Abstract
We have performed a series of first-principles electronic structure calculations to examine the reaction pathways and the corresponding free energy barriers for the ester hydrolysis of protonated cocaine in its chair and boat conformations. The calculated free energy barriers for the benzoyl ester hydrolysis of protonated chair cocaine are close to the corresponding barriers calculated for the benzoyl ester hydrolysis of neutral cocaine. However, the free energy barrier calculated for the methyl ester hydrolysis of protonated cocaine in its chair conformation is significantly lower than for the methyl ester hydrolysis of neutral cocaine and for the dominant pathway of the benzoyl ester hydrolysis of protonated cocaine. The significant decrease of the free energy barrier, ~ 4 kcal/mol, is attributed to the intramolecular acid catalysis of the methyl ester hydrolysis of protonated cocaine, because the transition state structure is stabilized by the strong hydrogen bond between the carbonyl oxygen of the methyl ester moiety and the protonated tropane N. The relative magnitudes of the free energy barriers calculated for different pathways of the ester hydrolysis of protonated chair cocaine are consistent with the experimental kinetic data for cocaine hydrolysis under physiologic conditions. Similar intramolecular acid catalysis also occurs for the benzoyl ester hydrolysis of (protonated) boat cocaine in the physiologic condition, although the contribution of the intramolecular hydrogen bonding to transition state stabilization is negligible. Nonetheless, the predictability of the intramolecular hydrogen bonding could be useful in generating antibody-based catalysts that recruit cocaine to the boat conformation and an analog that elicited antibodies to approximate the protonated tropane N and the benzoyl O more closely than the natural boat conformer might increase the contribution from hydrogen bonding. Such a stable analog of the transition state for intramolecular catalysis of cocaine benzoyl-ester hydrolysis was synthesized and used to successfully elicit a number of anti-cocaine catalytic antibodies.
Introduction
Cocaine addiction and overdose are major medical and public health problems that continue to defy treatment.1,2 Cocaine reinforces self-administration in relation to the peak serum concentration of the drug, the rate of rise to the peak and the degree of change of the serum level. Potent central nervous system stimulation is followed by depression.3 With overdose of the drug, respiratory depression, cardiac arrhythmia and acute hypertension are common effects. The disastrous medical and social consequences of cocaine addiction, such as violent crime, loss in individual productivity, illness and death, have made the development of an effective pharmacological treatment a high priority.4 However, cocaine mediates its reinforcing and toxic effects by blocking neurotransmitter reuptake and the classical pharmacodynamic approach has failed to yield small-molecule receptor antagonists due to the difficulties inherent in blocking a blocker.1–4 An alternative to receptor-based approaches is to interfere with the delivery of cocaine to its receptors and accelerate its clearance from the body.5 For this purpose, we have developed anti-cocaine catalytic antibodies with the capacity to bind and degrade cocaine.6,7
Anti-cocaine catalytic antibodies are a novel class of artificial enzymes with unique potential as therapeutic agents for cocaine overdose and addiction. This novel class of artificial enzymes, elicited by immunization with transition-state analogs of cocaine benzoyl-ester hydrolysis, have unique potential as therapeutic artificial enzymes due to their biocompatibility and extended plasma half-life. The design of a transition-state analog that would elicit a catalytic antibody8 is based on the mechanism of the corresponding non-enzymatic reaction, specifically the transition-state structure for the rate-determining step. Hence, a more complete understanding of the mechanisms of cocaine hydrolysis under physiologic condition could provide additional insights into the rational design of more effective transition-state analogs.
A detailed mechanistic understanding of the hydrolysis of cocaine can be obtained from an appropriate use of state-of-the-art first-principle computational techniques as a complement to experimental studies. Earlier electronic structure calculations of cocaine hydrolysis focused on the first step of the cocaine benzoyl-ester hydrolysis.9,10 Recently, the reaction pathways, solvent effects, and energy barriers were determined for alkaline hydrolysis of the benzoyl-ester and methyl-ester groups of neutral cocaine and some smaller alkyl esters in aqueous solution through a series of first-principle electronic structure calculations.11 The reaction coordinate calculations indicate that both the benzoyl-ester hydrolysis and the methyl-ester hydrolysis occur through a two-step process known for the majority of alkyl esters, i.e. the formation of a tetrahedral intermediate by the attack of hydroxide oxygen at the carbonyl carbon (first step) followed by the decomposition of the tetrahedral intermediate to products (second step). The decomposition of the tetrahedral intermediate requires a proton transfer from the hydroxide/hydroxyl oxygen to the ester oxygen, as the C–O bond between carbonyl carbon and ester oxygen gradually breaks. Two competing pathways for the second step of cocaine hydrolysis were examined: one associated with the direct proton transfer from the hydroxide/hydroxyl oxygen to the ester oxygen; and the other associated with a water-assisted proton transfer.11 The energy barrier calculated for the second step of the direct proton transfer is higher, whereas for benzoyl- and methyl-ester hydrolyses with water-assisted proton transfer the energy barriers for the second step are lower than for the first step. Thus, the first step should be rate-determining for the hydrolysis of both esters in aqueous solution, providing theoretical support for the design of stable analogs of the first transition state that elicited anti-cocaine catalytic antibodies.
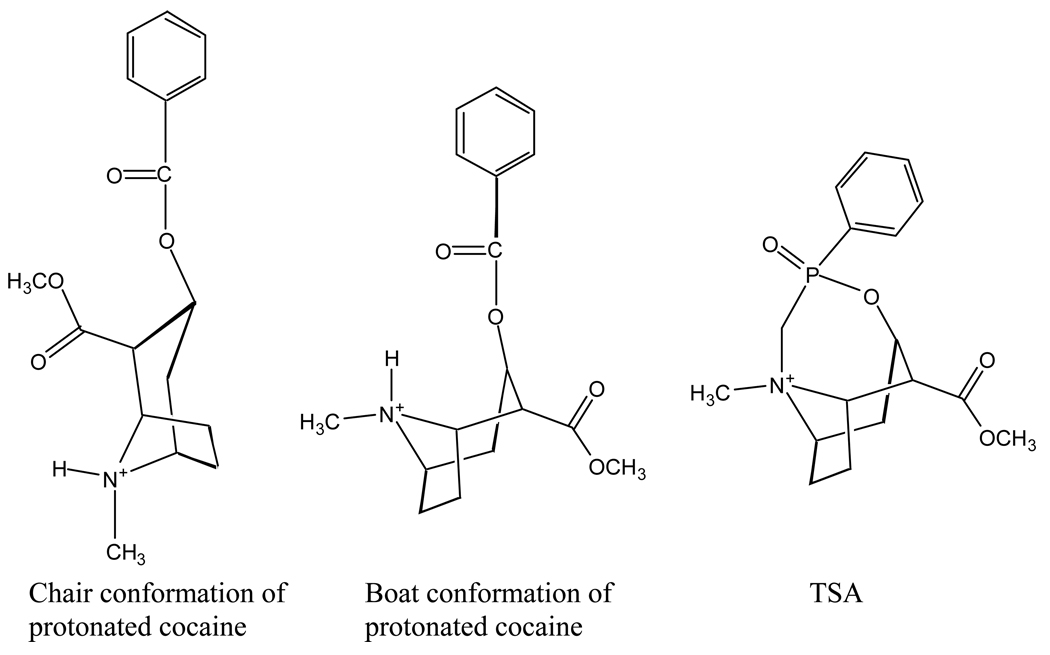
Under physiologic conditions (pH = 7.4) cocaine (pKa = 8.6) exists mainly as the protonated amine. The pathways for alkaline ester hydrolysis of neutral cocaine predict similar rates of reaction for methyl ester and benzoyl-ester hydrolysis. However, the methyl ester rapidly hydrolyzes in vivo and in aqueous solution at neutral pH. Our kinetic studies12 suggested that internal participation of the protonated amine in the alkaline hydrolysis of the cocaine methyl ester could account for its lability relative to the benzoyl-ester. For antibody catalysis the methyl ester is too small to be an effective epitope but we considered participation could be induced if an antibody were able to recruit cocaine from the chair conformation to the less stable boat form (see Chart 1 for the structures) and reorient the syn-protonated amine and benzoyl ester into proximity. Antibodies can provide significant binding energy and in principle antibody binding could effect conformer selection and promotion of substrate-assisted catalysis. To examine this idea, we performed both experimental studies and a detailed computational analysis of the energetics of this reaction for the design of the TSA for the alkaline hydrolysis of boat cocaine. Here we report the computational studies, whereas the corresponding experimental studies have been reported elsewhere.13a,13b
Chart 1.
A series of first-principle electronic structure calculations were carried out to study the competing reaction pathways and the corresponding free energy barriers for the ester hydrolysis of protonated cocaine in both chair and boat conformations. These calculations were performed to make a global judgement: Is the internally assisted reaction of boat cocaine plausible? The calculated results led to an affirmative answer to this question and inspired a novel TSA structure designed to elicit antibodies promoting substrate assistance. This TSA has yielded anti-cocaine catalytic antibodies with high efficiency.
Computational methods
All geometries of the reactants (i.e. hydroxide ion and the chair or boat conformation of protonated cocaine) and transition states were first fully optimized by employing density functional theory (DFT) using Becke’s three parameter hybrid exchange functional and the Lee-Yang-Parr correlation functional14 (B3LYP) with the 6-31G basis set, and were then refined at the B3LYP/6-31+G(d) level of theory. Vibrational frequency calculations were carried out to confirm the optimized transition states and stable structures and to perform zero-point vibration thermal corrections to the Gibbs free energies. Intrinsic reaction coordinate (IRC)15 calculations were performed to verify the expected connections of the first-order saddle points with the local minima found on the potential energy surface. The gas phase energy changes from the reactants to the corresponding transition states were determined by employing the geometries optimized at the B3LYP/6-31+G(d) level to carry out second-order Møller-Plesset (MP2) single-point energy calculations with the 6-31+G(d) basis set. Numerical results obtained for the hydroxide ion-catalyzed hydrolysis of methyl acetate in the gas phase indicate that the B3LYP/6-31+G(d) geometry optimization followed by the MP2/6-31+G(d) single-point energy calculation is adequate for studying the energy profile of ester hydrolysis.16
Self-consistent reaction field (SCRF) energy calculations were performed to calculate solvent shifts of the free energies by using the geometries optimized at the B3LYP/6-31+G(d) level in the gas phase. The free energy barrier for reaction in aqueous solution was taken as a sum of the free energy change calculated at the MP2/6-31+G(d)//B3LYP/6-31+G(d) level in the gas phase and the corresponding solvent shift determined by the SCRF calculations at the HF/6-31+G(d) level. In general, the solute-solvent interaction can be divided into a long-range electrostatic interaction and short-range non-electrostatic interactions (such as cavitation, dispersion, and Pauli repulsion).17 The dominant long-range electrostatic interaction was evaluated by using the recently developed GAMESS18 implementation of the surface and volume polarization for electrostatic interactions (SVPE).19 The SVPE model is also known as the fully polarizable continuum model (FPCM)20 because it fully accounts for both surface and volume polarization effects in the SCRF calculation. In other SCRF implementations, volume polarization effects are ignored or approximately modelled by modifying the surface polarization charge distribution through a simulation and/or charge renormalization,21,22,23,24,25,26,27,28,29 or the solute charge distribution is simply represented by a set of point charges at the solute nuclei.30,31
Since the solute cavity surface is defined as a solute electron charge isodensity contour determined self-consistently during the SVPE iteration process, the SVPE results (converged to the exact solution of Poisson’s equation with a given numerical tolerance) depend only on the contour value at a given dielectric constant and a certain quantum chemical calculation level.19a Our previous computational studies involving SVPE calculations have demonstrated that the SVPE calculations using the 0.002 a.u. contour led to the calculated energy barriers in good agreement with the corresponding experimental data for the hydroxide ion-catalyzed ester hydrolysis reactions.32,33,34 In addition, Bentley recently employed the minimum in the electron density function between pairs of interacting molecules to estimate molecular sizes, and found that the molecular surfaces identified by such a procedure are in excellent agreement with the 0.002 a.u. isodensity contour.35 Therefore the 0.002 a.u. contour was used in this study.
The current version19a of the SVPE implementation has its own limitations. In particular, the analytic energy derivatives required for geometry optimization and the calculation of the short-range non-electrostatic interactions have not been implemented yet. It has been shown that the short-range non-electrostatic interactions have significant contributions to the absolute hydration free energy of a charged species.36 Nevertheless, for a given chemical reaction step, the short-range non-electrostatic contributions to the energy change roughly cancel out.33 Previous computational studies demonstrate that, whether the short-range non-electrostatic interactions are included or not, the first-principles electronic structure calculations using the SVPE method with 0.002 a.u. contour can consistently predict energy barriers and free energy barriers in excellent agreement with the corresponding experimental data for the alkaline hydrolyses of carboxylic acid esters, phosphate esters, and their structural variants.32,33,34 To further examine this issue the contributions of short-range non-electrostatic interactions to the free energy barriers were estimated by using the polarizable continuum model (PCM)37 implemented in the Gaussian98 program38 with the default choices of the program for the recommended standard parameters. The total solvent shift should be a sum of the long-range electrostatic interaction contribution determined by the SVPE calculations and the total contribution of the short-range non-electrostatic interactions determined by the PCM calculations. We note that PCM calculations with the Gaussian98 program evaluate the electrostatic and non-electrostatic interactions separately; there is no coupling between the electrostatic and non-electrostatic interactions.
The SVPE solvation calculations were performed using a local version19a of GAMESS and all other results were obtained using Gaussian98 on SGI Origin 200 multiprocessor computers.
Results and discussion
Alkaline hydrolysis of chair cocaine
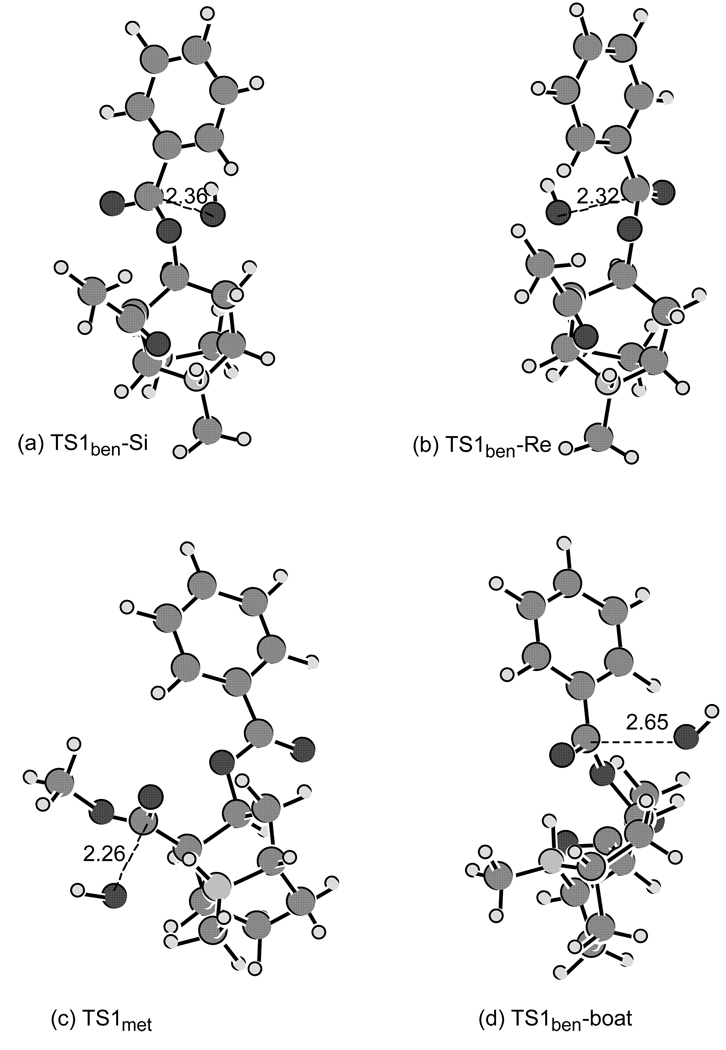
Depicted in Figure 1 are the geometries of the transition states optimized at the B3LYP/6-31+G* level. The Cartesian coordinates of these geometries and the corresponding reactants are given as supporting materials. For benzoyl ester hydrolysis of protonated cocaine in its chair conformation, the nucleophilic hydroxide ion can approach from two faces, denoted by Si and Re, of the carbonyl to form two stereoisomer tetrahedral intermediates (R and S). The two transition state structures, optimized at the B3LYP/6-31+G(d) level for the two competing pathways of the first step of the benzoyl ester hydrolysis, are denoted by TS1ben-Si and TS1ben-Re. The transition state for the first step of the cocaine methyl ester hydrolysis is denoted by TS1met. These three transition state structures optimized for the hydrolysis of protonated cocaine are similar to the corresponding transition state structures optimized for the alkaline hydrolysis of neutral cocaine.11 A remarkable difference is that the internuclear distances between the hydroxide oxygen and the carbonyl carbon become significantly shorter for the protonated cocaine: 2.36 Å, 2.32 Å, and 2.26 Å in TS1ben-Si, TS1ben-Re, and TS1met, respectively, for protonated cocaine hydrolysis compared to the corresponding distances 2.62 Å, 2.57 Å, and 3.15 Å for neutral cocaine.11 These results verify earlier predictions made using SM339 semiempirical methods, where PM340 geometry optimisations in aqueous solution resulted in internuclear hydroxide oxygen-carbonyl carbon distances of 2.23Å for TS1ben-Si and 2.13Å for TS1ben-Re.9
Figure 1.
Transition state structures optimized at the B3LYP/6-31+G* level for the ester hydrolysis of protonated cocaine: (a) and (b) for the benzoyl ester hydrolysis of the chair cocaine; (c) for the methyl ester hydrolysis of the chair cocaine; and (d) for the benzoyl ester hydrolysis of the boat cocaine.
The alkaline hydrolysis of carboxylic acid esters, including cocaine, proceed through a bimolecular base-catalyzed acyl-oxygen cleavage (BAC2) process and the rate-determining step is the first step, i.e. the formation of the tetrahedral intermediate.11,32 Therefore in this study we focus on the critical first step of the BAC2 process. The calculated energetic results are summarized in Table 1. It should be pointed out that the ΔG(gas) values listed in Table 1 are simply the Gibbs free energy changes from the separated reactants to the corresponding transition states when the solvent effects are ignored, but these values are not the free energy barriers for the corresponding reaction in the gas phase. This is because previous studies32,34 have demonstrated that for the reaction of an ester (no matter whether it is a carboxylic or phosphate ester), the ester and hydroxide ion first form a hydrogen-bonded complex in the gas phase (a local minimum on the potential energy surface) before going to the transition state, whereas such a hydrogen-bonded complex does not exist in aqueous solution. So, the free energy barrier for the imaginary reaction in the gas phase should be the free energy change from the hydrogen-bonded complex to the first transition state.32,34
Table 1.
Calculated Gibbs free energies (ΔG in kcal/mol, at T = 298.15 K and P = 1 atm) of the transition states relative to the corresponding separated reactants for the ester hydrolyses of protonated cocaine in solution.a
| Transition state | ΔG(gas)b | Solvent shiftc | ΔG(solution) e | ||
|---|---|---|---|---|---|
| Electrostatic (SVPE) |
Non- electrostatic (PCM)d |
Without non- electrostatic |
With non- electrostatic |
||
| TS1ben-Si | −82.7 | 101.8 | 0.5 | 19.1 | 19.6 |
| TS1ben-Re | −85.3 | 102.1 | 0.1 | 16.8 | 16.9 |
| TS1met | −100.4 | 112.9 | −1.4 | 12.5 | 11.1 |
| TS1ben-boat | −81.7 | 98.3 | 0.2 | 16.6 | 16.8 |
All calculations used geometries optimized at the B3LYP/6-31+G(d) level in gas phase. The reactants are hydroxide ion and the protonated cocaine in its chair or boat conformation.
Calculated at the MP2/6-31+G(d)//B3LYP/6-31+G(d) level in gas phase, including zero-point vibration and thermal corrections.
The solvent shifts were determined by performing the SVPE and PCM calculations at the HF/6-31+G(d) level.
Total contribution of short-range non-electrostatic solute-solvent interactions.
Gibbs free energy barrier in aqueous solution calculated as the ΔG(gas) value plus the electrostatic solvent shift determined by the SVPE calculation, without or with the non-electrostatic contributions determined by the PCM calculation.
As seen in Table 1, the solvent effects are crucial for calculating realistic free energy barriers and, not surprisingly, the calculated solvent shifts are dominated by the solute-solvent electrostatic interactions. The estimated short-range non-electrostatic contributions to the free energy barriers are negligible compared to the electrostatic contributions to the solvent shifts.
The calculated free energy barriers (at T = 298.15 K and P = 1 atm) associated with transition states TS1ben-Si and TS1ben-Re for the benzoyl ester hydrolysis of protonated cocaine are 19.1 and 16.8 kcal/mol, respectively. Thus, the reaction pathway for hydroxide attacking from the Re face of the carbonyl should be dominant, which is consistent with the conclusion obtained from the energy barriers calculated for neutral cocaine hydrolysis. This is not surprising because the proton attached to the tropane N atom does not participate in the benzoyl ester hydrolysis of chair cocaine. So, the effects of the cocaine protonation on the energy barriers for the benzoyl ester hydrolysis of chair cocaine should be insignificant. Previous similar computations11 on neutral cocaine hydrolysis predicted the energy barriers (i.e. the free energy barriers at T = 0 K) to be 8.5 and 7.6 kcal/mol corresponding to the transition states TS1ben-Si and TS1ben-Re, respectively. The corresponding free energy barriers calculated at T = 0 K for the benzoyl ester hydrolysis of protonated cocaine are 9.9 and 7.0 kcal/mol. These two values become 19.1 and 16.8 kcal/mol, respectively, at T = 298.15 K and P = 1 atm. The differences between the calculated free energy barriers at T = 0 K and the corresponding free energy barriers at T = 298.15 K are primarily attributed to entropic effects, particularly the translational entropy changes from the separated reactants to the transition states.
However, the free energy barrier calculated for the methyl ester hydrolysis of protonated cocaine (2.5 kcal/mol at T = 0 K and 12.5 kcal/mol at T = 298.15 K and P = 1 atm) is significantly lower than that for the dominant pathway of the benzoyl-ester hydrolysis (7.0 kcal/mol at T = 0 K and 16.8 kcal/mol at T = 298.15 K and P = 1 atm). It is also significantly lower than that for the methyl ester hydrolysis of neutral cocaine (7.0 kcal/mol at T = 0 K).11 The significant decrease of the free energy barrier, ~ 4 kcal/mol, can be attributed to the intramolecular acid catalysis of alkaline hydrolysis of the cocaine methyl ester. This catalysis results from the interplay between two opposing factors. First, the carbonyl oxygen of the methyl ester moiety hydrogen-bonds to the tropane N through the proton at the N atom in the transition state (TS1met) and the corresponding reactant (cocaine). The optimized internuclear distance between the carbonyl oxygen of the methyl ester moiety and the hydrogen on the tropane N is 1.801 Å in the reactant and 1.932 Å in the transition state. This NH⋯⋯O distance slightly increases going from the reactant to the transition state, as the hydroxide oxygen gradually approaches the carbonyl carbon to form a tetrahedral intermediate. On the other hand, during the conversion of reactants to transition state TS1met, the partial negative charge at the carbonyl oxygen becomes progressively larger. According to the natural population analysis (NPA) at the B3LYP/6-31+G* level, the net atomic charge at the carbonyl oxygen of the methyl ester moiety is −0.649 in the reactant and −0.703 in the transition state. So, there are two opposite factors affecting the change of the NH⋯⋯O hydrogen bond strength in going from the reactant to the transition state: one is the increase of the bond distance and the other is the increase of the negative charge on the oxygen atom when the changes of the charges on the N and H atoms are negligible. The aforementioned decrease (~ 4 kcal/mol) in the free energy barrier implies that the increase of the negative charge on the oxygen atom is predominant, making the NH⋯⋯O hydrogen bonding slightly stronger in the transition state. The stronger intramolecular hydrogen bonding should contribute more effectively to TS stabilization which explains the decrease in the free energy barrier. Furthermore, for ester hydrolysis of neutral cocaine (without a proton at the tropane N atom), the energy barrier calculated for the methyl ester hydrolysis of neutral cocaine is almost the same as that for the dominant pathway of the benzoyl ester hydrolysis.11 With the tropane N atom being protonated, the proton is involved in the bond formation and breaking process such that the barrier becomes ~ 4 kcal/mol lower for the methyl ester hydrolysis of protonated cocaine. The calculated relative magnitudes of the free energy barriers for the hydrolysis of the protonated cocaine are qualitatively consistent with the recently reported experimental results12 of the investigations on the hydrolysis kinetics of cocaine under physiologic conditions, because the cocaine methyl ester hydrolysis was found to be faster than the cocaine benzoyl ester hydrolysis.
Alkaline hydrolysis of boat cocaine
The transition state structure, denoted by TS1ben-boat, optimized at the B3LYP/6-31+G(d) level for the benzoyl ester hydrolysis of boat cocaine is also depicted in Figure 1. Because the carbonyl oxygen of the benzoyl ester moiety also hydrogen-bonds to the tropane N atom through the proton at the N for boat cocaine, one might also expect similar intramolecular acid catalysis of the benzoyl ester hydrolysis of boat cocaine as seen in the methyl ester hydrolysis of chair cocaine discussed above. The free energy barrier (7.2 kcal/mol at T = 0 K and 16.6 kcal/mol at T = 298.15 K and P = 1 atm) calculated for the benzoyl ester hydrolysis of boat cocaine is significantly higher than that for the methyl ester hydrolysis of chair cocaine and, at T = 298.15 K and P = 1 atm, is only 0.2 kcal/mol lower than that for the dominant pathway of the benzoyl ester hydrolysis of chair cocaine. This is because the optimized distance between the carbonyl oxygen of the benzoyl ester moiety and the hydrogen on the tropane N significantly increases from 1.632 Å in the reactant (boat cocaine) to 2.027 Å in the transition state TS1ben-boat while the net negative charge (NPA charge) at the carbonyl oxygen of the benzoyl ester moiety increases slightly from −0.657 in the reactant to −0.670 in the transition state. Note that the atomic charges determined by NPA or any other theoretical approach may only be used to qualitatively assess the change of the charge, as the absolute charges calculated are closely dependent on the theoretical approach used in the calculation. Qualitatively, the calculated increase of the negative charge from the reactant to the transition state for the benzoyl ester hydrolysis of the boat cocaine is smaller than that calculated for the methyl ester hydrolysis of chair cocaine, implying that the factor of the charge increase for the benzoyl ester hydrolysis of the boat cocaine is less significant than that for the methyl ester hydrolysis of chair cocaine. Overall, the effects of the two opposite factors (i.e. the increase of the NH⋯⋯O distance and increase of the negative charge on the O atom) on the free energy barrier nearly cancel for the methyl ester hydrolysis of chair cocaine. Alternatively, we can describe the intramolecular catalysis of boat cocaine as a process that overcomes the unfavourable steric interactions produced by crowding when the more favourable chair conformation converts to the boat conformation. Intramolecular catalysis of this more crowded species has roughly the same free energy of activation as does regular intermolecular catalysis of the benzoyl ester.
Of greater interest is that the free energy barrier for benzoyl hydrolysis of boat cocaine is no higher than for benzoyl hydrolysis of chair cocaine. This result implies that a TSA for intramolecular hydrolysis might yield catalysts that would recruit a functional group from the substrate. Thus these theoretical calculations answer a global question: Is the benzoyl ester hydrolysis of boat cocaine even plausible? The similar free energy barriers calculated for the benzoyl ester hydrolysis of the chair and boat cocaine structures support this concept. Intramolecular hydrogen bonding could be useful in generating antibody-based catalysts that recruit cocaine to the boat conformation, and an analog that elicited antibodies to approximate the protonated tropane N and the benzoyl O more closely than the natural boat conformer might increase the contribution from hydrogen bonding. Such a TSA structure (depicted in Chart 1) was synthesized and 85 cocaine esterases out of 450 anti-analog antibodies were elicited — a performance markedly superior to that of a previously employed simple phosphonate ester as a stable analog of the transition state structure for the benzoyl ester hydrolysis of chair cocaine.6,7,9 In turn, the encouraging experimental results13 support the thrust of these computations.
Conclusion
A series of first-principle electronic structure calculations were performed to examine the reaction pathways and the corresponding free energy barriers for the ester hydrolysis of protonated cocaine (pH 7.4) in both chair and boat conformations. The transition state structures optimized for the ester hydrolysis of protonated cocaine in its chair conformation are similar to the corresponding transition state structures optimized for the alkaline hydrolysis of neutral cocaine, but the optimized internuclear distances between the hydroxide oxygen and carbonyl carbon are all significantly shorter for protonated cocaine.
The calculated free energy barriers for benzoyl ester hydrolysis of protonated chair cocaine are close to the corresponding barriers calculated for the benzoyl ester hydrolysis of neutral cocaine, because the proton attached to the tropane N atom does not participate in the benzoyl ester hydrolysis of chair cocaine. However, the free energy barrier calculated for the methyl ester hydrolysis of protonated cocaine in its chair conformation is significantly lower than for the methyl ester hydrolysis of neutral cocaine and for the dominant pathway of the benzoyl ester hydrolysis of protonated cocaine. The significant decrease of the free energy barrier, ~ 4 kcal/mol, is attributed to intramolecular acid catalysis: the transition state structure is stabilized by strong hydrogen bonding between the carbonyl oxygen of the methyl ester moiety and the protonated tropane N. The calculated relative free energy barriers for the ester hydrolysis of protonated chair cocaine are qualitatively consistent with the experimental results reported for the hydrolysis kinetics of cocaine under physiologic conditions.
Similar intramolecular acid catalysis also exists in the benzoyl ester hydrolysis of protonated boat cocaine, because the carbonyl oxygen of the benzoyl ester moiety and the protonated tropane N are in contact through a hydrogen bond. The contribution of the intramolecular hydrogen bonding to transition-state stabilization makes up for the unfavourable steric interactions present in the boat conformer of cocaine. The free energy barrier calculated for the benzoyl ester hydrolysis of boat cocaine is 0.2 kcal/mol lower than that of benzoyl hydrolysis for chair cocaine. The calculated results provide a solid basis for rational design of transition state analogs to elicit anti-cocaine catalytic antibodies with high efficiency. Finally, this work supports the general potential for substrate-assisted antibody catalysis. For a substrate possessing a catalytically useful, well-positioned functional group, that group can be predictably induced to participate in the intramolecular transformation of another substrate group by a conformational change induced by antibody binding.
Supplementary Material
Acknowledgments
This work was supported in part by a grant from the Office of National Drug Control Policy (DWL), NIH/NIDA grant R01DA013930 (CGZ), and NIH, ACS/PRF, and NSF grant CHE-0116435 (GCG).
Footnotes
Supplementary Information Available. Cartesian coordinates of the geometries optimized at the B3LYP/6-31+G* level and the corresponding energies.
References
- 1.(a) Gawin FH, Ellinwood EH., Jr N. Eng. J. Med. 1988;318:1173. doi: 10.1056/NEJM198805053181806. [DOI] [PubMed] [Google Scholar]; (b) Landry DW. Scientific American. 1997;276:28. doi: 10.1038/scientificamerican0297-42. [DOI] [PubMed] [Google Scholar]
- 2.Singh S. Chem. Rev. 2000;100:925. doi: 10.1021/cr9700538. [DOI] [PubMed] [Google Scholar]
- 3.Sparenborg S, Vocci F, Zukin S. Drug Alcohol Depend. 1997;48:149. doi: 10.1016/s0376-8716(97)00072-0. [DOI] [PubMed] [Google Scholar]
- 4.Gorelick DA. Drug Alcohol Depend. 1997;48:159. doi: 10.1016/s0376-8716(97)00119-1. [DOI] [PubMed] [Google Scholar]
- 5.Zhan C-G, Zheng F, Landry DW. J. Am. Chem. Soc. 2003;125:2462. doi: 10.1021/ja020850+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Landry DW, Zhao K, Yang GX-Q, Glickman M, Georgiadis TM. Science. 1993;259:1899. doi: 10.1126/science.8456315. [DOI] [PubMed] [Google Scholar]
- 7.(a) Yang G, Chun J, Arakawa-Uramoto H, Wang X, Gawinowicz MA, Zhao K, Landry DW. J. Am. Chem. Soc. 1996;118:5881. [Google Scholar]; (b) Mets B, Winger G, Cabrera C, Seo S, Jamdar S, Yang G, Zhao K, Briscoe RJ, Almonte R, Woods JH, Landry DW. Proc. Natl. Acad. Sci. USA. 1998;95:10176. doi: 10.1073/pnas.95.17.10176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lerner RA, Benkovic SJ, Schultz PG. Science. 1991;252:659. doi: 10.1126/science.2024118. [DOI] [PubMed] [Google Scholar]
- 9.(a) Sherer EC, Turner GM, Lively TN, Landry DW, Shields GC. J. Mol. Model. 1996;2:62. [Google Scholar]; (b) Sherer EC, Yang G, Turner GM, Shields GC, Landry DW. J. Phys. Chem. A. 1997;101:8526. [Google Scholar]
- 10.(a) Sherer EC, Turner GM, Shields GC. Int. J. Quantum Chem. Quantum Biol. Symp. 1995;22:83. [Google Scholar]; (b) Turner GM, Sherer EC, Shields GC. Int. J. Quantum Chem. Quantum Biol. Symp. 1995;22:103. [Google Scholar]
- 11.Zhan C-G, Landry DW. J. Phys. Chem. A. 2001;105:1296. [Google Scholar]
- 12.Li P, Zhao K, Deng S, Landry DW. Helvetica Chim. Acta. 1999;82:85. [Google Scholar]
- 13. Deng S, Bharat N, de Prada P, W. Landry DW. Org. Biomol. Chem. 2004;2:288. doi: 10.1039/b314264g. (b) The computational studies described in this report were actually completed far before the experimental studies described in ref.13a, but the submission of this report for publication was delayed considerably due to some unexpected reason.
- 14.(a) Becke AD. J. Chem. Phys. 1993;98:5648. [Google Scholar]; (b) Lee C, Yang W, Parr RG. Phys. Rev. B. 1988;37:785. doi: 10.1103/physrevb.37.785. [DOI] [PubMed] [Google Scholar]; (c) Stephens PJ, Devlin FJ, Chabalowski CF, Frisch MJ. J. Phys. Chem. 1994;98:11623. [Google Scholar]
- 15.(a) Gonzalez C, Schlegel HB. J. Chem. Phys. 1989;90:2154. [Google Scholar]; (b) Gonzalez C, Schlegel HB. J. Phys. Chem. 1990;94:5523. [Google Scholar]
- 16.Zhan C-G, Landry DW, Ornstein RL. J. Am. Chem. Soc. 2000;122:1522. [Google Scholar]
- 17.(a) Tomasi J, Persico M. Chem. Rev. 1994;94:2027. [Google Scholar]; (b) Cramer CJ, Truhlar DG. In: Solvent Effects and Chemical Reactions. Tapia O, Bertran J, editors. Dordrecht: Kluwer; 1996. p. 1. [Google Scholar]; (c) Cramer CJ, Truhlar DG. Chem. Rev. 1999;99:2161. doi: 10.1021/cr960149m. [DOI] [PubMed] [Google Scholar]; (d) Chipman DM. J. Chem. Phys. 1997;106:10194. [Google Scholar]; (e) Chipman DM. J. Chem. Phys. 1999;110:8012. [Google Scholar]
- 18.Schmidt MW, Baldridge KK, Boatz JA, Elbert ST, Gordon MS, Jensen JH, Koseki S, Matsunaga N, Nguyen KA, Su SJ, Windus TL, Dupuis M, Montgomery JA. J. Comput. Chem. 1993;14:1347. [Google Scholar]
- 19.(a) Zhan C-G, Bentley J, Chipman DM. J. Chem. Phys. 1998;108:177. [Google Scholar]; (b) Zhan C-G, Chipman DM. J. Chem. Phys. 1998;109:10543. [Google Scholar]; (c) Zhan C-G, Chipman DM. J. Chem. Phys. 1999;110:1611. [Google Scholar]
- 20.(a) Zhan C-G, Norberto de Souza O, Rittenhouse R, Ornstein RL. J. Am. Chem. Soc. 1999;121:7279. [Google Scholar]; (b) Zhan C-G, Zheng F. J. Am. Chem. Soc. 2001;123:2835. doi: 10.1021/ja005529a. [DOI] [PubMed] [Google Scholar]; (c) Zheng F, Zhan C-G, Ornstein RL. J. Phys. Chem. B. 2002;106:717. [Google Scholar]; (d) Zhan C-G, Dixon DA, Sabri MI, Kim M-S, Spencer PS. J. Am. Chem. Soc. 2002;124:2744. doi: 10.1021/ja0113394. [DOI] [PubMed] [Google Scholar]; (e) Dixon DA, Feller D, Zhan C-G, Francisco SF. J. Phys. Chem. A. 2002;106:3191. [Google Scholar]; (f) Dixon DA, Feller D, Zhan C-G, Francisco SF. Int. J. Mass Spectrom. 2003;227:421. [Google Scholar]; (g) Zhan C-G, Dixon DA, Spencer PS. J. Phys. Chem. B. 2003;107:2853. [Google Scholar]; (h) Chen X, Zhan C-G. J. Phys. Chem. A. 2004;108:3789. [Google Scholar]; (i) Zhan C-G, Dixon DA, Spencer PS. J. Phys. Chem. B. 2004;108:6098. [Google Scholar]; (j) Chen X, Zhan C-G. J. Phys. Chem. A. 2004;108:6407. [Google Scholar]; (k) Xiong Y, Zhan C-G. J. Org. Chem. 2004;69:8451. doi: 10.1021/jo0487597. [DOI] [PubMed] [Google Scholar]
- 21.Tomasi J, Persico M. Chem. Rev. 1994;94:2027. [Google Scholar]
- 22.Mejias JA, Lago S. J. Chem. Phys. 2000;113:7306. [Google Scholar]
- 23.Cramer CJ, Truhlar DG. In: Solvent Effects and Chemical Reactions. Tapia O, Bertran J, editors. Dordrecht: Kluwer; 1996. p. 1. [Google Scholar]
- 24.Chipman DM. J. Chem. Phys. 2000;112:5558. [Google Scholar]
- 25.Barone V, Cossi M, Tomasi J. J. Chem. Phys. 1997;107:3210. [Google Scholar]
- 26.Tomasi J, Mennucci B, Cances E. J. Mol. Struct. (Theochem) 1999;464:211. [Google Scholar]
- 27.Cancès E, Mennucci B. J. Chem. Phys. 2001;114:4744. [Google Scholar]
- 28.Cossi M, Rega N, Scalmani G, Barone V. J. Chem. Phys. 2001;114:5691. [Google Scholar]
- 29.Chipman DM. J. Chem. Phys. 2002;116:10129. [Google Scholar]
- 30.Tawa GJ, Topol IA, Burt SK, Caldwell RA, Rashin AA. J. Chem. Phys. 1998;109:4852. [Google Scholar]
- 31.Topol IA, Tawa GJ, Burt SK, Rashin AA. J. Chem. Phys. 1999;111:10998. [Google Scholar]
- 32.Zhan C-G, Landry DW, Ornstein RL. J. Am. Chem. Soc. 2000;122:2621. [Google Scholar]
- 33.Zhan C-G, Landry DW, Ornstein RL. J. Phys. Chem. A. 2000;104:7672. [Google Scholar]
- 34.Zheng F, Zhan C-G, Ornstein RL. J. Chem. Soc. Perkin Trans. 2001;2:2355. [Google Scholar]
- 35.Bentley J. J. Phys. Chem. A. 1998;102:6043. [Google Scholar]
- 36.(a) Zhan C-G, Dixon DA. J. Phys. Chem. A. 2001;105:11534. [Google Scholar]; (b) Zhan C-G, Dixon DA. J. Phys. Chem. A. 2002;106:9737. [Google Scholar]; (c) Zhan C-G, Dixon DA. J. Phys. Chem. A. 2003;107:4403. [Google Scholar]; (d) Zhan C-G, Dixon DA. J. Phys. Chem. A. 2004;108:2020. [Google Scholar]
- 37.(a) Miertus S, Scrocco E, Tomasi J. Chem. Phys. 1981;55:117. [Google Scholar]; (b) Miertus S, Tomasi J. Chem. Phys. 1982;65:239. [Google Scholar]; (c) Cossi M, Barone V, Cammi R, Tomasi J. Chem. Phys. Lett. 1996;255:327. [Google Scholar]
- 38.Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Zakrzewski VG, Montgomery JA, Stratmann RE, Burant JC, Dapprich S, Millam JM, Daniels AD, Kudin KN, Strain MC, Farkas O, Tomasi J, Barone V, Cossi M, Cammi R, Mennucci B, Pomelli C, Adamo C, Clifford S, Ochterski J, Petersson GA, Ayala PY, Cui Q, Morokuma K, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Cioslowski J, Ortiz JV, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Gomperts R, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Gonzalez C, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Andres JL, Gonzalez AC, Head-Gordon M, Replogle ES, Pople JA. Gaussian. Vol. 98. Pittsburgh PA: Gaussian, Inc.; 1998. Revision A.6. [Google Scholar]
- 39.Cramer CJ, Truhlar DG. J. Comp. Chem. 1992;13:1089. [Google Scholar]
- 40.Stewart JJP. J. Comp. Chem. 1989;10:209. 221. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.