Abstract
The mechanical properties of dentin and enamel affect the reliability and wear properties of a tooth. This study investigated the influence of clinical dental treatments and procedures, such as whitening treatments or etching prior to restorative procedures. Both autoclaved and non-autoclaved teeth were studied in order to allow for both comparison with published values and improved clinical relevance. Nanoindentation analysis with the Oliver-Pharr model provided elastic modulus and hardness across the dentin-enamel junction (DEJ). Large increases were observed in the elastic modulus of enamel in teeth that had been autoclaved (52.0GPa versus 113.4GPa), while smaller increases were observed in the dentin (17.9GPa versus 27.9GPa). Likewise, there was an increase in the hardness of enamel (2.0GPa versus 4.3GPa) and dentin (0.5GPa versus 0.7GPa) with autoclaving. These changes suggested that the range of elastic modulus and hardness values previously reported in literature may be partially due to the sterilization procedures. Treatment of the exterior of non-autoclaved teeth with Crest Whitestrips™, Opalescence™ or UltraEtch™ caused changes in the mechanical properties of both the enamel and dentin. Those treated with Crest Whitestrips™ showed a reduction in the elastic modulus of enamel (55.3GPa to 32.7GPa) and increase in the elastic modulus of dentin (17.2GPa to 24.3GPa). Opalescence™ treatments did not significantly affect the enamel properties, but did result in a decrease in modulus of dentin (18.5GPa to 15.1GPa). Additionally, as expected, UltraEtch™ treatment decreased the modulus and hardness of enamel (48.7GPa to 38.0GPa and 1.9GPa to 1.5GPa, respectively) and dentin (21.4GPa to 15.0GPa and 1.9GPa to 1.5GPa, respectively). Changes in the mechanical properties were linked to altered protein concentration within the tooth, as evidenced by fluorescence microscopy and Fourier transform infrared spectroscopy.
1. Introduction
The dentin-enamel junction (DEJ) is a highly specialized interface offering reliability, toughness and crack deflection, and thus is often the subject of interest for the development of biomimetic models uniting dissimilar materials (Marshall et al. 2001). Both enamel and dentin are composed of crystalline calcium phosphate, primarily hydroxylapatite, as well as protein and water in various concentration. The mechanical properties are controlled by the ratio and interface between these components, as well as the composite structure. Previous research has focused on measurement of mechanical properties of dental hard tissues to understand vertical crack propagation found in teeth (Marshall et al. 2003) and the mechanical abrasion of teeth due to external loading such as that caused by mastication or improper brushing (Luiz et al. 2007), both of which result in the loss of hard dental tissues. Further research on the mechanical properties of these hard tissues will allow for the development of a model of effective stress transfer between dissimilar materials, improved dental treatments, and more reliable restorative materials that mimic the resistance to deformation and stress dissipation of natural tooth (Marshall et al. 2003).
The mechanical properties of dentin and enamel, as well as those across the DEJ, have been studied using nanoindentation (Angker et al. 2006, Habelitz et al. 2001). Investigations involving an array of loading parameters, sample sources, and preparation methods have established the hardness of dentin and enamel to be 0.2–2.5 GPa and 16.3–29.8 GPa, and the elastic modulus to be 1.3–4.9 GPa and 39.5–108.2 GPa (Angker et al. 2006), respectively. These ranges reflect variability in the sample preparation, orientation of enamel and dentin structures, type of tooth, as well as the tissue source. Previous studies typically measured the mechanical properties of dentin, enamel and the DEJ, and have focused on the effects of aging (Park et al. 2008), orientation (Ferguson et al. 2004), and storage procedures (Guidoni et al. 2006). Teeth exhibited consistent trends across the DEJ, regardless of age, though increasing elastic modulus of enamel was observed with increasing age of the donor. The suggested explanation for such an observation was altered crystallography and chemistry (Park et al. 2008).
Limited investigations into the effects of whitening agents have indicated mechanical changes with treatments (Hairul Nizam et al. 2005). Evidence has been provided, indicating that there is an alteration in the nanomechanical properties of teeth with exposure to chemical agents, including common storage solutions (Habelitz et al 2002). Furthermore, Hairul Nizam et al. suggested that hydrogen peroxide, which is often used in dental whitening agents, alters the mechanical behavior of teeth. In this study, human premolars were sliced then soaked in 30% hydrogen peroxide for 24hrs. Dentin demonstrated a decrease in mean hardness of 29–55% and decrease of Young’s modulus of 19–43% with exposure to hydrogen peroxide. Enamel exhibited a decrease of hardness of 13–23% and decrease of Young’s modulus by 18–32% (Hairul Nizam et al. 2005). While these results are compelling, it is difficult to extrapolate to what degree peroxides soften will dentin and enamel clinically. Whitening treatments are applied to the outside enamel surface only and not directly to the dentin. These are applied for shorter periods of time and usually contain lower percentages of peroxide. In addition, most commercially available treatments are not comprised of peroxides alone but contain other additives including fluoride, which is known to harden enamel (Dickinston et al. 2005). Therefore, more work is needed to determine the effects of popular dental whitening treatments on the mechanical properties of teeth.
The majority of previous studies of mechanical properties of teeth does not consider sterilization treatment effects, do not use internal controls, nor follow manufacturer recommended instructions for dental treatments. The aim of this study was to clarify the effects of dental treatments and procedures on physical and mechanical properties of dentin and enamel. This study takes into consideration the use of common cosmetic and clinical restorative preparation dental treatments, such as whitening and etching, and monitors the changes in mechanical properties with alterations in chemical composition and structure. The whitening treatments used were Crest Whitestrips™ Premium, a 10% hydrogen peroxide treatment, Opalescence™, a 20% carbamide peroxide treatment. In addition, UltraEtch™, a 35% phosphoric acid etchant, was used as a positive control comparison. Etching by phosphoric acid is known to reduce dental mechanical properties and increase surface roughness (Barbour et al. 2007). UltraEtch™ is used prior to restorative treatments to help the restorative materials better adhere to the tooth. Unlike previous studies, all treatments in this study were applied according to the manufacturer recommended instructions. In addition, this study had a secondary goal of determining how common experimental preparation and storage conditions affect measured dental mechanical properties. White et al. 1994 reported that four types of sterilization methods were commonly used in dental research, including autoclaving. In this study, both autoclaved teeth and non-autoclaved teeth were tested with each treatment, permitting comparison with previously published mechanical property data (Fong et al. 2000, Zhou et al. 2006) as well as improved clinical relevance. Each sample was compared to a portion of similar structural orientation from the same tooth, minimizing the effects of extraneous factors, such as age of tooth, type of tooth, prism direction, and health of the donor.
2. Materials and Methods
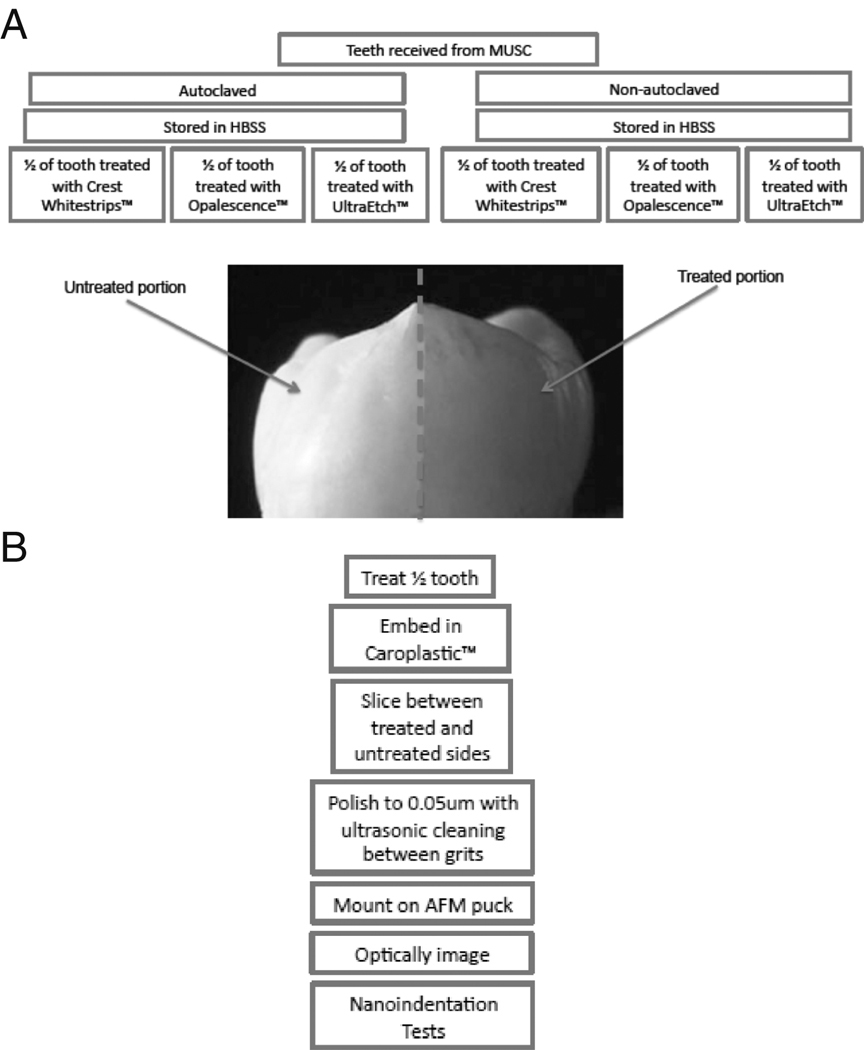
Human teeth were obtained from the Medical University of South Carolina, where the teeth were extracted due to vertical cracking or localized caries. Specimens with abnormal features, such as cracks, cavities, fillings, etc. in the vicinity of the testing area were eliminated. A total of 12 teeth were used in this study. Half of the teeth were disinfected using a standard chlorine treatment (Cuny and Carpenter, 1997) and then autoclaved, while the others received neither sterilization treatment. For clarity, the teeth that received the standard chlorine treatment and subsequent autoclaving processing will be referred to as “autoclaved” and those that received neither sterilization treatment will be referred to as “non-autoclaved”. All teeth were then stored in Hank’s Balanced Salt Solution (HBSS), which has been recommended for the storage of teeth, as there is minimal change in elastic modulus, as compared to samples stored in deionized water or CaCl(2)-solution (Guidoni et al. 2006). Sample treatment and preparation for mechanical testing are outlined in Figure 1 A and B.
Figure 1.
A flowchart of the tooth sample treatment (A) and preparation for mechanical testing (B).
A set of autoclaved and non-autoclaved teeth were subjected to dental treatments after being removed from the HBSS and briefly air-dried for 30 seconds. A dental treatments was applied to half of the outer surface of each tooth according to the manufacturer recommended directions: one set of teeth was treated with a layer UltraEtch™ for 30 seconds, the second set was coated with Opalescence™ and wrapped in Parafilm™ for one hour, the third set was wrapped in Crest Whitestrips™ Premium for 30 minutes. The other half of each tooth was left untreated as a control. After each treatment, the teeth were rinsed with water, air dried and then embedded in Caroplastic™, a non-infiltrating polyester resin. After approximately 24 hours curing time, the teeth were bisected using a 0.8mm thick diamond blade. Samples were then mounted to reflect similar tubule orientation between each control and corresponding treated sample. All samples were then sliced into 2mm thick sections to conform to the sample size needed for nanoindentation, then ground and then polished with 0.05 µm alumina slurry for 30 minutes (Habelitz et al. 2002). Between each polishing step, the slices were ultrasonically cleaned in deionized water (Marshall et al. 2003 and Habelitz et al. 2001). Ultrasonic cleaning for 10 seconds was found to be sufficient to remove polishing particles while not disrupting dental structure and properties. The tooth slices were then removed from the Caroplastic™ mechanically, etched with 0.005M citric acid, as per a previously established preparation method for mechanical testing of teeth (Habelitz et al. 2001), and then rinsed for 5 seconds with deionized water.
Contact AFM with a Veeco NP non-conductive silicon nitride tip was used to map the topography of the DEJ prior to polishing. Images were analyzed to determine root-mean-squared (RMS) roughness of treated and untreated samples. This was done to ensure that indentation depth was sufficient to overcome surface roughness. Backscatter SEM was used to acquire additional images of the tooth samples, identifying potential surface disturbances due to sample preparation.
The mechanical properties across the DEJ of each tooth slice were then measured using a Hysitron™ Triboscope transducer with a modified Veeco™ Multimode AFM. All indentation tests were performed statically with a two micron, 90° conical, diamond tip. Optical microscopy was used to facilitate consistent mechanical testing locations between each control sample and corresponding treated sample. Indentation was performed under a constant loading rate to a maximum force of 5000µN with 10µm spacing between indents to prevent effects of plastic deformation of previous indentations. A holding segment was enforced at the maximum load to allow for creep relaxation.
Chemical characterization was done to investigate a possible correlation between the mechanical properties and composition. FTIR and fluorescence microscopy were employed to further analyze apparent changes in composition. Samples were treated and prepared for chemical testing as described in Table 1. The exteriors of three autoclaved teeth were left untreated or treated with Crest Whitestrips™, Opalescence™ or UltraEtch™ according to the package directions. Dentin was separated from the enamel. The dentin was then formed into potassium bromide pellets then analyzed by IR spectroscopy under dry nitrogen.
Table 1.
Chart of preparation methods for chemical characterization. Three teeth were sterilized and received one of the dental treatments, Crest Whitestrips™, Opalescence™, or UltraEtch™. Three teeth were not sterilized but did receive one of the dental treatments. One tooth was autoclaved prior to slicing. One tooth was autoclaved prior to slicing and again after slicing. One tooth was treated with hydrogen peroxide.
| Tooth Treatment | FTIR | Autofluorescence | Monoclonal Anti-collagen |
|---|---|---|---|
| No treatment | ¶ | ¶ | ¶ |
| Autoclaving & Crest Whitestrips™ | ¶ | ¶ | |
| Autoclaving & Opalescence™ | ¶ | ||
| Autoclaving & UltraEtch™ | ¶ | ¶ | |
| Crest Whitestrips™ of ½ tooth | ¶ | ||
| Opalescence™ of ½ tooth | ¶ | ||
| UltraEtch™ of ½ tooth | ¶ | ||
| Autoclaved as whole tooth | ¶ | ||
| Autoclaved as whole tooth and secondary autoclaving after slicing | ¶ | ||
| Hydrogen peroxide treatment of ½ tooth | ¶ |
Fluorescence microscopy was used to image the protein content of teeth with exposure to Crest Whitestrips™, Opalescence™ or UltraEtch™. Four teeth were prepared for examining autofluorescence. The exteriors of three autoclaved teeth were treated according to the manufacturer recommended directions and one was left untreated. The samples were then sliced longitudinally into 2mm sections. The samples were then exposed to light of approximately 550nm and fluorescence was observed.
To further clarify if there was a change in concentration of collagen Type 1 with dental treatments, it was beneficial to use fluorescence microscopy for samples stained with a monoclonal collagen Type 1 antibody (Clone Col-1, Sigma-Aldrich, St. Louis, MO). It should be noted that this primary antibody does not bind to heat denatured collagen. An additional batch of teeth was prepared. Non-autoclaved teeth were bisected, with half treated with Crest Whitestrips™, Opalescence™, or UltraEtch™, while the other half of each tooth was untreated. To further isolate the effect of hydrogen peroxide, the active ingredient in Crest Whitestrips™, a slice of a non-autoclaved tooth was soaked in 3% hydrogen peroxide for 30 minutes. Additionally, one tooth that had been autoclaved prior to slicing, and one tooth that had been autoclaved both prior to slicing and after slicing were prepared to evaluate the effect of autoclaving on chemical composition. Rhodamine was elected as a secondary antibody to distinguish between selective binding and the autofluorescence of collagen in the green spectrum. These observations further characterized dental proteins’ response to chemical exposure.
The paired Student’s t-test with a p-value of 0.05 was used to calculate the statistical significance of changed in mechanical properties and surface roughness due to treatment. This method was elected because it allows for identification of changes within a population upon treatment, and is suitable for small sample sizes.
3. Results
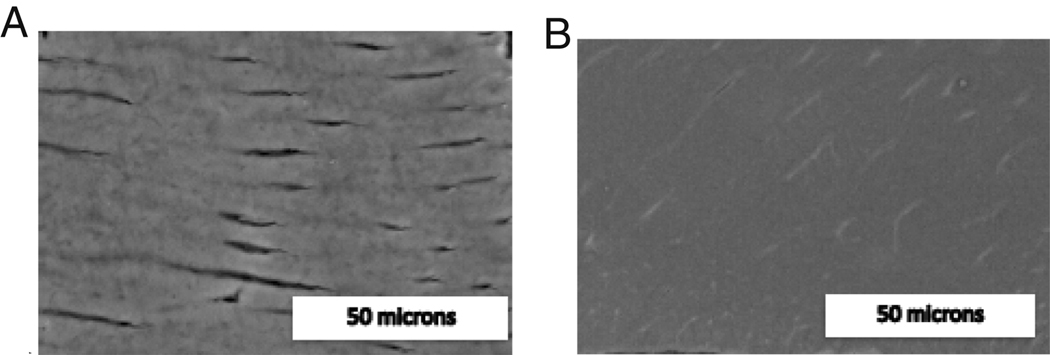
Preliminary work verified the importance of sample preparation. Long ultrasonic times were found to cause collapse of tubules, Figures 2 A and B. Habelitz et al. previously noted a similar disruption of the microstructure, mentioning that ultrasonication longer than 30s may alter the surface enamel’s mechanical behavior. Another degradation potentially resulting in the breakdown of the hydroxylapatite matrix was that of corrosion when the samples were left in an ionic storage solution in the presence of metal AFM sample pucks, Figure 3. Therefore, for all the results reported here, teeth were mounted on pucks immediately prior to testing.
Figure 2.
SEM image of dentin with 10 seconds (A) and 3 minutes (B) of ultrasonication.
Figure 3.
An illustration of the significant loss of mechanical integrity accompanying long-term storage in HBSS while mounted to AFM puck.
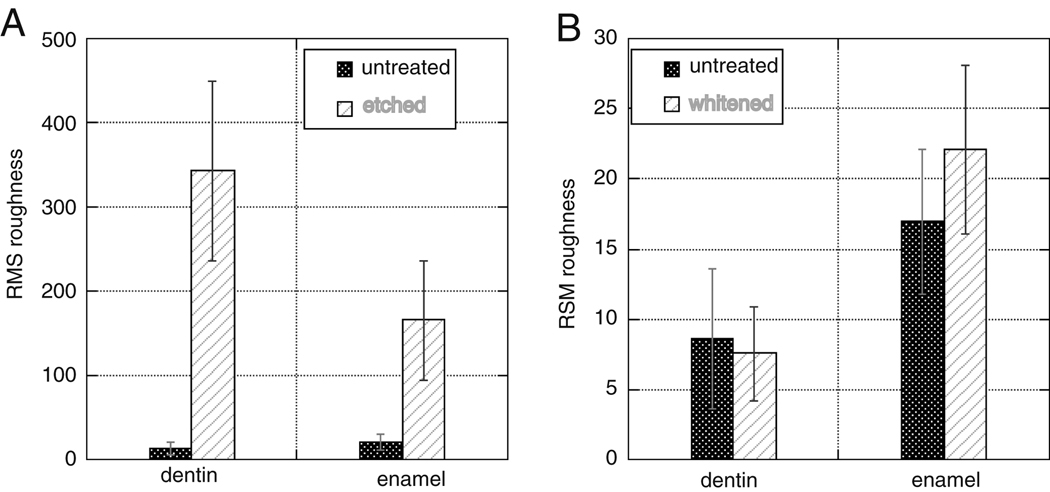
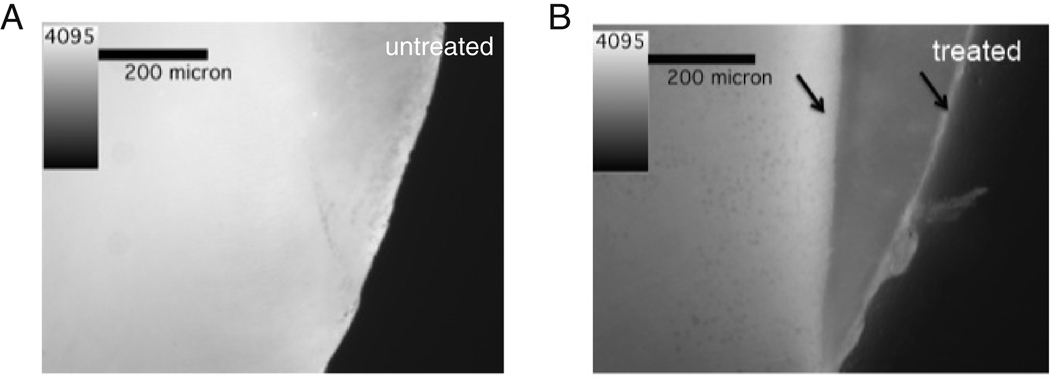
AFM was used to determine the roughness of dentin and enamel before and after treatment with UltraEtch™ and Opalescence™. The RMS roughness of enamel increased from 12.97±7.34 nm to 342.92±106.95 nm and dentin increased from 21.10±9.95nm to 165.99±70.86nm due to etching with UltraEtch™(Figure 4 A) (p≪0.01, Student’s t-test). AFM was also used to determine the effect of Opalescence™ treatment (Figure 4 B) on dentin and enamel. AFM indicated that whitening with Opalescence™ did not significantly change the RMS roughness of enamel (p=0.057, Student’s t-test), but did significantly increase the RMS roughness of dentin from 16.93±5.12nm to 22.06±5.98nm (p=4.2E-7, Student’s t-test).
Figure 4.
An illustration of the change in roughness of dentin and enamel upon treatment with UltraEtch™ (A) or Opalescence™ (B).
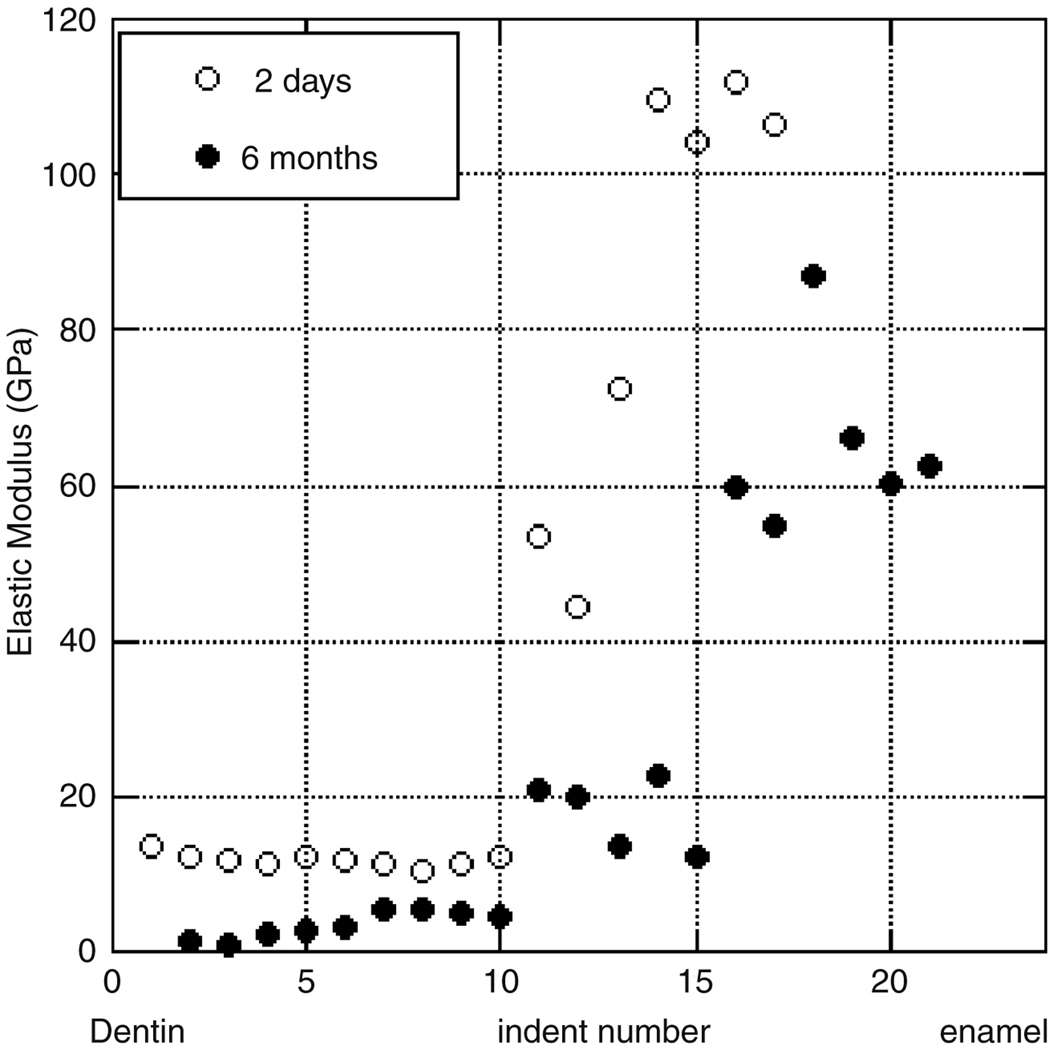
For the untreated autoclaved teeth, the average elastic modulus of dentin was 31.6±6.5 GPa and enamel was 111.7±16.0 GPa (Figure 6). The hardness value for dentin was found to be 1.0±0.3 GPa and that of enamel was 4.3±0.4 GPa. These values are consistent with those of previously published studies, in which hardness values of enamel are found to be between 3 GPa and 6 GPa, with elastic modulus of 70–120 GPa (Watari 2005, Park et al. 2007, Willems et al. 1993, Meredith et al. 1996, Xu et al. 1998, Habelitz et al. 2001, Mann and Dickinson, 2006, Staines et al. 1981, and Balooch et al. 2004), while the elastic modulus of dentin is reported to be 16.33–37 GPa with a hardness of 0.74–1.09 GPa and (Marshall et al. 2001, Oliveira et al. 2003, Angker et al. 2003, Kinney et al. 1996, and Fong et al. 2000).
Figure 6.
An illustration of the general trend and variation among the modulus of an individual untreated tooth.
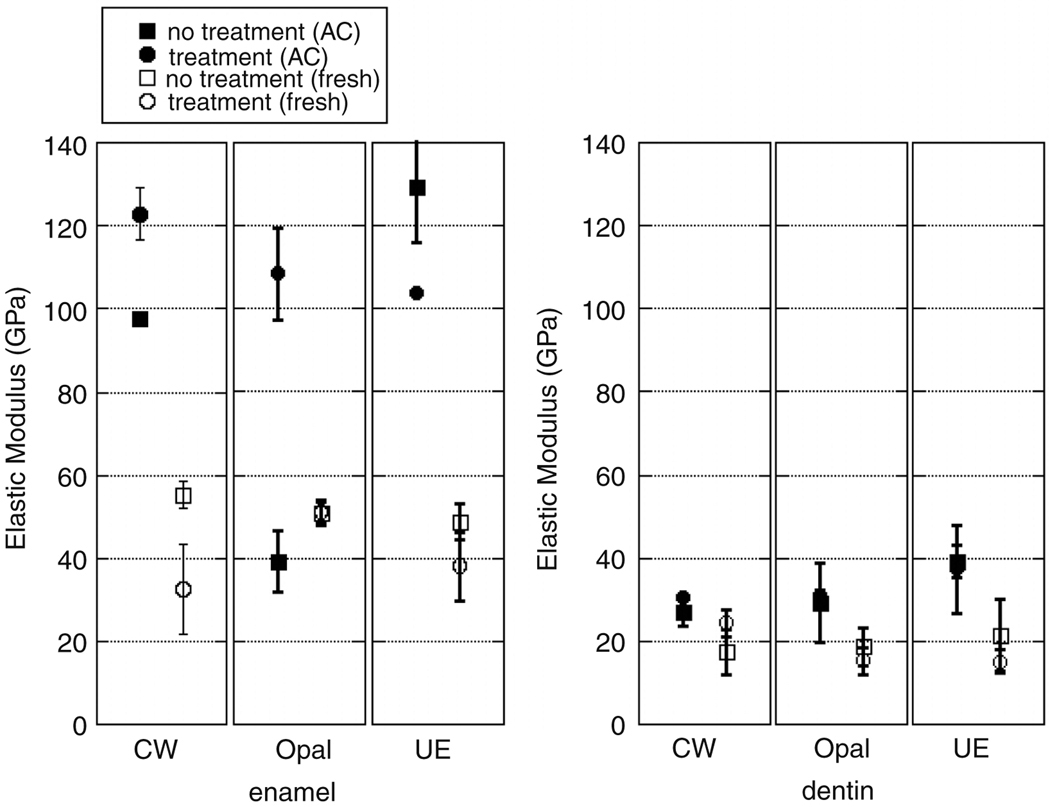
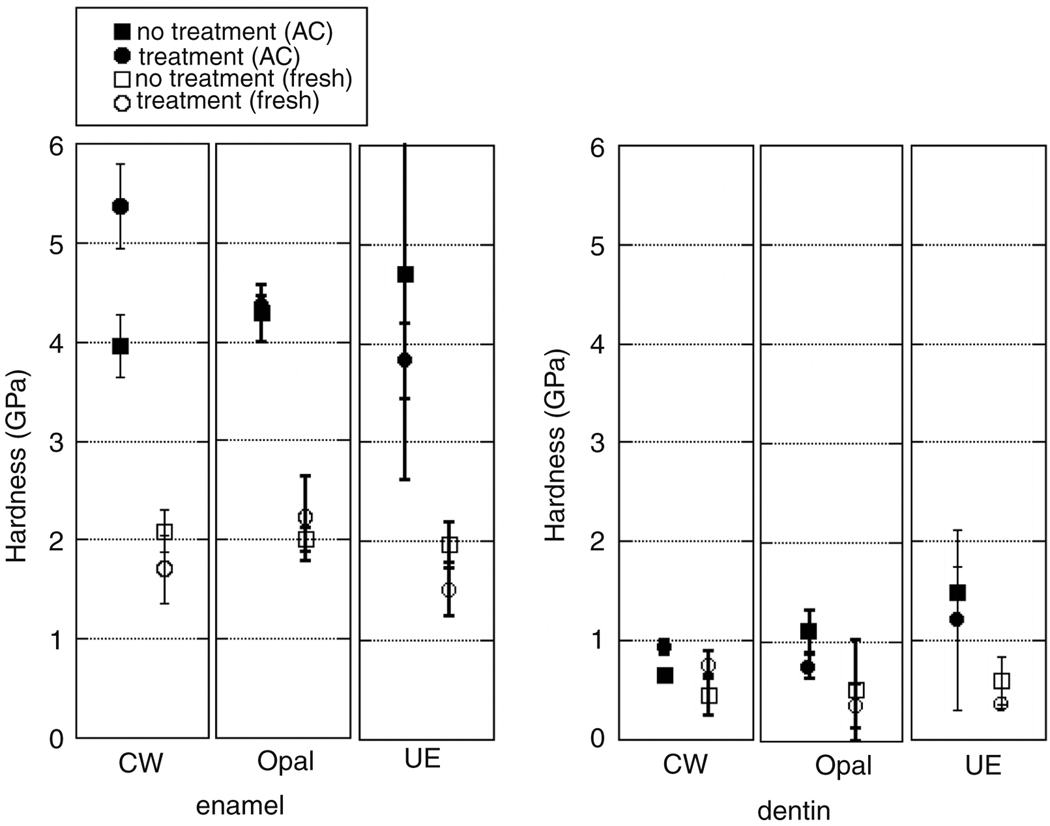
The effects of dental preparation and treatments on autoclaved and non-autoclaved teeth’s mechanical properties are summarized Figure 7 and Figure 8. The enamel and dentin elastic modulus and hardness of teeth treated with the Crest Whitestrips™ were significantly increased (p=3.3E-4, p=9.8E-6, p=4.3E-4 and p=9.8E-6 respectively). Whitening of autoclaved teeth due to Opalescence™ decreases modulus of dentin (p=0.0079) but has no significant effect on enamel (p=0.308), though only the exterior enamel was treated. Treatment with Opalescence™ also resulted in a decrease in the hardness of dentin (p=6.9E-3). Etching with UltraEtch™, a 35% phosphoric acid etchant, did significantly decrease the modulus and hardness of enamel (p=1.4E-3, p=0.14E-2 and p=2.0E-3) and the modulus of dentin, but did not significantly change the hardness of dentin (p=0.132) for autoclaved teeth.
Figure 7.
A comparison of the elastic modulus of autoclaved and non-autoclaved enamel and dentin with and without treatment.
Figure 8.
A comparison of the hardness of autoclaved and non-autoclaved enamel and dentin with and without treatment.
Teeth that did not receive the autoclaving and chlorine sterilization demonstrated lower elastic modulus and hardness. Treatment of non-autoclaved teeth with Crest Whitestrips™ resulted in a significant decrease in the elastic modulus and hardness of enamel (p=5.8E-3 and p=4.9E-3) and increase in that of dentin (p=4.7E-2). Treatment of non-autoclaved teeth with Opalescence™ resulted in a decrease in elastic modulus of the dentin (p=1.3E-2), though the hardness of dentin, as well as hardness and modulus of enamel remained unchanged (p=0.461, p=0.406, and p=0.168 respectively). Treatment of non-autoclaved teeth with UltraEtch™ resulted in a decrease of modulus and hardness of dentin (p=1.0E-3 and p=3.4E-3) and enamel (p=1.2E-6 and p=1.8E-2)
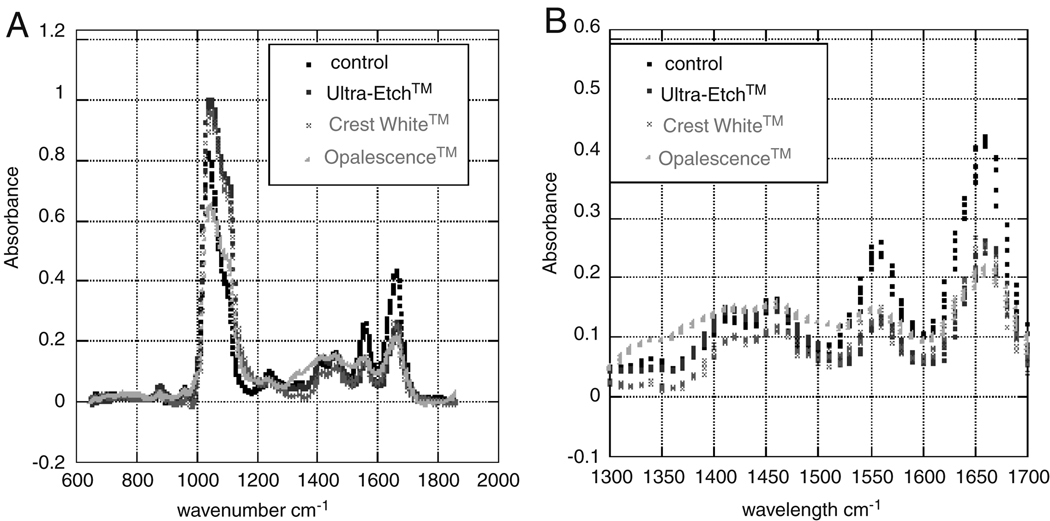
Protein concentration was investigated by means of Fourier transform infrared spectroscopy, Figure 9. FTIR has been useful in comparing natural and deproteinated dentin and enamel (Fattibene et al, 2005). Their study showed a decrease in the amine bonds (1550cm−1) was associated with a decreased in organic material. In this case, concentrations of organic components that may have been affected by sodium hydroxide, potassium hydroxide, or phosphoric acid were of interest. Analysis of IR spectra of dentin in this study revealed notable changes in absorbance of amide bond with dental treatments around the 1550cm−1 thus indicating a possible loss or denaturing of protein. The decrease in 1645 cm−1 may indicate either defluorination or the decrease of amide bonds (Fattibene et al, 2005). Further research is necessary to investigate the relationship between absorbance due to denaturing of proteins and fluoride content. That may be evidenced by increased absorbance at other wavenumbers and build on the work by Hammari et al. 2004 and Rintoul et al. 2007. For example, Rintoul et al. used 3500cm−1 to estimate the amount of fluoride ion in hydroxylapatite samples. Whereas Hammari et al. tracked the 750cm−1 to monitor fluoride adsorptions.
Figure 9.
An FTIR spectrum (A) of teeth subjected to various treatments indicates a reduction of amide bonds (B) as shown by the decrease in absorbance from the control. This further suggests a loss or denaturing of protein with treatments.
Florescence microscopy in the green spectrum showed a loss of collagen in teeth that were treated with Crest Whitestrips™ and UltraEtch™ compared with those that were not treated. There was an apparent loss of fluorescence along the DEJ with Crest Whitestrips™ treatment and a loss of fluorescence within the dentin with UltraEtch™ treatment. However, denatured collagen may also exhibit some autofluorescence, so collagen specific labeling protocol was necessary.
Florescence staining of intact collagen Type 1 with monoclonal anti-collagen Type 1 indicated the presence of proteins, highlighting the apparent overall reduction or denaturing of collagen and increased definition of DEJ, which accompanies treatment with Crest Whitestrips™. There was a loss of fluorescence, indicative of a loss or denaturing of protein, in the outer enamel of teeth treated with Opalescence™. There was also a noticeable loss of fluorescence in the dentin and enamel and increase definition of the DEJ in those teeth that have been treated with UltraEtch™ (Figure 10). An overall loss of florescence was observed with hydrogen peroxide, the active ingredient in Crest Whitestrips™ treatment. The secondary autoclaving caused almost complete loss of florescence and increased definition along the DEJ. This lack of protein was most obvious on the surface of the enamel teeth dental treatment.
Figure 10.
An example of apparent compositional change within a tooth. Fluorescence staining for type I collagen of A) control sample and B) sample treated with UltraEtch™.
4. Discussion
The Oliver-Pharr model was used to analyze variations in the hardness and elastic modulus of enamel and dentin (Nizam et al. 2005 and Fischer-Cripps, 2004). The Oliver-Pharr Model also assumes homogeneous deformation in the area below the indenter tip. Enamel and dentin tubules were oriented approximately perpendicular to the direction of indentation. However, the small size of the indents in this study relative to the inter-tubule spacing validated the assumption of homogeneity. A topographical image of the surface around the indentation area was obtained prior to testing, and efforts were made to avoid indenting into a pore or tubule to prevent breaking the indenter tip and allow the sample to be treated as a uniform material.
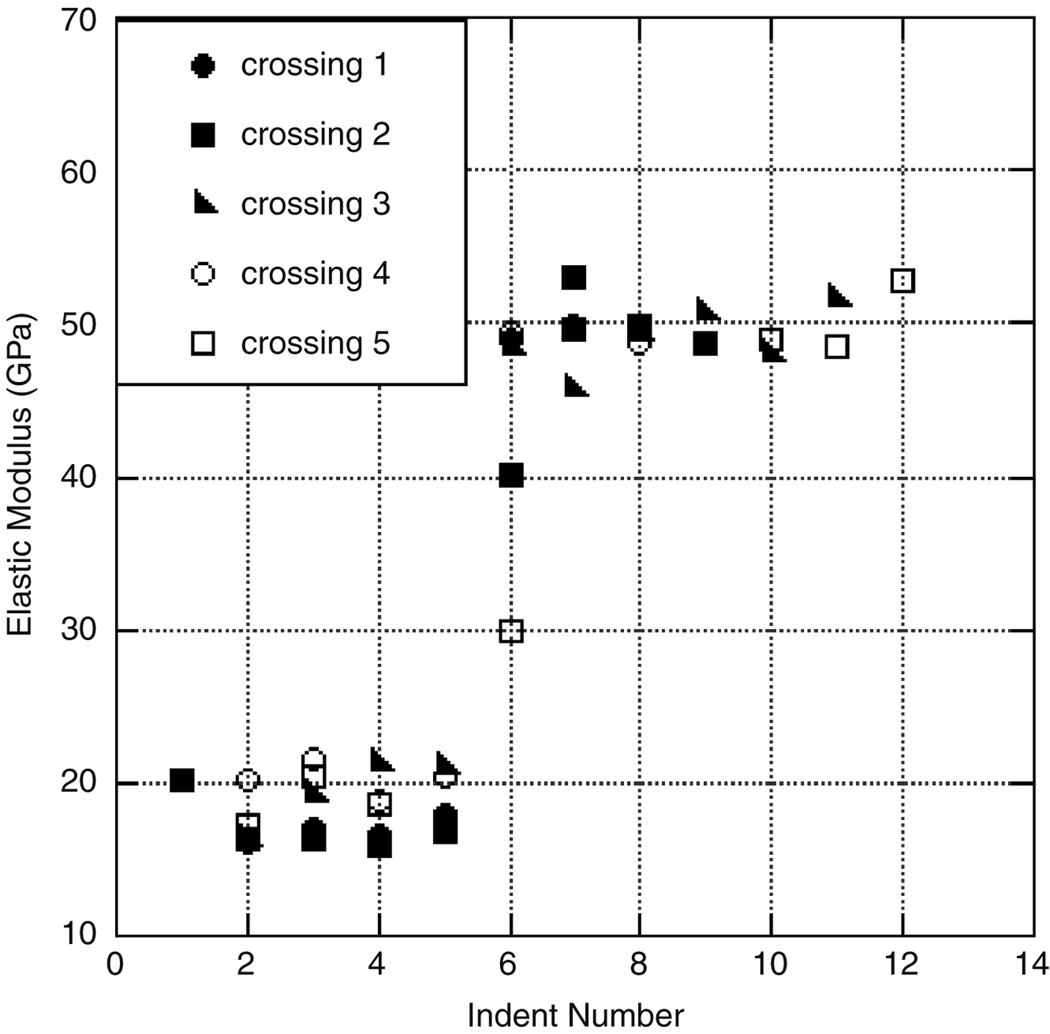
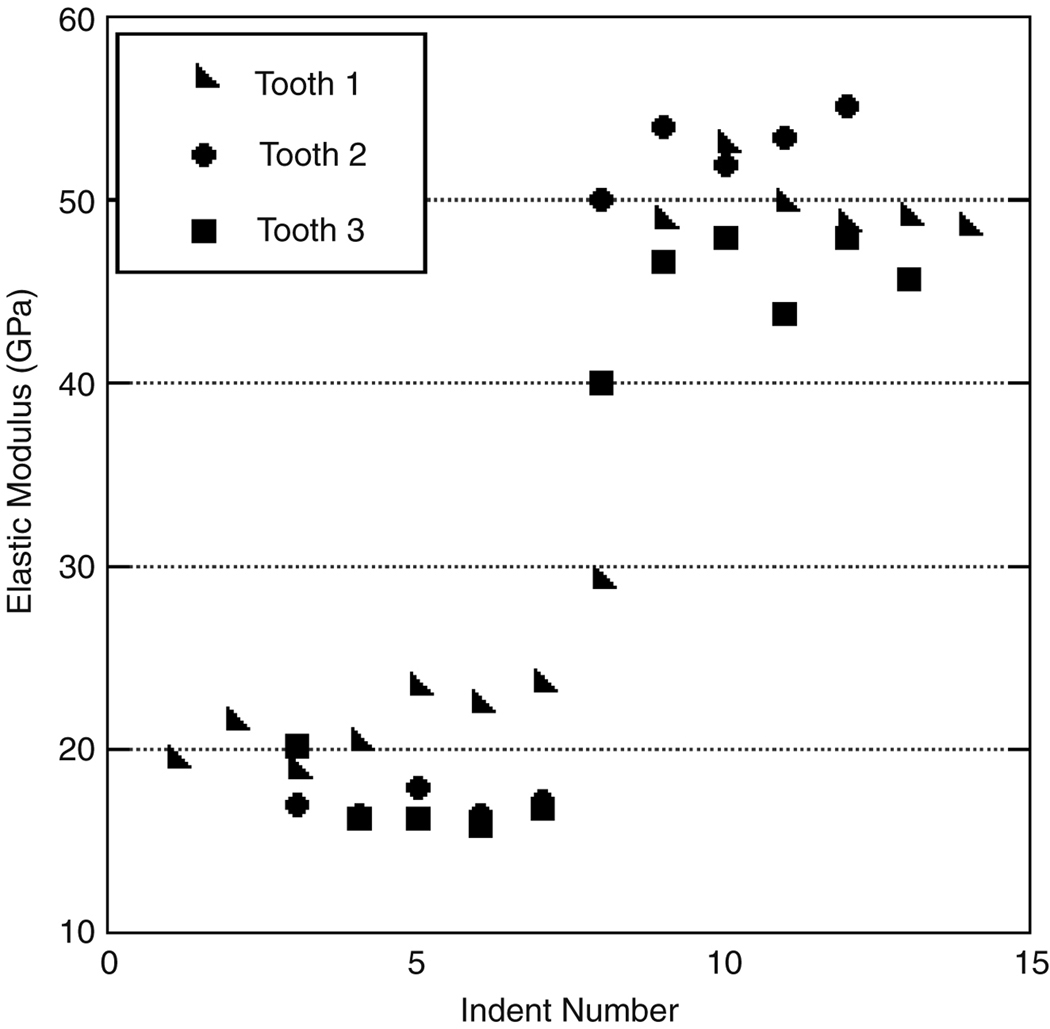
Preliminary tests indicated that the respective load for nanoindentation of dentin and enamel yielded no noticeable pile-up. Untreated teeth exhibit similar trends in hardness and elastic modulus across the DEJ, though there was fluctuation among teeth. Previous research has highlighted variation in mechanical properties due to the age, location, or type of tooth (Park et al. 2008), thus all testing was completed on similar locations on all teeth. Variation of mechanical properties for three distinct teeth are shown in Figure 5, illustrating three indentation sets across the DEJ. All sets were taken in relative proximity to each other and show variation within a single tooth. Note that the variation between teeth is greater than the variation between indents taken from the same section of teeth. To reduce the deviation in this study due to age and type of tooth, the teeth prepared to study the common dental treatments were only treated on one side and sectioned to allow for comparison of treatment and control.
Figure 5.
An illustration of the general trend and variation among the modulus of three different untreated teeth subjected to autoclaving.
The Student’s t-test indicated that UltraEtch™ did increase RMS roughness according to the trend expected, based on optical images and previously published results regarding lower concentrations of the active ingredient, phosphoric acid (Oliveira et al. 2003). The initial roughness values were comparable to those published in the literature (Marshall et al. 2001). Mechanical properties data of autoclaved samples was comparable to published values. Teeth that had been autoclaved demonstrated a significantly reduced elastic modulus and hardness, relative to samples that were not sterilized by autoclaving or chlorine treatment. This is consistent with previous findings showing decrease of modulus and hardness of bone following autoclaving (Todoh et al. 2006). In addition, this alteration in dental mechanical properties with autoclaving suggests that a possible reason for the large range of published mechanical behavior data is due to sample handling. Effects of external variables were well controlled by the comparison of each treated sample with an untreated portion of the same tooth. Treatments were applied only to the exterior of an intact tooth, for the manufacturer recommended duration. Changes observed with nanoindentation within the dentin suggest that the treatment time is adequate for penetration into at least the first 100 µm of the dentin. The increase in mechanical properties of samples treated with Crest Whitestrips™ may have been due to the addition of fluoride, offsetting the effects of the peroxide.
Changes in mechanical properties were presumed to be due to compositional or structural changes within the sample. Protein concentration was investigated as a potential contributor, revealing a loss or denaturing of collagen with each of the three investigated treatments. These concentrations were monitored by relative comparison of the amide bonding region absorption of FTIR spectra. The most abundant protein in teeth is Type I collagen, which autofluoresces at approximately 550 nm when excited in the 470–490 nm wavelength (Abrahãol 2006). Compositional changes were apparent using fluorescence microscopy of auto-fluorescing protein, and further verified by fluorescence microscopy with a collagen Type 1 specific labeling protocol.
5. Conclusion
Development of a standard testing technique is warranted for tooth preparation (enamel and dentin), storage and testing. Autoclaving was generally found to increase the elastic modulus and hardness of teeth. Although only surface of each tooth was treated, changes were noted in both the enamel and dentin. Non-autoclaved teeth treated with Crest Whitestrips™ showed a reduction in the elastic modulus of enamel and increase in the elastic modulus of dentin. Opalescence™ treatments did not significantly affect the enamel properties, but did result in a decrease in modulus of dentin. Additionally, as expected, the phosphoric acid UltraEtch™ treatment decreased the modulus and hardness of enamel and dentin. Changes in mechanical properties of teeth may be due in part to the loss or denaturing of protein, particularly collagen type I, as evident with optical imaging, fluorescent microscopy, and FTIR.
Acknowledgments
Financial support was provided by the National Science Foundation's Division of Materials Research REU program under grant number 0453554, an NSF Graduate Research Fellowship (Datko), and an NIH K25 HL092228. SC LIFE and SSBR. The authors would like to thank K. Ivey for FTIR characterization and L. Jenkins for consultation on teeth preparation and stabilization.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abrahãol IJ. Collagen analysis in human tooth germ papillae. Braz. Dent. J. 2006;17(3):208–212. doi: 10.1590/s0103-64402006000300006. [DOI] [PubMed] [Google Scholar]
- Angker L, Swain MV. Nanoindentation: application to dental hard tissue investigations. J. Mater. Res. 2006;21(8):893–1905. [Google Scholar]
- Angker L, Swain MV. Micro-mechanical characterization of the properties of primary tooth dentine. J. Dent. 2003;31:261–267. doi: 10.1016/s0300-5712(03)00045-9. [DOI] [PubMed] [Google Scholar]
- Balooch G, Marshall GW, Marshall SJ, Warren OL. Evaluation of a new modulus mapping technique to investigate microstructural features of human teeth. J. Biomech. 2004;37:1223–1232. doi: 10.1016/j.jbiomech.2003.12.012. [DOI] [PubMed] [Google Scholar]
- Barbour ME, Shellis RP. An investigation using atomic force microscopy nanoindentation of dental enamel demineralization as a function of undissociated acid concentration and differential buffer capacity. Phys. Med. Biol. 2007;52:899–910. doi: 10.1088/0031-9155/52/4/003. [DOI] [PubMed] [Google Scholar]
- Cuny E, Carpenter WM. Extracted teeth: decontamination, disposal and use. J. Calif. Dent. Assoc. 1997;25:801–804. [PubMed] [Google Scholar]
- Dickinson M, Mann A. Nanomechanics and chemistry of caries-like lesions in dental enamel. Materials Research Society Proceedings. 2004:29. [Google Scholar]
- Fattibene P, Carosi A, De Coste V, Sacchetti A, Nuvara A, Postorino P, Dore PA. Comparative epr, infrared and raman study of natural and deproteinated tooth enamel and dentin. Phys. Med. Biol. 2005;50:1095–1108. doi: 10.1088/0031-9155/50/6/004. [DOI] [PubMed] [Google Scholar]
- Ferguson VL, Boyde A, Bushby A. Elastic modulus of dental enamel: effect of enamel prism orientation and mineral content. J. Mater. Res. 2004;19:249–259. [Google Scholar]
- Fischer-Cripps AC. Nanoindentation. 2nd ed. New York: Spring-Verlang; 2004. [Google Scholar]
- Fong H, Sarikaya M, White SN, Snead ML. Nano-mechanical properties profiles across dentin-enamel junction of human incisor teeth. Mater. Sci. Eng. C. 2000;7(2):119–128. [Google Scholar]
- Guidoni G, Denkmayr J, Schoberl T, Jager I. Nanoindentation in teeth: influence of experimental conditions on local mechanical properties. Philos. Mag. 2006;86(33):5705–5714. [Google Scholar]
- Habelitz S, Marshall GW, Balloch M, Marshall SJ. Nanoindentation and storage of teeth. J. Biomech. 2002;35:995–998. doi: 10.1016/s0021-9290(02)00039-8. [DOI] [PubMed] [Google Scholar]
- Habelitz S, Marshall SJ, Marshall GW, Balooch M. Mechanical properties of human dental enamel on the nanometer scale. Arch. Oral. Bio. 2001;46(2):173–183. doi: 10.1016/s0003-9969(00)00089-3. [DOI] [PubMed] [Google Scholar]
- Hammari LEL, Laghzizil A, Barboux P, Lahlil K, Saoiabi A. Retention of fluoride ions from aqueous solution using porous hydroxyapatite structure and conduction properties. J. Haz. Mat. B. 2004;114:41–44. doi: 10.1016/j.jhazmat.2004.06.032. [DOI] [PubMed] [Google Scholar]
- Kinney JH, Balooch M, Marshall SJ, Marshall GW, Weihs TP. Atomic force microscope measurements of the hardness and elasticity of peritubular and intertubular human dentin. J. Biomech. Eng. 1996;118(1):133–135. doi: 10.1115/1.2795939. [DOI] [PubMed] [Google Scholar]
- Luiz B, Amboni R, Prates LH, Bertolino JR, Pires A. Influence of drinks on resin composite: evaluation of degree of cure and color change parameters. Polym. Test. 2007;26:438–444. [Google Scholar]
- Mann AB, Dickinson ME. Nanomechanics, chemistry and structure at the enamel surface. Monogr. Oral. Sci. 2006;19:105–131. doi: 10.1159/000090588. [DOI] [PubMed] [Google Scholar]
- Marshall GW, Balooch M, Gallagher R, Gansky SA, Marshall SJ. Properties of the dentinoenamel junction: AFM studies of nanohardness, elastic modulus and fracture. J. Biomed. Mater. Res. 2001;54:87–95. doi: 10.1002/1097-4636(200101)54:1<87::aid-jbm10>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Marshall SJ, Balooch M, Habelitz S, Balooch G, Gallagher R, Marshall GW. The dentin–enamel junction—a natural, multilevel interface. J Eur. Ceram. Soc. 2003;23:2897–2904. [Google Scholar]
- Meredith N, Sherriff M, Setchell DJ, Swanson SA. Measurement of the microhardness and young's modulus of human enamel and dentine using an indentation technique. Arch. Oral Biol. 1996;41:539–545. doi: 10.1016/0003-9969(96)00020-9. [DOI] [PubMed] [Google Scholar]
- Nizam BRH, Lim CT, Chang HK, Yap AUJ. Nanoindentation study of human premolars subjected to bleaching agent. J. Biomech. 2005;38:2204–2211. doi: 10.1016/j.jbiomech.2004.09.023. [DOI] [PubMed] [Google Scholar]
- Oliveira SS, Pugach MK, Hilton JF, Watanabe LG, Marshall SJ, Marshall GW. The influence of the dentin smear layer on adhesion: a self-etching primer vs. a total-etch system. Dent. Mater. 2003;19:758–757. doi: 10.1016/s0109-5641(03)00023-x. [DOI] [PubMed] [Google Scholar]
- Palosaari H. Matrix metalloproteinases (mmps) and their specific tissue inhibitors (timps) in mature human odontoblasts and pulp tissue. Doctoral Dissertation. Acta. Univ. Oul. D. 2003:739. http://herkules.oulu.fi/isbn9514270789/index.html?lang=en.
- Park S, Wang DH, Zhang D, Arola D. Property gradients in human enamel: A nanoscopic evaluation. Proc. SEM Annu. Conf. Expo. Exp. Appl. Mech. 2007;22:1225–1229. [Google Scholar]
- Park S, Wang DH, Zhang D, Romberg E. Mechanical properties of human enamel as a function of age and location in the tooth. J. Mater. Sci. Mater. Med. 2008;19(6):2317–2324. doi: 10.1007/s10856-007-3340-y. [DOI] [PubMed] [Google Scholar]
- Rintoul L, Wentrup-Bryne E, Suzuki S, Grondahl L. FT-IR spectroscopy of fluoro-substituted hydroxyapatite: strength and limitations, J. Mater. Sci: Mater Med. 2007:1701–1709. doi: 10.1007/s10856-007-3052-3. [DOI] [PubMed] [Google Scholar]
- Staines M, Robinson WH, Hood JA. Spherical indentation of tooth enamel. J. Mater. Sci. 1981;16:2551–2556. [Google Scholar]
- Todoh M, Tadano S, Imari Y. Effect of collagen matrix on mechanical properties of bone. J. Biomech. 2006;39:S471. [Google Scholar]
- Watari F. In situ quantitative analysis of etching process of human teeth by atomic force microscopy. J. Electron Microsc. 2005;54:299–308. doi: 10.1093/jmicro/dfi056. [DOI] [PubMed] [Google Scholar]
- White JM, Goodie HE, Marshall SJ, Marshall GW. Sterilization of Teeth by Gamma Radiation. J. Dent. Res. 1994;73:1560–1569. doi: 10.1177/00220345940730091201. [DOI] [PubMed] [Google Scholar]
- Willems G, Celis JP, Lambrechts P, Braem M, Vanherele G. Hardness and Young's modulus determined by nanoindentation technique of filler particles of dental restorative materials compared with human enamel. J. Biomed. Mater. Res. 1993;27:747–755. doi: 10.1002/jbm.820270607. [DOI] [PubMed] [Google Scholar]
- Xu H, Smith DT, Jahanmir S, Romberg E, Kelly JR, Thompson VP, Rekow ED. Indentation damage and mechanical properties of human enamel and dentin. J. Dent. Res. 1998;77:472–480. doi: 10.1177/00220345980770030601. [DOI] [PubMed] [Google Scholar]
- Zhou J, Hsiung LL. Depth-dependent mechanical properties of enamel by nanoindentation. J. of Biomed. Mater. Res. 2006;Part A:66–74. doi: 10.1002/jbm.a.31012. [DOI] [PubMed] [Google Scholar]