Abstract
Eusocial animal societies, as diverse as those found in the ants, bees, wasps, shrimp and naked mole-rats, are structured around one or few reproductive females. The remaining females are helpers called ‘workers’ that are mostly sterile. A paradigm in studies of eusociality is that worker sterility is a key to societal functions because advanced sociality cannot be achieved when there is conflict over reproduction. Yet, traits such as sensory responsiveness, foraging and hoarding behaviour that change between female reproductive life stages also vary between workers. This variation is central to worker division of labour, a complex social trait believed to be instrumental for the ecological success of animal societies. Thus, we took a step back from established views on worker sterility and societal functions, and hypothesized that division of labour can be better understood if adaptive variation in worker behaviour is seen as emerging from pre-existing mechanisms associated with female reproduction. In exploring this reproductive ground plan hypothesis (RGPH) in honeybee workers, we established that variation in foraging division of labour correlates with ovary size and is affected by expression changes in vitellogenin, an egg yolk protein precursor. Here, we explain and reconcile the RGPH with data on honeybee sensory sensitivity, genomic mapping, transcript and endocrine profiling, and link our discussion with Ihle et al. (2010, this issue, pp. xx-xx). The findings bring together mechanistic and evolutionary explanations of honeybee worker behaviour. This essay suggests that a broader view on worker reproductive traits can increase the understanding of animal social behaviour.
Keywords: insulin-like signaling, juvenile hormone, nuclear hormone receptor, ovary, pollen hoarding, reproductive behaviour, social evolution, vitellogenin, worker behaviour
Background
Eusocial animal societies are characterized by reproductive division of labour between primary reproductives and mostly sterile helpers called workers. Workers display different biases in the kinds of behavioural tasks they perform, which are often associated with changes in physiology that are correlated with age, as well as differences in adult morphology. This striking level of social organization is believed to be the cause of the enormous ecological and evolutionary success of social species, including the advanced societies of ants, bees, wasps and termites (Oster & Wilson 1978).
Recently, Hölldobler & Wilson (2008) resurrected the early 20th century metaphor of the insect society as a superorganism (Wheeler 1911), conjuring images of a distributed organism with systems equivalent to physiology, reproduction, communication and information processing (nervous system). The superorganism metaphor works well at the phenomenological level of the colony but does not explain the genetics or developmental biology of social evolution. There is no single ‘superorganismal’ genome that natural selection can act on. Instead, each individual in a colony is a product of development derived from that individual’s genome. Natural selection must change this genome to influence development and behaviour. The challenge is to understand how natural selection on colonies changes genomes, development and individual behaviour (i.e. to reveal the developmental evolution of the social structure; Page & Amdam 2007).
There are many definitions of the superorganism (Page & Mitchell 1991; Mitchell 2003), but central to all is the reproductive division of labour between the fertile reproductives and the workers. The definitions imply that developmental evolution of worker sterility is key to social harmony because advanced sociality cannot be achieved when there is conflict over reproduction (Wilson & Sober 1989). In ants, bees and wasps, which make up the majority of the social insect taxa, division of labour is exclusively female, and the female reproductive process is a potential source of conflict. To reduce conflict, natural selection has acted on reproductive gene networks, reducing or eliminating their functionality in workers relative to their fertile sisters, the queens (Wilson 1971; Khila & Abouheif 2008). Functional systems of worker reproductive biology, consequently, are seen as something natural selection will abolish as societies evolve more complexity and become more ‘superorganismal’.
In this essay, we discuss a different view. We place worker reproductive biology at the forefront of developmental evolution of complex social behaviour, an approach taken previously towards reproductive division of labour between queens and workers, and towards worker age polyethism (temporal change in task performance) by West-Eberhard (1987, 1996). The regulatory networks of reproductive maturation and plasticity that coordinate expression of female physiology and behaviour are exploited by natural selection to adapt social structures. This is possible because correlation of female physiology and behaviour is central not only to successful reproduction in solitary species (Klowden 1990; Clements 1992; Atchley et al. 2005), but also to worker division of labour (Seeley 1982; Hölldobler & Wilson 1990).
We focus on the honeybee, Apis mellifera, the best-studied social insect (Honey Bee Genome Sequencing Consortium 2006). We describe how artificial selection on stored colony food resources altered the social foraging behaviour of honeybees through effects on worker reproductive biology. We believe this response to colony-level selection exemplifies how developmental evolution of complex social structures can occur: the influence of reproductive biology on female food-related behaviour, an apparently ubiquitous trait in animals (Clements 1992; Clarke & Ossenkopp 1998; Atchley et al. 2005), can be evolutionary co-opted to produce division of labour between workers with different behavioural biases in food collection and food hoarding (Amdam et al. 2004b, 2006). This reproductive ground plan hypothesis (RGPH, see below for details) refocuses the discussion regarding the role of worker reproduction from one where worker ovaries lead to competition and discord to one where the reproductive system of workers has been co-opted and is now a facilitator of cooperation and social organization.
Honeybee Division of Labour and Stored Colony Resources of Nectar and Pollen
Honeybee workers demonstrate a striking division of labour that is physiologically based, in which bees of different ages perform different tasks (Seeley 1982; Robinson 1992). Younger bees perform tasks within the nest, such as feeding larvae, constructing and maintaining the nest and processing honey, while older bees forage. This division of labour is further divided into specialists that perform some tasks more frequently than other individuals. For example, foragers can specialize on collecting pollen, a protein source, or nectar, a source of carbohydrate. This specialization is best observed as a foraging bias or ‘preference’, measured as the ratio of the two substances collected by the individual bee: some bees collect relatively more pollen, others more nectar (Page et al. 2000). The collective activities of foraging workers provide food for adults and developing larvae within the nest and results in the adaptive storage of surplus honey (from nectar) and pollen by colonies.
Artificial Selection on Stored Pollen: Effects on Social Behaviour
We (Page & Fondrk 1995) conducted a bidirectional selection programme for high and low levels of surplus pollen storage by honeybee colonies (pollen hoarding) and demonstrated a strong response to selection. We looked at individual behavioural traits that changed as a consequence of selection on the colony-level phenotype. We found that workers from the strain selected for increased pollen storage (high pollen-hoarding strain) initiate foraging about 10 days earlier in life than low pollen-hoarding strain bees (Pankiw & Page 2001). High strain bees are more likely to bias their foraging (specialize) towards pollen, while low strain bees are more likely to specialize on nectar (Page & Fondrk 1995; Page et al. 1995; Fewell & Page 2000; Pankiw & Page 2001). Furthermore, when high strain bees forage for nectar, they accept nectar with lower concentrations of sugar, and also respond to lower concentrations of sucrose solution when analysed with a proboscis extension response (PER) test (see also Fig. 1).
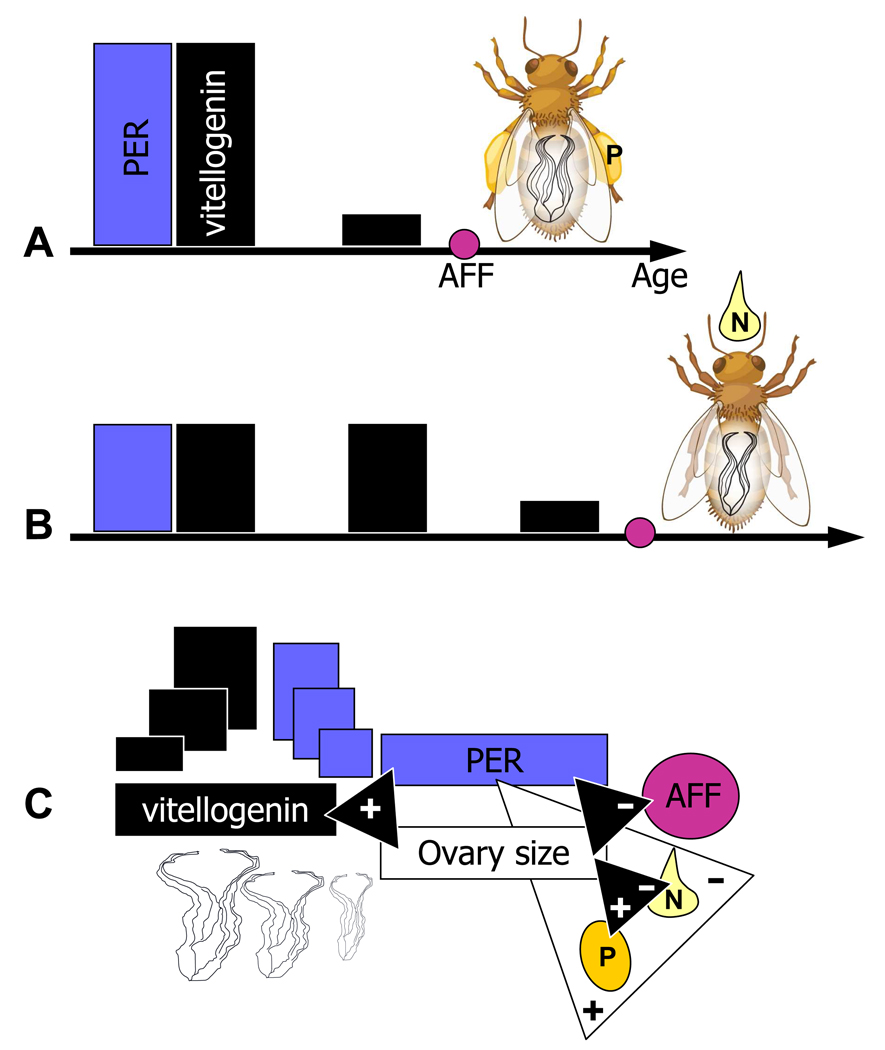
Figure 1.
Variation in foraging behaviour correlates with differences in sensory and reproductive traits in worker honeybees. Trait associations in selected high (a) and low (b) pollen-hoarding strain bees. Horizontal arrows show the timeline of worker ontogeny (Age). High strain bees emerge as adults with a larger ovary (white line-drawings inside bees), elevated sucrose responsiveness measured by the proboscis extension reflex (PER, blue bars), and develop a higher peak titre of vitellogenin yolk protein (black bars) as young adults compared with low strain bees. The vitellogenin level of high strain bees then drops rapidly and workers initiate foraging earlier in life than bees with low strain genotype. This difference in ‘age at first foraging’ (AFF) is indicated by violet circles, panel (a) versus (b). As foragers, high strain workers bias their collecting towards pollen (‘P’, bee in (a)), while low strain workers are biased towards nectar (‘N’, bee in (b)). (c) The corresponding trait correlations in wild-type (unselected) worker bees, which show considerable phenotypic variation (illustrated by ovary, PER, and vitellogenin symbols of various sizes). Black, connecting triangles indicate positive (+) correlations between vitellogenin expression, PER and ovary size, and between ovary size and pollen foraging, and negative correlations (−) between PER and age at foraging onset, and between ovary size and foraging onset. The white triangle specifies that PER is also correlated with foraging choice directly. These associations in wild-type bees reflect the same relationships as those seen in selected pollen-hoarding strain workers.
Pollen-hoarding strain phenotypes reflect genotypic differences, validated by cross-fostering experiments where high strain bees were reared by low strain colonies and vice versa (Calderone & Page 1992) and by co-fostering where strains were reared together by ‘wild-type’ (unselected commercial) honeybees (Pankiw & Page 2001; Scheiner et al. 2001). The genotypes, however, are not fixed. The breeding scheme includes planned outcrosses to the original source population (California, U.S.A., Page & Fondrk 1995) and maintains within-strain variability. Recent genome sequencing of two low strain sisters covering 92% of AT-rich and 96% of GC-rich DNA, revealed heterozygosity for 596 668 and 601 709 single nucleotide polymorphisms (SNP), respectively. Out of these SNP, 215 471 were unique between the two bees (G. V. Amdam, unpublished data). Studies of naturally occurring interindividual variation between wild-type worker bees, furthermore, have verified that the behavioural correlations seen in pollen-hoarding strains, that is, between foraging onset, foraging bias, nectar concentration collected and PER, are general to workers (Page et al. 1998; Pankiw & Page 2000; Amdam et al. 2006). Thus, our artificial selection on colony-level pollen hoarding appeared to have acted on a suite of associated behavioural traits that is readily present in worker honeybees.
Artificial Selection on Stored Pollen: Effects on Reproductive Biology Linked to Behaviour
We conducted several physiological screens of the high and low pollen-hoarding strain bees and found significant differences in traits associated with reproductive processes (Amdam et al. 2004b, 2006, 2007; Wang et al. 2009). The reproductive physiology of honeybee workers is largely defined by the ovary, which consists of ovarian filaments called ovarioles (Snodgrass 1956), and by vitellogenin, the major egg yolk protein of oviparous animals (Engels 1974). Honeybee ovariole number is determined during larval life and is constant thereafter, while the vitellogenin protein is dynamically expressed during adulthood and reaches peak levels in young, preforaging bees; see Amdam et al. (2009) for a review. Our studies showed that high pollen-hoarding strain workers are characterized by a larger ovary (more ovarioles) than low strain bees. After adult emergence, high strain workers also experience a more rapid increase in vitellogenin (vg) gene expression, a higher preforaging peak titre of vitellogenin protein, and an earlier decline in the vitellogenin level compared to low strain bees (Fig. 1). In a test of worker fecundity in the absence of the queen (queen pheromones inhibit workers from making eggs; Hoover et al. 2003), we also discovered that queenless high strain bees can produce eggs sooner than their low strain counterparts (Amdam et al. 2006).
The positive association between worker ovariole number and potential fecundity in queenless colonies was established later in wild-type bees (Makert et al. 2006), but even more intriguingly, correlations between adaptive social behaviour and reproductive traits were confirmed in workers from intact societies: wild-type worker bees with more ovarioles forage earlier in life, show preference towards pollen collection and a preference for lower concentrations of nectar. In addition, they are more sensitive to sucrose in PER tests, and they express the vg gene at higher levels as young adults (Tsuruda et al. 2008; see Fig. 1 for a summary). These findings strongly suggest that selection on colony pollen hoarding acted on a pre-existing suite of behavioural traits including sensory perception, food seeking and food hoarding, and also on a related suite of physiological traits associated with the workers’ reproductive system. We reasoned that, if food-related behaviour is connected to the reproductive physiology of worker bees, then colonies could respond to selection on food hoarding through a change in worker development that influenced the ovary and vg expression.
A Hypothesis Explaining the Role of Ovaries in Developmental Evolution of Social Structure
To explain our results, we put forth the RGPH (Amdam et al. 2004b). This ground plan hypothesis suggests that female reproductive biology is a source of behavioural variation that can be used to attain advanced foraging division of labour and specialization in workers.
The RGPH emerged through the interactions of the two of us (Amdam and Page) when we discovered that we were looking at different sides of the same behavioural question. Page was struggling to find physiological explanations for the behavioural traits discussed above, while Amdam was trying to make sense of how vitellogenin could influence honeybee foraging behaviour (Amdam & Omholt 2003). Together, we took a step back from the dogma that natural selection on sociality acts to reduce the functionality of reproductive gene and endocrine networks in workers. We were inspired by broadly accepted effects of ovarian signalling on physiology and behaviour in other animal systems (Clements 1992; Clarke & Ossenkopp 1998; Chakraborty & Gore 2004; Simonet et al. 2004; Atchley et al. 2005), and by Hölldobler & Wilson (1990), West-Eberhard (1987, 1996) and others (reviewed in: Engels 1990; West-Eberhard 1996), who described correlations between stages of ovarian development and patterns of age polyethism in many species of wasps, ants and bees: young nest workers can have swollen ovaries or lay trophic eggs used in social food provisioning, while older individuals with poorly developed ovaries forage in the field. We asked whether associations between behaviour and reproductive traits in worker honeybees could be explained by adaptive modifications of reproductive regulatory networks functioning in division of labour (Amdam et al. 2004b).
In insects, ovarian signals, such as the release of ecdysteroid hormones from the gonad, act together with the systemic juvenile hormone (JH) to modulate behavioural and sensory states that shift between the different reproductive life stages (Simonet et al. 2004). Such major endocrine signals are sensitive to changes in the insects’ environment and nutrition, and influence the switch from nectar to blood-host foraging in female Culex nigripalpus mosquitoes (Hancock & Foster 2000), the shift from feeding and sexual behaviour to fasting and parental activity in the earwig Labidura riparia (Vancassel et al. 1984), the initiation of oviposition in crickets (Cayre et al. 1996), the initiation of sexual behaviour of male Agrotis ipsilon moths (Duportets et al. 1998), and shifts between periods of intense flight activity and reproductive behaviour in several taxa (Dingle & Winchell 1997).
From these data, the RGPH proposes that adaptive division of labour in insect societies, such as the honeybee, can emerge through natural selection on reproductive biology. This biological ground plan provides a regulatory scaffold that, in solitary ancestors, could coordinate the expression of female reproductive physiology and behaviour in response to changes in ambient conditions, nesting environment and nutrient availability. We assumed that a regulatory association between ovarian signals, yolk protein synthesis and timely hoarding of nest provisions of pollen for feeding larvae was central to reproduction in ancestors of honeybees (Amdam et al. 2004b). Also, the females of extant solitary bees will feed on nectar and pollen, while storage of pollen in the nest is specific to their larval provisioning behaviour, the only exception being taxa of parasitic bees that exploit nest resources of other species (Engels 1990). If this regulatory scaffold was conserved rather than diminished by social evolution, then colonies could respond to artificial selection for pollen hoarding by exploiting ancestral gene networks that linked ovarian and yolk protein physiology to behaviour (Fig. 2).
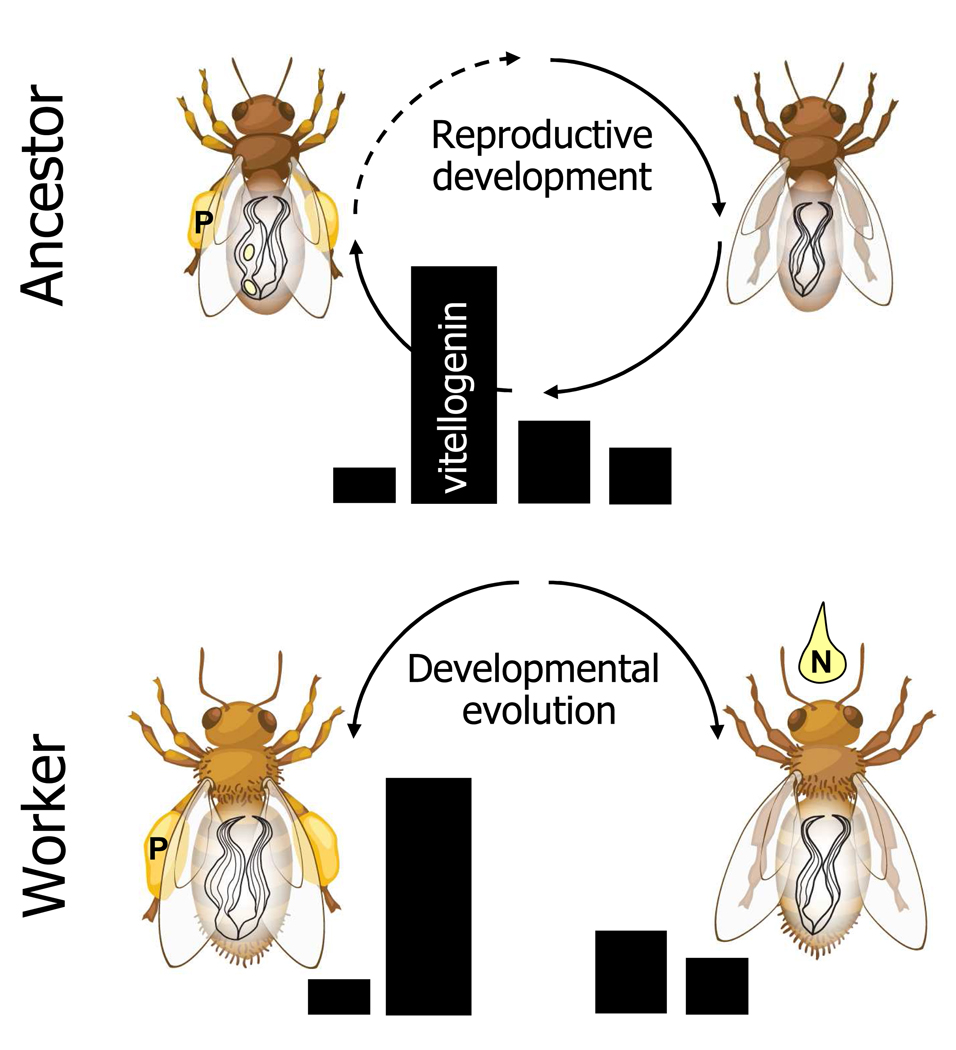
Figure 2.
The reproductive ground plan hypothesis (RGPH) outlines how female reproductive biology may have been co-opted in the division of labour between foraging worker honeybees. Top panel: in ancestors of honeybees, the progression of reproductive development was linked to changes in female food-related behaviour. During periods of no active reproduction (top, right), the ovary was undeveloped (inactive, not enlarged with yolk) and the vitellogenin titre (black bars) was low. During reproduction, ovary activity and vitellogenin protein expression increased prior to oocyte yolk deposition, before vitellogenin returned to baseline (top, left), and only this maturational stage, or phase of the reproductive cycle, would be associated with pollen collection (‘P’) for nest provisioning of young. Below: similar to phenotypic aspects of the reproductive physiology of ancestors, high pollen-hoarding strain bees show rapid modulation (up–down) of vitellogenin (bottom, left), while low strain bees have more moderate, constant levels (bottom, right). The RGPH proposes that this differential physiology is linked via a pleiotropic gene network to the corresponding behaviour of the ancestor, explaining how pollen foraging is more prevalent in high strain workers compared to low strain bees. Full-line arrows in the top panel specify that reproduction perhaps was maturational in the ancestor (i.e. with one reproductive period). Dashed-line arrow indicates the alternative; a reproductive cycle that could be repeated.
Regulatory mechanisms of solitary bee behaviour are poorly understood, but if the genetics of reproductive behaviour was better known for such species, the ancestral condition that applies to the honeybee could still not be directly tested. Nevertheless, relationships between female reproductive biology and food-related behaviour appear to be ubiquitous also beyond insects (Clements 1992; Clarke & Ossenkopp 1998; Atchley et al. 2005). The existence of a pleiotropic gene network that may confer this association in bees is supported by quantitative trait loci (QTL) genome mapping of backcrosses between the pollen-hoarding strains. This gene network links worker foraging behaviour to the ovary, and emerges as four major, interacting QTL (pln1–4) with effects on foraging choice (nectar versus pollen), nectar load weight, age at foraging onset, PER and ovary size (Hunt et al. 1995; Page et al. 2000; Rueppell et al. 2004a, b; Wang et al. 2009). Positional candidate genes and further inference from the pln1–4 regions is discussed below (see Molecular Regulation of Behaviour below).
vg, a Gene Linking Social Behaviour to Worker Reproductive Physiology
Ten years ago, the only specific function attributed to the honeybee vitellogenin protein was participation in egg yolk formation (Engels et al. 1990). A pattern of synthesis in fat body (analogous to vertebrate liver and adipose tissue) and deposition in oocytes fitted well with the high expression levels in queen bees, but it was difficult to explain the dynamic expression seen in workers (Rutz & Lüscher 1974). In 2003, Amdam and coworkers discovered that vitellogenin was used in worker brood-rearing behaviour (Amdam et al. 2003a). Since then, the understanding of how honeybee vitellogenin functions in adult workers has changed entirely (Brandt et al. 2005; Corona et al. 2007; Marco Antonio et al. 2008). Contrasting the more traditional view of the role of yolk proteins and peptides, honeybee vitellogenin is now recognized as a behavioural affector protein that builds a direct connection between worker reproductive physiology and foraging behavioural control (Amdam et al. 2009).
To enable functional research on vitellogenin, we (Amdam et al. 2003b) pioneered the use of RNA interference (RNAi) as a gene knockdown tool for adult honeybees. With this method, we demonstrated that the level of vg gene expression during early life influences the timing of foraging onset, as well as the subsequent foraging preference of worker honeybees (Nelson et al. 2007). When vg is knocked down, workers initiate foraging earlier in life and show a preference for nectar collection. The behavioural response of early foraging onset supported a theoretical model proposed by Amdam & Omholt (2003), the double repressor hypothesis (DRH), in which declining vitellogenin levels trigger the transition from nest bees to foragers (Fig. 3). In addition, the effect on foraging onset was consistent with the phenotype of high pollen-hoarding strain bees: an early drop in vitellogenin levels correlating with early onset of foraging behaviour compared to low strain bees (Amdam et al. 2007). The knockdowns’ bias towards nectar, moreover, was in line with the foraging preference of low strain bees that, similar to vg gene knockdowns, do not receive high vitellogenin exposure during early life (Fig. 3). Following the same logic, Ihle et al. (2010, this issue, pp. xx-xx) revealed that further down-regulation of vg expression does not influence the foraging choice of low strain bees, which already have moderate vitellogenin levels, while the high strain bees respond with a significant shift in behaviour towards a preference for nectar loading that is indistinguishable from the phenotype of low strain bees.
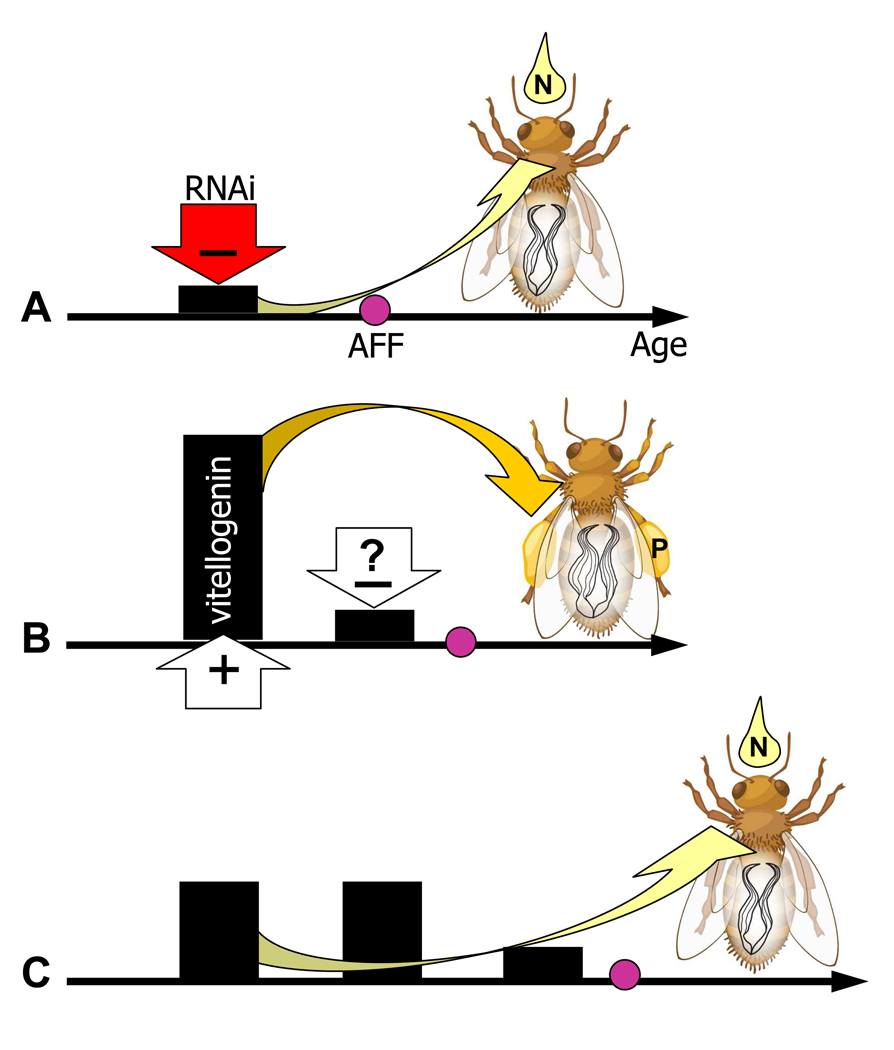
Figure 3.
The phenotype of wild-type vitellogenin (vg) gene knockdown workers is consistent with trait associations in high and low pollen-hoarding strain bees (Nelson et al. 2007). (a) RNAi-mediated knockdown of the vg gene (red (−) arrow) results in repressed vitellogenin protein levels (black bars) from early life, lowering the bees’ age at first foraging (AFF). This behaviour is similar to high strain bees (b) that show early decline of vitellogenin and low AFF during ontogeny. Thereafter, knockdown workers bias their foraging effort towards nectar (‘N’), similar to low strain bees (c) that do not experience high vitellogenin exposure as young adults. Thus, the early-life vitellogenin level predicts the foraging bias of worker bees, as indicated by the arrows from vitellogenin to the nectar and pollen (‘P’) symbols in the three panels. The white (+) and (−) arrows in (b) refer to ovarian signalling. They suggest that the larger ovary of high strain bees may explain the rapid up–down modulation and high peak levels of vitellogenin in this genotype, but a connection between large ovary size and early vitellogenin decline (the white (- ?) arrow) is not experimentally confirmed.
The expression of vg can correlate with ovary size (Tsuruda et al. 2008): young (5–7 days old) wild-type bees with many ovarioles show elevated levels of vg transcript. In explaining this association, we hypothesize that endocrine factors released by ovarian tissue cause vg expression to increase, as seen during early reproductive maturation in mosquitoes (Clements 1992). During the early life of workers, large ovaries (more ovarian tissue) may drive this increase more effectively (Fig. 2). In later stages of the insect reproductive process, a negative feedback signal from the ovary can follow (Clements 1992). This feedback, which coordinates the cessation of vitellogenin production with the completion of oocyte yolk deposition, may have been a trait of honeybee ancestors (Fig. 2). We speculate that in workers, large ovaries also confer the negative feedback more effectively, in sum explaining the correlation between many ovarioles, early peak titres and early cessation of vitellogenin synthesis in high strain bees (Fig 2, Fig 3). This untested hypothesis draws some indirect empirical support from previous results (Amdam et al. 2007). In young (7-day-old) high strain workers, Amdam and coworkers found a positive association between ovary size and the endocrine feedback response to vg downregulation. The feedback was detected as an increase in JH titre and expression of ultraspiracle (USP), a nuclear hormone receptor gene orthologue of vertebrate retinoid X receptor, RxR (Guidugli et al. 2005; Velarde et al. 2009). No equivalent response was detected in the low strain bees.
Challenge to the RGPH
Our hypothesis that the worker ovary is a source of adaptive behavioural control has been challenged. Oldroyd & Beekman (2008) compared ovary size, ovary activation and foraging behaviour of wild-type bees with a strain of bees selected for ‘anarchistic’ behaviour. Anarchistic workers lay eggs in the presence of a functional queen with a higher frequency than normal. This behaviour suggests that anarchistic workers are more resistant to inhibition of oviposition conferred by pheromones from queen and brood (Barron et al. 2001; Oldroyd et al. 2001). Similar resistance is not a normal characteristic of wild-type bees or the strains selected for pollen hoarding (Amdam & Page 2008).
Oldroyd & Beekman reasoned that the anarchistic strain was more reproductive than wild type because workers with anarchistic genotype are more likely to lay eggs in the presence of the queen. They proposed that if the RGPH holds then anarchistic bees should display the behavioural phenotype associated with pollen preference, like that seen in high pollen-hoarding strain bees. However, the anarchistic workers did not show foraging bias towards pollen and initiated foraging later in life than the wild-type bees. Oldroyd & Beekman (2008, page 6) concluded that they doubted ‘… the validity of a general association between reproductive potential and division of labor when foraging, modulated by the production of vitellogenin’. Interestingly, however, their wild-type genotype that foraged early in life had more ovarioles than the anarchistic bees. Thus, although we cannot explain the separate syndromes of pheromonal resistance or anarchistic egg laying, it is encouraging that the correlation of foraging onset and ovariole number is consistent between such diverse stocks of bees (Amdam & Page 2008; Tsuruda et al. 2008).
Molecular Regulation of Behaviour
Through our studies of honeybees, we have demonstrated that selection on a colony-level trait, the amount of stored pollen, acts on entrenched relationships of behavioural physiology that centre on ovaries and vitellogenin levels in workers. Colonies selected to store more pollen have workers with larger ovaries that collect more pollen, and the pollen foraging bias correlates with ovary size in wild-type bees. Variation in ovary size correlates with levels of vg expression in wild type, and ovary size can correlate with the endocrine response of JH and USP that feeds back to curtail vitellogenin synthesis. Effects of vitellogenin on foraging behaviour have been demonstrated directly by RNAi-mediated gene knockdown. In light of the evolutionary process we describe in this essay, these connections make sense, however, how are they explained mechanistically?
Genetic effects explain only a fraction of behaviour, including the pollen-hoarding behaviour of honeybees (Amdam et al. 2009). Environmental influences play major roles in behavioural expression, and correspondingly, the ovary, vitellogenin titre, and JH level of worker bees respond to changing environmental conditions (Huang & Robinson 1995; Amdam et al. 2004a; Linksvayer et al. 2009). Stochastic processes also affect individuals, and can contribute to division of labour (Amdam & Omholt 2003). Thus, genotypic differences such as those exemplified by pollen-hoarding strains provide but one of several sources of behavioural variation. Pollen hoarding is, nevertheless, robustly influenced by four major QTL, pln1–4, with pleiotropic effects on sensory sensitivity, foraging behaviour and ovariole number (see above). In a recent experiment (Wang et al. 2009), we studied the expression of five genes in the QTL regions based on a positional candidate gene list (Hunt et al. 2007). We found that two of these genes, a nuclear hormone receptor-like gene homologue HR46 (pln2) and the phosphoinositide 3-kinase encoding gene PDK1 (pln3), showed consistent, tissue-specific expression differences between bees of the high and low pollen-hoarding strains as well as between workers with large (‘high’) and small (‘low’) ovaries derived from a backcross between the strains. Our backcross experiment demonstrated a direct genetic linkage between few ovarioles and elevated fat body HR46 expression at adult emergence, and between many ovarioles, increased PDK1 expression in fat body, and pollen foraging behaviour in mature adults.
Wang and coworkers hypothesized that the link between HR46, ovary size and worker behaviour could emerge from an influence of HR46 on the developmental mechanism that determines ovariole number in honeybees. This number is increased by JH (Schmidt-Capella & Hartfelder 1998), which may rely on USP for DNA binding (Barchuk et al. 2004). In Drosophila, HR46 (also called dHR3) is induced by ecdysone signalling when USP is present (Schubiger & Truman 2000). Yet, USP and dHR3 may interact competitively (Lam et al. 1997), perhaps contributing to an inverse relationship between JH and ecdysone signalling that also characterizes honeybees (Zufelato et al. 2000; Barchuk et al. 2002). Wang and coworkers, furthermore, attributed the genetic association between ovary size, foraging behaviour and PDK1 transcript abundance to a causal link between insulin-like signalling (nutrient sensing that includes PDK1 phosphorylation) and larval ovarian differentiation, and between ovarian regulation of fat body insulin-like signalling and adult behaviour (Wang et al. 2009). A direct effect of insulin-like signalling on honeybee ovary size and foraging behaviour was recently confirmed by RNAi-mediated knockdown of the insulin receptor substrate encoding gene, IRS (N. S. Mutti, Y. Wang & G. V. Amdam, unpublished data). IRS is upstream of PDK1 in insulinlike pathways, and is one of only four positional candidate genes in the genomic region of pln4 (Hunt et al. 2007). Taken together, these results suggest that the honeybee worker division of labour is influenced by a genetic architecture that governs endocrine signal transmission, via nuclear hormone receptors, and nutritional responsiveness, acting via insulin-like pathway conductivity.
Two Hypotheses Explaining Worker Behaviour: the RGPH and the DRH
Integration of endocrine physiology, endogenous resource sensing and behaviour is a hallmark of reproductive gene networks (Amdam et al. 2004b; Flatt et al. 2005). In the distantly related Drosophila, JH is positively influenced by insulin-like signalling and has a positive effect on ovariole number (Tu & Tatar 2003), yolk synthesis, oocyte maturation and adult reproductive behaviour (Tatar 2004; Flatt et al. 2005). However, although JH is a positive affector of ovary size also in honeybees (Schmidt-Capella & Hartfelder 1998), the association between JH and yolk synthesis has changed. Application of JH analogue suppresses vitellogenin expression in workers (Pinto et al. 2000). Honeybee vitellogenin, reciprocally, can suppress JH (see above). This regulatory constellation creates a feedback system revealed by vg knockdown (Guidugli et al. 2005; Amdam et al. 2007): vg downregulation triggers increased JH signalling, enhanced USP expression, further suppression of the vitellogenin level, and results in the release of foraging behaviour. The feedback architecture between vitellogenin and JH and its effect on worker division of labour was first proposed Amdam & Omholt (2003), who outlined a ‘double repressor’ model (or DR hypothesis, the DRH) for regulation of foraging onset in honeybees. The DRH was subsequently expanded upon, to include USP at the intersection between vg and JH (Guidugli et al. 2005), placement of vitellogenin upstream of insulin-like signals that act on JH (Seehuus et al. 2006; Corona et al. 2007; Hunt et al. 2007), a positive relationship between ovary size and endocrine sensitivity including JH and USP (Amdam et al. 2007), positive correlation between ovary size and vg expression (Tsuruda et al. 2008), and negative versus positive genetic linkage between ovary size and HR46 versus PDK1 transcript abundance, respectively (Wang et al. 2009). This regulatory structure and its effect on foraging behaviour are summarized in Fig. 4.
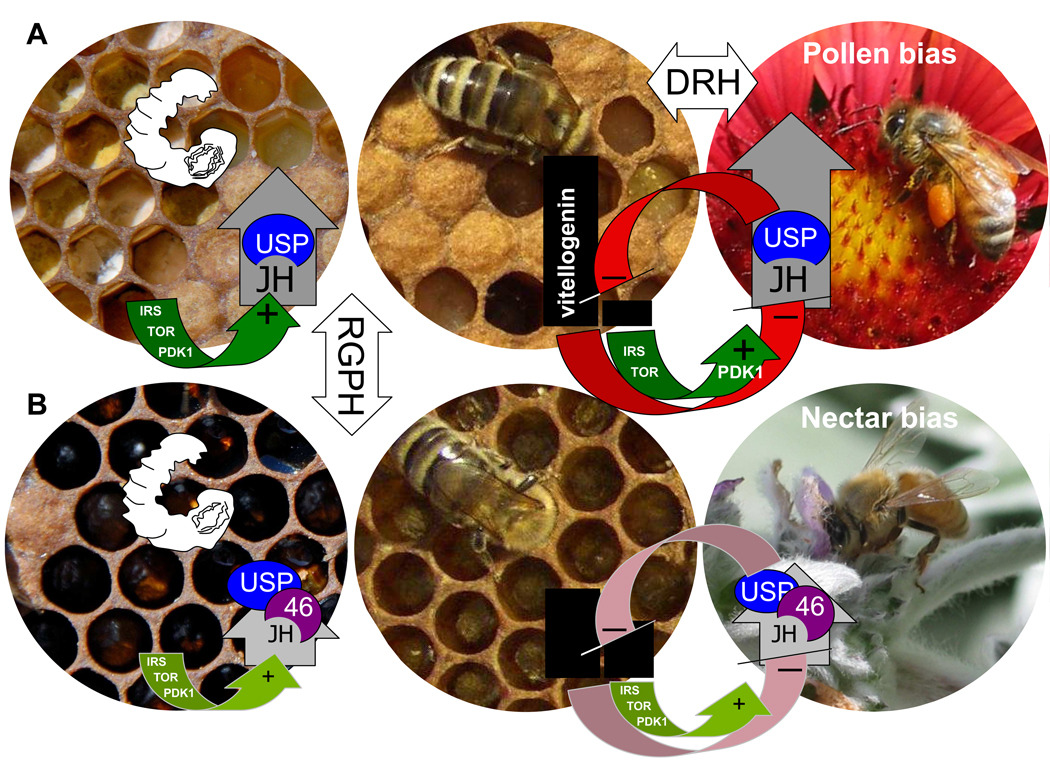
Figure 4.
The reproductive ground plan hypothesis (RGPH) underscores that vitellogenin (black bars) and juvenile hormone (JH, grey arrow-bars) modules are inherent to insect reproduction (Amdam et al. 2007). Reproductive biology is influenced by environmental factors such as food availability sensed by nutrient-sensitive pathways (green). These pathways include the insulin receptor substrate (IRS) and target of rapamycin (TOR); while PDK1 is a phosphorylation target downstream of both IRS and TOR. The response is integrated by endocrine signal transmission via systemic hormones like JH and nuclear hormone receptors such as USP (blue) and HR46 (46, purple). The double repressor hypothesis (DRH) (Amdam & Omholt 2003) proposes that transition from nursing (mid panels) to foraging behaviour (right panels) in honeybees is influenced by a feedback loop (red (−) arrows) between vitellogenin and JH. Vitellogenin inhibits JH synthesis, USP expression and insulin-like signalling (Guidugli et al. 2005; Hunt et al. 2007; Ament et al. 2008) that are increased in foragers. When vitellogenin levels drop, JH can be triggered to increase and further suppress expression of its own repressor. We propose that bidirectional selection on pollen-hoarding behaviour acted on these relationships. (a) In high pollen-hoarding strain bees, nutrient sensing and reproductive endocrine signal transmission is increased, resulting in developmental retention of a larger ovary (filament structures inside larva, top left panel) and dynamic interplay between the ovary, vitellogenin, and adult behaviour (top mid (nurse) versus top right (forager) panel). (b) In low strain bees, nutrient sensing and reproductive endocrine signal transmission is less active (shown as less intense green and red indicators), resulting in reduced ovary size (fewer filaments retained inside larva, bottom left panel) and less sensitivity of interplay between ovary, vitellogenin, and behaviour in adults (bottom mid versus bottom right panel). Increased HR46 expression (Wang et al. 2009) might be central to the low strain phenotype, as this receptor has potential to competitively inhibit the signal transmission of JH and USP.
The mechanistic structure of the DRH, thereby, contains elements central to the RGPH. Yet initially, the two frameworks were not experimentally reconciled (Toth & Robinson 2007). This gap is closed by Ihle et al. (2010, this issue, pp. xx-xx). Ihle and coworkers show that the effect of vitellogenin on the nectar and pollen-loading choices of workers, as explained by the RGPH, is conditional on the JH suppressor function of vitellogenin that explains age at foraging onset via the double repressor mechanism. Their study confirms experimentally that vitellogenin must access and affect the endocrine response system of worker honeybees to change foraging behaviour (see Ihle et al. 2010 for further details).
It is well known that animal behaviour is influenced by genetic and endocrine mechanisms that pace and affect the reproductive progression of females. We provide evidence, hypotheses, and insight about how the reproductive regulatory networks of facultatively sterile worker honeybees have been co-opted by natural (and artificial) selection resulting in adaptive modification of behaviour associated with foraging division of labour. The extent to which the models we present here hold for other social insects remains to be demonstrated. However, supporting results have emerged from recent studies of the ant Pogonomyrmex californicus (Dolezal et al. 2009) and the bee Apis cerana (Rueppell et al. 2008). We hope this essay will provide inspiration for future research that centres on understanding the evolution and current regulation of sociality.
Acknowledgments
We thank K. Ihle for comments, and S. Deviche and J. Sahertian for assistance with artwork. G.V.A. was supported by the Norwegian Research Council (175413, 180504, 185306, and 191699), U.S. National Science Foundation (0615502) and the PEW Foundation. R.E.P. was supported by the U.S. Department of Agriculture (NRI-CSREES 2003-01620) and the National Institute on Aging (NIA P01 AG22500).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amdam GV, Omholt SW. The hive bee to forager transition in honeybee colonies: the double repressor hypothesis. Journal of Theoretical Biology. 2003;223:451–464. doi: 10.1016/s0022-5193(03)00121-8. [DOI] [PubMed] [Google Scholar]
- Amdam GV, Page RE. Oldroyd and Beekman do not test ground plan hypothesis that explains origins of social behavior. PLoS Biology. 2008;6 e56r2248. [Google Scholar]
- Amdam GV, Norberg K, Hagen A, Omholt SW. Social exploitation of vitellogenin. Proceedings of the National Academy of Sciences, U.S.A. 2003a;100:1799–1802. doi: 10.1073/pnas.0333979100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amdam GV, Simões ZLP, Guidugli KR, Norberg K, Omholt SW. Disruption of vitellogenin gene function in adult honeybees by intra-abdominal injection of double-stranded RNA. BMC Biotechnology. 2003b;3:1–8. doi: 10.1186/1472-6750-3-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amdam GV, Hartfelder K, Norberg K, Hagen A, Omholt SW. Altered physiology in worker honey bees (Hymenoptera: Apidae) infested by the mite Varroa destructor (Acari: Varroidae): a factor in colony loss during over-wintering? Journal of Economic Entomology. 2004a;97:741–747. doi: 10.1093/jee/97.3.741. [DOI] [PubMed] [Google Scholar]
- Amdam GV, Norberg K, Fondrk MK, Page RE. Reproductive ground plan may mediate colony-level selection effects on individual foraging behavior in honey bees. Proceedings of the National Academy of Sciences, U.S.A. 2004b;101:11350–11355. doi: 10.1073/pnas.0403073101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amdam GV, Csondes A, Fondrk MK, Page RE. Complex social behavior derived from maternal reproductive traits. Nature. 2006;439:76–78. doi: 10.1038/nature04340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amdam GV, Nilsen KA, Norberg K, Fondrk MK, Hartfelder K. Variation in endocrine signaling underlies variation in social life history. American Naturalist. 2007;170:37–46. doi: 10.1086/518183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amdam GV, Ihle KE, Page RE. Regulation of honey bee (Apis mellifera) life histories by vitellogenin. In: Pfaff D, Arnold A, Etgen A, Fahrbach S, Rubin R, editors. Hormones, Brain and Behavior. San Diego: Elsevier Academic Press; 2009. [Google Scholar]
- Ament SA, Corona M, Pollock HS, Robinson GE. Insulin signaling is involved in the regulation of worker division of labor in honey bee colonies. Proceedings of the National Academy of Sciences, U.S.A. 2008;105:4226–4231. doi: 10.1073/pnas.0800630105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atchley DP, Weaver KL, Eckel LA. Taste responses to dilute sucrose solutions are modulated by stage of the estrous cycle and fenfluramine treatment in female rats. Physiology & Behavior. 2005;86:265–271. doi: 10.1016/j.physbeh.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Barchuk AR, Bitondi MMG, Simões ZLP. Effects of juvenile hormone and ecdysone on the timing of vitellogenin appearance in hemolymph of queen and worker pupae of Apis mellifera. Journal of Insect Science. 2002;2.1:1–8. doi: 10.1673/031.002.0101. Available online: insectscience.org/2.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barchuk AR, Maleszka R, Simões ZLP. Apis mellifera ultraspiracle: cDNA sequence and rapid up-regulation by juvenile hormone. Insect Molecular Biology. 2004;13:459–467. doi: 10.1111/j.0962-1075.2004.00506.x. [DOI] [PubMed] [Google Scholar]
- Barron AB, Oldroyd BP, Ratnieks FLW. Worker reproduction in honey-bees (Apis) and the anarchic syndrome: a review. Behavioral Ecology and Sociobiology. 2001;50:199–208. [Google Scholar]
- Brandt BW, Zwaan BJ, Beekman M, Westendorp RGJ, Slagboom PE. Shuttling between species for pathways of lifespan regulation: a central role for the vitellogenin gene family? Bioessays. 2005;27:339–346. doi: 10.1002/bies.20161. [DOI] [PubMed] [Google Scholar]
- Calderone NW, Page RE. Effects of interactions among genotypically diverse nestmates on task specialization by foraging honey bees (Apis mellifera) Behavioral Ecology and Sociobiology. 1992;30:219–226. [Google Scholar]
- Cayre M, Strambi C, Charpin P, Augier R, Renucci M, Strambi A. Inhibition of polyamine biosynthesis alters oviposition behavior in female crickets. Behavioral Neuroscience. 1996;110:1117–1125. [PubMed] [Google Scholar]
- Chakraborty TR, Gore AC. Aging-related changes in ovarian hormones, their receptors, and neuroendocrine function. Experimental Biology and Medicine. 2004;229:977–987. doi: 10.1177/153537020422901001. [DOI] [PubMed] [Google Scholar]
- Clarke SN, Ossenkopp KP. Taste reactivity responses in rats: influence of sex and the estrous cycle. American Journal of Physiology. 1998;274:R718–R724. doi: 10.1152/ajpregu.1998.274.3.R718. [DOI] [PubMed] [Google Scholar]
- Clements AN. The Biology of Mosquitoes: Volume 1: Development, Nutrition and Reproduction. New York: Chapman & Hall; 1992. [Google Scholar]
- Corona M, Velarde RA, Remolina S, Moran-Lauter A, Wang Y, Hughes KA, Robinson GE. Vitellogenin, juvenile hormone, insulin signaling, and queen honey bee longevity. Proceedings of the National Academy of Sciences, U.S.A. 2007;104:7128–7133. doi: 10.1073/pnas.0701909104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingle H, Winchell R. Juvenile hormone as a mediator of plasticity in insect life histories. Archives of Insect Biochemistry and Physiology. 1997;35:359–373. [Google Scholar]
- Dolezal A, Brent C, Gadau J, Hölldobler B, Amdam GV. Endocrine physiology of division of labor in Pogonomyrmex californicus founding queens. Animal Behaviour. 2009;77:1005–1010. [Google Scholar]
- Duportets L, Dufour MC, Couillaud F, Gadenne C. Biosynthetic activity of corpora allata, growth of sex accessory glands and mating in the male moth Agrotis ipsilon (Hufnagel) Journal of Experimental Biology. 1998;201:2425–2432. doi: 10.1242/jeb.201.16.2425. [DOI] [PubMed] [Google Scholar]
- Engels W. Occurrence and significance of vitellogenins in female castes of social hymenoptera. American Zoologist. 1974;14:1229–1237. [Google Scholar]
- Engels W. Social Insects: an Evolutionary Approach to Castes and Reproduction. Berlin: Springer-Verlag; 1990. [DOI] [PubMed] [Google Scholar]
- Engels W, Kaatz H, Zillikens A, Simões ZLP, Truve A, Braun RP, Dittrich F. Honey bee reproduction: vitellogenin and caste-specific regulation of fertility. In: Hoshi M, Yamashita O, editors. Advances in Invertebrate Reproduction. Amsterdam: Elsevier Science; 1990. pp. 495–502. [Google Scholar]
- Fewell JH, Page RE. Colony-level selection effects on individual and colony foraging task performance in honeybees, Apis mellifera L. Behavioral Ecology and Sociobiology. 2000;48:173–181. [Google Scholar]
- Flatt T, Tu MP, Tatar M. Hormonal pleiotropy and the juvenile hormone regulation of Drosophila development and life history. BioEssays. 2005;27:999–1010. doi: 10.1002/bies.20290. [DOI] [PubMed] [Google Scholar]
- Guidugli KR, Nascimento AM, Amdam GV, Barchuk AR, Omholt SW, Simões ZLP, Hartfelder K. Vitellogenin regulates hormonal dynamics in the worker caste of a eusocial insect. FEBS Letters. 2005;579:4961–4965. doi: 10.1016/j.febslet.2005.07.085. [DOI] [PubMed] [Google Scholar]
- Hancock RG, Foster WA. Exogenous Juvenile hormone and methoprene, but not male accessory gland substances or ovariectomy, affect the blood/nectar choice of female Culex nigiripalpus mosquitoes. Medical and Veterinary Entomology. 2000;14:373–382. doi: 10.1046/j.1365-2915.2000.00253.x. [DOI] [PubMed] [Google Scholar]
- Hölldobler B, Wilson EO. The Ants. Cambridge, Massachusetts: Belknap Press of Harvard University Press; 1990. [Google Scholar]
- Hölldobler B, Wilson EO. The Superorganism: the Beauty, Elegance, and Strangeness of Insect Societies. New York: W. W. Norton; 2008. [Google Scholar]
- Honey Bee Genome Sequencing Consortium. Insights into social insects from the genome of the honeybee Apis mellifera. Nature. 2006;443:931–949. doi: 10.1038/nature05260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover SER, Keeling CI, Winston ML, Slessor KN. The effect of queen pheromones on worker honey bee ovary development. Naturwissenschaften. 2003;90:477–480. doi: 10.1007/s00114-003-0462-z. [DOI] [PubMed] [Google Scholar]
- Huang Z-Y, Robinson GE. Seasonal changes in juvenile hormone titers and rates of biosynthesis in honey bees. Journal of Comparative Physiology B. 1995;165:18–28. doi: 10.1007/BF00264682. [DOI] [PubMed] [Google Scholar]
- Hunt GJ, Page RE, Fondrk MK, Dullum CJ. Major quantitative trait loci affecting honey-bee foraging behavior. Genetics. 1995;141:1537–1545. doi: 10.1093/genetics/141.4.1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt GJ, Amdam GV, Schlipalius D, Emore C, Sardesai N, Williams CE, Rueppell O, Guzmán-Novoa E, Arechavaleta-Velasco M, Chandra S, Fondrk MK, Beye M, Page RE., Jr Behavioral genomics of honeybee foraging and nest defense. Naturwissenschaften. 2007;94:247–267. doi: 10.1007/s00114-006-0183-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihle KE, Page RE, Jr, Frederick K, Fondrk MK, Amdam GV. Genotype effect on regulation of behaviour by vitellogenin supports reproductive origin of honeybee foraging bias. Animal Behaviour. 2010;79:xx-xx. doi: 10.1016/j.anbehav.2010.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khila A, Abouheif E. Reproductive constraint is a developmental mechanism that maintains social harmony in advanced ant societies. Proceedings of the National Academy of Sciences, U.S.A. 2008;105:17884–17889. doi: 10.1073/pnas.0807351105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klowden MJ. The endogenous regulation of mosquito reproductive behavior. Experientia. 1990;46:660–670. doi: 10.1007/BF01939928. [DOI] [PubMed] [Google Scholar]
- Lam GT, Jiang C, Thummel CS. Coordination of larval and prepupal gene expression by the DHR3 orphan receptor during Drosophila metamorphosis. Development. 1997;124:1757–1769. doi: 10.1242/dev.124.9.1757. [DOI] [PubMed] [Google Scholar]
- Linksvayer TA, Fondrk MK, Page RE., Jr Honeybee social regulatory networks are shaped by colony-level selection. American Naturalist. 2009;173:E99–E107. doi: 10.1086/596527. [DOI] [PubMed] [Google Scholar]
- Makert GR, Paxton RJ, Hartfelder K. Ovariole number: a predictor for differential reproductive success among worker subfamilies in queenless honey bee (Apis mellifera L.) colonies. Behavioral Ecology and Sociobiology. 2006;60:815–825. [Google Scholar]
- Marco Antonio DS, Guidugli-Lazzarini KR, Nascimento AM, Simões ZLP, Hartfelder K. RNAi-mediated silencing of vitellogenin gene function turns honeybee (Apis mellifera) workers into extremely precocious foragers. Naturwissenschaften. 2008;95:953–961. doi: 10.1007/s00114-008-0413-9. [DOI] [PubMed] [Google Scholar]
- Mitchell SD. Biological Complexity and Integrative Pluralism. Cambridge, Massachusetts: Cambridge University Press; 2003. [Google Scholar]
- Nelson CM, Ihle K, Amdam GV, Fondrk MK, Page RE. The gene vitellogenin has multiple coordinating effects on social organization. PLoS Biology. 2007;5:673–677. doi: 10.1371/journal.pbio.0050062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldroyd BP, Wossler T, Ratnieks FLW. Regulation of ovary activation in worker honey-bees (Apis mellifera): larval signal production and adult response thresholds differ between anarchistic and wild-type bees. Behavioral Ecology and Sociobiology. 2001;50:366–370. [Google Scholar]
- Oldroyd BP, Beekman M. Effects of selection for honey bee worker reproduction on foraging traits. PLoS Biology. 2008;6:e56. doi: 10.1371/journal.pbio.0060056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oster GF, Wilson EO. Caste and Ecology in the Social Insects. Princeton, New Jsersey: Princeton University Press; 1978. [PubMed] [Google Scholar]
- Page RE, Amdam GV. The making of a social insect: developmental architectures of social design. BioEssays. 2007;29:334–343. doi: 10.1002/bies.20549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page RE, Fondrk MK. The effects of colony-level selection on the social organization of honey bee (Apis mellifera L.) colonies: colony-level components of pollen hoarding. Behavioral Ecology and Sociobiology. 1995;36:135–144. [Google Scholar]
- Page RE, Mitchell SD. Self organization and adaptation in insect societies. In: Fine A, Forbes M, Wessels L, editors. PSA. East Lansing, Michigan: Philosophy of Science Association; 1991. pp. 289–298. [Google Scholar]
- Page RE, Robinson GE, Fondrk MK, Nasr ME. Effects of worker genotypic diversity on honey bee colony development and behavior (Apis mellifera L.) Behavioral Ecology and Sociobiology. 1995;36:387–396. [Google Scholar]
- Page RE, Erber J, Fondrk MK. The effect of genotype on response thresholds to sucrose and foraging behavior of honey bees (Apis mellifera L.) Journal of Comparative Physiology A. 1998;182:489–500. doi: 10.1007/s003590050196. [DOI] [PubMed] [Google Scholar]
- Page RE, Fondrk MK, Hunt GJ, Guzman-Novoa E, Humphries MA, Nguyen K, Greene AS. Genetic dissection of honeybee (Apis mellifera L.) foraging behavior. Journal of Heredity. 2000;91:474–479. doi: 10.1093/jhered/91.6.474. [DOI] [PubMed] [Google Scholar]
- Pankiw T, Page RE. Response thresholds to sucrose predict foraging division of labor in honeybees. Behavioral Ecology and Sociobiology. 2000;47:265–267. [Google Scholar]
- Pankiw T, Page RE. Genotype and colony environment affect honeybee (Apis mellifera L.) development and foraging behavior. Behavioral Ecology and Sociobiology. 2001;51:87–94. [Google Scholar]
- Pinto LZ, Bitondi MMG, Simões ZLP. Inhibition of vitellogenin synthesis in Apis mellifera workers by a juvenile hormone analogue, pyriproxyfen. Journal of Insect Physiology. 2000;46:153–160. doi: 10.1016/s0022-1910(99)00111-0. [DOI] [PubMed] [Google Scholar]
- Robinson GE. Regulation of division of labor in insect societies. Annual Review of Entomology. 1992;37:637–665. doi: 10.1146/annurev.en.37.010192.003225. [DOI] [PubMed] [Google Scholar]
- Rueppell O, Pankiw T, Nielson D, Fondrk MK, Beye M, Page RE. The genetic architecture of the behavioral ontogeny of honey bee workers. Genetics. 2004a;167:1767–1779. doi: 10.1534/genetics.103.021949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueppell O, Pankiw T, Page RE. Pleiotropy, epistasis and new QTL: the genetic architecture of honey bee foraging behavior. Journal of Heredity. 2004b;95:481–491. doi: 10.1093/jhered/esh072. [DOI] [PubMed] [Google Scholar]
- Rueppell O, Hunggims E, Tingek S. Association between larger ovaries and pollen foraging in queenless Apis cerana workers supports the reproductive ground-plan hypothesis of social evolution. Journal Insect Behaviour. 2008;21:317–321. [Google Scholar]
- Rutz W, Lüscher M. The occurrence of vitellogenin in workers and queens of Apis mellifica and the possibility of its transmission to the queen. Journal of Insect Physiology. 1974;20:897–909. doi: 10.1016/0022-1910(74)90179-6. [DOI] [PubMed] [Google Scholar]
- Scheiner R, Page RE, Erber J. The effects of genotype, foraging role, and sucrose responsiveness on the tactile learning performance of honey bees (Apis mellifera L.) Neurobiology of Learning and Memory. 2001;76:138–150. doi: 10.1006/nlme.2000.3996. [DOI] [PubMed] [Google Scholar]
- Schmidt-Capella IC, Hartfelder K. Juvenile hormone effect on DNA synthesis and apoptosis in caste-specific differentiation of the larval honey bee (Apis mellifera L.) ovary. Journal of Insect Physiology. 1998;44:385–391. doi: 10.1016/s0022-1910(98)00027-4. [DOI] [PubMed] [Google Scholar]
- Schubiger M, Truman JW. The RXR ortholog USP suppresses early metamorphic processes in Drosophila in the absence of ecdysteroids. Development. 2000;127:1151–1159. doi: 10.1242/dev.127.6.1151. [DOI] [PubMed] [Google Scholar]
- Seehuus SC, Norberg K, Gimsa U, Krekling T, Amdam GV. Reproductive protein protects sterile honey bee workers from oxidative stress. Proceedings of the National Academy of Sciences, U.S.A. 2006;103:962–967. doi: 10.1073/pnas.0502681103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley TD. Adaptive significance of the age polyethism schedule in honeybee colonies. Behavioral Ecology and Sociobiology. 1982;11:287–293. [Google Scholar]
- Simonet G, Poels J, Claeys I, Van Loy T, Franssens V, De Loof A, Broeck JV. Neuroendocrinological and molecular aspects of insect reproduction. Journal of Neuroendocrinology. 2004;16:649–659. doi: 10.1111/j.1365-2826.2004.01222.x. [DOI] [PubMed] [Google Scholar]
- Snodgrass RE. Anatomy of the Honey Bee. New York: Comstock; 1956. [Google Scholar]
- Tatar M. The neuroendocrine regulation of Drosophila aging. Experimental Gerontology. 2004;39:1745–1750. doi: 10.1016/j.exger.2004.06.024. [DOI] [PubMed] [Google Scholar]
- Toth AL, Robinson GE. Evo-devo and the evolution of social behavior. Trends in Genetics. 2007;23:334–341. doi: 10.1016/j.tig.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Tsuruda J, Amdam GV, Page RE. Sensory response system of social behavior tied to female reproductive traits. PLoS One. 2008;3:e3397. doi: 10.1371/journal.pone.0003397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu MP, Tatar M. Juvenile diet restriction and the aging and reproduction of adult Drosophila melanogaster. Aging Cell. 2003;2:327–333. doi: 10.1046/j.1474-9728.2003.00064.x. [DOI] [PubMed] [Google Scholar]
- Vancassel M, Foraste M, Strambi A, Strambi C. Normal and experimentally induced changes in hormonal hemolymph titers during parental behavior of the earwig Labidura riparia. General and Comparative Endocrinology. 1984;56:444–456. doi: 10.1016/0016-6480(84)90087-x. [DOI] [PubMed] [Google Scholar]
- Velarde RA, Robinson GE, Fahrbach SE. Coordinated responses to developmental hormones in the Kenyon cells of the adult worker honey bee brain (Apis mellifera L.) Journal of Insect Physiology. 2009;55:59–69. doi: 10.1016/j.jinsphys.2008.10.006. [DOI] [PubMed] [Google Scholar]
- Wang Y, Amdam GV, Rueppell O, Wallrichs MA, Fondrk MK, Kaftanoglu O, Page RE., Jr PDK1 and HR46 gene homologs tie social behavior to ovary signals. PLoS One. 2009;4:e4899. doi: 10.1371/journal.pone.0004899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West-Eberhard MJ. Flexible strategy and social evolution. In: Itô Y, Brown JL, Kikkawa J, editors. Animal Societies: Theories and Fact. Tokyo: Japan Science Society Press; 1987. pp. 35–51. [Google Scholar]
- West-Eberhard MJ. Wasp societies as microcosms for the study of development and evolution. In: Turillazzi S, West-Eberhard MJ, editors. Natural History and Evolution of Paper Wasp. New York: Oxford University Press; 1996. pp. 290–317. [Google Scholar]
- Wheeler WM. The ant-colony as an organism. Morphology. 1911;22:307–325. [Google Scholar]
- Wilson DS, Sober E. Reviving the superorganism. Journal of Theoretical Biology. 1989;136:337–356. doi: 10.1016/s0022-5193(89)80169-9. [DOI] [PubMed] [Google Scholar]
- Wilson EO. The Insect Societies. Cambridge, Massachusetts: Belknap Press of Harvard University Press; 1971. [Google Scholar]
- Zufelato MS, Bitondi MMG, Simões ZLP, Hartfelder K. The juvenile hormone analog pyriproxyfen affects ecdysteroid-dependent cuticle melanization and shifts the pupal ecdysteroid peak in the honey bee (Apis mellifera) Arthropod Structure & Development. 2000;29:111–119. doi: 10.1016/s1467-8039(00)00023-2. [DOI] [PubMed] [Google Scholar]