Abstract
After a period of forced overfeeding, many individuals actively compensate for this weight gain by reducing food intake and maintaining this state of hypophagia well into the post-overfeeding period. Our central goal is to define the mechanism underlying this adaptive reduction in food intake. When male Long Evans rats were implanted with indwelling gastric cannula and overfed a liquid low-fat (10% fat) diet for 17 days, overfed rats exhibited increased weight gain (P < 0.01) but decreased food intake, and this hypophagia persisted for 4-6 days post-overfeeding (P < 0.05). Leptin levels were increased 8-fold by overfeeding (P < 0.01), yet returned to baseline within 2 days post-overfeeding, despite the persistent hypophagia. Energy expenditure and oxygen consumption (VO2) were increased on the first day post-overfeeding (P < 0.05), but subsequently normalized prior to the normalization of food intake. Lastly, in leptin receptor deficient Obese Zucker (fa/fa) rats, overfeeding produced a significant decrease in food intake during active overfeeding. However, food intake returned to near baseline levels within one day post-overfeeding. Contrastingly, food intake remained suppressed in lean controls for 6 days post-overfeeding. Thus intact leptin signaling is not required for the decrease in food intake that occurs during overfeeding, but the ability to maintain this hypophagia is substantially impaired in the absence of leptin signaling. In addition, this post-overfeeding leptin effect appears to occur despite the fact that leptin levels normalize relatively rapidly post-overfeeding.
Keywords: Positive energy balance, weight loss, energy expenditure, ghrelin, PYY, leptin, overfeeding
Introduction
The existence of regulatory systems which act to defend against weight loss and starvation is well supported. Periods of energy restriction induce alterations in energy metabolism and behavior that collectively attenuate further loss of body energy reserves and rapidly restore energy stores once food again becomes available. Although a variety of circulating cues contribute to this adaptive response to negative energy balance, a reduction in circulating leptin appears to be a critical signal for many of these changes, including alterations in energy expenditure, reproductive function, thyroid function, immune function, and neuroendocrine hormone secretion [1], as well as neural and behavioral changes associated with an increased motivation to procure and consume food [2-4].
In contrast to this well described response to negative energy balance, there is substantially less information regarding adaptive responses to positive energy balance. Indeed, the very existence of such a regulatory response seems uncertain considering that many humans and animals readily gain weight and adiposity when exposed to highly palatable, energy dense diets. Yet not all individuals gain weight when faced with a high-fat diet, and several lines of evidence suggest that genetic or developmentally programmed differences in the weight regulatory system contribute to the considerable variability between individuals in their propensity to gain weight in an obesigenic environment [5-18]. When animals are overfed either via gavage or intragastric infusion, a marked reduction of voluntary food intake occurs in addition to changes in metabolism [19-22]. More importantly, the reduction in voluntary food intake persists for days or even weeks beyond the cessation of overfeeding, suggesting that regulatory systems detect weight gain and react to normalize body weight. Yet there is a distinct lack of information regarding the endocrine and neural mechanisms mediating this adaptive hypophagia, despite its relevance to obesity. Overfeeding does alter ghrelin, insulin and leptin levels [21, 23-25], but the degree to which these hormones contribute to the persistent hypophagia and weight loss following the cessation of overfeeding is unclear. Similar evidence implicates neurons within the hypothalamus, particularly hypothalamic melanocortin signaling, as overfeeding increased POMC expression while antagonism of melanocortin receptors following overfeeding attenuated the spontaneous hypophagia [26]. The mechanisms by which POMC neurons detect positive energy balance remain unclear.
The current work focuses on the period of voluntarily hypophagia that follows overfeeding. This model system produces a unique window of time in which animals voluntarily choose to reduce food intake and lose weight, and thus provides a unique opportunity within which to identify physiological regulatory systems which effectively defend against weight gain and induce weight loss. The primary goals of this experiment were to: 1) test whether circulating levels of leptin, insulin, PYY or ghrelin were persistently altered during this window of hypophagia, 2) whether the persistent decreases in food intake were accompanied by adaptive changes in energy metabolism or metabolic gene expression, and 3) determine whether intact leptin signaling is required for this adaptive response.
Materials and Methods
Animals and surgical description
Male Long Evans rats (Harlan Laboratories, Indianapolis, IN) weighing approximately 330g were used for all studies, except those involving Lean Zucker (Fa/Fa, ∼275g) or Obese Zucker (fa/fa, ∼ 411g) rats purchased from Charles River (Raleigh, NC). Animals were housed individually in shoebox cages under a 12:12hr light:dark cycle with ad libitum access to standard rodent chow and water. All procedures were approved by the institutional animal care and use committee at Pennington Biomedical Research Center.
Rats were fasted overnight prior to the surgical implantation of the intragastric cannula. They were anesthetized with isoflurane and received a pre-operative antibiotic injection (Baytril). A midline ventral abdominal incision was made caudal to the rib cage, and the stomach exteriorized. A 5 French polyurethane catheter (Instech Laboratory, Plymouth Meeting, PA) was inserted into a small incision made in the avascular portion of the stomach. The catheter has two moveable syringe bulbs that were positioned on either side of the stomach wall to hold it in place, and the catheter was further secured in the stomach with a purse string suture using 4-0 Vicryl to prevent slipping or leaking. Approximately 1 cm of the catheter projected into the stomach. The remaining catheter was passed subcutaneously to the dorsal midline, where it was exteriorized and secured using a purse string suture (4-0 silk). The abdominal wall incision was closed using Vicryl and the ventral abdomen incision closed using Michel clips which were removed after 7 days. The exteriorized portion of the catheter was attached via a stainless steel coupler to additional tubing which was threaded through a covance animal infusion harness and spring tether system. The spring tether was attached to a swivel and counter-balanced lever arm, which allowed the animal full range of movement within its home cage. All harness and tether equipment was purchased from Instech Laboratories.
Standard Overfeeding and Pair-Feeding Protocol
The gastric cannula was attached, via the tubing extension, to syringe pumps (Harvard Apparatus, Holliston, MA) which allowed the chronic administration of either water (control), or a low-fat liquid diet containing 1.0 Kcal/ml, of which 21.0% is protein, 10.0% is fat, and 69% is carbohydrate (Cat #:710140: Dyets, Inc; Bethlehem, PA). Immediately following surgery animals were placed within the harness system, but were infused with a very low rate of water (0.5 ml/hr) to maintain cannula patency while animals recovered from surgery. After 4 days of recovery, the overfeeding protocol was initiated. The infusion volume was gradually increased over a 15-17 day period of overfeeding. Because the diet contained 1 Kcal/ml, the volume of the infusion was equal to the Kcals infused as presented on Figure 1. Water infused controls were infused with a volume of water that was at all times equal to that of the overfed group. The use of water as a volume control is consistent with previous experiments [21, 26]. At the end of the overfeeding period, the infusion pumps were turned off and the rats were removed from the tether system but remained within their harness to protect the gastric cannula. Pair-feeding was also initiated at this point in appropriate groups. Once per day (morning), each Pair-fed animal was offered an amount of food equal to the average intake of the overfed group, and thus were in effect restricted to the intake of the formerly overfed rats for the duration of the post-overfeeding period.
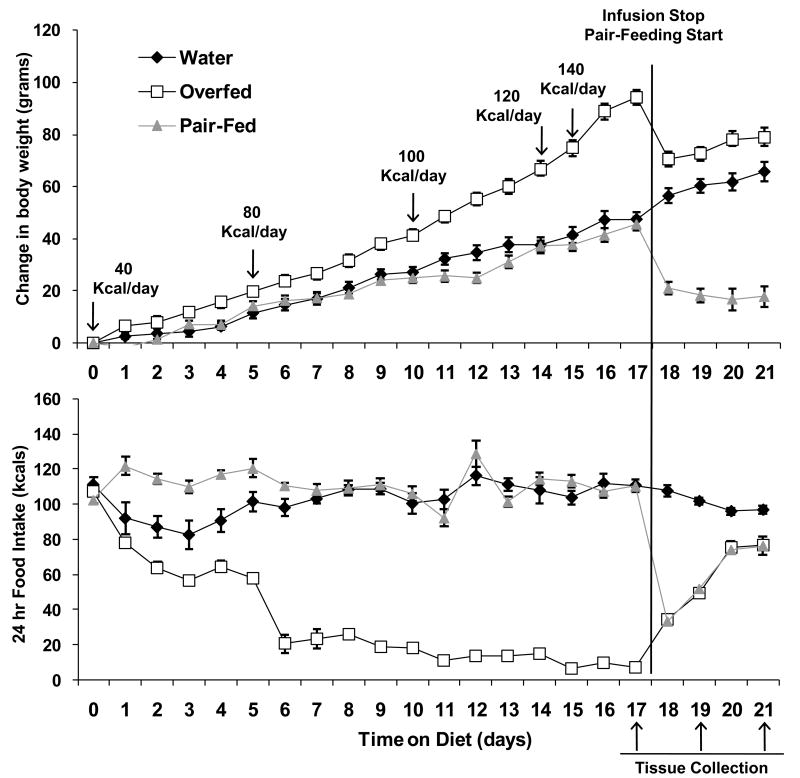
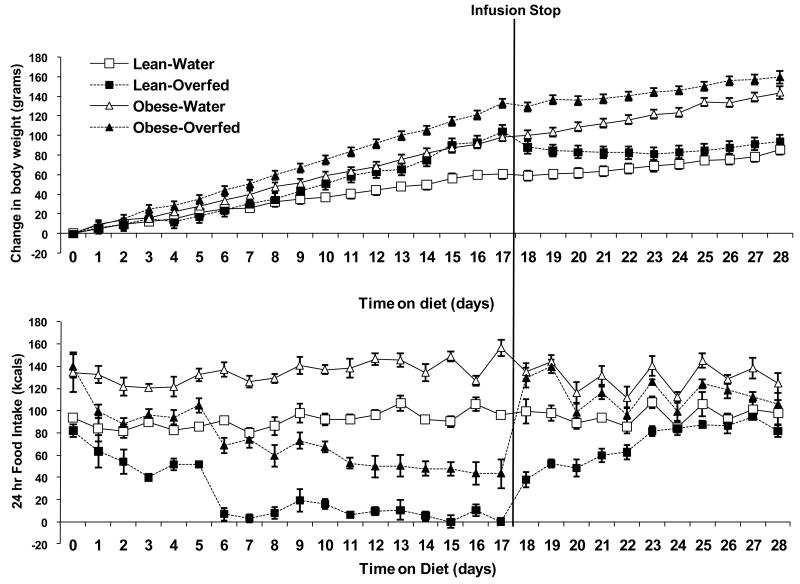
Figure 1. Body weight gain and food intake in response to overfeeding.
Male Long Evans rats were implanted with indwelling intragastric cannula and infused with water (Water) or a low fat liquid diet (Overfed) for 17 days, at which point the intragastric infusion ended and animals were tracked for an additional 4 days. Pair-Fed animals were single housed and not manipulated for the first 17 days of the experiment, but beginning on Day 17 were pair-fed to the spontaneous intake of the Overfed animals. Body Weight gain (top panel) and spontaneous food intake (lower panel) were assessed daily for the duration of the experiment. Subgroups (8-10 rats/group) were sacrificed on Day 17 (final day of overfeeding), Day 19 and Day 21 of the experiment. Body weight gain and food intake differed between Overfed and Water controls beginning on days 3 and 1 respectively (P < 0.05), and these differences persisted throughout the study.
Experiment 1. Effects of overfeeding on food intake, body weight, and circulating nutritional signals
35 Long Evans rats, implanted with a gastric cannula, were randomly assigned to either overfed or water (control) groups. Overfeeding progressed for 17 days, during which time food intake and body weight were assessed daily. Throughout the study, the volume of water infused into the control group was matched to the volume of the liquid diet. After 17 days of overfeeding or water infusion, the infusion protocol was halted, and animals were allowed to consume food ad libitum for 4 additional days. During this post-overfeeding period, subgroups (n=6) of animals were sacrificed either on the final day of overfeeding (Day 17), or two (Day 19) or four days (Day 21) following the cessation of overfeeding. In addition, a third group of 17 rats (Pair-Fed; PF) was included which was neither cannulated nor harnessed, and was allowed to eat ad libitum throughout the overfeeding period. However, upon cessation of overfeeding (Day 17) this group was pair fed (restricted) to the caloric intake of the overfed group to provide a control for their acute reduction in energy intake. The PF rats were sacrificed at Day 19 and Day 21 of the post-overfeeding protocol. At the time of sacrifice trunk blood was collected to assay circulating hormones via radioimmunoassay, and liver, white adipose and brown adipose tissue (WAT and BAT) were collected and rapidly frozen for RNA extraction and determination of metabolic gene expression.
Experiment 2: Effects of overfeeding on metabolic gene expression, energy expenditure and fat oxidation
To more directly assess energy expenditure and substrate oxidation, 12 Long Evans rats were randomly assigned to water (control), overfed or pair-fed groups as described in Experiment 1 above. At the cessation of overfeeding, animals were transferred to Oxymax metabolic chambers (Columbus Instruments, Columbus, OH) to assess oxygen consumption and carbon dioxide production during the post-overfeeding period. As with the Experiment 1, pair feeding was initiated at this same time, such that upon entering the metabolic chambers the PF rats consumed an amount of food that was identical to the previously overfed group (i.e., paired to their spontaneous hypophagia). To ensure viable data following transfer to the metabolic chambers, rats were adapted to the chambers prior to gastric cannulation surgery and for periodic 4-hour periods during the active overfeeding protocol. Animals were maintained in the metabolic chambers for 6 days post-overfeeding, with food intake and body weight measured daily. Values for VO2, CO2, RER, and direct energy expenditure were collected on each animal every 10 minutes throughout the study, but the values for each animal were averaged across each 24-hour period, and then analyzed via repeated measures ANOVA over the 6-day post-overfeeding period. VO2 is presented both as raw levels of oxygen consumption, and are normalized to the daily body weights (BW0.75) for each animal. Direct energy expenditure is presented as kcals/hr, and calculated as: VO2 × (3.815 + (1.232 × RER).
Experiment 3: Role of leptin in the response to overfeeding
9 lean (Fa/Fa) and 12 obese (fa/fa) Zucker rats were cannulated, harnessed, and overfed via an identical protocol to that previously described in Experiment 1. Daily food intake and body weight were recorded during the overfeeding period, and for an additional 11 days following the cessation of overfeeding. Animals had ad libitum access to rodent chow during and following the overfeeding protocol.
Hormone Analysis
Trunk blood was collected at sacrifice and allowed to clot at 4°C overnight, centrifuged at 3000×g for 30 minutes, and serum collected and stored at -80°C. Serum levels of leptin, insulin, PYY (total) and ghrelin (total) were measured via radioimmunoassay kit (Millipore, St. Charles, MO for leptin, insulin and PYY; Phoenix Pharmaceuticals, Burlingame, CA for ghrelin), according to the manufacturer's instructions.
RNA extraction and real-time PCR
Liver, white adipose and brown adipose tissue was collected at sacrifice, and total RNA extracted using Tri-Reagent (Molecular Research Center, Cincinnati, OH), according to the manufacturer's instructions. Confirmation of intact 18S and 28S RNA bands was achieved by ethidium bromide staining after electrophoresis of samples in 1% agarose/formaldehyde gels. Samples were quantified by spectrophotometry then mRNA was reversed transcribed into cDNA and mRNA expression determined using the SYBR green methodology in optical 384-well plates in an ABI PRISM 7900 sequence detector (Applied Biosystems, Branchburg, NJ). All expression data were normalized to cyclophilin mRNA levels. Primers are as follows:
| Gene | Forward Sequence | Reverse Sequence |
| CYC | GGAGATGGCACAGGAGGAAA | CCCATAGTGCTTCAGCTTGAAGTT |
| FAS | AGGATGTCAACAAGCCCAA | ACAGAGGAGAAGGCCACAAA |
| PEPCK | GCAGAGCATAAGGGCAAGGT | GCCGAAGTTGTAGCCAAAGAA |
| LEP | AGACCATTGTCACCAGGATCAAT | GGTGAAGCCCAGGAATGAAG |
| UCP1 | GGACCTACAATGCTTACAGAGTTATAGC | TCGTCCCTTTCCACAGTGTTG |
Statistical Analysis
Data were analyzed using the SAS software package (SAS V9, SAS Institute, Cary NC) using a two-tailed t-test or ANOVA using the general linear model (GLM) procedure. Repeated measures analysis were conducted when appropriate, using the GLM procedure and ID(treatment) as the error term for the main effect of treatment. When experiment wide tests were significant, post-hoc comparisons were made using the LSMEANS statement with the PDIFF option, and thus represent least significant differences tests for preplanned comparisons. For real-time PCR, expression levels were normalized to cyclophilin prior to analysis. All data are expressed as mean ± SEM, with a probability value of 0.05 considered statistically significant.
Results
Experiment 1: Effects of prior overfeeding on food intake, body weight, metabolic gene expression and feeding relevant hormones
Intragastric infusion of a low-fat, liquid diet produced a significant, progressive increase in body weight over the duration of the experiment (P < 0.05, Fig. 1). Overfeeding also decreased voluntary food intake, with the reduction in food intake being detectable within 1 day (P < 0.01, Fig 1.) Food intake in the overfed animals continued to decrease relative to water infused controls throughout the duration of the study in concert with the increased delivery of calories. By the end of the overfeeding period (Day 15-17), overfed animals were only consuming ∼1.7g of chow per day; 6.5% of baseline food intake (P < 0.001). Infusion of an equal volume of water had no effect on body weight or food intake throughout the overfeeding protocol, as water infused animals were no different from unharnessed, uninfused animals (i.e., the pairfed group on days 1-17, prior to the initiation of pair feeding; P > 0.05). Therefore, neither the increasing volume of infusion nor the harness and tether had a marked impact on food intake or body weight gain.
As expected, the overfeeding induced decrease in food intake persisted well beyond the cessation of overfeeding, such that the formerly overfed rats were consuming significantly less than water infused controls both 2 (12.1 vs 25.0g; P < 0.001; Fig 1), and 4 (18.8 vs. 23.8g; P = 0.02) days after the cessation of overfeeding. These decreases in food intake were associated with a progressive normalization of body weight post-overfeeding, although initial decreases in weight are likely due changes in gut-fill and four days was insufficient for a complete restoration of body weight.
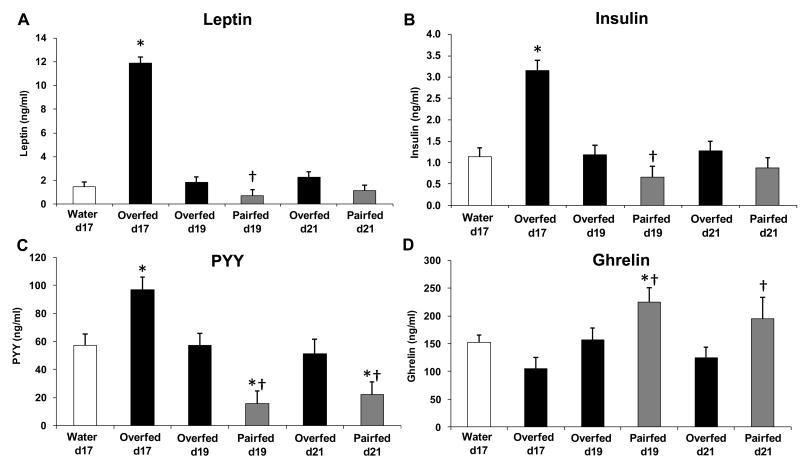
At the maximal point of overfeeding (Day 17), levels of leptin, insulin and PYY were all markedly increased (P < 0.0001 vs water infused controls; Figure 2), with leptin levels increased over 8-fold. Ghrelin levels were also decreased, although the decrease did not reach statistical significance (P = 0.165). However, two days following the cessation of overfeeding (Day 19), all of these changes were normalized, such that levels of leptin, insulin, ghrelin, and PYY were no longer different between the formerly overfed rats and water infused rats, despite the persistent, voluntary hypophagia in the overfed animals.
Figure 2. Levels of circulating hormones during the post-overfeeding period.
Animals described in Figure 1 were sacrificed after 17 days of overfeeding, or 2 (Day 19) or 4 (Day 21) days after the cessation of overfeeding. Pair-fed animals were not manipulated for the first 17 days of the study, but were pair-fed (restricted) to food intake of the overfed group beginning on Day 17. Trunk blood was collected at sacrifice and circulating levels of leptin, insulin, PYY and ghrelin were determined via RIA. *P < 0.05 vs Water; †P < 0.10 vs Overfed on the same day.
To control for the acute (albeit spontaneous) reduction in food intake in these formerly overfed rats, an additional group of animals were pair-fed to the spontaneous hypophagia of the formerly overfed animals (Pair-Fed; PF). In relation to these pair-fed animals, circulating leptin, insulin and PYY levels were higher (P < 0.05) in the overfed rats while ghrelin levels were lower (P < 0.05) on Day 19, two days after the cessation of overfeeding (Figure 2). This pattern continues for an additional 2 days (Day 21), although insulin and leptin are no longer statistically different between overfed vs. pair-fed groups by Day 21.
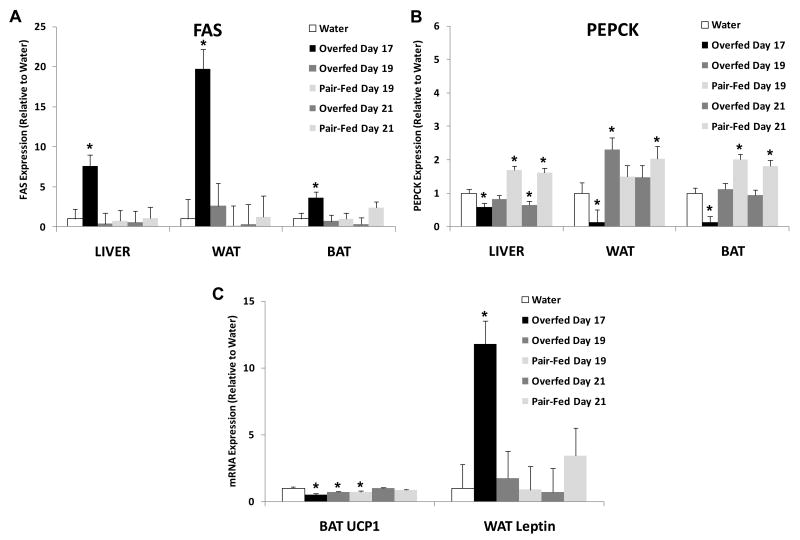
Expression of fatty acid synthase (FAS) and phosphoenolpyruvate carboxykinase (PEPCK) were measured in liver, brown and white adipose tissue on Days 17, 19 and 21 (Figure 3), in order to assess fluctuations in carbohydrate and lipid metabolism. Expression of FAS was significantly increased by overfeeding (Day 17) in all tissues measured (P < 0.01), but these increases were normalized (compared to water infused controls) within two days following the cessation of overfeeding (Day 19). No clear pattern of FAS expression between the overfed and pair-fed groups was apparent. PEPCK was significantly decreased by the activate overfeeding (Day 17) in all tissues (P < 0.01), but again these changes were largely normalized (compared to water infused controls) within two days (Day 19). However, PEPCK levels were significantly higher in the pair-fed as compared to formerly overfed rats within BAT and liver (P < 0.05), but not in WAT, on days 19 and 21. Levels of BAT UCP1 were significantly decreased by the active overfeeding (Day 19), and remained similar to or lower than water infused controls both two (Day 19) and four (Day 21) days following the end of the overfeeding. Lastly, leptin mRNA expression within the retroperitoneal fat pad was significantly increased by overfeeding (P < 0.001), but within two days was normalized, consistent with observed changes in circulating leptin protein (Figure 2).
Figure 3. Effect of overfeeding on metabolic gene expression in brown fat, white fat and liver.
Animals described in Figure 1 were sacrificed after 17 days of overfeeding, or 2 (Day 19) or 4 (Day 21) days after the cessation of overfeeding. Pair-Fed animals were not manipulated for the first 17 days of the experiment, but beginning on Day 17 were pair-fed to the spontaneous intake of the Overfed animals. Real-time PCR was used to assess the mRNA levels of fatty acid synthase (FAS; Figure 3A) and phosphoenolpyruvate carboxykinase (PEPCK; Figure 3B) within intrascapular brown adipose tissue (BAT), liver, and retroperitoneal white fat (WAT), which were isolated at sacrifice. In addition, uncoupling protein 1 (UCP1) in BAT and leptin expression in WAT were also determined (Figure 3C). *P < 0.05 vs. Water.
Experiment 2: Effects of a prior period of overfeeding on energy expenditure and fat oxidation
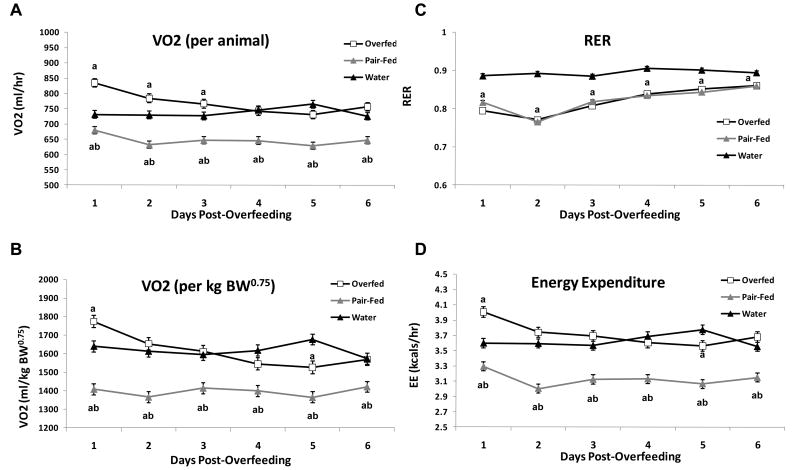
To more directly assess energy expenditure and substrate oxidation during the post-overfeeding period, control (water), overfed and PF animals were immediately placed into metabolic cages following overfeeding, with pair-feeding started at this same time point (as in Figure 1). Intragastric overfeeding again increased body weight while simultaneously decreasing voluntary food intake compared to water infused controls during the period of active overfeeding (P < 0.05; data not shown), and voluntary food intake remained significantly suppressed for approximately 4 days following the cessation of overfeeding. Overfed rats exhibited a significant increase in oxygen consumption (VO2) relative to water infused controls on the first day post-overfeeding (P < 0.05), with VO2 levels gradually returning to normal over the post-overfeeding period. This increase was detectable regardless of whether the data were expressed on a per animal basis (Figure 4A) or normalized to body weight (BW0.75; Figure 4B), although the effect is more marked on a whole animal basis. Contrastingly, the pair-fed group exhibited a marked and significant reduction in VO2 throughout the post-feeding period. Measures of direct energy expenditure, which account for changes in both VO2 and RER, indicate a similar increase in energy expenditure in the overfed animals on the first day of overfeeding, followed by a rapid normalization.
Figure 4. Effect of prior overfeeding on oxygen consumption, respiratory exchange ratio and energy expenditure.
Upon cessation of overfeeding, animals were immediately transferred to metabolic chambers to assess energy expenditure and respiratory exchange ratio for 6 days post-overfeeding. Pair feeding began when animals were placed in the metabolic chambers, such that pair-fed animals consumed the same amount of food as the overfed group. Average 24-hour values for VO2 (A and B), RER (C), and direct energy expenditure (D) for the six subsequent days post-overfeeding are reported. VO2 is expressed on both a per animal basis (A) and a per gram body weight basis (BW0.75; B). aP < 0.05 vs Water; bP < 0.05 overfed vs. pair-fed
Respiratory exchange ratio (RER) was also measured throughout the 6 days following the overfeeding (Figure 4C). In comparison to water infused controls, formerly overfed rats exhibited a marked decrease in RER throughout the post-overfeeding period (P < 0.01), although the magnitude of this change waned over time. However, RER between the formerly overfed and their pair-fed controls was identical throughout the study, indicating that this marked shift toward fat oxidation can be explained almost exclusively by the decrease in food intake.
Experiment 3: Role of leptin in the response to overfeeding
To more directly determine whether intact leptin signaling is required for the persistent, adaptive hypophagia that follows forced overfeeding, Obese Zucker rats, which lack functional leptin receptors, were used to determine whether the absence of leptin signaling alters the response to overfeeding. Lean Zucker rats exhibited a significant (P < 0.01), progressive increase in body weight during the overfeeding, followed by an adaptive normalization of body weight post-overfeeding (Figure 6). Body weights in the lean overfed animals were no longer statistically different from water controls by day 24 (7 days following the cessation of overfeeding). This normalization of body weight in lean Zucker rats was associated with a persistent reduction in food intake, with food intake remaining significantly reduced (P < 0.05) in the overfed animals as compared to water infused animals for 6 days post-overfeeding (Figure 6).
Contrastingly, Obese (leptin receptor mutant) Zucker rats exhibited a different pattern of body weight and food intake. Obese Zucker rats exhibited a greater rate of weight gain as compared to Lean Zuckers when infused with water, and their rate of weight gain was increased further by overfeeding (P < 0.01; Figure 6). In addition, when the overfeeding ended, obese Zuckers did not sharply reduce body weight, but instead their body weights remained elevated compared to the water infused controls for the duration of the study. Obese Zucker rats were hyperphagic at baseline (Lean-Water vs. Obese-Water; P < 0.001), consistent with the established phenotype of these animals. Overfeeding reduced food intake in Obese rats during the active overfeeding period, though the baseline difference in food intake also persisted. However, while Lean-Overfed rats maintained a reduction in voluntary food intake for 6 days post-overfeeding (P < 0.05), voluntary food intake returned to baseline in less than 1 day in Obese-Overfed rats. Thus, the ability to maintain a persistent, adaptive reduction in food intake following overfeeding was markedly impaired in the Obese Zucker rats. However, it should be noted that average daily food intake in the Obese Zucker rats did remain slightly less than their water infused controls during the post-overfeeding period (30.9 ± 1.1g per day vs. 27.3 ± .9 g/day; P = 0.03), indicating that other signals are contributing to a subtle reduction of food intake even in the absence of leptin signaling.
Because Lean and Obese Zucker rats exhibit differences in baseline food intake, overfeeding by a fixed caloric amount (described above) results in the Obese Zucker rats being slightly underfed compared to the Lean Zucker relative to their baseline intakes. To confirm that differences in the degree of overfeeding do not underlie the impaired response in the Obese Zucker, an additional group of lean Zucker rats were overfed by the same relative amount as the Obese, resulting in their receiving less total calories than the original lean group described in Figure 6. In these animals, food intake was significantly suppressed relative to water infused controls during overfeeding (95.2 ± 3.98 vs. 30.9 ± 1.22 kcals on Day 17; P < 0.001; data not shown). In addition, these rats also exhibited a clear post-overfeeding hypophagia, with food intake remaining suppressed below water controls for 2 days post-overfeeding (P < 0.01), and tending to be decreased on the third day (P = 0.09; data not shown). Thus reducing the rate of overfeeding reduced the magnitude of post-overfeeding hypophagia, but did not prevent it from occurring. As such, the marked impairment of post-overfeeding hypophagia in the Obese Zucker rats cannot be explained by differences in relative amount of overfeeding, but more likely reflects a constitutive defect in their regulatory response.
Discussion
In rodents and non-human primates, periods of forced overfeeding lead to persistent decreases in food intake that last days or weeks beyond the cessation of overfeeding [19-22]. Very little is known about the underlying metabolic, neuroendocrine or behavioral mechanisms that mediate this spontaneous hypophagia, although available evidence implicates the hypothalamus, particularly hypothalamic melanocortin signaling [21, 26]. The current manuscript assesses the hormonal and metabolic response to overfeeding, particularly focusing on the period of post-overfeeding hypophagia. Based on their established role in the regulation of energy balance, we hypothesized that circulating leptin and insulin would contribute to this post-overfeeding hypophagia, and expected that they would increase during overfeeding and remain elevated post-overfeeding in concordance with the persistent hypophagia. Contrastingly, because the gut hormones PYY and ghrelin are ostensibly regulated by the presence of nutrients in the stomach, we hypothesized that PYY and ghrelin levels would rapidly normalize once the overfeeding ended. However, leptin and insulin did not remain elevated as expected, as each hormone rapidly normalized relative to water infused controls in the post-overfeeding period; well before the normalization of food intake. Thus while active overfeeding alters circulating nutritional signals, their rapid normalization, prior to the normalization of food intake, suggests that none of these hormones contributes to the persistent hypophagia observed post-overfeeding.
Yet circulating hormones in the formerly overfed rats were also different from their pair-fed controls, which exhibited marked decreases in leptin, insulin and PYY and increases in ghrelin as compared to water-infused controls, consistent with their caloric restriction. The formerly overfed rats do not exhibit these changes. For instance, while leptin levels return to baseline (water control) levels on Day 19, they remained higher than leptin levels in the Pair-fed animals. Thus the hormonal pattern of the formerly overfed rats is not reflective of their state of acute negative energy balance, and there is no evidence of a counter regulatory resistance to the ongoing weight loss. This condition is perhaps expected considering that weight loss in this setting is physiologically appropriate. In addition, because leptin levels fall more rapidly than body weight, these observations are consistent with the hypothesis that circulating leptin levels are not exclusively tied to body adiposity, but instead reflect a more acute nutritional status of the organism [27-30]. Lastly, it should be noted that the current observations are not specific for the biologically active forms of PYY and ghrelin, and do not account for changes in leptin binding proteins. While total levels of these hormones tend to be correlated to active levels [31-33], it remains possible that the active hormones could be altered in pattern that is different from the total levels assessed here.
While a persistent reduction in food intake is a primary mediator of weight loss during the post-overfeeding period, it is also possible that adaptive changes in metabolism and energy expenditure contribute to post-overfeeding weight loss. Oxygen consumption and energy expenditure in the overfed rats were increased on the first day post-overfeeding, but this increase was short-lived and normalized well before food intake. In addition, a proportion of the increased energy expenditure during overfeeding may reflect the thermic effect of food [34-36]. We also failed to detect any increase in BAT UCP1 expression in the overfed animals, and feed efficiency (gain:fed ratio) was actually increased (data not shown). Thus these data do not support a persistent increase in energy expenditure post-overfeeding. Yet VO2 and energy expenditure were consistently higher in the overfed group as compared to the pair-fed group. Thus despite the fact that the overfed animals are eating less and losing weight, energy expenditure never fell below baseline as in the pair-fed rats. These changes are mirrored in the changes in metabolic gene expression. Marked increases in FAS expression and concomitant decreases in PEPCK expression were observed in all tissues (liver, WAT, BAT) during overfeeding, but both were largely normalized within two days post-overfeeding, despite the fact that these animals remained actively hypophagic. Thus physiological responses consistent with caloric restriction (increased PEPCK and decreased energy expenditure) are not being engaged. Lastly, the overfed group exhibited a marked decrease in the respiratory exchange ratio RER, consistent with a shift toward fat oxidation. However, because this reduction in RER was fully replicated in the pair fed animals, it appears that this shift toward fat oxidation was primarily a consequence of the reduction in food intake and does not reflect an inherent metabolic response. Taken together, these observations fail to provide clear support for an increase in energy expenditure as a significant mediator of weight loss during the post-overfeeding period, and instead suggest that a voluntary decrease in food intake is the primary driver of post-overfeeding weight loss. However, formerly overfed animals do not exhibit hormonal and metabolic changes characteristic of caloric restriction (i.e. the pair-fed group), despite their substantial hypophagia.
The above data supports previous work demonstrating that rats voluntarily reduce their food intake in order to lose weight following overfeeding. However, because leptin levels normalize well before the normalization of food intake, it is unclear whether leptin plays any role in this adaptive response. To more directly test leptin's role in this post-overfeeding hypophagia, we assessed the response of Obese-Zucker (fa/fa) rats to overfeeding. These rats bear a spontaneous mutation in the leptin receptor that impairs its function, and as a consequence are obese and hyperphagic in comparison to their lean littermates [37, 38]. Interestingly, active overfeeding produced a significant decrease in food intake in the Obese-Zucker rats, demonstrating that intact leptin signaling is not required for the suppression of food intake that accompanies active overfeeding. Because the current experiments did not focus on the period of active overfeeding, the identity of these leptin-independent signals remains unclear, though they are undoubtedly linked to the presence of excess nutrients in the gut and/or the resulting gastric distension. Yet while the Obese rats were capable of detecting active overfeeding, their ability to persistently reduce food intake following overfeeding was almost completely lost. Food intake returned to near baseline levels within the first day post-overfeeding in the Obese-Overfed group, such that only a subtle decrease in average daily food intake was detected during the post-overfeeding period. This loss of adaptive hypophagia was accompanied by a greatly blunted normalization of body weight in the Obese-Overfed as compared to the Lean-Overfed groups.
While our data clearly demonstrate that Obese (fa/fa) Zucker rats have an impaired feeding response during the post-overfeeding period, several possible interpretations may explain this defect. The most straight-forward interpretation is that intact leptin is required to adaptively alter food intake following a period of overfeeding. Leptin is well established to contribute to the adaptive response to weight loss, and the current data indicate that leptin may also be required to effectively respond to weight gain. Yet how can leptin suppress food intake when its levels are no longer elevated? It is possible that the increase in circulating leptin produces a persistent change in downstream regulatory systems. Yet this explanation seems unlikely considering that acute injections of leptin suppress food intake for relatively short periods of time (1-2 days), but food intake remains suppressed for as much as 6 days post-overfeeding in the current study, and for even longer in separate studies involving longer periods of overfeeding [19, 22]. An alternative explanation is that the marked fluctuations in circulating leptin are accompanied by marked changes in leptin sensitivity. For instance, it is possible that the rapid fall in leptin levels post-overfeeding results in acute increases in leptin sensitivity. If so, it is possible that the persistent decrease in food intake post-overfeeding is due primarily to a persistent increase in leptin sensitivity, and that the gradual normalization of food intake is driven by a normalization of leptin sensitivity, despite a relatively constant level of circulating leptin. Future studies will assess leptin sensitivity during and post-overfeeding, both to determine if leptin sensitivity changes post-overfeeding and to test whether leptin resistance even develops in animals that gain weight in the absence of a high-fat diet.
However, other possible scenarios could explain the impaired feeding behavior in the Obese Zucker rats post-overfeeding. First, it is possible that other signals of nutritional status drive the acute hypophagia, but do so via a mechanism that requires intact leptin signaling. Many satiety signals interact with leptin action, such that their efficacy is enhanced by leptin but impaired in the absence of leptin [39-42]. Obese-Zucker rats are also resistant to the anorectic effects of insulin [43]. Thus it remains possible that leptin is not critically involved in driving post-overfeeding hypophagia, but that the absence of leptin signaling (and the resulting obesity) so fundamentally alters this regulatory mechanism that the underlying signaling components (i.e. insulin) cannot produce their effect. Considering the evidence that the development of food regulatory circuits is impaired in the absence of leptin [44], it is further possible that developmental alterations within the hypothalamus may contribute to the phenotype, independent of the presence or absence of leptin post-overfeeding. While the current work suggests leptin signaling is necessary to maintain post-overfeeding hypophagia, additional work is required to determine the specific mechanisms and site of action through which leptin signaling is required for post-overfeeding hypophagia, as well as whether additional signaling systems contribute to this response.
In summary, the data presented herein demonstrate that involuntary overfeeding induces adaptive, persistent decreases in food intake that lead to a subsequent normalization of body weight once the overfeeding ends. While the post-overfeeding period is associated with significant changes in energy expenditure and fat oxidation, these changes are largely dependent on the voluntary hypophagia and do not reflect compensatory responses to the weight gain. Rats lacking functional leptin receptor signaling (Obese Zucker fa/fa rats) suppressed their food intake during overfeeding, but exhibited an impaired ability to maintain this decrease in food intake in the post-overfeeding period, such that only a subtle decrease in average daily food intake was detected. Taken together, these data indicate that decreased food intake during overfeeding is driven by a leptin-independent mechanism, but that constitutive loss of leptin signaling severely impairs the ability to maintain this decrease in food intake and successfully normalize body weight post-overfeeding. Lastly, these data also highlight the unique power of this model system to identify behavioral and neuroendocrine systems which promote voluntary, spontaneous reductions in food intake and body weight.
Figure 5. Effects of overfeeding on body weight and food intake in Lean and Obese Zucker rats.
Obese Zucker rats bearing mutations in the leptin receptor, and their wildtype controls (Lean), were implanted with indwelling gastric cannula and overfed with a liquid low-fat diet or water for 17 days, at which point the overfeeding ended. Body weight gain (Top Panel) and Food Intake (Bottom Panel) were measured daily throughout the overfeeding period and for an additional 11 days post-overfeeding. In lean controls, overfeeding produced a significant increase in body weight and decrease in food intake by days 10 and 2, respectively (P < 0.05); while Obese Zuckers exhibited a significant increase in body weight gain and decrease in food intake by days 6 and 1 respectively (P < 0.05). Upon cessation of overfeeding, food intake remained reduced in Lean-Overfed animals for 6 days post-overfeeding (P < 0.05), but normalized within one day post overfeeding in Obese-Overfed rats.
Acknowledgments
Grants: This project was supported by National Institutes of Health grants R03-NS051570 and P20-RR021945 to CDM, and by the Metabolic and Genomics core facilities at PBRC, which are supported in part by COBRE (NIH P20-RR021945) and CNRU (NIH 1P30-DK072476) center grants.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ahima RS. Leptin and the neuroendocrinology of fasting. Front Horm Res. 2000;26:42–56. doi: 10.1159/000061014. [DOI] [PubMed] [Google Scholar]
- 2.Figlewicz DP, MacDonald Naleid A, Sipols AJ. Modulation of food reward by adiposity signals. Physiol Behav. 2007;91:473–8. doi: 10.1016/j.physbeh.2006.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baicy K, London ED, Monterosso J, Wong ML, Delibasi T, Sharma A, Licinio J. Leptin replacement alters brain response to food cues in genetically leptin-deficient adults. Proc Natl Acad Sci U S A. 2007;104:18276–9. doi: 10.1073/pnas.0706481104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Farooqi IS, Bullmore E, Keogh J, Gillard J, O'Rahilly S, Fletcher PC. Leptin regulates striatal regions and human eating behavior. Science. 2007;317:1355. doi: 10.1126/science.1144599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jebb SA, Siervo M, Fruhbeck G, Goldberg GR, Murgatroyd PR, Prentice AM. Variability of appetite control mechanisms in response to 9 weeks of progressive overfeeding in humans. Int J Obes (Lond) 2006;30:1160–2. doi: 10.1038/sj.ijo.0803194. [DOI] [PubMed] [Google Scholar]
- 6.Blundell JE, et al. Resistance and susceptibility to weight gain: individual variability in response to a high-fat diet. Physiol Behav. 2005;86:614–22. doi: 10.1016/j.physbeh.2005.08.052. [DOI] [PubMed] [Google Scholar]
- 7.Levin BE, Dunn-Meynell AA, Balkan B, Keesey RE. Selective breeding for diet-induced obesity and resistance in Sprague-Dawley rats. Am J Physiol. 1997;273:R725–30. doi: 10.1152/ajpregu.1997.273.2.R725. [DOI] [PubMed] [Google Scholar]
- 8.Levin BE, Hogan S, Sullivan AC. Initiation and perpetuation of obesity and obesity resistance in rats. Am J Physiol. 1989;256:R766–71. doi: 10.1152/ajpregu.1989.256.3.R766. [DOI] [PubMed] [Google Scholar]
- 9.Lewis SR, Dym C, Chai C, Singh A, Kest B, Bodnar RJ. Genetic variance contributes to ingestive processes: a survey of eleven inbred mouse strains for fat (Intralipid) intake. Physiol Behav. 2007;90:82–94. doi: 10.1016/j.physbeh.2006.08.028. [DOI] [PubMed] [Google Scholar]
- 10.Schemmel R, Mickelsen O, Gill JL. Dietary obesity in rats: Body weight and body fat accretion in seven strains of rats. J Nutr. 1970;100:1041–8. doi: 10.1093/jn/100.9.1041. [DOI] [PubMed] [Google Scholar]
- 11.Smith BK, Andrews PK, West DB. Macronutrient diet selection in thirteen mouse strains. Am J Physiol Regul Integr Comp Physiol. 2000;278:R797–805. doi: 10.1152/ajpregu.2000.278.4.R797. [DOI] [PubMed] [Google Scholar]
- 12.West DB, Boozer CN, Moody DL, Atkinson RL. Dietary obesity in nine inbred mouse strains. Am J Physiol. 1992;262:R1025–32. doi: 10.1152/ajpregu.1992.262.6.R1025. [DOI] [PubMed] [Google Scholar]
- 13.Bouchard C, et al. Overfeeding in identical twins: 5-year postoverfeeding results. Metabolism. 1996;45:1042–50. doi: 10.1016/s0026-0495(96)90277-2. [DOI] [PubMed] [Google Scholar]
- 14.Bouchard C, et al. Sensitivity to overfeeding: the Quebec experiment with identical twins. Prog Food Nutr Sci. 1988;12:45–72. [PubMed] [Google Scholar]
- 15.Bouret SG, Simerly RB. Developmental programming of hypothalamic feeding circuits. Clin Genet. 2006;70:295–301. doi: 10.1111/j.1399-0004.2006.00684.x. [DOI] [PubMed] [Google Scholar]
- 16.Gluckman PD, Hanson MA, Beedle AS, Raubenheimer D. Fetal and neonatal pathways to obesity. Front Horm Res. 2008;36:61–72. doi: 10.1159/000115337. [DOI] [PubMed] [Google Scholar]
- 17.Levin BE. Why some of us get fat and what we can do about it. J Physiol. 2007;583:425–30. doi: 10.1113/jphysiol.2007.135434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Speakman JR. A nonadaptive scenario explaining the genetic predisposition to obesity: the “predation release” hypothesis. Cell Metab. 2007;6:5–12. doi: 10.1016/j.cmet.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 19.Harris RB, Kasser TR, Martin RJ. Dynamics of recovery of body composition after overfeeding, food restriction or starvation of mature female rats. J Nutr. 1986;116:2536–46. doi: 10.1093/jn/116.12.2536. [DOI] [PubMed] [Google Scholar]
- 20.Harris RB, Martin RJ. Changes in lipogenesis and lipolysis associated with recovery from reversible obesity in mature female rats. Proc Soc Exp Biol Med. 1989;191:82–9. doi: 10.3181/00379727-191-42893. [DOI] [PubMed] [Google Scholar]
- 21.Seeley RJ, Matson CA, Chavez M, Woods SC, Dallman MF, Schwartz MW. Behavioral, endocrine, and hypothalamic responses to involuntary overfeeding. Am J Physiol. 1996;271:R819–23. doi: 10.1152/ajpregu.1996.271.3.R819. [DOI] [PubMed] [Google Scholar]
- 22.Jen KL, Hansen BC. Feeding behavior during experimentally induced obesity in monkeys. Physiol Behav. 1984;33:863–9. doi: 10.1016/0031-9384(84)90220-8. [DOI] [PubMed] [Google Scholar]
- 23.Williams DL, Grill HJ, Cummings DE, Kaplan JM. Overfeeding-induced weight gain suppresses plasma ghrelin levels in rats. J Endocrinol Invest. 2006;29:863–8. doi: 10.1007/BF03349188. [DOI] [PubMed] [Google Scholar]
- 24.Deng QG, She H, Cheng JH, French SW, Koop DR, Xiong S, Tsukamoto H. Steatohepatitis induced by intragastric overfeeding in mice. Hepatology. 2005;42:905–14. doi: 10.1002/hep.20877. [DOI] [PubMed] [Google Scholar]
- 25.Harris RB, Ramsay TG, Smith SR, Bruch RC. Early and late stimulation of ob mRNA expression in meal-fed and overfed rats. J Clin Invest. 1996;97:2020–6. doi: 10.1172/JCI118637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hagan MM, Rushing PA, Schwartz MW, Yagaloff KA, Burn P, Woods SC, Seeley RJ. Role of the CNS melanocortin system in the response to overfeeding. J Neurosci. 1999;19:2362–7. doi: 10.1523/JNEUROSCI.19-06-02362.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Trayhurn P, Thomas ME, Duncan JS, Rayner DV. Effects of fasting and refeeding on ob gene expression in white adipose tissue of lean and obese (oblob) mice. FEBS Lett. 1995;368:488–90. doi: 10.1016/0014-5793(95)00719-p. [DOI] [PubMed] [Google Scholar]
- 28.Daniel JA, Whitlock BK, Baker JA, Steele B, Morrison CD, Keisler DH, Sartin JL. Effect of body fat mass and nutritional status on 24-hour leptin profiles in ewes. J Anim Sci. 2002;80:1083–9. doi: 10.2527/2002.8041083x. [DOI] [PubMed] [Google Scholar]
- 29.Havel PJ. Role of adipose tissue in body-weight regulation: mechanisms regulating leptin production and energy balance. Proc Nutr Soc. 2000;59:359–71. doi: 10.1017/s0029665100000410. [DOI] [PubMed] [Google Scholar]
- 30.Dubuc GR, Phinney SD, Stern JS, Havel PJ. Changes of serum leptin and endocrine and metabolic parameters after 7 days of energy restriction in men and women. Metabolism. 1998;47:429–34. doi: 10.1016/s0026-0495(98)90055-5. [DOI] [PubMed] [Google Scholar]
- 31.Korner J, Bessler M, Cirilo LJ, Conwell IM, Daud A, Restuccia NL, Wardlaw SL. Effects of Roux-en-Y gastric bypass surgery on fasting and postprandial concentrations of plasma ghrelin, peptide YY, and insulin. J Clin Endocrinol Metab. 2005;90:359–65. doi: 10.1210/jc.2004-1076. [DOI] [PubMed] [Google Scholar]
- 32.le Roux CW, et al. Attenuated peptide YY release in obese subjects is associated with reduced satiety. Endocrinology. 2006;147:3–8. doi: 10.1210/en.2005-0972. [DOI] [PubMed] [Google Scholar]
- 33.Marzullo P, et al. The relationship between active ghrelin levels and human obesity involves alterations in resting energy expenditure. J Clin Endocrinol Metab. 2004;89:936–9. doi: 10.1210/jc.2003-031328. [DOI] [PubMed] [Google Scholar]
- 34.Stock MJ. Gluttony and thermogenesis revisited. Int J Obes Relat Metab Disord. 1999;23:1105–17. doi: 10.1038/sj.ijo.0801108. [DOI] [PubMed] [Google Scholar]
- 35.van Baak MA. Meal-induced activation of the sympathetic nervous system and its cardiovascular and thermogenic effects in man. Physiol Behav. 2008;94:178–86. doi: 10.1016/j.physbeh.2007.12.020. [DOI] [PubMed] [Google Scholar]
- 36.Joosen AM, Westerterp KR. Energy expenditure during overfeeding. Nutr Metab (Lond) 2006;3:25. doi: 10.1186/1743-7075-3-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Iida M, Murakami T, Ishida K, Mizuno A, Kuwajima M, Shima K. Substitution at codon 269 (glutamine --> proline) of the leptin receptor (OB-R) cDNA is the only mutation found in the Zucker fatty (fa/fa) rat. Biochem Biophys Res Commun. 1996;224:597–604. doi: 10.1006/bbrc.1996.1070. [DOI] [PubMed] [Google Scholar]
- 38.Phillips MS, Liu Q, Hammond HA, Dugan V, Hey PJ, Caskey CJ, Hess JF. Leptin receptor missense mutation in the fatty Zucker rat. Nat Genet. 1996;13:18–9. doi: 10.1038/ng0596-18. [DOI] [PubMed] [Google Scholar]
- 39.Emond M, Schwartz GJ, Ladenheim EE, Moran TH. Central leptin modulates behavioral and neural responsivity to CCK. Am J Physiol. 1999;276:R1545–9. doi: 10.1152/ajpregu.1999.276.5.R1545. [DOI] [PubMed] [Google Scholar]
- 40.Morton GJ, et al. Leptin action in the forebrain regulates the hindbrain response to satiety signals. J Clin Invest. 2005;115:703–10. doi: 10.1172/JCI200522081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schwartz GJ, Moran TH. Leptin and neuropeptide y have opposing modulatory effects on nucleus of the solitary tract neurophysiological responses to gastric loads: implications for the control of food intake. Endocrinology. 2002;143:3779–84. doi: 10.1210/en.2002-220352. [DOI] [PubMed] [Google Scholar]
- 42.Williams DL, Baskin DG, Schwartz MW. Leptin regulation of the anorexic response to glucagon-like peptide-1 receptor stimulation. Diabetes. 2006;55:3387–93. doi: 10.2337/db06-0558. [DOI] [PubMed] [Google Scholar]
- 43.Intraventricular insulin reduces food intake and body weight of lean but not obese Zucker rats. Appetite. 1986;7:381–6. doi: 10.1016/s0195-6663(86)80006-x. [DOI] [PubMed] [Google Scholar]
- 44.Bouret SG, Draper SJ, Simerly RB. Trophic action of leptin on hypothalamic neurons that regulate feeding. Science. 2004;304:108–10. doi: 10.1126/science.1095004. [DOI] [PubMed] [Google Scholar]