaths occurred for parallel analyses in women and men, that the inverse gradient of mortality across exercise capacity is similar for the 2 sexes.20,21,28,31 We do not have enough information for Duke treadmill score calculations. The present study did not have information on medication usage, dietary habits, hemoglobin A1C levels, or presence of end-organ damage to include these in our analysis. Although mortality data were primarily obtained from the National Death Index, which has established validity, the possibility still exists of misclassification of CVD and CHD deaths. However, these issues of death certificate analyses are not relevant for all-cause mortality, which in the present study showed results similar to CVD and CHD mortality. The consistency of results for all-cause, CVD, and CHD mortality is reassuring, and therefore, we do not think these issues would cause major misinterpretation of the data. Finally, we do not have available information on the sensitivity of the ECG test. Future studies are needed to include these data to confirm the findings reported here.
In conclusion, abnormal exercise ECG response is a significant determinant of CHD mortality in men with diabetes mellitus. Assessment of exercise ECG by maximal stress testing provides important prognostic information independent of CRF level and traditional CHD risk factors. Exercise stress testing to determine both ECG responses and CRF may be used as a tool for potential CHD risk stratification in men with diabetes mellitus. We encourage clinicians to further consider the diagnostic value of maximal exercise ECG tests for this high-risk group of men.
CLINICAL PERSPECTIVE
Although exercise testing is proven to have prognostic value, studies that examine the relationship between exercise ECG (E-ECG) responses and coronary heart disease (CHD) mortality risk in asymptomatic men with existing cardiovascular disease risk factors are inconsistent. Most studies have shown a positive association in high-risk subgroups, although others have not. However, these studies have not focused on individuals with diabetes mellitus, which is considered a CHD risk equivalent. Therefore, the primary aim of our study was to evaluate the relationship between E-ECG testing and CHD mortality in a large population of asymptomatic men with diabetes mellitus. We examined the association between E-ECG responses and mortality in men with documented diabetes who completed a maximal treadmill exercise test and were without a previous cardiovascular disease event at baseline. We observed a direct gradient of mortality risk across normal, equivocal, and abnormal E-ECG groups that remained significant after adjustment for age, smoking, family history of cardiovascular disease or diabetes mellitus, abnormal resting ECG responses, fasting glucose level, cardiorespiratory fitness, and factors that may be intermediate in the causal pathway between E-ECG and CHD (body mass index, hypertension, and hypercholesterolemia). A second major finding was that the association between E-ECG result and CHD generally was consistent within strata of other CHD predictors. A third noteworthy issue was that fit men had a higher survival rate than unfit men within each category of E-ECG outcome. Our data suggest that abnormal E-ECG response is a significant determinant of CHD mortality in men with diabetes mellitus.
Acknowledgments
We thank the Cooper Clinic physicians and technicians for collecting the baseline data, staff at the Cooper Institute for data entry and data management, and Gaye Christmus for editorial assistance.
Sources of Funding
The Aerobics Center Longitudinal Study was supported by National Institutes of Health grants AG06945 and HL62508.
Footnotes
Disclosures
None.
References
- 1. [Accessed March 25, 2007];World Health Organization Fact Sheet N°312. 2006 September; Available at: http://www.who.int/mediacentre/factsheets/fs312/en/index.html.
- 2.Church TS, LaMonte MJ, Barlow CE, Blair SN. Cardiorespiratory fitness and body mass index as predictors of cardiovascular disease mortality among men with diabetes. Arch Intern Med. 2005;165:2114–2120. doi: 10.1001/archinte.165.18.2114. [DOI] [PubMed] [Google Scholar]
- 3.Larsen J, Brekke M, Sandvik L, Arnesen H, Hanssen KF, Dahl-Jorgensen K. Silent coronary atheromatosis in type 1 diabetic patients and its relation to long-term glycemic control. Diabetes. 2002;51:2637–2641. doi: 10.2337/diabetes.51.8.2637. [DOI] [PubMed] [Google Scholar]
- 4.Laukkanen JA, Kurl S, Lakka TA, Tuomainen TP, Rauramaa R, Salonen R, Eränen J, Salonen J. Exercise-induced silent myocardial ischemia and coronary morbidity and mortality in middle-aged men. J Am Coll Cardiol. 2001;38:72–79. doi: 10.1016/s0735-1097(01)01311-0. [DOI] [PubMed] [Google Scholar]
- 5.Balady GJ, Larson MG, Vasan RS, Leip EP, O’Donnell CJ, Levy D. Usefulness of exercise testing in the prediction of coronary disease risk among asymptomatic persons as a function of the Framingham risk score. Circulation. 2004;110:1920–1925. doi: 10.1161/01.CIR.0000143226.40607.71. [DOI] [PubMed] [Google Scholar]
- 6.Bruce RA, DeRouen TA, Hossack KF, Blake B, Hofer V. Value of maximal exercise tests in risk assessment of primary coronary heart disease events in healthy men. Am J Cardiol. 1980;46:371–378. doi: 10.1016/0002-9149(80)90003-x. [DOI] [PubMed] [Google Scholar]
- 7.Rywik TM, O’Connor FC, Gittings NS, Wright JG, Khan AA, Fleg JL. Role of nondiagnostic exercise-induced ST-segment abnormalities in predicting future coronary events in asymptomatic volunteers. Circulation. 2002;106:2787–2792. doi: 10.1161/01.cir.0000039329.47437.3b. [DOI] [PubMed] [Google Scholar]
- 8.Bodegard J, Erikssen G, Bjornholt JV, Gjesdal K, Liestol K, Erikssen J. Reasons for terminating an exercise test provide independent prognostic information: 2014 apparently healthy men followed for 26 years. Eur Heart J. 2005;26:1394–1401. doi: 10.1093/eurheartj/ehi278. [DOI] [PubMed] [Google Scholar]
- 9.Redberg RF, Greenland P, Fuster V, Pyörälä K, Blair SN, Folsom AR, Newman AB, O’Leary DH, Orchard TJ, Psaty B, Schwartz JS, Starke R, Wilson P. Prevention Conference VI: Diabetes and Cardiovascular Disease: Writing Group III: risk assessment in persons with diabetes. Circulation. 2002;105:e144–e152. doi: 10.1161/01.cir.0000013955.34262.af. [DOI] [PubMed] [Google Scholar]
- 10.Callaham PR, Froelicher VF, Klein J, Risch M, Dubach P, Friis R. Exercise-induced silent ischemia: age, diabetes mellitus, previous myo-cardial infarction and prognosis. J Am Coll Cardiol. 1989;14:1175–1180. doi: 10.1016/0735-1097(89)90413-0. [DOI] [PubMed] [Google Scholar]
- 11.Weiner DA, Ryan TJ, Parsons L, Fisher LD, Chaitman BR, Sheffield L, Tristani FE. Significance of silent myocardial ischemia during exercise testing in patients with diabetes mellitus: a report from the Coronary Artery Surgery Study (CASS) Registry. Am J Cardiol. 1991;68:729–734. doi: 10.1016/0002-9149(91)90644-z. [DOI] [PubMed] [Google Scholar]
- 12.Gibbons LW, Mitchell TL, Wei M, Blair SN, Cooper KH. Maximal exercise test as a predictor of risk for mortality from coronary heart disease in asymptomatic men. Am J Cardiol. 2000;86:53–558. doi: 10.1016/s0002-9149(00)00827-4. [DOI] [PubMed] [Google Scholar]
- 13.The Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 1997;20:1183–1197. doi: 10.2337/diacare.20.7.1183. [DOI] [PubMed] [Google Scholar]
- 14.Pickering TG, Hall JE, Appel LJ, Falkner BE, Graves J, Hill MN, Jones DW, Kurtz T, Sheps SG, Roccella EJ. Recommendations for blood pressure measurement in humans and experimental animals: part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Hypertension. 2005;45:142–161. doi: 10.1161/01.HYP.0000150859.47929.8e. [DOI] [PubMed] [Google Scholar]
- 15.Rosamond W, Flegal K, Friday G, Furie K, Go A, Greenlund K, Haase N, Ho M, Howard V, Kissela B, Kittner S, Lloyd-Jones D, McDermott M, Meigs J, Moy C, Nichol G, O’Donnell CJ, Roger V, Rumsfeld J, Sorlie P, Steinberger J, Thom T, Wasserthiel-Smoller S, Hong Y. Heart disease and stroke statistics-2007 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2007;115:e69–e171. doi: 10.1161/CIRCULATIONAHA.106.179918. [DOI] [PubMed] [Google Scholar]
- 16.Balke B, Ware RW. An experimental study of physical fitness in Air Force personnel. US Armed Forces Med J. 1959;10:675–688. [PubMed] [Google Scholar]
- 17.Gibbons L, Blair SN, Kohl HW, Cooper K. The safety of maximal exercise testing. Circulation. 1989;80:846–852. doi: 10.1161/01.cir.80.4.846. [DOI] [PubMed] [Google Scholar]
- 18.Pollock ML, Bohannon RL, Cooper KH, Ayres JJ, Ward A, White SR, Linnerud AC. A comparative analysis of four protocols for maximal treadmill stress testing. Am Heart J. 1976;92:39–46. doi: 10.1016/s0002-8703(76)80401-2. [DOI] [PubMed] [Google Scholar]
- 19.American College of Sports Medicine. ACSM’s Guidelines For Exercise Testing And Prescription. 7th ed. Philadelphia, Pa: Lippincott Williams & Wilkins; 2005. [Google Scholar]
- 20.Blair SN, Kampert JB, Kohl HW, III, Barlow CE, Macera CA, Paffen-barger RS, Gibbons LW. Influences of cardiorespiratory fitness and other precursors on cardiovascular disease and all-cause mortality in men and women. JAMA. 1996;276:205–210. [PubMed] [Google Scholar]
- 21.Kampert JB, Blair SN, Barlow CE, Kohl HW., III Physical activity, physical fitness, and all-cause and cancer mortality: a prospective study of men and women. Ann Epidemiol. 1996;6:452–457. doi: 10.1016/s1047-2797(96)00059-2. [DOI] [PubMed] [Google Scholar]
- 22.Sui X, LaMonte MJ, Blair SN. Cardiorespiratory fitness as a predictor of nonfatal cardiovascular events in asymptomatic women and men. Am J Epidemiol. 2007;165:1413–1423. doi: 10.1093/aje/kwm031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.May O, Arildsen H, Damsgaard EM, Mickley H. Prevalence and prediction of silent ischaemia in diabetes mellitus: a population-based study. Cardiovasc Res. 1997;34:241–247. doi: 10.1016/s0008-6363(97)00046-1. [DOI] [PubMed] [Google Scholar]
- 24.Elhendy A, Arruda AM, Mahoney DW, Pellikka PA. Prognostic stratification of diabetic patients by exercise echocardiography. J Am Coll Cardiol. 2001;37:1551–1557. doi: 10.1016/s0735-1097(01)01199-8. [DOI] [PubMed] [Google Scholar]
- 25.Lee DP, Fearon WF, Froelicher VF. Clinical utility of the exercise ECG in patients with diabetes and chest pain. Chest. 2001;119:1576–1581. doi: 10.1378/chest.119.5.1576. [DOI] [PubMed] [Google Scholar]
- 26.Prakash M, Myers J, Froelicher VF, Marcus R, Do D, Kalisetti D, Atwood JE. Clinical and exercise test predictors of all-cause mortality: results from >6,000 consecutive referred male patients. Chest. 2001;120:1003–1013. doi: 10.1378/chest.120.3.1003. [DOI] [PubMed] [Google Scholar]
- 27.Wei M, Gibbons LW, Kampert JB, Nichaman MZ, Blair SN. Low cardiorespiratory fitness and physical inactivity as predictors of mortality in men with type 2 diabetes. Ann Intern Med. 2000;132:605–611. doi: 10.7326/0003-4819-132-8-200004180-00002. [DOI] [PubMed] [Google Scholar]
- 28.Church TS, Cheng YJ, Earnest CP, Barlow CE, Gibbons LW, Priest EL, Blair SN. Exercise capacity and body composition as predictors of mortality among men with diabetes. Diabetes Care. 2004;27:83–88. doi: 10.2337/diacare.27.1.83. [DOI] [PubMed] [Google Scholar]
- 29.Harris MI, Hadden WC, Knowler WC, Bennett PH. Prevalence of diabetes and impaired glucose tolerance and plasma glucose levels in US population aged 20–74 yr. Diabetes. 1987;36:523–534. doi: 10.2337/diab.36.4.523. [DOI] [PubMed] [Google Scholar]
- 30.Centers for Disease Control and Prevention. National Diabetes Fact Sheet: United States, 2005. Atlanta, Ga: Centers for Disease Control and Prevention; 2006. [Google Scholar]
- 31.Marquis P, Fayol C, Joire JE, Leplege A. Psychometric properties of a specific quality of life questionnaire in angina pectoris patients. Qual Life Res. 1995;4:540–546. doi: 10.1007/BF00634749. [DOI] [PubMed] [Google Scholar]